RETRACTED: Hybrid Application of Nanoparticles and Polymer in Enhanced Oil Recovery Processes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Fluids
2.1.2. Sandpacks
2.2. Methods
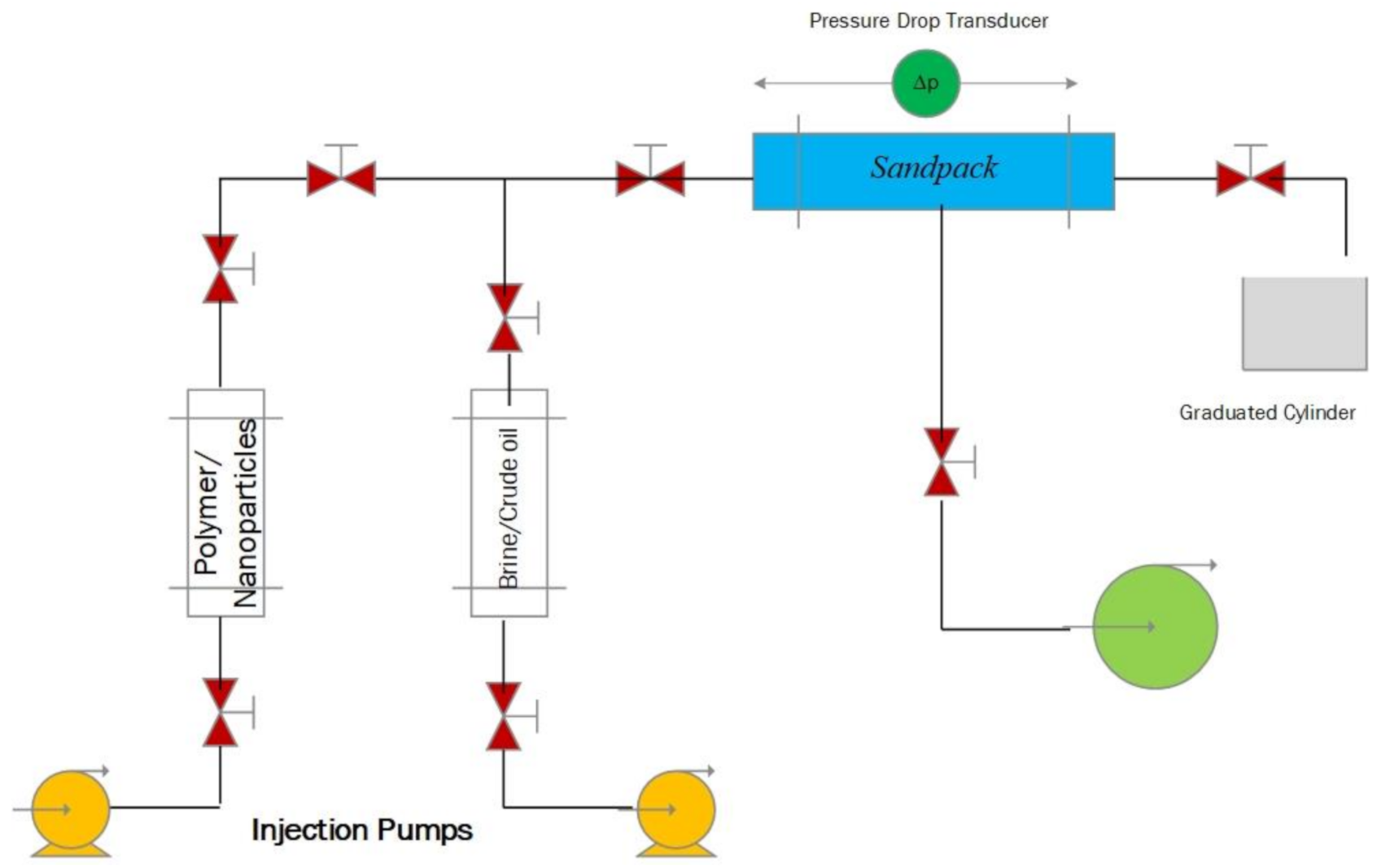
2.2.1. Experimental Apparatus
2.2.2. Interfacial Tension
2.2.3. Residual Resistance Factor
2.2.4. Relative Permeability Curves
2.2.5. Contact Angle
2.2.6. Apparent Viscosity Measurement
3. Results and Discussion
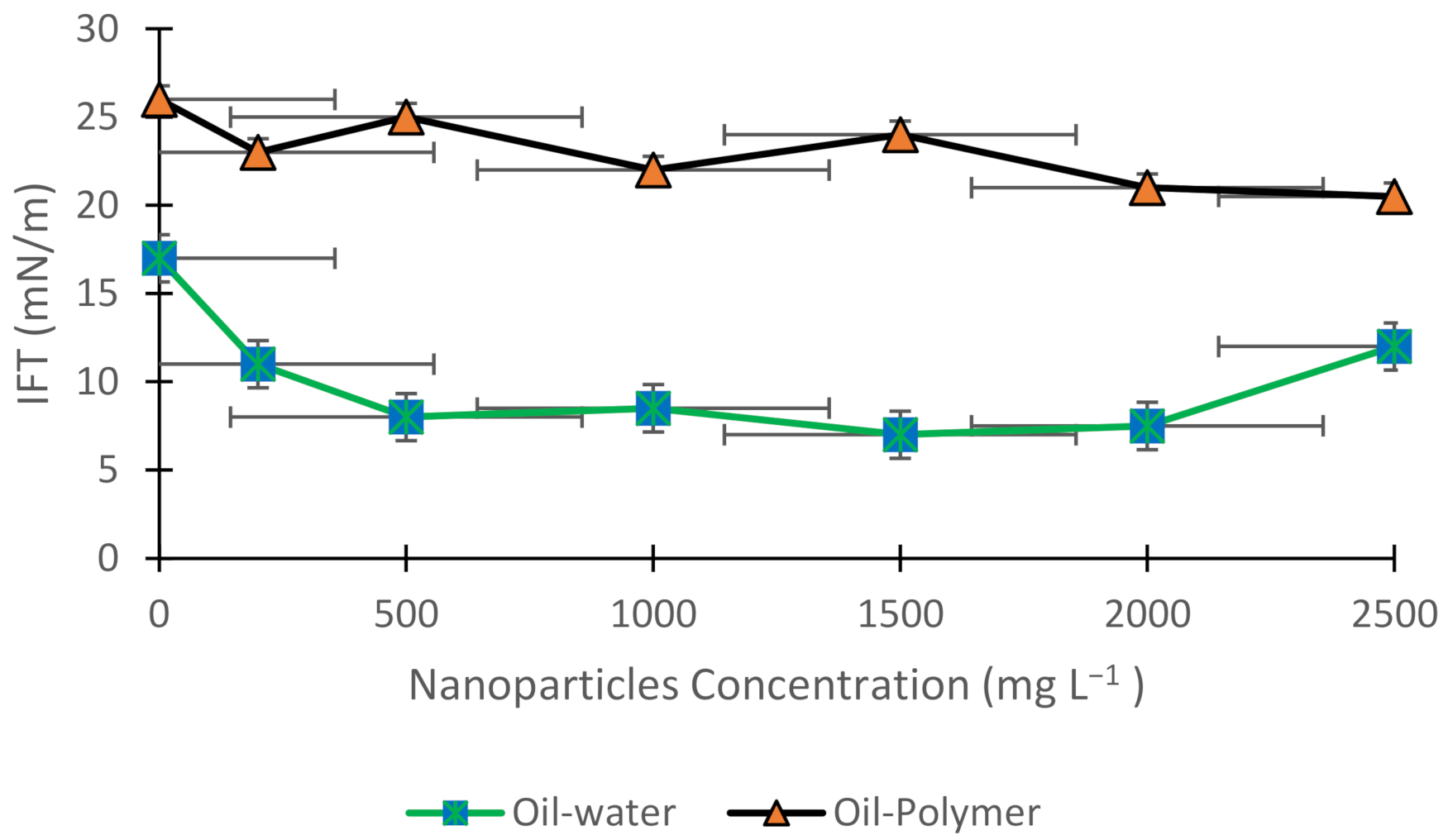
3.1. Interfacial Tension
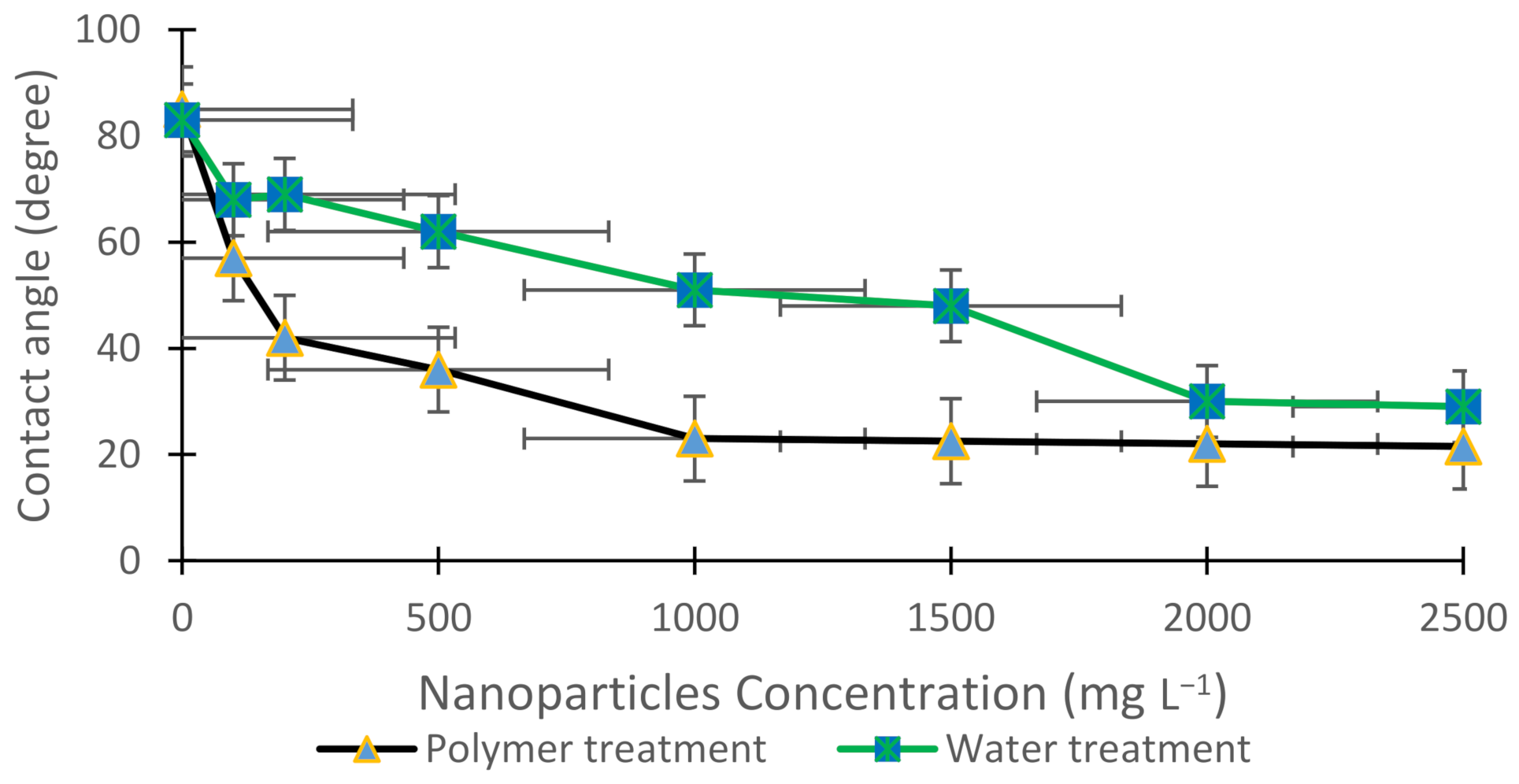
3.2. Contact Angle
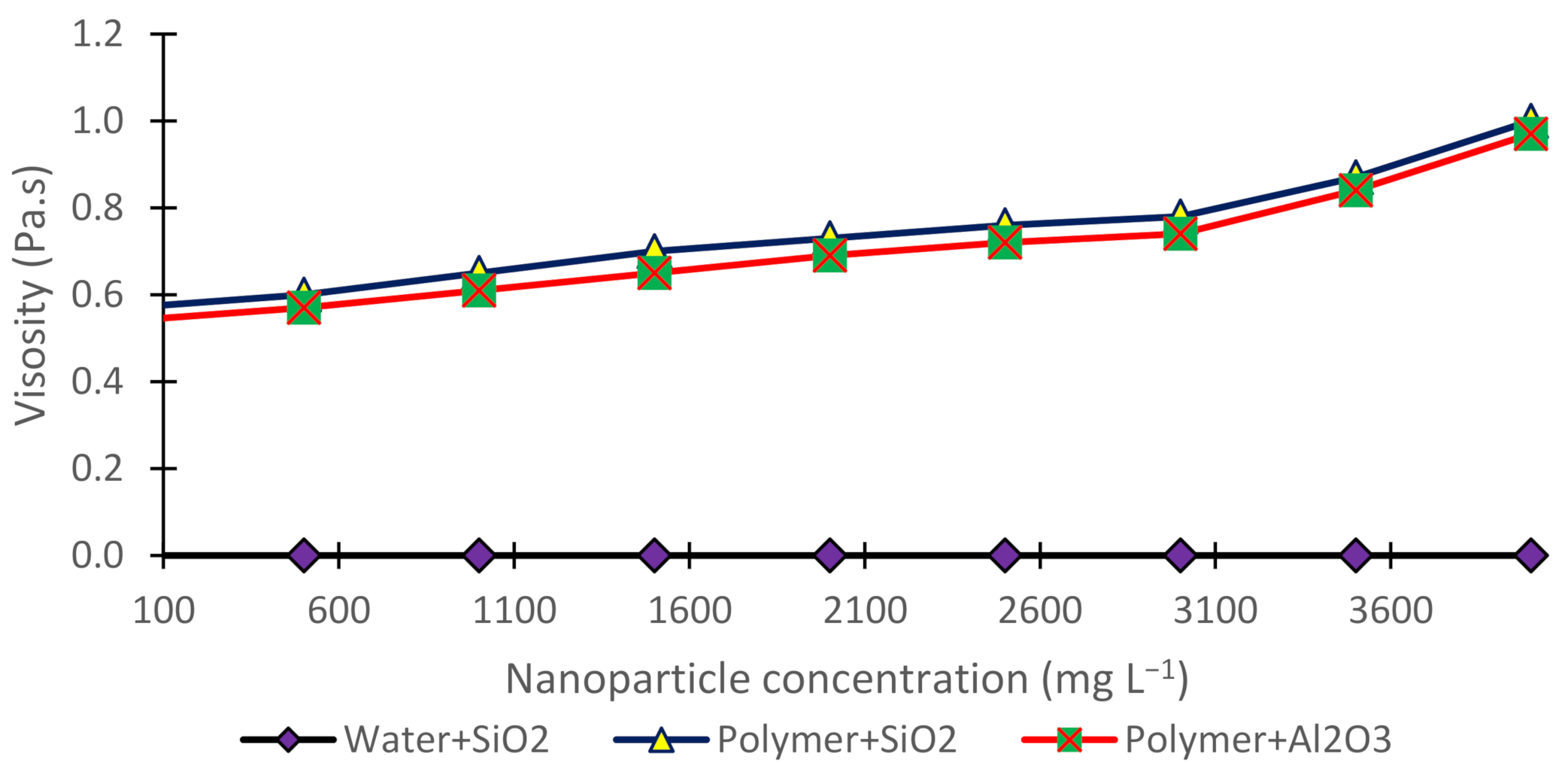
3.3. Viscosity
3.4. Relative Permeability Curves
3.5. Residual Resistance Factor
3.6. Pressure Drop
3.7. Oil Recovery Factor
3.8. Summary
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, M.R.; Deng, L.; Liu, G.C.; Wen, L.; Wang, J.G.; Huang, K.B.; Tang, H.T.; Pan, Y.M. Porous organic polymer-derived nanopalladium catalysts for chemoselective synthesis of antitumor benzofuro[2,3-b]pyrazine from 2-Bromophenol and Isonitriles. Org. Lett. 2019. [Google Scholar] [CrossRef] [PubMed]
- Zuo, C.; Chen, Q.; Tian, L.; Waller, L.; Asundi, A. Transport of intensity phase retrieval and computational imaging for partially coherent fields: The phase space perspective. Opt. Lasers Eng. 2015. [Google Scholar] [CrossRef]
- Zhang, H.; Guan, W.; Zhang, L.; Guan, X.; Wang, S. Degradation of an Organic Dye by Bisulfite Catalytically Activated with Iron Manganese Oxides: The Role of Superoxide Radicals. ACS Omega 2020. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Sun, M.; Song, L.; Guo, J.; Zhang, L. Fate of NaClO and membrane foulants during in-situ cleaning of membrane bioreactors: Combined effect on thermodynamic properties of sludge. Biochem. Eng. J. 2019. [Google Scholar] [CrossRef]
- Sun, M.; Yan, L.; Zhang, L.; Song, L.; Guo, J.; Zhang, H. New insights into the rapid formation of initial membrane fouling after in-situ cleaning in a membrane bioreactor. Process Biochem. 2019. [Google Scholar] [CrossRef]
- Zhang, K.; Huo, Q.; Zhou, Y.Y.; Wang, H.H.; Li, G.P.; Wang, Y.W.; Wang, Y.Y. Textiles/Metal-Organic Frameworks Composites as Flexible Air Filters for Efficient Particulate Matter Removal. ACS Appl. Mater. Interfaces 2019. [Google Scholar] [CrossRef]
- Duan, Z.; Li, C.; Zhang, Y.; Dong, L.; Bai, X.; Yang, M.; Jia, D.; Li, R.; Cao, H.; Xu, X. Milling surface roughness for 7050 aluminum alloy cavity influenced by nozzle position of nanofluid minimum quantity lubrication. Chin. J. Aeronaut. 2020. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, W.; Li, C.; Yang, M.; Zhang, Y.; Jia, D.; Hou, Y.; Li, R.; Cao, H.; Ali, H.M. Convective Heat Transfer Coefficient Model Under Nanofluid Minimum Quantity Lubrication Coupled with Cryogenic Air Grinding Ti–6Al–4V. Int. J. Precis. Eng. Manuf. Green Technol. 2020. [Google Scholar] [CrossRef]
