Poly(Vinyl Chloride) Spheres Coated with Graphene Oxide Sheets: From Synthesis to Optical Properties and Their Applications as Flame-Retardant Agents
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Influence of GO Sheets on PVC Sphere Size
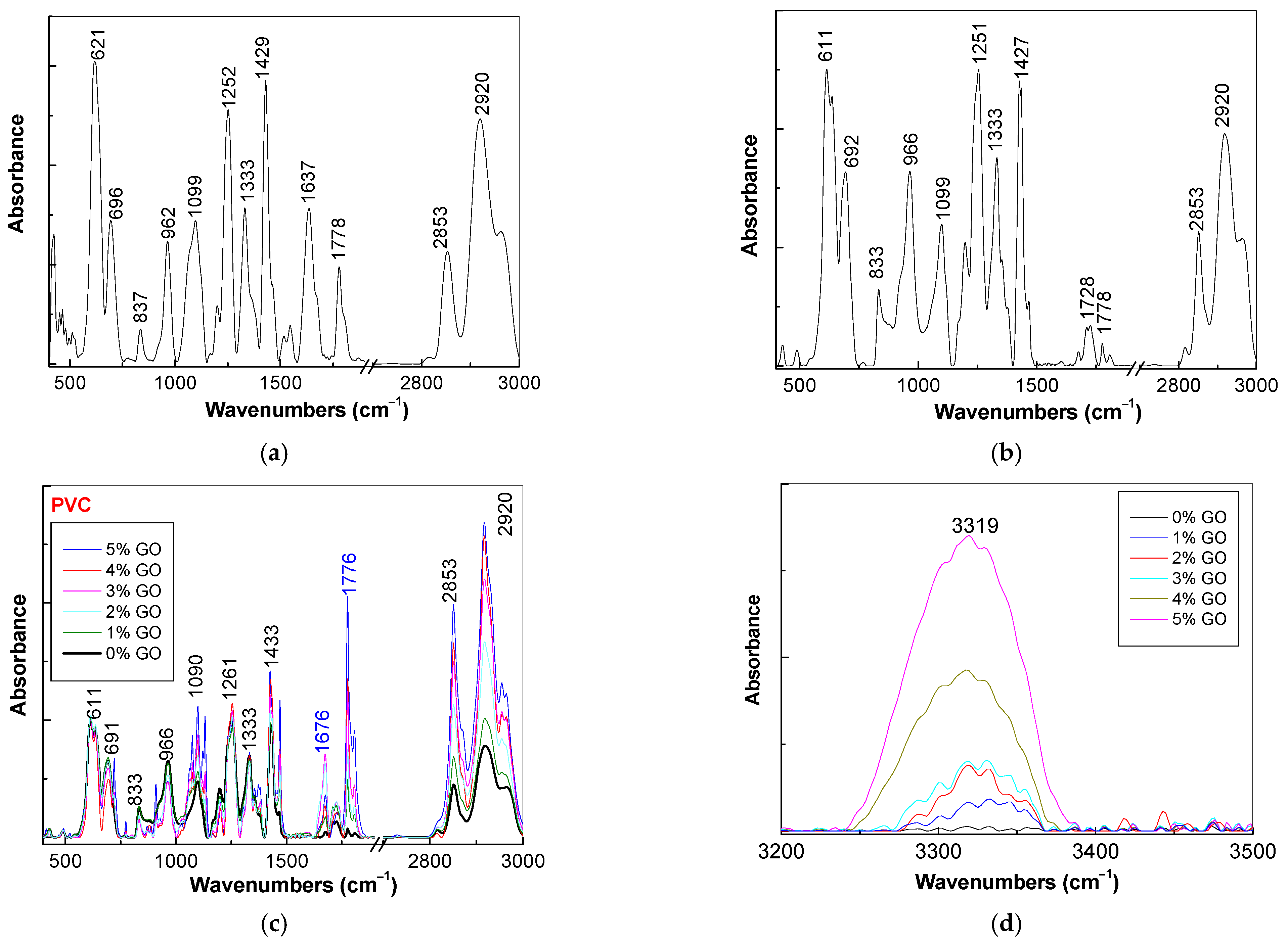
3.2. Vibrational Properties of PVC Spheres and Their Composites with GO Sheets
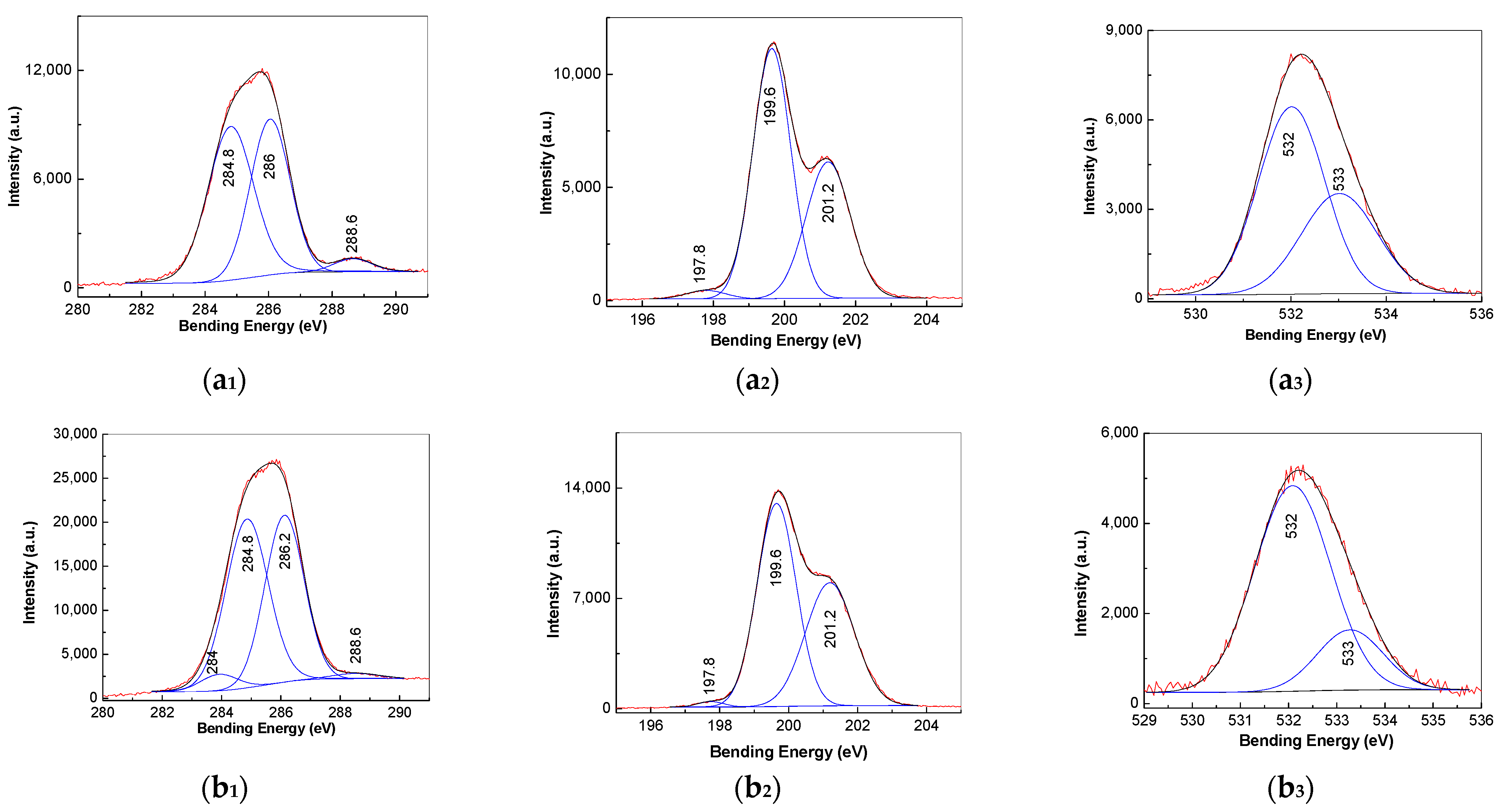
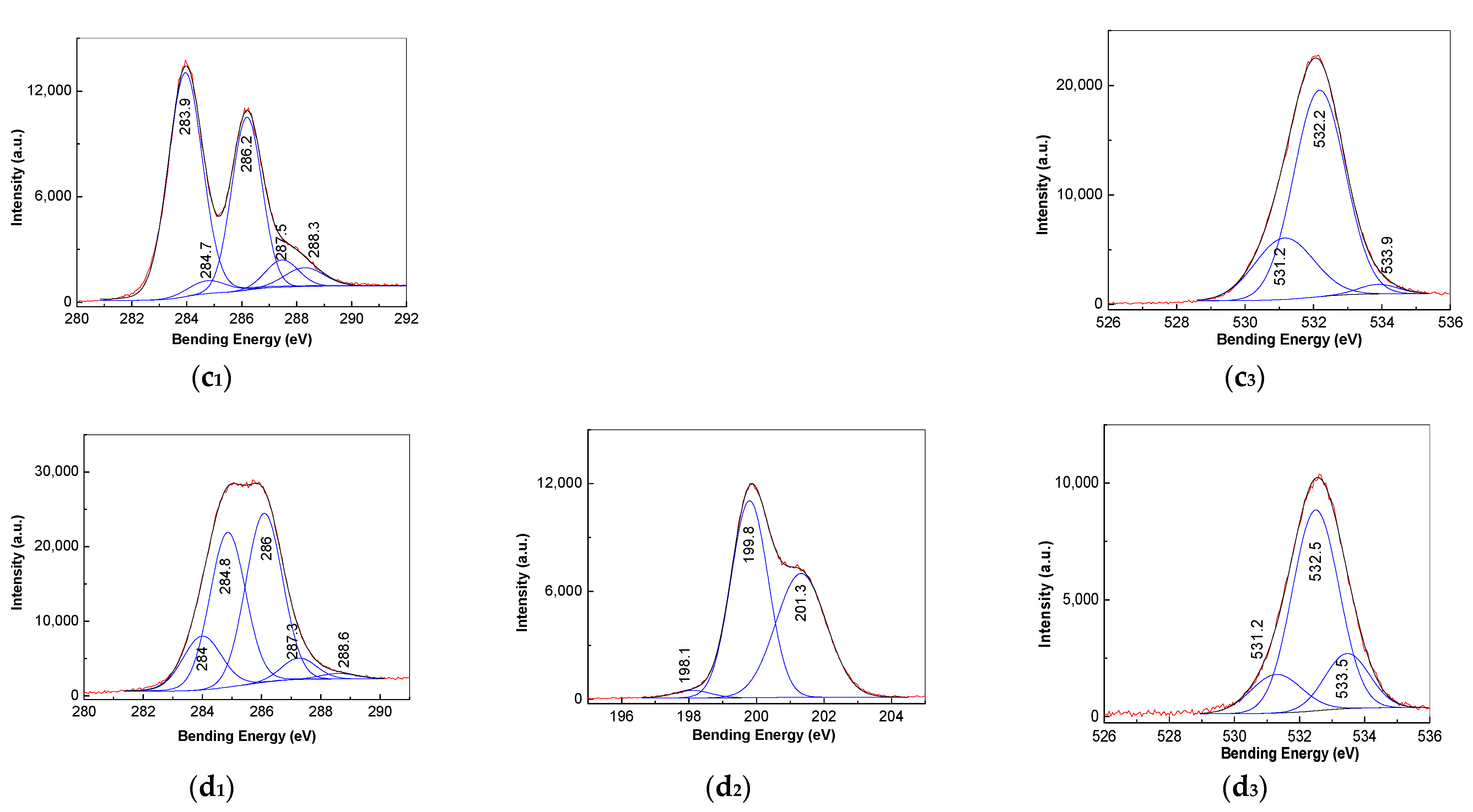
3.3. XPS Spectra of PVC Spheres and Their Composites with GO Sheets
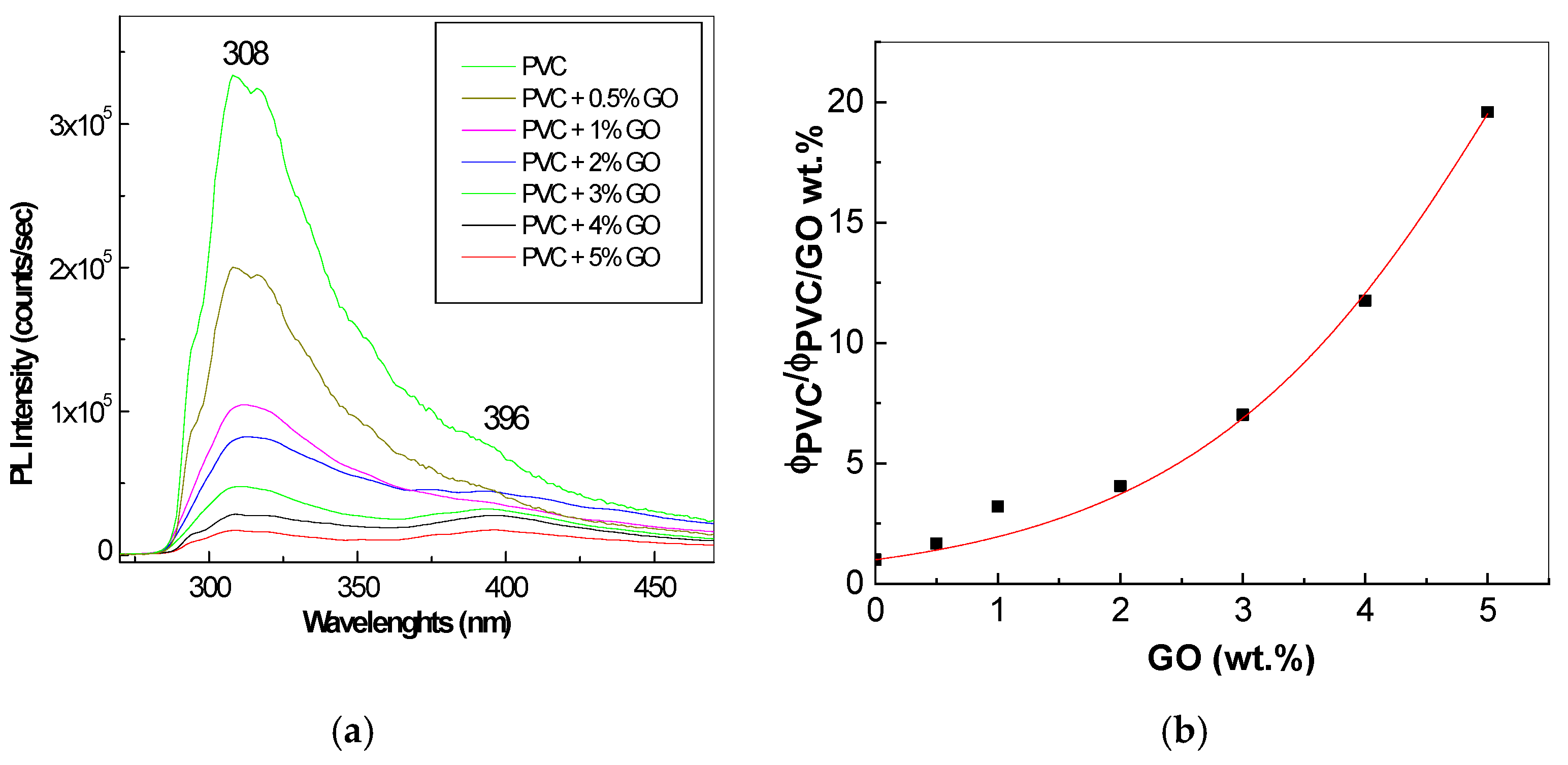
3.4. Photoluminescence of PVC Spheres and Their Composites with GO Sheets
3.5. Potential of PVC Spheres Coated with GO Sheets as Flame-Retardant Materials
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Deshmukh, K.; Joshi, G.M. Thermo-mechanical properties of poly (vinyl chloride)/graphene oxide as high performance nanocomposites. Polym. Test. 2014, 34, 211–219. [Google Scholar] [CrossRef]
- Sun, L. Structure and synthesis of graphene oxide. Chin. J. Chem. Eng. 2019, 27, 2251–2260. [Google Scholar] [CrossRef]
- Konios, D.; Stylianakis, M.M.; Stratakis, E.; Kymakis, E. Dispersion behaviour of graphene oxide and reduced graphene oxide. J. Colloid Interface Sci. 2014, 430, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Salavagione, H.J.; Martínez, G. Importance of covalent linkages in the preparation of effective reduced graphene oxide-poly(vinyl chloride) nanocomposites. Macromolecules 2011, 44, 2685–2692. