Polymer-Based Smart Drug Delivery Systems for Skin Application and Demonstration of Stimuli-Responsiveness
Abstract
1. Introduction
2. The Skin, A Well-Established Route for Drug Delivery But Still Challenging
3. Polymer-Based Smart Drug Delivery Systems for Topical Applications
3.1. Types of SDDS Formulations for Topical Applications
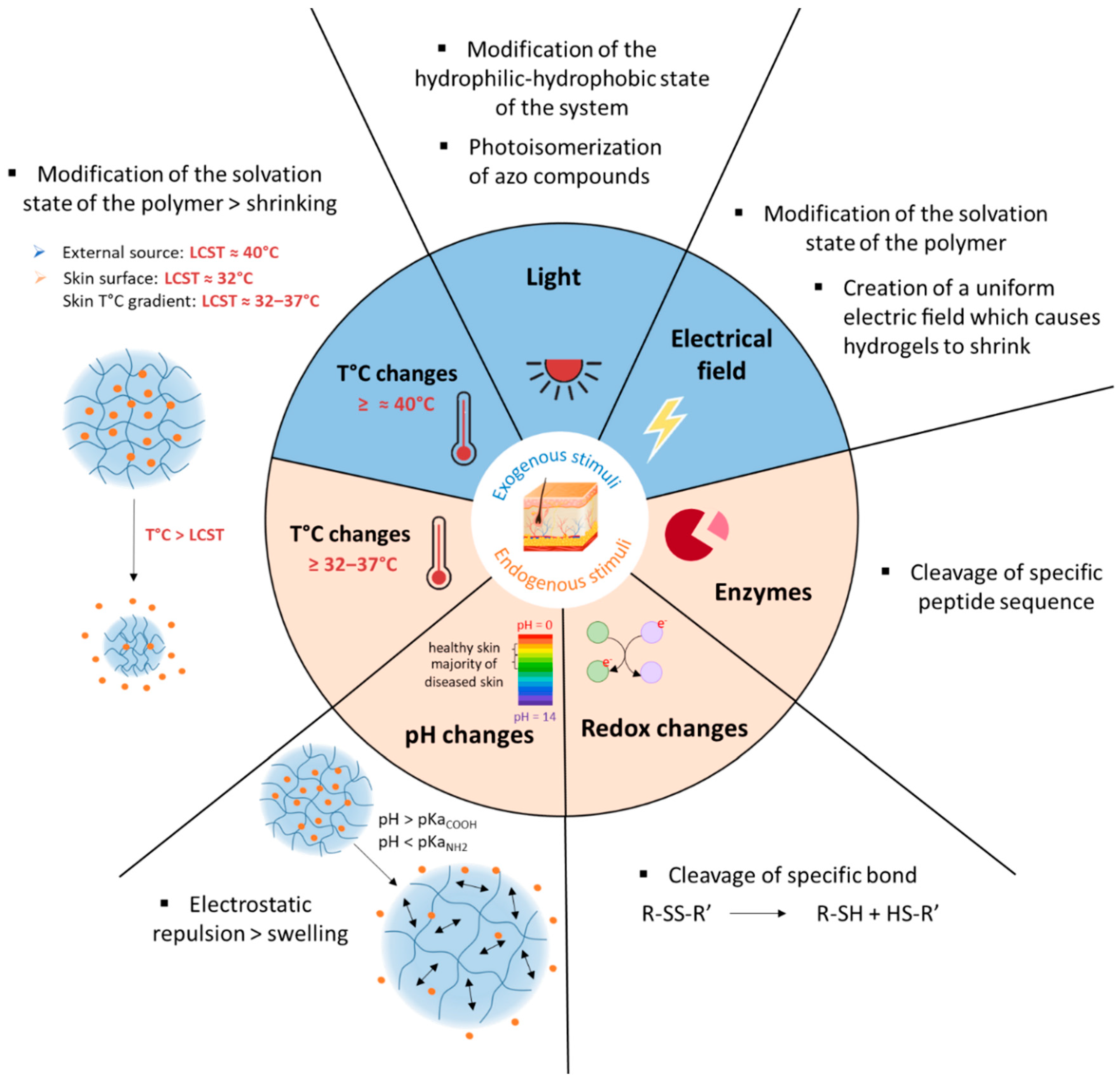
3.2. pH-Responsive Delivery Systems
3.2.1. Skin pH and Its Variations
3.2.2. Mechanisms of pH-Responsiveness in Smart Polymeric Systems
3.2.3. pH-Responsive Systems Based on Anionic Polymers
3.2.4. pH-Responsive Systems Based on Cationic Polymers
3.3. Thermoresponsive Delivery Systems
3.3.1. Application Strategies of a Thermoresponsive System for Skin Delivery
- Using the thermal gradient of the skin (32–37 °C) to deliver drugs. This strategy is usually adapted to nanosystems. The surface temperature (32 °C) prevents immediate drug release, and it is only when nanovectors finally reach deeper layers of the SC (37 °C) that the drug is released [58,74,75,76].