- Gao, T.; Li, C.; Jia, D.; Zhang, Y.; Yang, M.; Wang, X.; Cao, H.; Li, R.; Ali, H.M.; Xu, X. Surface morphology assessment of CFRP transverse grinding using CNT nanofluid minimum quantity lubrication. J. Clean. Prod. 2020. [Google Scholar] [CrossRef]
- Sui, M.; Li, C.; Wu, W.; Yang, M.; Ali, H.M.; Zhang, Y.; Jia, D.; Hou, Y.; Li, R.; Cao, H. Temperature of Grinding Carbide with Castor Oil-Based MoS2 Nanofluid Minimum Quantity Lubrication. J. Therm. Sci. Eng. Appl. 2021. [Google Scholar] [CrossRef]
- Mao, Q.F.; Shang-Guan, Z.F.; Chen, H.L.; Huang, K. Immunoregulatory role of IL-2/STAT5/CD4+ CD25+ Foxp3 Treg pathway in the pathogenesis of chronic osteomyelitis. Ann. Transl. Med. 2019, 7. [Google Scholar] [CrossRef]
- Huang, K.; Ge, S. The anti-CXCL4 antibody depletes CD4 (+) CD25 (+) FOXP3 (+) regulatory T cells in CD4+ T cells from chronic osteomyelitis patients by the STAT5 pathway. Ann. Palliat. Med. 2020, 9, 2723–2730. [Google Scholar] [CrossRef]
- Zheng, Y.; Yu, Y.; Lin, W.; Jin, Y.; Yong, Q.; Huang, C. Enhancing the enzymatic digestibility of bamboo residues by biphasic phenoxyethanol-acid pretreatment. Bioresour. Technol. 2021, 325, 124691. [Google Scholar] [CrossRef]
- Peng, X.; He, H.; Liu, Q.; She, K.; Zhang, B.; Wang, H.; Pan, Y. Photocatalyst-Controlled and Visible Light-Enabled Selective Oxidation of Pyridinium Salts. Sci. China Chem. 2021. [Google Scholar] [CrossRef]
- Huang, C.; Zheng, Y.; Lin, W.; Shi, Y.; Huang, G.; Yong, Q. Removal of fermentation inhibitors from pre-hydrolysis liquor using polystyrene divinylbenzene resin. Biotechnol. Biofuels 2020, 13, 1–14. [Google Scholar] [CrossRef]
- Lin, W.; Xing, S.; Jin, Y.; Lu, X.; Huang, C.; Yong, Q. Insight into understanding the performance of deep eutectic solvent pretreatment on improving enzymatic digestibility of bamboo residues. Bioresour. Technol. 2020, 306, 123163. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, C.; Jia, D.; Zhang, D.; Zhang, X. Experimental evaluation of the lubrication performance of MoS2/CNT nanofluid for minimal quantity lubrication in Ni-based alloy grinding. Int. J. Mach. Tools Manuf. 2015. [Google Scholar] [CrossRef]
- Zuo, C.; Sun, J.; Li, J.; Zhang, J.; Asundi, A.; Chen, Q. High-resolution transport-of-intensity quantitative phase microscopy with annular illumination. Sci. Rep. 2017. [Google Scholar] [CrossRef]
- Huang, W.Y.; Wang, G.Q.; Li, W.H.; Li, T.T.; Ji, G.-j.; Ren, S.-C.; Jiang, M.; Yan, L.; Tang, H.-T.; Pan, Y.-M.; et al. Porous Ligand Creates New Reaction Route: Bifunctional Single-Atom Palladium Catalyst for Selective Distannylation of Terminal Alkynes. Chem 2020. [Google Scholar] [CrossRef]
- Qiao, Y.-X.; Sheng, S.-L.; Zhang, L.-M.; Chen, J.; Yang, L.-L.; Zhou, H.-L.; Wang, Y.-X.; Li, H.-B.; Zheng, Z.-B. Friction and wear behaviors of a high nitrogen austenitic stainless steel Fe-19Cr-15Mn-0.66N. J. Min. Metall. Sect. B Metall. 2021. [Google Scholar] [CrossRef]