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, H.; Xia, J.; Zhang, F.; Li, F.; Xia, Y.I.; Li, Y. Novel GO-blended PVDF ultrafiltration membranes. Desalination 2012, 299, 50–54. [Google Scholar] [CrossRef]
- Aljaafari, A.A.; Ibrahim, S.S.; El-Brolossy, T.A. Thermophysical and electrical characterization of PVC-SWNT nanocomposites. Compos. Part A Appl. Sci. Manuf. 2011, 42, 394–399. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Jashni, E.; Habibi, M.; Nemati, M.; Van der Bruggen, B. Evaluating the ion transport characteristics of novel graphene oxide nanoplates entrapped mixed matrix cation exchange membranes in water deionizatio. J. Memb. Sci. 2017, 541, 641–652. [Google Scholar] [CrossRef]
- Deshmukh, K.; Ahamed, M.B.; Shah, A.H.; Pandey, M.; Joshi, G.M. Morphology, Ionic Conductivity, and Impedance Spectroscopy Studies of Graphene Oxide-Filled Polyvinylchloride Nanocomposites. Polym.-Plast. Technol. Eng. 2015, 54, 1743–1752. [Google Scholar] [CrossRef]
- Mindivan, A.P.F. The Synthesis, Thermal and Structural Characterization of Polyvinylchloride / Graphene Oxide ( PVC / GO ) Composites, International scientific conference. Mater. Sci. Non-Equilib. Phase Transform. 2015, 48, 45–48. [Google Scholar]