- Using the temperature imbalance between healthy skin and injured skin to trigger the release of active ingredients, specifically on the injured site concerned, by application of the active ingredient [77]. For example, it was shown that chronically infected wounds show a temperature 3 °C to 4 °C higher than normal skin [4].
- Elevating the temperature of the region to be treated artificially using an external thermal trigger, e.g., heating patch or infrared lamp [10].
3.3.2. Mechanisms of the Temperature Responsiveness of Smart Polymeric Systems
3.3.3. Thermoresponsive SDDS for Cutaneous Administration
3.4. Other Stimuli-Responsive Delivery Systems
3.4.1. Redox-Responsive Systems
3.4.2. Enzyme-Cleavable Systems
3.4.3. Electro-Sensitive Systems
3.5. Dual Stimuli-Responsive Systems
4. Proof of Concept: Demonstration of the Stimuli-Responsiveness of the SDDS
4.1. Physico-Chemical Characterization of the Stimuli-Responsiveness
4.1.1. Characterization Methods Specific to Thermoresponsive SDDS
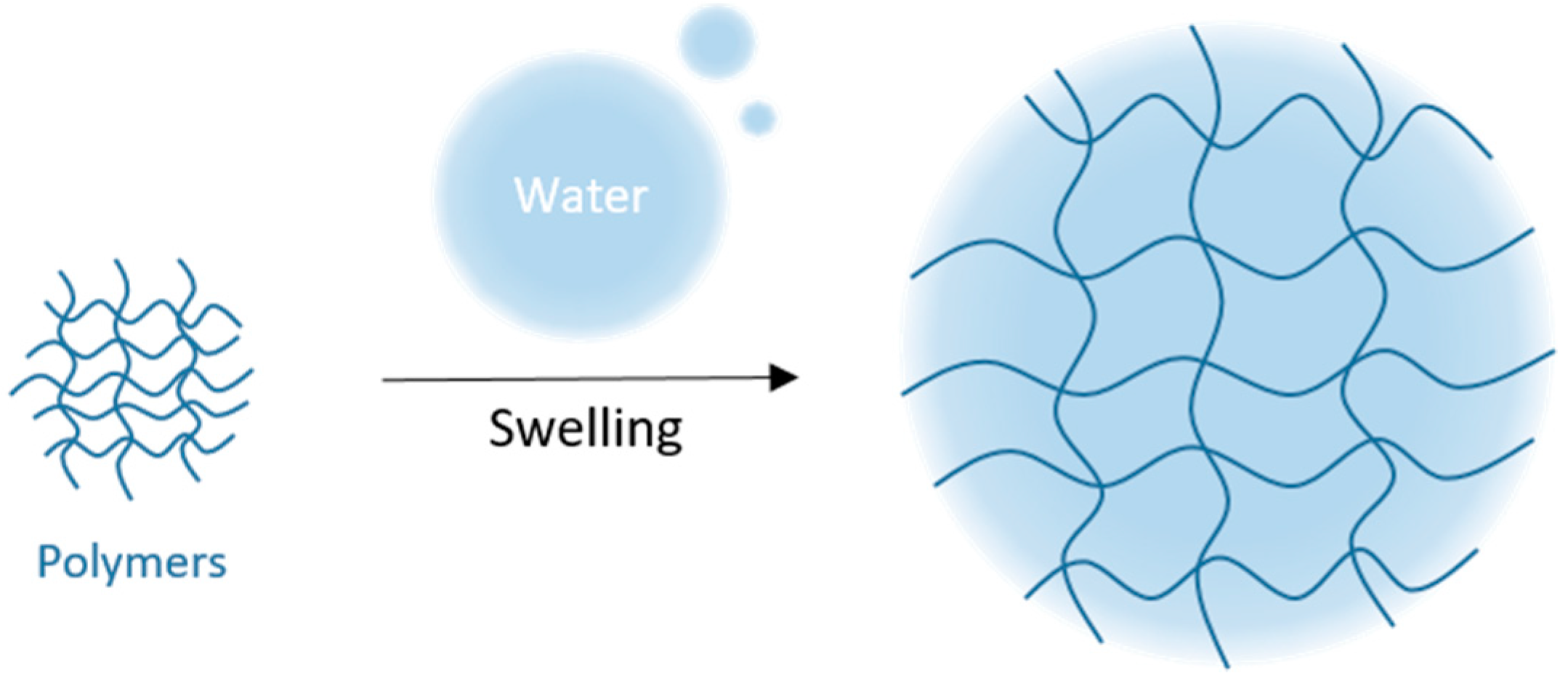
4.1.2. Swelling/Shrinking Studies
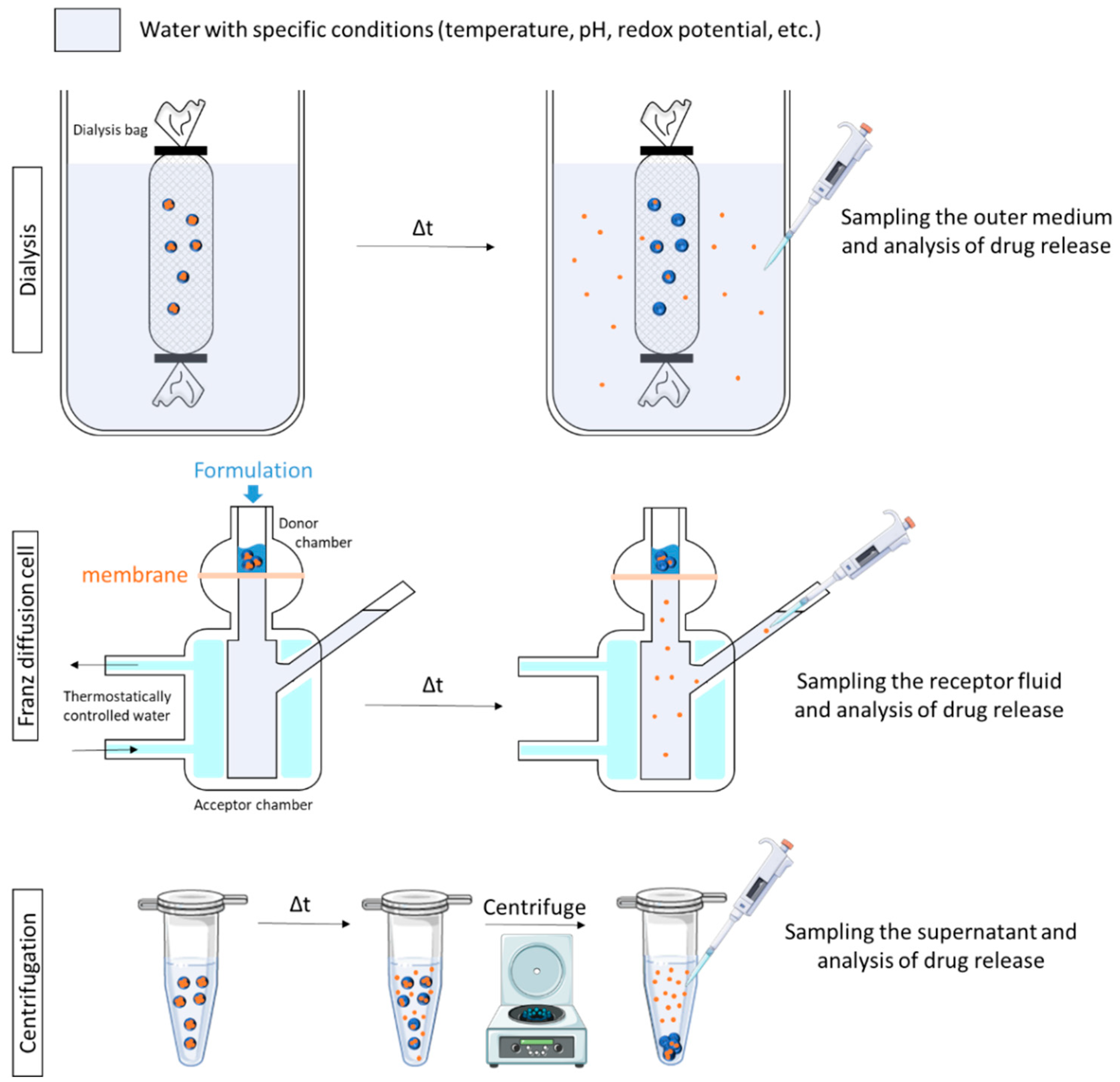
4.2. In Vitro Triggered Drug Release Studies
4.2.1. Proof of Concept Using Qualitative Methods