- Wang, P.; Li, Z.; Xie, Q.; Duan, W.; Zhang, X.; Han, H. A Passive Anti-icing Strategy Based on a Superhydrophobic Mesh with Extremely Low Ice Adhesion Strength. J. Bionic. Eng. 2021. [Google Scholar] [CrossRef]
- Kazemi, A.; Yang, S. Effects of magnesium dopants on grain boundary migration in aluminum-magnesium alloys. Comput. Mater. Sci. 2021. [Google Scholar] [CrossRef]
- Kazemi, A.; Yang, S. Atomistic Study of the Effect of Magnesium Dopants on the Strength of Nanocrystalline Aluminum. JOM 2019. [Google Scholar] [CrossRef]
- Zheng, L.; Yu, P.; Zhang, Y.; Wang, P.; Yan, W.; Guo, B.; Huang, C.; Jiang, Q. Evaluating the bio-application of biomacromolecule of lignin-carbohydrate complexes (LCC) from wheat straw in bone metabolism via ROS scavenging. Int. J. Biol. Macromol. 2021. [Google Scholar] [CrossRef]
- Davarpanah, A.; Mirshekari, B.; Jafari Behbahani, T.; Hemmati, M. Integrated production logging tools approach for convenient experimental individual layer permeability measurements in a multi-layered fractured reservoir. J. Pet. Explor. Prod. Technol. 2018. [Google Scholar] [CrossRef]
- Hu, X.; Li, M.; Peng, C.; Davarpanah, A. Hybrid Thermal-Chemical Enhanced Oil Recovery Methods; An Experimental Study for Tight Reservoirs. Symmetry 2020, 12, 947. [Google Scholar] [CrossRef]
- Davarpanah, A. Parametric study of polymer-nanoparticles-assisted injectivity performance for axisymmetric two-phase flow in EOR processes. Nanomaterials 2020, 10, 1818. [Google Scholar] [CrossRef]
- Hu, X.; Xie, J.; Cai, W.; Wang, R.; Davarpanah, A. Thermodynamic effects of cycling carbon dioxide injectivity in shale reservoirs. J. Pet. Sci. Eng. 2020. [Google Scholar] [CrossRef]
- Davarpanah, A. A feasible visual investigation for associative foam >\ polymer injectivity performances in the oil recovery enhancement. Eur. Polym. J. 2018. [Google Scholar] [CrossRef]
- Yang, Y.; Yao, J.; Wang, C.; Gao, Y.; Zhang, Q.; An, S.; Song, W. New pore space characterization method of shale matrix formation by considering organic and inorganic pores. J. Nat. Gas. Sci. Eng. 2015. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, J.; Ma, X.; Yao, C.; Zhang, L.; Yang, Y.; Wang, J.; Yao, J.; Zhao, H. History Matching of Naturally Fractured Reservoirs Using a Deep Sparse Autoencoder. SPE J. 2021. [Google Scholar] [CrossRef]
- Nesic, S.; Zolotukhin, A.; Mitrovic, V.; Govedarica, D.; Davarpanah, A. An Analytical Model to Predict the Effects of Suspended Solids in Injected Water on the Oil Displacement Efficiency during Waterflooding. Processes 2020, 8, 659. [Google Scholar] [CrossRef]
- Davarpanah, A.; Mirshekari, B. Experimental Investigation and Mathematical Modeling of Gas Diffusivity by Carbon Dioxide and Methane Kinetic Adsorption. Ind. Eng. Chem. Res. 2019. [Google Scholar] [CrossRef]
- Mazarei, M.; Davarpanah, A.; Ebadati, A.; Mirshekari, B. The feasibility analysis of underground gas storage during an integration of improved condensate recovery processes. J. Pet. Explor. Prod. Technol. 2019. [Google Scholar] [CrossRef]