- Zhao, Y.; Lu, J.; Liu, X.; Wang, Y.; Lin, J.; Peng, N.; Li, J.; Zhao, F. Performance enhancement of polyvinyl chloride ultrafiltration membrane modified with graphene oxide. J. Colloid Interface Sci. 2016, 480, 1–8. [Google Scholar] [CrossRef]
- Hwang, T.; Frank, Z.; Neubauer, J.; Kim, K.J. High-performance polyvinyl chloride gel artificial muscle actuator with graphene oxide and plasticizer. Sci. Rep. 2019, 9, 9658. [Google Scholar] [CrossRef]
- Moradi, H.; Parvin, P.; Shahi, F.; Ojaghloo, A. Fiber optic Fabry-Perot acoustic sensor using PVC and GO diaphragms. Osa Contin. 2020, 3, 943–951. [Google Scholar] [CrossRef]
- Khakpour, S.; Jafarzadeh, Y.; Yegani, R. Incorporation of graphene oxide/nanodiamond nanocomposite into PVC ultrafiltration membranes. Chem. Eng. Res. Des. 2019, 152, 60–70. [Google Scholar] [CrossRef]
- Cotet, L.C.; Magyari, K.; Todea, M.; Dudescu, M.C.; Danciu, V.; Baia, L. Versatile self-assembled graphene oxide membranes obtained under ambient conditions by using a water-ethanol suspension. J. Mat. Chem. A 2017, 5, 2132–2142. [Google Scholar] [CrossRef]
- Pérez Del Pino, A.; György, E.; Cotet, L.C.; Baia, L.; Logofatu, C. Laser-induced chemical transformation of free-standing graphene oxide membranes in liquid and gas ammonia environments. RSC Adv. 2016, 6, 50034–50042. [Google Scholar] [CrossRef]
- Stroe, M.; Cristea, M.; Matei, E.; Galateanu, A.; Cotet, L.C.; Pop, L.C.; Baia, M.; Danciu, V.; Anghel, I.; Baia, L.; et al. Optical properties of composites based on graphene oxide and polystyrene. Molecules 2020, 25, 2419. [Google Scholar] [CrossRef] [PubMed]