4.2.2. Release Kinetics Studies
4.3. Triggered Skin Penetration Studies
4.3.1. Types of Skin Models
4.3.2. Mapping the Active Ingredient 2D/3D Distribution in Skin
4.3.3. Quantitative Techniques
- Samples of receptor fluid are taken at regular intervals and replaced with an equal volume of fresh medium. The samples are then analyzed by appropriate techniques (e.g., HPLC) for AI determination. The cumulative amounts of the drug are then plotted against the time, showing the permeation behavior [34,73,81,96].
- At a specific time, at the end of the permeation study or before, the skin is removed from the FD cells, and the stratum corneum is wiped clean. The amount of AI retained in the entire skin or in each layer is determined after appropriate extraction [79]. The skin can be sectioned using different methods (e.g., cryo-sections [34,37,76], heating [73], forceps [80], or go through tape-stripping [46,48,75]) before AI determination in each layer.
4.4. In Vivo Efficacy
5. Discussion
- Most materials are synthesized under poorly reproducible conditions, and the methods to prepare smart SDDS are not standardized.
- The developed polymer-based SDDS are considered to be ‘‘new excipients’’, and thus toxicity, biocompatibility and biodegradability are major issues that take time to be elucidated. That is why, in particular, alternatives to thermoresponsive NIPAM, which is not biodegradable, are studied, like ethylene glycol methacrylate or N-vinylcaprolactam. The use of natural polymers is also an interesting way to tackle the issue. Some authors started to slightly modify natural polymers to make them responsive to pH or temperature. This shift towards naturalness is all the more marked in the field of cosmetics.
- Another important point is the cost of production and evaluation of smart products, compared with already established dermocosmetics products. This is not discussed in the literature yet but has an important impact on the industrial feasibility of the systems. Our recent results indicated that the SDDS made of only 33% of the redox responsive mPEG-SS-PLA polymers were active in vitro [124]. Thus, the use of a mixture of stimuli-responsive and neutral polymers could be a way to control the SDDS cost.
- Different from traditional dosage forms, the impact of the stimulus-responsiveness on the release kinetics or efficacy of the SDDS has to be attested; therefore, there is a need for developing suitable analytical techniques.