- Pan, F.; Zhang, Z.; Zhang, X.; Davarpanah, A. Impact of anionic and cationic surfactants interfacial tension on the oil recovery enhancement. Powder Technol. 2020. [Google Scholar] [CrossRef]
- Jia, K.; Feng, Q.; Davarpanah, A. Effect of anionic and non-anionic surfactants on the adsorption density. Pet. Sci. Technol. 2021. [Google Scholar] [CrossRef]
- Esfandyari, H.; Moghani, A.; Esmaeilzadeh, F.; Davarpanah, A. A Laboratory Approach to Measure Carbonate Rocks’ Adsorption Density by Surfactant and Polymer. Math. Probl. Eng. 2021. [Google Scholar] [CrossRef]
- Sepahvand, T.; Etemad, V.; Matinizade, M.; Shirvany, A. Symbiosis of AMF with growth modulation and antioxidant capacity of Caucasian Hackberry (Celtis Caucasica L.) seedlings under drought stress. Cent. Asian J. Environ. Sci. Technol. Innov. 2021, 2. [Google Scholar] [CrossRef]
- Jalali Sarvestani, M.; Charehjou, P. Fullerene (C20) as a potential adsorbent and sensor for the removal and detection of picric acid contaminant: DFT Studies. Cent. Asian J. Environ. Sci. Technol. Innov. 2021, 2. [Google Scholar] [CrossRef]
- Awan, B.; Sabeen, M.; Shaheen, S.; Mahmood, Q.; Ebadi, A.; Toughani, M. Phytoextraction of zinc contaminated water by Tagetes minuta L. Cent. Asian J. Environ. Sci. Technol. Innov. 2020, 1, 150–158. [Google Scholar] [CrossRef]
- Bafkar, A. Kinetic and equilibrium studies of adsorptive removal of sodium-ion onto wheat straw and rice husk wastes. Cent. Asian J. Environ. Sci. Technol. Innov. 2020, 1. [Google Scholar] [CrossRef]
- Maina, Y.; Kyari, B.; Jimme, M. Impact of household fuel expenditure on the environment: The quest for sustainable energy in Nigeria. Cent. Asian J. Environ. Sci. Technol. Innov. 2020, 1, 109–118. [Google Scholar] [CrossRef]
- Nwankwo, C.; EGobo, A.; Israel-Cookey, C.; AAbere, S. Effects of hazardous waste discharge from the activities of oil and gas companies in Nigeria. Cent. Asian J. Environ. Sci. Technol. Innov. 2020, 1, 119–129. [Google Scholar] [CrossRef]
- Qayyum, S.; Khan, I.; Meng, K.; Zhao, Y.; Peng, C. A review on remediation technologies for heavy metals contaminated soil. Cent. Asian J. Environ. Sci. Technol. Innov. 2020, 1, 21–29. [Google Scholar] [CrossRef]
- Ebadi, A.; Toughani, M.; Najafi, A.; Babaee, M. A brief overview on current environmental issues in Iran. Cent. Asian J. Environ. Sci. Technol. Innov. 2020, 1, 1–11. [Google Scholar] [CrossRef]
- Nnaemeka, A. Environmental pollution and associated health hazards to host communities (Case study: Niger delta region of Nigeria). Cent. Asian J. Environ. Sci. Technol. Innov. 2020, 1, 30–42. [Google Scholar] [CrossRef]
- Firozjaii, A.M.; Saghafi, H.R. Review on chemical enhanced oil recovery using polymer flooding: Fundamentals, experimental and numerical simulation. Petroleum 2020. [Google Scholar] [CrossRef]