- Cotet, L.C.; Cadar, C.; Mihis, A.; Magyari, K.; Muresan-Pop, M.; Pop, L.C.; Mihaila, A.; Szekely, I.; Dragan, S.; Dudescu, M.; et al. Mixture of graphene oxide/phosphoric acid/melamine as coating for improved fire protective performance and enhancement of surface electrical properties on wood chipboard. J. Nanosci. Nanotechnol. 2021, 21, 2312–2322. [Google Scholar] [CrossRef] [PubMed]
- Chuquitarqui, A.; Cotet, L.C.; Baia, M.; Gyorgy, E.; Magyari, K.; Barbu-Tudoran, L.; Baia, L.; Díaz-González, M.; Fernandez Sanchez, C.; Perez del Pino, A. New fabrication method for producing reduced graphene oxide flexible electrodes by using low-power visible laser diode engraving system. Nanotechnology 2020, 31, 325402. [Google Scholar] [CrossRef]
- Endo, K. Synthesis and structure of poly(vinyl chloride). Prog. Polym. Sci. 2002, 27, 2021–2054. [Google Scholar] [CrossRef]
- Lyon, R.E.; Walters, R.N.; Stoliarov, S.I.; Safronava, N. Principles and Practice of Microscale Combustion Calorimetry; Federal Aviation Administration: Atlantic City International Airport, NJ, USA, 2013. [Google Scholar]
- Standard test method for determining flammability characteristics of plastics and other solid materials using microscale combustion calorimetry. ASTM D7309-13. 2013.