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, D.; Yang, F.; Xiong, F.; Gu, N. The Smart Drug Delivery System and Its Clinical Potential. Theranostics 2016, 6, 1306–1323. [Google Scholar] [CrossRef]
- Alvarez-Lorenzo, C.; Concheiro, A. Smart Drug Delivery Systems: From Fundamentals to the Clinic. Chem. Commun. 2014, 50, 7743–7765. [Google Scholar] [CrossRef]
- Sanchez-Moreno, P.; Ortega-Vinuesa, J.L.; Peula-Garcia, J.M.; Marchal, J.A.; Boulaiz, H. Smart Drug-Delivery Systems for Cancer Nanotherapy. CDT 2018, 19, 339–359. [Google Scholar] [CrossRef]
- Fierheller, M.; Sibbald, R.G. A Clinical Investigation into the Relationship between Increased Periwound Skin Temperature and Local Wound Infection in Patients with Chronic Leg Ulcers. Adv. Skin Wound Care 2010, 23, 369–379. [Google Scholar] [CrossRef]
- Pastore, S.; Korkina, L. Redox Imbalance in T Cell-Mediated Skin Diseases. Mediat. Inflamm. 2010, 2010, 1–9. [Google Scholar] [CrossRef]
- Rippke, F.; Schreiner, V.; Doering, T.; Maibach, H.I. Stratum Corneum PH in Atopic Dermatitis: Impact on Skin Barrier Function and Colonization with Staphylococcus Aureus. Am. J. Clin. Dermatol. 2004, 5, 217–223. [Google Scholar] [CrossRef]
- Wagener, F.; Carels, C.; Lundvig, D. Targeting the Redox Balance in Inflammatory Skin Conditions. Int. J. Mol. Sci. 2013, 14, 9126–9167. [Google Scholar] [CrossRef]
- Dai, Y.-Q.; Qin, G.; Geng, S.-Y.; Yang, B.; Xu, Q.; Wang, J.-Y. Photo-Responsive Release of Ascorbic Acid and Catalase in CDBA-Liposome for Commercial Application as a Sunscreen Cosmetic. RSC Adv. 2012, 2, 3340. [Google Scholar] [CrossRef]
- Huang, X.; Wang, X.; Wang, S.; Yang, J.; Zhong, L.; Pan, J. UV and Dark-Triggered Repetitive Release and Encapsulation of Benzophenone-3 from Biocompatible ZnO Nanoparticles Potential for Skin Protection. Nanoscale 2013, 5, 5596. [Google Scholar] [CrossRef] [PubMed]
- Rancan, F.; Giulbudagian, M.; Jurisch, J.; Blume-Peytavi, U.; Calderón, M.; Vogt, A. Drug Delivery across Intact and Disrupted Skin Barrier: Identification of Cell Populations Interacting with Penetrated Thermoresponsive Nanogels. Eur. J. Pharm. Biopharm. 2017, 116, 4–11. [Google Scholar] [CrossRef]
- Javadzadeh, Y.; Azharshekoufeh Bahari, L. Therapeutic Nanostructures for Dermal and Transdermal Drug Delivery. In Nano- and Microscale Drug Delivery Systems; Elsevier: Oxford, UK, 2017; pp. 131–146. ISBN 978-0-323-52727-9. [Google Scholar]
- Bos, J.D.; Meinardi, M.M.H.M. The 500 Dalton Rule for the Skin Penetration of Chemical Compounds and Drugs: The 500 Dalton Rule for Skin Penetration of Chemical Compounds and Drugs. Exp. Dermatol. 2000, 9, 165–169. [Google Scholar] [CrossRef]
- Hadgraft, J. Skin Deep. Eur. J. Pharm. Biopharm. 2004, 58, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, U.; Ahmad, Z.; Khan, A.; Akhtar, J.; Singh, S.; Ahmad, F. Strategies in Development and Delivery of Nanotechnology Based Cosmetic Products. Drug Res. 2018, 68, 545–552. [Google Scholar] [CrossRef]