- Mandal, A. Chemical flood enhanced oil recovery: A review. Int. J. Oil Gas Coal Technol. 2015. [Google Scholar] [CrossRef]
- Gurgel, A.; Moura, M.C.P.A.; Dantas, T.N.C.; Neto, E.B.; Neto, A.D. A Review on Chemical Flooding Methods Applied in Enhanced Oil Recovery. Braz. J. Pet. Gas 2008. [Google Scholar] [CrossRef]
- Davarpanah, A.; Mirshekari, B. Mathematical modeling of injectivity damage with oil droplets in the waste produced water re-injection of the linear flow. Eur. Phys. J. Plus 2019. [Google Scholar] [CrossRef]
- Davarpanah, A.; Shirmohammadi, R.; Mirshekari, B.; Aslani, A. Analysis of hydraulic fracturing techniques: Hybrid fuzzy approaches. Arabian J. Geosci. 2019. [Google Scholar] [CrossRef]
- Esfandyari, H.; Moghani Rahimi, A.; Esmaeilzadeh, F.; Davarpanah, A.; Mohammadi, A.H. Amphoteric and cationic surfactants for enhancing oil recovery from carbonate oil reservoirs. J. Mol. Liq. 2020. [Google Scholar] [CrossRef]
- Davarpanah, A.; Mirshekari, B. Numerical simulation and laboratory evaluation of alkali–surfactant–polymer and foam flooding. Int. J. Environ. Sci. Technol. 2019. [Google Scholar] [CrossRef]
- Davarpanah, A.; Mirshekari, B. Experimental study of CO2 solubility on the oil recovery enhancement of heavy oil reservoirs. J. Therm. Anal. Calorim. 2019. [Google Scholar] [CrossRef]
- Esfandyari, H.; Shadizadeh, S.R.; Esmaeilzadeh, F.; Davarpanah, A. Implications of anionic and natural surfactants to measure wettability alteration in EOR processes. Fuel 2020. [Google Scholar] [CrossRef]
- Davarpanah, A.; Mirshekari, B. A mathematical model to evaluate the polymer flooding performances. Energy Rep. 2019. [Google Scholar] [CrossRef]
- Esfandyari, H.; Hoseini, A.H.; Shadizadeh, S.R.; Davarpanah, A. Simultaneous evaluation of capillary pressure and wettability alteration based on the USBM and imbibition tests on carbonate minerals. J. Pet. Sci. Eng. 2020. [Google Scholar] [CrossRef]
- Hu, Y.; Cheng, Q.; Yang, J.; Zhang, L.; Davarpanah, A. A laboratory approach on the hybrid-enhanced oil recovery techniques with different saline brines in sandstone reservoirs. Processes 2020, 8, 1051. [Google Scholar] [CrossRef]
- Davarpanah, A.; Shirmohammadi, R.; Mirshekari, B. Experimental evaluation of polymer-enhanced foam transportation on the foam stabilization in the porous media. Int. J. Environ. Sci. Technol. 2019. [Google Scholar] [CrossRef]
- Davarpanah, A.; Akbari, E.; Doudman-Kushki, M.; Ketabi, H.; Hemmati, M. Simultaneous feasible injectivity of foam and hydrolyzed polyacrylamide to optimize the oil recovery enhancement. Energy Explor. Exploit. 2019. [Google Scholar] [CrossRef]
- Haiyan, Z.; Davarpanah, A. Hybrid Chemical Enhanced Oil Recovery Techniques: A Simulation Study. Symmetry 2020, 12, 1086. [Google Scholar] [CrossRef]
- Sheng, J.J. Modern Chemical Enhanced Oil Recovery. Mod. Chem. Enhanc. Oil Recover. 2011. [Google Scholar] [CrossRef]