- Ng, Y.H.; Zope, I.S.; Dasari, A.; Tan, K.H. Correlating and performance of a fire-retardant coating across different scales of testing. Polymers 2020, 12, 2271. [Google Scholar] [CrossRef]
- Prokhorov, K.A.; Aleksandrova, D.A.; Sagitova, E.A.; Nikolaeva, G.Y.; Vlasova, T.V.; Pashinin, P.P.; Jones, C.A.; Shilton, S.J. Raman spectroscopy evaluation of polyvinylchloride structure. J. Phyiscs Conf. Ser. 2016, 691, 012001. [Google Scholar] [CrossRef]
- Qiu, F.; He, G.; Hao, M.; Zhang, G. Enhancing the mechanical and electrical properties of poly(vinyl chloride)-based conductive nanocomposites by Zinc oxide nanorods. Materials 2018, 11, 2139. [Google Scholar] [CrossRef] [PubMed]
- Hankett, J.M.; Zhang, C.; Chen, Z. Sum frequency generation and coherent anti-Stokes Raman spectroscopic studies on plasma-treated plasticized polyvinyl chloride films. Langmuir 2012, 28, 4654–4662. [Google Scholar] [CrossRef]
- Stingescu, L.; Cadar, C.; Cotet, L.C.; Baia, L.; Saszet, K.; Magyari, K.; Mihis, A.G.; Fort, C.I.; Stroe, M.; Matei, E.; et al. Morphological and structural investigation of the poly(vinyl chloride)/graphene oxide composites. Studia Ubb Chem. Lxv 2020, 3, 245–258. [Google Scholar] [CrossRef]
- Abdel-Fattah, A.; Alharthi, A.; Fahmy, T. Spectroscopic, optical and thermal characterization of polyvinyl chloride-based plasma-functionalized MWCNTs composite thin films. Appl. Phys. A. 2019, 125, 475. [Google Scholar] [CrossRef]
- Wilczewski, S.; Skorczewska, K.; Tomaszewska, J.; Lewandowski, K. Structural and properties of poly(vinyl chloride)/graphene nanocomposites. Polym. Test. 2020, 81, 106282. [Google Scholar] [CrossRef]
- Eigler, S.; Dotzer, C.; Hirsch, A. Visualization of defect densities in reduced graphene oxide. Carbon 2002, 50, 3666–3673. [Google Scholar] [CrossRef]
- Chatterjee, N.; Basu, S.; Palit, S.K.; Maiti, M.M. A reexamination of the degradation of polyvinylchloride by thermal analysis. J. Polymer Sci. Part A Polym. Chem. 1994, 32, 1225–1236. [Google Scholar] [CrossRef]
- Kositchaiyong, A.; Rosarpitak, V.; Hamada, H.; Sombatsompop, N. Anti-fungal performance and mechanical-morphological properties of PVC and wood/PVC composites under UV-wathering aging and soil-burial exposure. Int. Biodeterior. Biodegrad. 2014, 91, 128–137. [Google Scholar] [CrossRef]
- Ramesh, S.; Chai, M.F. Conductivity, dielectric behavior and FTIR studies of high molecular weight poly(vinylchloride)-lithium triflate polymer electrolytes. Mat. Sci. Eng. B 2007, 139, 240–245. [Google Scholar] [CrossRef]