- Alkilani, A.; McCrudden, M.T.; Donnelly, R. Transdermal Drug Delivery: Innovative Pharmaceutical Developments Based on Disruption of the Barrier Properties of the Stratum Corneum. Pharmaceutics 2015, 7, 438–470. [Google Scholar] [CrossRef]
- Ramadon, D.; McCrudden, M.T.C.; Courtenay, A.J.; Donnelly, R.F. Enhancement Strategies for Transdermal Drug Delivery Systems: Current Trends and Applications. Drug Deliv. Transl. Res. 2021. [Google Scholar] [CrossRef]
- Dhamecha, D.; Rajendra, V.; Rathi, A.; Ghadlinge, S.; Saifee, M.; Dehghan, M. Physical Approaches to Penetration Enhancement. Int. J. Health Res. 2011, 3, 57–70. [Google Scholar] [CrossRef]
- Draelos, Z.K. Cosmetic Dermatology: Products and Procedures; Wiley Blackwell: Oxford, UK, 2016; ISBN 978-1-118-65546-7. [Google Scholar]
- Bharkatiya, M.; Nema, R. Skin Penetration Enhancement Techniques. J Young Pharm. 2009, 1, 110. [Google Scholar] [CrossRef]
- Kováčik, A.; Kopečná, M.; Vávrová, K. Permeation Enhancers in Transdermal Drug Delivery: Benefits and Limitations. Expert Opin. Drug Deliv. 2020, 17, 145–155. [Google Scholar] [CrossRef]
- Sood, N.; Bhardwaj, A.; Mehta, S.; Mehta, A. Stimuli-Responsive Hydrogels in Drug Delivery and Tissue Engineering. Drug Deliv. 2016, 23, 748–770. [Google Scholar] [CrossRef]
- Mota, A.H.; Rijo, P.; Molpeceres, J.; Reis, C.P. Broad Overview of Engineering of Functional Nanosystems for Skin Delivery. Int. J. Pharm. 2017, 532, 710–728. [Google Scholar] [CrossRef]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an Emerging Platform for Cancer Therapy. Nat. Nanotechnol. 2007, 2, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Abasian, P.; Ghanavati, S.; Rahebi, S.; Nouri Khorasani, S.; Khalili, S. Polymeric Nanocarriers in Targeted Drug Delivery Systems: A Review. Polym. Adv. Technol. 2020, 31, 2939–2954. [Google Scholar] [CrossRef]
- Soni, K.S.; Desale, S.S.; Bronich, T.K. Nanogels: An Overview of Properties, Biomedical Applications and Obstacles to Clinical Translation. J. Control Release 2016, 240, 109–126. [Google Scholar] [CrossRef]
- Lambers, H.; Piessens, S.; Bloem, A.; Pronk, H.; Finkel, P. Natural Skin Surface PH Is on Average below 5, Which Is Beneficial for Its Resident Flora. Int. J. Cosmet. Sci. 2006, 28, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Danby, S.G.; Cork, M.J. pH in Atopic Dermatitis. In Current Problems in Dermatology; Surber, C., Abels, C., Maibach, H., Eds.; S. Karger AG: Basel, Switzerland, 2018; Volume 54, pp. 95–107. ISBN 978-3-318-06384-4. [Google Scholar]
- Eberlein-König, T.; Schäfer, J.; Huss, B. Skin Surface PH, Stratum Corneum Hydration, Trans-Epidermal Water Loss and Skin Roughness Related to Atopic Eczema and Skin Dryness in a Population of Primary School Children: Clinical Report. Acta Derm. Venereol. 2000, 80, 188–191. [Google Scholar] [CrossRef]
- Sparavigna, A.; Setaro, M.; Gualandri, V. Cutaneous PH in Children Affected by Atopic Dermatitis and in Healthy Children: A Multicenter Study. Skin Res. Technol. 1999, 5, 221–227. [Google Scholar] [CrossRef]