- Hu, Z.; Haruna, M.; Gao, H.; Nourafkan, E.; Wen, D. Rheological Properties of Partially Hydrolyzed Polyacrylamide Seeded by Nanoparticles. Ind. Eng. Chem. Res. 2017. [Google Scholar] [CrossRef]
- Agista, M.N.; Guo, K.; Yu, Z. A state-of-the-art review of nanoparticles application in petroleum with a focus on enhanced oil recovery. Appl. Sci. 2018, 8, 871. [Google Scholar] [CrossRef]
- Shamsijazeyi, H.; Miller, C.A.; Wong, M.S.; Tour, J.M.; Verduzco, R. Polymer-coated nanoparticles for enhanced oil recovery. J. Appl. Polym. Sci. 2014. [Google Scholar] [CrossRef]
- Ali, J.A.; Kolo, K.; Manshad, A.K.; Mohammadi, A.H. Recent advances in application of nanotechnology in chemical enhanced oil recovery: Effects of nanoparticles on wettability alteration, interfacial tension reduction, and flooding. Egypt J. Pet. 2018. [Google Scholar] [CrossRef]
- Cheraghian, G.; Hendraningrat, L. A review on applications of nanotechnology in the enhanced oil recovery part B: Effects of nanoparticles on flooding. Int. Nano Lett. 2016. [Google Scholar] [CrossRef]
- Ali, J.A.; Kalhury, A.M.; Sabir, A.N.; Ahmed, R.N.; Ali, N.H.; Abdullah, A.D. A state-of-the-art review of the application of nanotechnology in the oil and gas industry with a focus on drilling engineering. J. Pet. Sci. Eng. 2020. [Google Scholar] [CrossRef]
- Ju, B.; Fan, T.; Ma, M. Enhanced oil recovery by flooding with hydrophilic nanoparticles. China Particuology 2006. [Google Scholar] [CrossRef]
- Ogolo, N.A.; Olafuyi, O.A.; Onyekonwu, M.O. Enhanced oil recovery using nanoparticles. Soc. Pet. Eng. SPE Saudi Arab. Sect. Tech. Symp. Exhib. 2012. [Google Scholar] [CrossRef]
- Piñerez Torrijos, I.D.; Puntervold, T.; Strand, S.; Austad, T.; Bleivik, T.H.; Abdullah, H.I. An experimental study of the low salinity Smart Water—Polymer hybrid EOR effect in sandstone material. J. Pet. Sci. Eng. 2018. [Google Scholar] [CrossRef]
- Omidi, A.; Manshad, A.K.; Moradi, S.; Ali, J.A.; Sajadi, S.M.; Keshavarz, A. Smart- and nano-hybrid chemical EOR flooding using Fe3O4/eggshell nanocomposites. J. Mol. Liq. 2020. [Google Scholar] [CrossRef]
- Shabib-Asl, A.; Abdalla Ayoub, M.; Abdalla Elraies, K. Combined low salinity water injection and foam flooding in sandstone reservoir rock: A new hybrid EOR. In Proceedings of the SPE Middle East Oil and Gas Show and Conference, Manama, Bahrain, 18–21 March 2019. [Google Scholar] [CrossRef]
- Rezvani, H.; Panahpoori, D.; Riazi, M.; Parsaei, R.; Tabaei, M.; Cortés, F.B. A novel foam formulation by Al2O3/SiO2 nanoparticles for EOR applications: A mechanistic study. J. Mol. Liq. 2020. [Google Scholar] [CrossRef]
- Maghzi, A.; Mohebbi, A.; Kharrat, R.; Ghazanfari, M.H. An experimental investigation of silica nanoparticles effect on the rheological behavior of polyacrylamide solution to enhance heavy oil recovery. Pet. Sci. Technol. 2013. [Google Scholar] [CrossRef]