- Zhou, J.; Gui, B.; Qiao, Y.; Zhang, J.; Wang, W.; Yao, H.; Yu, Y.; Xu, M. Understanding the pyrolysis mechanism of polyvinylchloride (PVC) by characterizing the chars produced in a wire-mesh reactor. Fuel 2016, 166, 526–532. [Google Scholar] [CrossRef]
- Salavagione, H.J.; Martinez, G.; Marco, C. A polymer/solvent synergetic effect to improve the solubility of modified multi-walled carbon nanotubes. J. Mater. Chem. 2012, 22, 7020–7027. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Bassler, G.C.; Morrill, T.C. Spectrometric Identification of Organic Compounds, 4th ed.; John Wiley and Sons: New York, NY, USA, 1981. [Google Scholar]
- Jontoria-Lucas, C.; Lopez-Peinado, A.K.; Lopez-Gonzalez, J.D.; Rojas-Cervantes, M.L.; Martin-Aranda, R.M. Study of oxygen-containing groups in a series of graphite oxides: Physical and chemical characterization. Carbon 1995, 33, 1585–1592. [Google Scholar] [CrossRef]
- Miranda, M.R.; Pakdel, H.; Roy, C.; Darmstadt, H.; Vasile, C. Vacuum pyrolysis of PVCII: Product analysis. Polym. Degrad. Stabil. 1999, 66, 107–125. [Google Scholar] [CrossRef]
- Atzei, D.; Elsener, B.; Manfredini, M.; Marchetti, A.; Malagoli, M.; Galavotti, F.; Rossi, A. Radiation-induced migration of additives in PVC-based biomedical disposable devices Part 2. Surface analysis by XPS. Surf. Interface Anal. 2003, 35, 673–681. [Google Scholar] [CrossRef]
- Fu, R.; Dong, C.; Zhang, Y.; Sun, C.; Qu, R.; Ji, C.; Zhang, Y. PPTA-oligomer functionalized multiwalled carbon nanotubes synthesized by “one-pot” method for reinforcement of polyvinyl chloride. J. Mater. Sci. 2019, 54, 11804–11817. [Google Scholar] [CrossRef]
- del Pino, A.P.; Villaroya, A.M.; Chuquitarqui, A.; Logofatu, C.; Tonti, D.; Gyorgy, E. Reactive laser synthesis of nitrogen-doped hybrid graphene-based electrodes for energy storage. J. Mater. Chem. A 2018, 6, 16074–16086. [Google Scholar] [CrossRef]
- Ivan, R.; de Pino, A.P.; Yousef, U.; Logofatu, C.; Gyorgy, E. Laser synthesis of TiO2—Carbon nanomaterial layers with enhanced photodegration effeiciency towards antibiotics and dyes. J. Photochem. Photobiol. A Chem. 2020, 399, 112616. [Google Scholar] [CrossRef]
- Soudais, Y.; Moga, L.; Blazek, J.; Lemort, F. Coupled DTA-TGA-FT-IR investigation of pyrolytic decomposition of EVA, PVC and cellulose. J. Anal. Appl. Pyrolysis 2007, 78, 46–57. [Google Scholar] [CrossRef]