- Seidenari, S.; Francomano, M.; Mantovani, L. Baseline Biophysical Parameters in Subjects with Sensitive Skin. Contact Dermat. 1998, 38, 311–315. [Google Scholar] [CrossRef]
- Runeman, J.; Faergemann, O.; Larkö, B. Experimental Candida Albicans Lesions in Healthy Humans: Dependence on Skin PH. Acta Derm. Venereol. 2000, 80, 421–424. [Google Scholar] [CrossRef]
- Schürer, N. pH and Acne. In Current Problems in Dermatology; Surber, C., Abels, C., Maibach, H., Eds.; S. Karger AG: Basel, Switzerland, 2018; Volume 54, pp. 115–122. ISBN 978-3-318-06384-4. [Google Scholar]
- Schneider, L.A.; Korber, A.; Grabbe, S.; Dissemond, J. Influence of PH on Wound-Healing: A New Perspective for Wound-Therapy? Arch. Dermatol. Res. 2007, 298, 413–420. [Google Scholar] [CrossRef]
- Sabitha, M.; Rejinold, N.S.; Nair, A.; Lakshmanan, V.-K.; Nair, S.V.; Jayakumar, R. Curcumin Loaded Chitin Nanogels for Skin Cancer Treatment via the Transdermal Route. Nanoscale 2012, 4, 239–250. [Google Scholar] [CrossRef]
- Sabitha, M.; Rejinold, N.S.; Nair, A.; Lakshmanan, V.-K.; Nair, S.V.; Jayakumar, R. Development and Evaluation of 5-Fluorouracil Loaded Chitin Nanogels for Treatment of Skin Cancer. Carbohydr. Polym. 2013, 91, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Sahu, P.; Kashaw, S.K.; Sau, S.; Kushwah, V.; Jain, S.; Agrawal, R.K.; Iyer, A.K. PH Responsive 5-Fluorouracil Loaded Biocompatible Nanogels For Topical Chemotherapy of Aggressive Melanoma. Colloids Surf. B Biointerfaces 2019, 174, 232–245. [Google Scholar] [CrossRef]
- Sahu, P.; Kashaw, S.K.; Sau, S.; Kushwah, V.; Jain, S.; Agrawal, R.K.; Iyer, A.K. PH Triggered and Charge Attracted Nanogel for Simultaneous Evaluation of Penetration and Toxicity against Skin Cancer: In-Vitro and Ex-Vivo Study. Int. J. Biol. Macromol. 2019, 128, 740–751. [Google Scholar] [CrossRef]
- Sahu, P.; Kashaw, S.K.; Jain, S.; Sau, S.; Iyer, A.K. Assessment of Penetration Potential of PH Responsive Double Walled Biodegradable Nanogels Coated with Eucalyptus Oil for the Controlled Delivery of 5-Fluorouracil: In Vitro and Ex Vivo Studies. J. Control Release 2017, 253, 122–136. [Google Scholar] [CrossRef]
- Sahu, P.; Kashaw, S.K.; Kushwah, V.; Sau, S.; Jain, S.; Iyer, A.K. PH Responsive Biodegradable Nanogels for Sustained Release of Bleomycin. Bioorg. Med. Chem. 2017, 25, 4595–4613. [Google Scholar] [CrossRef]
- Jung, S.-M.; Yoon, G.H.; Lee, H.C.; Jung, M.H.; Yu, S.I.; Yeon, S.J.; Min, S.K.; Kwon, Y.S.; Hwang, J.H.; Shin, H.S. Thermodynamic Insights and Conceptual Design of Skin-Sensitive Chitosan Coated Ceramide/PLGA Nanodrug for Regeneration of Stratum Corneum on Atopic Dermatitis. Sci. Rep. 2015, 5, 18089. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Hui, P.C.; Wat, E.; Kan, C.; Leung, P.-C.; Wang, W. Drug Delivery System of Dual-Responsive PF127 Hydrogel with Polysaccharide-Based Nano-Conjugate for Textile-Based Transdermal Therapy. Carbohydr. Polym. 2020, 236, 116074. [Google Scholar] [CrossRef]