- Gbadamosi, A.O.; Junin, R.; Manan, M.A.; Agi, A.; Oseh, J.O.; Usman, J. Effect of aluminium oxide nanoparticles on oilfield polyacrylamide: Rheology, interfacial tension, wettability and oil displacement studies. J. Mol. Liq. 2019. [Google Scholar] [CrossRef]
- Ahmed, A.; Saaid, I.M.; Ahmed, A.A.; Pilus, R.M.; Baig, M.K. Evaluating the potential of surface-modified silica nanoparticles using internal olefin sulfonate for enhanced oil recovery. Pet. Sci. 2020. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, Y.; Chen, G.; Gai, Z. Application of nanoparticles in enhanced oil recovery: A critical review of recent progress. Energies 2017, 10, 345. [Google Scholar] [CrossRef]
- Gbadamosi, A.O.; Junin, R.; Manan, M.A.; Yekeen, N.; Agi, A.; Oseh, J.O. Recent advances and prospects in polymeric nanofluids application for enhanced oil recovery. J. Ind. Eng. Chem. 2018. [Google Scholar] [CrossRef]
- Ali, H.; Soleimani, H.; Yahya, N.; Khodapanah, L.; Sabet, M.; Demiral, B.M.R.; Hussain, T.; Adebayo, L.L. Enhanced oil recovery by using electromagnetic-assisted nanofluids: A review. J. Mol. Liq. 2020. [Google Scholar] [CrossRef]
- Gbadamosi, A.O.; Junin, R.; Manan, M.A.; Agi, A.; Oseh, J.O.; Usman, J. Synergistic application of aluminium oxide nanoparticles and oilfield polyacrylamide for enhanced oil recovery. J. Pet. Sci. Eng. 2019. [Google Scholar] [CrossRef]
- Maurya, N.K.; Mandal, A. Studies on behavior of suspension of silica nanoparticle in aqueous polyacrylamide solution for application in enhanced oil recovery. Pet. Sci. Technol. 2016. [Google Scholar] [CrossRef]
- Cheraghian, G.; Khalili Nezhad, S.S.; Kamari, M.; Hemmati, M.; Masihi, M.; Bazgir, S. Adsorption polymer on reservoir rock and role of the nanoparticles, clay and SiO2. Int. Nano Lett. 2014. [Google Scholar] [CrossRef]
- Saha, R.; Uppaluri, R.V.S.; Tiwari, P. Impact of Natural Surfactant (Reetha), Polymer (Xanthan Gum), and Silica Nanoparticles to Enhance Heavy Crude Oil Recovery. Energy Fuels 2019. [Google Scholar] [CrossRef]
- Sharma, T.; Iglauer, S.; Sangwai, J.S. Silica Nanofluids in an Oilfield Polymer Polyacrylamide: Interfacial Properties, Wettability Alteration, and Applications for Chemical Enhanced Oil Recovery. Ind. Eng. Chem. Res. 2016. [Google Scholar] [CrossRef]
- Lee, J.; Huang, J.; Babadagli, T. Visual support for heavy-oil emulsification and its stability for cold-production using chemical and nano-particles. In Proceedings of the SPE Annual Technical Conference and Exhibition, Calgary, AB, Canada, 30 September–2 October 2019. [Google Scholar] [CrossRef]
- Cheraghian, G. Evaluation of clay and fumed silica nanoparticles on adsorption of surfactant polymer during enhanced oil recovery. J. Jpn. Pet. Inst. 2017. [Google Scholar] [CrossRef]
- Samba, M.A.; Hassan, H.A.; Munayr, M.S.; Yusef, M.; Eschweido, A.; Burkan, H.; Elsharafi, M.O. Nanoparticles EOR aluminum oxide (Al2O3) used as a spontaneous imbibition test for sandstone core. In Proceedings of the ASME International Mechanical Engineering Congress and Exposition, Salt Lake City, UT, USA, 11–14 November 2019. [Google Scholar] [CrossRef]