- Yu, J.; Sun, L.; Ma, C.; Qiao, Y.; Yao, H. Thermal degradation of PVC: A review. Water Manag. 2016, 48, 300–314. [Google Scholar] [CrossRef]
- Mc Neill, I.C.; Memetea, L.; Cole, W.J. A study of the products of PVC thermal degradation. Polym. Degrad. Stab. 1995, 49, 181–191. [Google Scholar] [CrossRef]
- Lee, T.; Lee, J.; Ok, Y.S.; Oh, J.I.; Lee, S.R.; Rinklebe, J.; Kwon, E.E. Utilizing CO2 to suppress the generation of harmful chemicals from thermal degradation of polyvinyl chloride. J. Clean. Prod. 2017, 162, 1465–1471. [Google Scholar] [CrossRef]
- Blazevska-Gilev, J.; Spaseska, D. Formal kinetic analysis of PVC thermal degradation. J. Univ. Chem. Technol. Metall. 2010, 45, 251–254. [Google Scholar]
- Lyon, R.E.; Speitel, L.; Walters, R.N.; Crowley, S. Fire-resistant elastomers. Fire Mater. 2003, 27, 195–208. [Google Scholar] [CrossRef]
- Cogen, J.M.; Lin, T.S.; Lyon, R.E. Correlations between pyrolysis combustion flow calorimetry and conventional flammability tests with halogen-free flame retardant polyolefin compounds. Fire Mater. Int. J. 2009, 33, 33–50. [Google Scholar] [CrossRef]
- Lin, T.S.; Cogen, J.M.; Lyon, R.E. Correlations between Microscale Combustion Calorimetry and Conventional Flammability Tests for Flame Retardant Wire and Cable Compounds. In Proceedings of the 56th International Wire and Cable Symposium (IWCS 2007), Lake Buena Vista, FL, USA, 11–14 November 2007; pp. 176–185. [Google Scholar]
- Lyon, R.; Walters, R.; Stoliarov, S. Thermal analysis of flammability. J. Therm. Anal. Calorim. 2007, 89, 441–448. [Google Scholar] [CrossRef]
- Lyon, R.E.; Walters, R.N. Pyrolysis Combustion Flow Calorimetry. J. Anal. Appl. Pyrolysis 2004, 71, 27. [Google Scholar] [CrossRef]

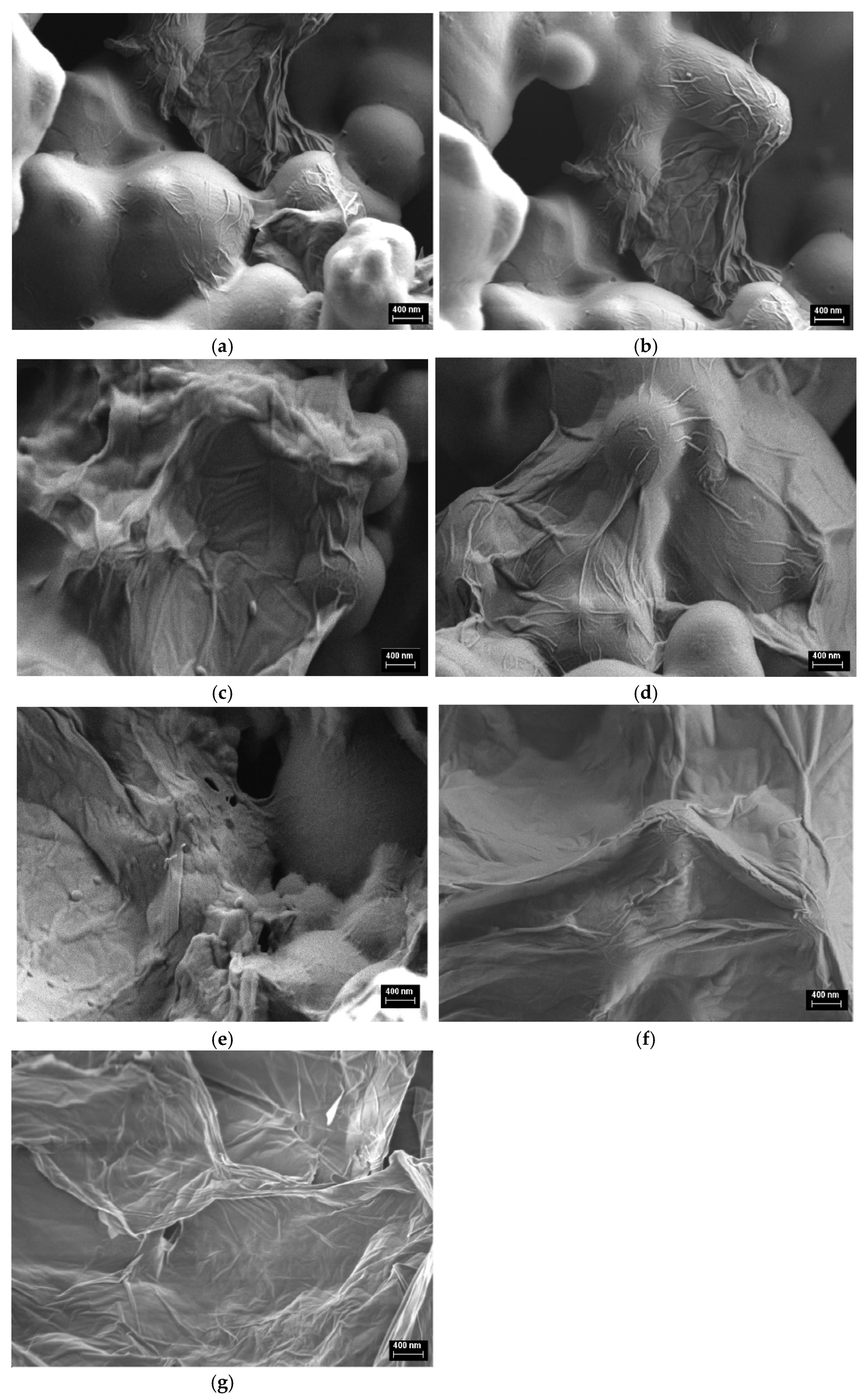
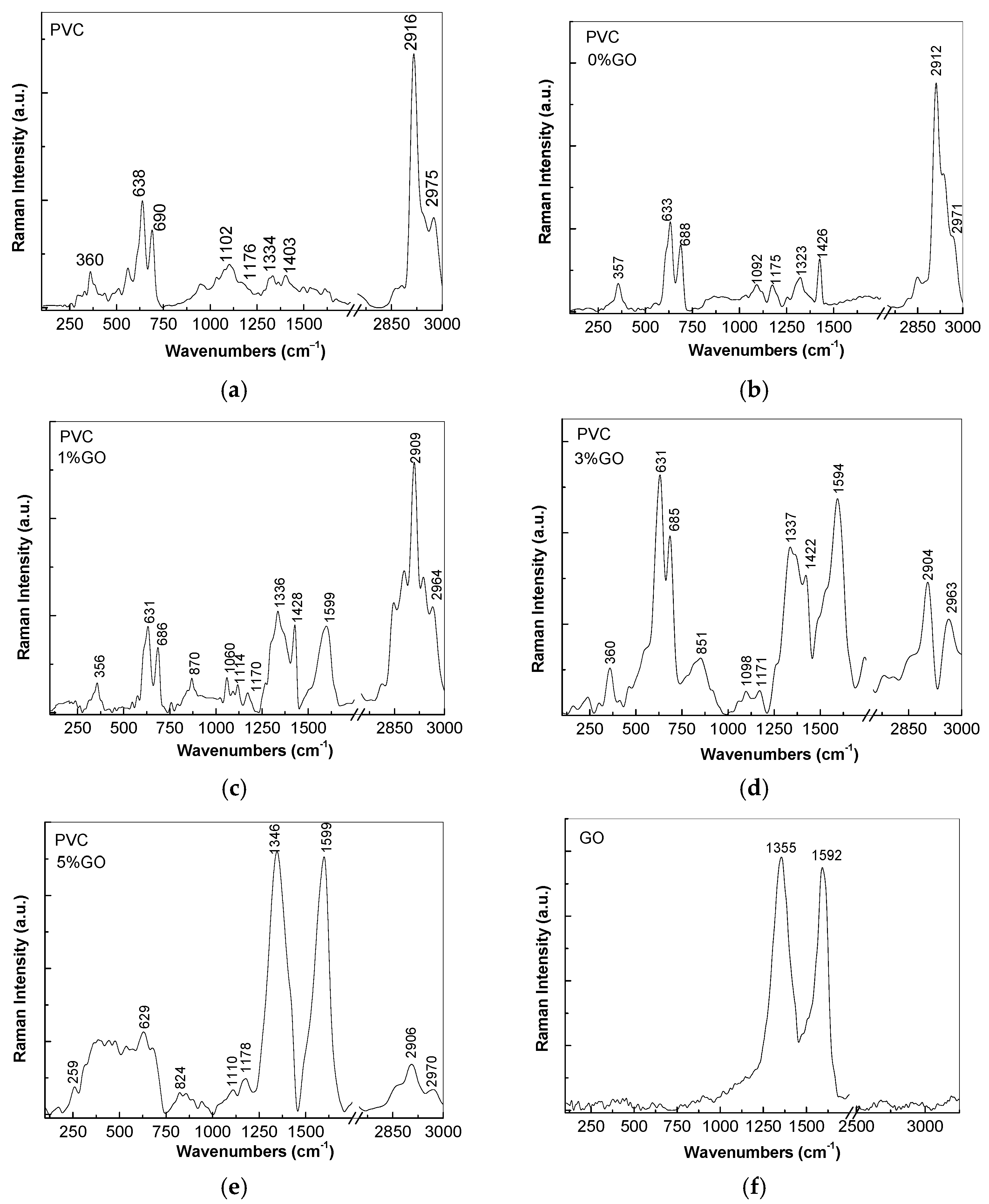
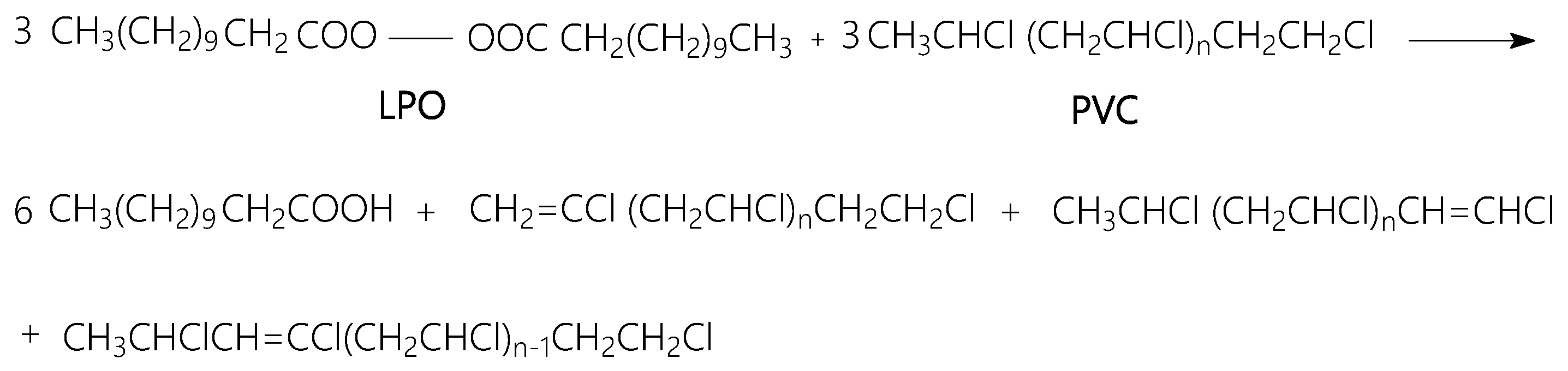
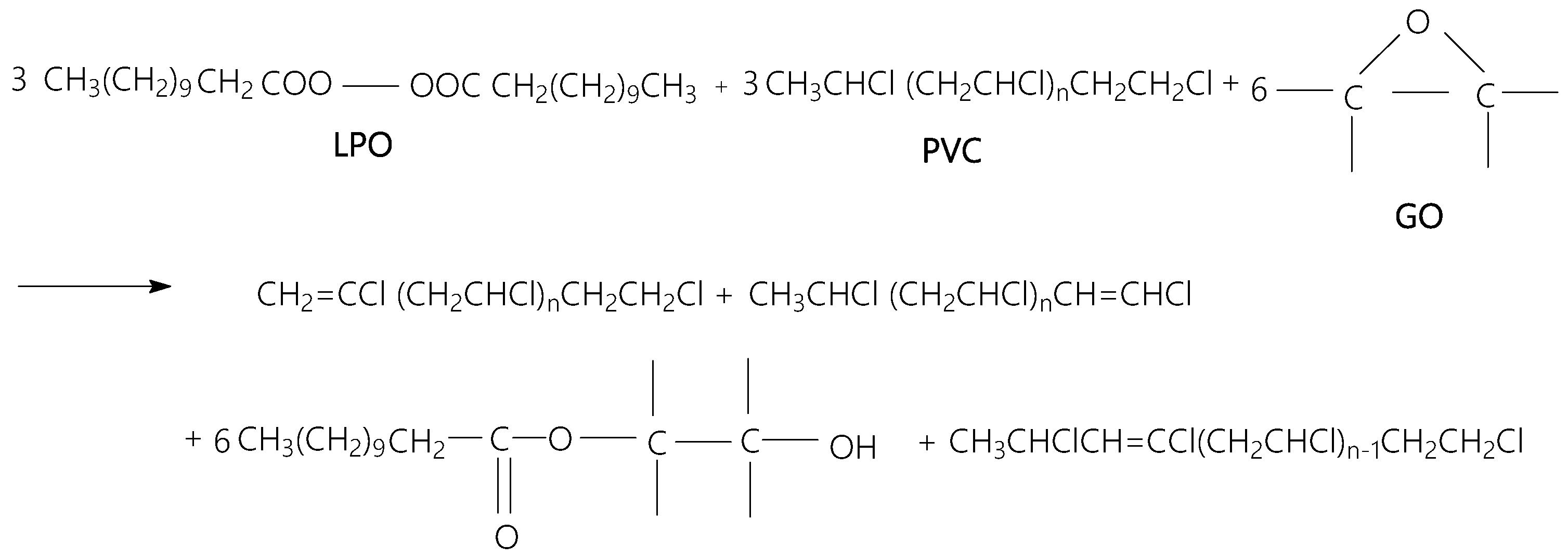


| ν (cm−1) | Assignments [22,23,24,25] |
|---|---|
| 360 | C–Cl in the trans configuration of polymer |
| 638–690 | C–Cl stretching |
| 1102–1179 | C–C stretching |
| 1334–1403 | C–H symmetrical stretching in CH2 group |
| 2916 | C–H asymmetrical stretching in CH2 group |
| 2975 | C–H asymmetrical stretching in CH3 group |
| ν (cm−1) | Assignments [30,31,32,33] |
|---|---|
| 621 | wagging of C–H bonds of syndiotactic structure of PVC |
| 696 | stretching C–Cl bond of isotactic structure of PVC |
| 837 | rocking of C–H bond in CH2 group |