- Daryayehsalameh, B.; Nabavi, M.; Vaferi, B. Modeling of CO2 capture ability of [Bmim][BF4] ionic liquid using connectionist smart paradigms. Environ. Technol. Innov. 2021, 22, 101484. [Google Scholar] [CrossRef]
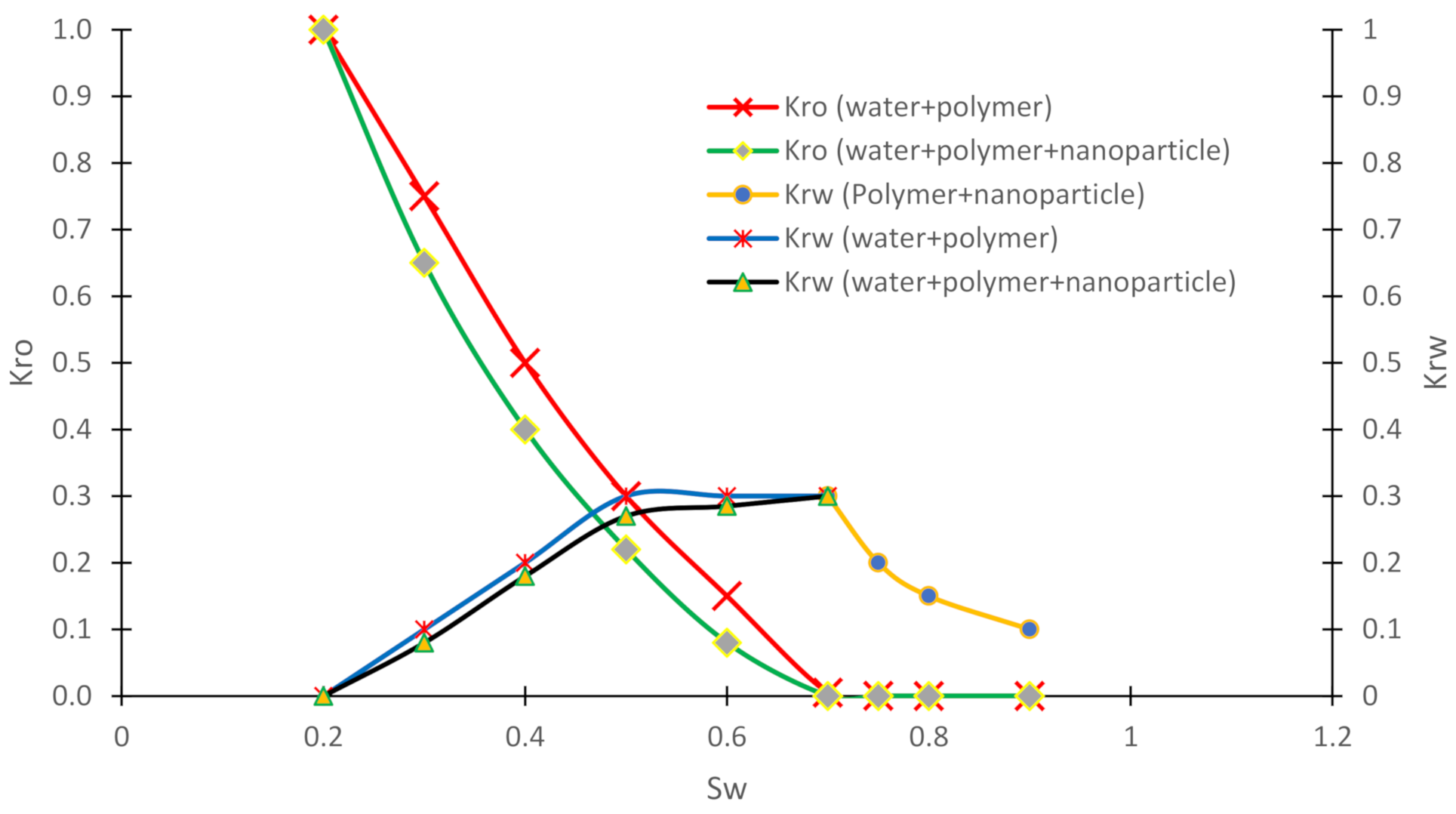
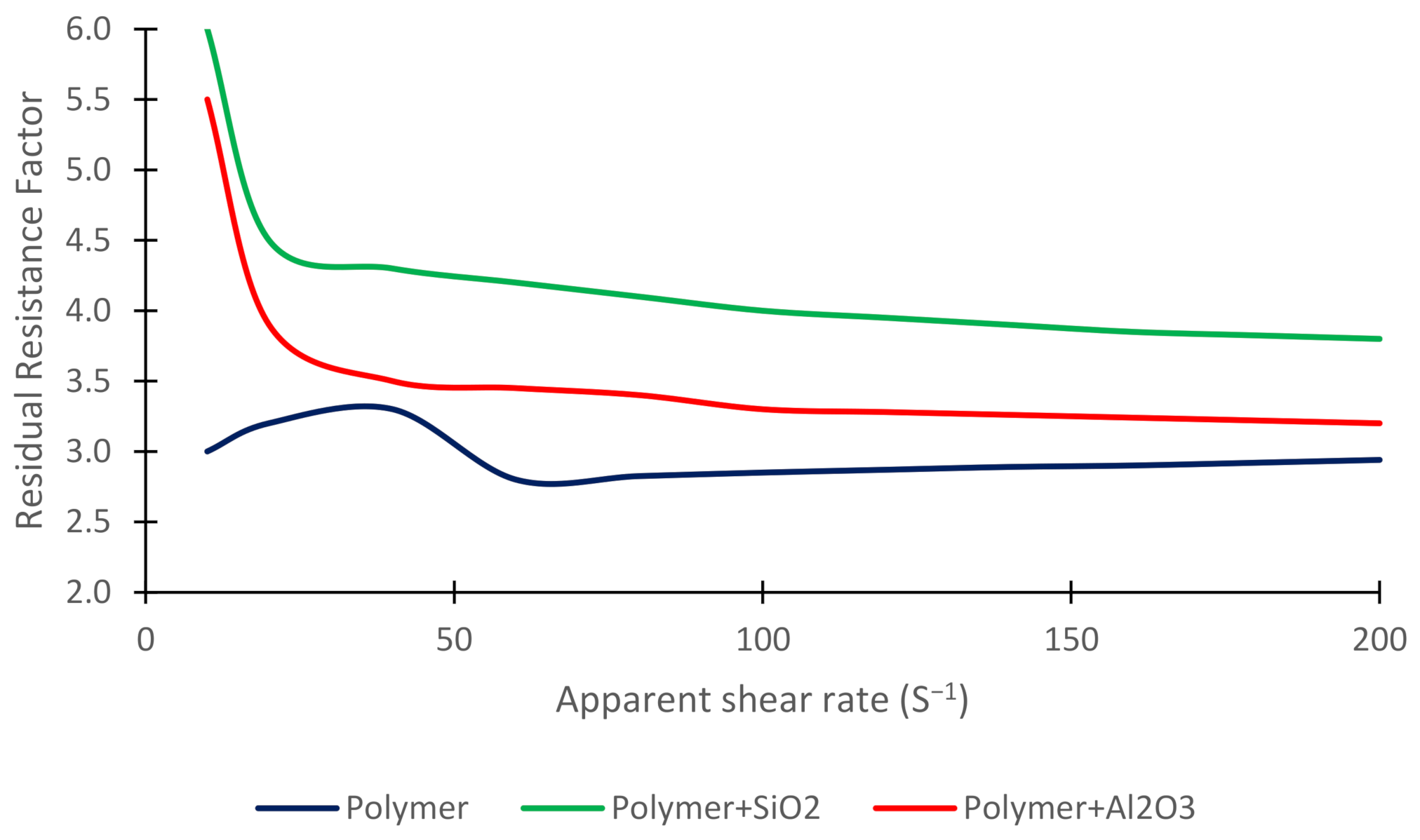
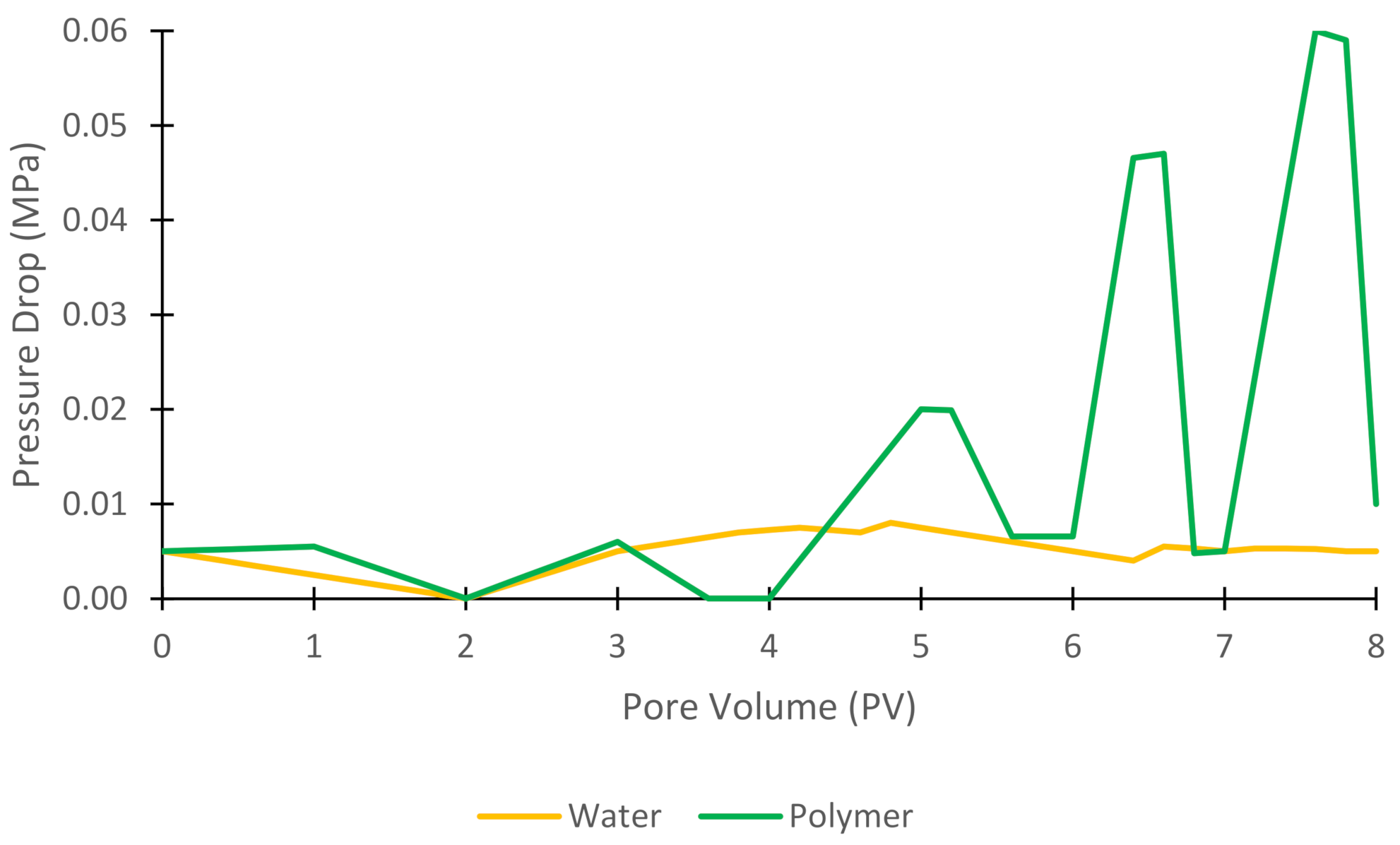
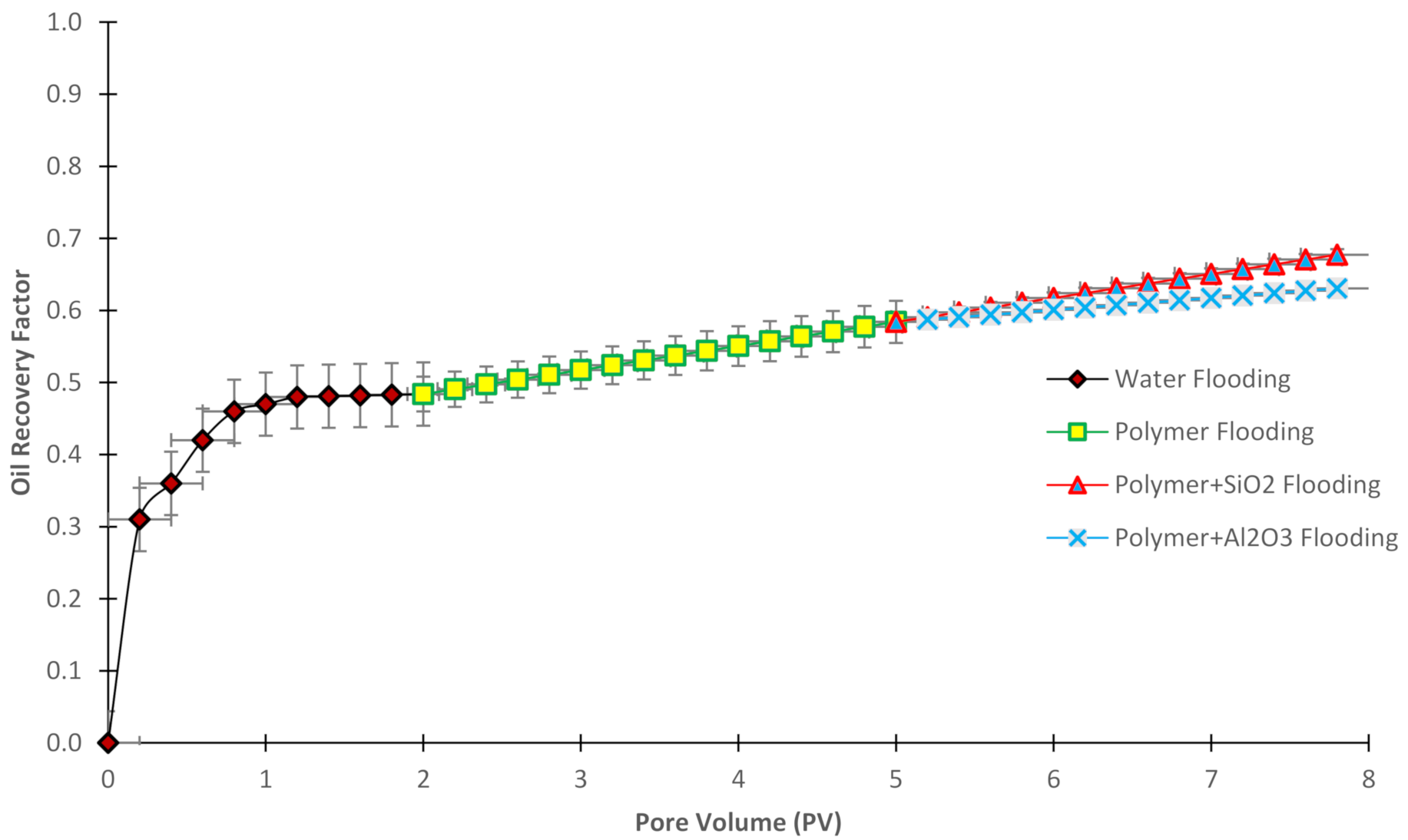
| Authors | Method | Objectives and Results |
|---|---|---|
| Hu et al. (2020) [58] | Saline brines-Foams in sandstone reservoirs | Foam injection after KCl brine has provided a higher recovery factor than another saline brines. It is due to the minimization of monovalent ions in brine. |
| Piñerez Torrijos et al. (2018) [71] | Hybrid injection of smart water and polymer | Tertiary Low salinity polymer injection has provided higher oil recovery rather than other injectivity scenarios. |
| Omidi et al. (2020) [72] | Hybrid nanoparticles and surfactant injection | Hybrid injection of nanoparticles and surfactant can provide the highest recovery factor. |
| Shabib-Asl et al. (2019) [73] | Hybrid injection of low salinity water and foam | Hybrid injection of low salinity water and foam can provide the highest recovery factor. |
| Rezvani et al. (2020) [74] | Foam stability by nanoparticles with the aim of oil recovery improvement | The foam stability has been improved by the addition of nanoparticles of SiO2 and Al2O3. Therefore, oil recovery has been increased as the foam has been stabilized. |
| Water Flooding | Polymer Flooding | Polymer Flooding + Al2O3 | Polymer Flooding + SiO2 | |
|---|---|---|---|---|
| Oil recovery factor (%) | 49% | 58% | 63% | 68% |
| Max Kro | 1 | / | / | / |
| Max Krw | / | 0.30 | / | 0.30 |
| Min Krw | / | 0.30 | / | 0.10 |
| Sro | / | 0.29 | / | 0.12 |
| Min RRF | / | 2.94 | 3.20 | 3.80 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.; Zhao, Z.; Dong, H.; Vladimirovna Mikhailova, M.; Davarpanah, A. RETRACTED: Hybrid Application of Nanoparticles and Polymer in Enhanced Oil Recovery Processes. Polymers 2021, 13, 1414. https://doi.org/10.3390/polym13091414
Hu Y, Zhao Z, Dong H, Vladimirovna Mikhailova M, Davarpanah A. RETRACTED: Hybrid Application of Nanoparticles and Polymer in Enhanced Oil Recovery Processes. Polymers. 2021; 13(9):1414. https://doi.org/10.3390/polym13091414
Chicago/Turabian StyleHu, Yanqiu, Zeyuan Zhao, Huijie Dong, Maria Vladimirovna Mikhailova, and Afshin Davarpanah. 2021. "RETRACTED: Hybrid Application of Nanoparticles and Polymer in Enhanced Oil Recovery Processes" Polymers 13, no. 9: 1414. https://doi.org/10.3390/polym13091414
APA StyleHu, Y., Zhao, Z., Dong, H., Vladimirovna Mikhailova, M., & Davarpanah, A. (2021). RETRACTED: Hybrid Application of Nanoparticles and Polymer in Enhanced Oil Recovery Processes. Polymers, 13(9), 1414. https://doi.org/10.3390/polym13091414