| 962 | wagging of C–H bond in polymer with trans configuration |
| 1099 | deformation of C-H bonds |
| 1252 | rocking of C–H bond in polymer with trans configuration |
| Sample | Mass (mg) | Residues (mg) | Char Yield (wt.%) | Decomposition Rate (%) | HRC (J g−1K−1) | THR (kJ g−1) | PHRR (W g−1) | TPHRR (°C) | Time (s) |
|---|---|---|---|---|---|---|---|---|---|
| PVC-GO 0 wt.% | 14.02 | 0.88 | 6.25 | 93.75 | 168.13 | 14.27 | 226.32 | 505.64 | 139.50 |
| PVC-GO 0.5 wt.% | 14.04 | 0.92 | 6.55 | 93.45 | 190.61 | 15.83 | 185.12 | 500.27 | 184.00 |
| PVC-GO 1 wt.% | 14.03 | 1.13 | 8.05 | 91.95 | 173.76 | 14.87 | 178.97 | 457.11 | 159.50 |
| PVC-GO 2 wt.% | 14.00 | 0.91 | 6.50 | 93.50 | 159.88 | 16.01 | 190.74 | 504.91 | 191.00 |
| PVC-GO 3 wt.% | 14.07 | 1.01 | 7.18 | 92.82 | 148.78 | 15.18 | 186.71 | 456.24 | 159.50 |
| PVC-GO 4 wt.% | 14.10 | 1.05 | 7.42 | 92.58 | 145.29 | 16.09 | 179.05 | 494.26 | 178.00 |
| PVC-GO 5 wt.% | 14.08 | 1.17 | 8.34 | 91.66 | 143.17 | 15.87 | 179.84 | 464.84 | 163.50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baibarac, M.; Stingescu, L.; Stroe, M.; Negrila, C.; Matei, E.; Cotet, L.C.; Anghel, I.; Şofran, I.E.; Baia, L. Poly(Vinyl Chloride) Spheres Coated with Graphene Oxide Sheets: From Synthesis to Optical Properties and Their Applications as Flame-Retardant Agents. Polymers 2021, 13, 565. https://doi.org/10.3390/polym13040565
Baibarac M, Stingescu L, Stroe M, Negrila C, Matei E, Cotet LC, Anghel I, Şofran IE, Baia L. Poly(Vinyl Chloride) Spheres Coated with Graphene Oxide Sheets: From Synthesis to Optical Properties and Their Applications as Flame-Retardant Agents. Polymers. 2021; 13(4):565. https://doi.org/10.3390/polym13040565
Chicago/Turabian StyleBaibarac, Mihaela, Luiza Stingescu, Malvina Stroe, Catalin Negrila, Elena Matei, Liviu C. Cotet, Ion Anghel, Ioana E. Şofran, and Lucian Baia. 2021. "Poly(Vinyl Chloride) Spheres Coated with Graphene Oxide Sheets: From Synthesis to Optical Properties and Their Applications as Flame-Retardant Agents" Polymers 13, no. 4: 565. https://doi.org/10.3390/polym13040565
APA StyleBaibarac, M., Stingescu, L., Stroe, M., Negrila, C., Matei, E., Cotet, L. C., Anghel, I., Şofran, I. E., & Baia, L. (2021). Poly(Vinyl Chloride) Spheres Coated with Graphene Oxide Sheets: From Synthesis to Optical Properties and Their Applications as Flame-Retardant Agents. Polymers, 13(4), 565. https://doi.org/10.3390/polym13040565