- Zhu, L.; Bratlie, K.M. PH Sensitive Methacrylated Chitosan Hydrogels with Tunable Physical and Chemical Properties. Biochem. Eng. J. 2018, 132, 38–46. [Google Scholar] [CrossRef]
- Klee, S.K.; Farwick, M.; Lersch, P. Triggered Release of Sensitive Active Ingredients upon Response to the Skin’s Natural PH. Colloids Surf. A Physicochem. Eng. Asp. 2009, 338, 162–166. [Google Scholar] [CrossRef]
- Jeong, H.J.; Nam, S.J.; Song, J.Y.; Park, S.N. Synthesis and Physicochemical Properties of PH-Sensitive Hydrogel Based on Carboxymethyl Chitosan/2-Hydroxyethyl Acrylate for Transdermal Delivery of Nobiletin. J. Drug Deliv. Sci. Technol. 2019, 51, 194–203. [Google Scholar] [CrossRef]
- Kwon, S.S.; Kong, B.J.; Park, S.N. Physicochemical Properties of PH-Sensitive Hydrogels Based on Hydroxyethyl Cellulose–Hyaluronic Acid and for Applications as Transdermal Delivery Systems for Skin Lesions. Eur. J. Pharm. Biopharm. 2015, 92, 146–154. [Google Scholar] [CrossRef]
- Kim, A.R.; Lee, S.L.; Park, S.N. Properties and in Vitro Drug Release of PH- and Temperature-Sensitive Double Cross-Linked Interpenetrating Polymer Network Hydrogels Based on Hyaluronic Acid/Poly (N-Isopropylacrylamide) for Transdermal Delivery of Luteolin. Int. J. Biol. Macromol. 2018, 118, 731–740. [Google Scholar] [CrossRef]
- Soriano-Ruiz, J.L.; Calpena-Campmany, A.C.; Silva-Abreu, M.; Halbout-Bellowa, L.; Bozal-de Febrer, N.; Rodríguez-Lagunas, M.J.; Clares-Naveros, B. Design and Evaluation of a Multifunctional Thermosensitive Poloxamer-Chitosan-Hyaluronic Acid Gel for the Treatment of Skin Burns. Int. J. Biol. Macromol. 2020, 142, 412–422. [Google Scholar] [CrossRef]
- Park, S.H.; Shin, H.S.; Park, S.N. A Novel PH-Responsive Hydrogel Based on Carboxymethyl Cellulose/2-Hydroxyethyl Acrylate for Transdermal Delivery of Naringenin. Carbohydr. Polym. 2018, 200, 341–352. [Google Scholar] [CrossRef]
- Sahle, F.F.; Gerecke, C.; Kleuser, B.; Bodmeier, R. Formulation and Comparative in Vitro Evaluation of Various Dexamethasone-Loaded PH-Sensitive Polymeric Nanoparticles Intended for Dermal Applications. Int. J. Pharm. 2017, 516, 21–31. [Google Scholar] [CrossRef]
- Ninan, N.; Forget, A.; Shastri, V.P.; Voelcker, N.H.; Blencowe, A. Antibacterial and Anti-Inflammatory PH-Responsive Tannic Acid-Carboxylated Agarose Composite Hydrogels for Wound Healing. ACS Appl. Mater. Interfaces 2016, 8, 28511–28521. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Zhang, H.; Song, T.; Liu, X.; Gao, Y.; Zhou, J.; Li, Y. Sustainable Dual Release of Antibiotic and Growth Factor from PH-Responsive Uniform Alginate Composite Microparticles to Enhance Wound Healing. ACS Appl. Mater. Interfaces 2019, 11, 22730–22744. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, M.E.; Cuestas, M.L.; Pérez, C.J.; Campo Dall′ Orto, V.; Copello, G.J. Smart Release of Antimicrobial ZnO Nanoplates from a PH-Responsive Keratin Hydrogel. J. Colloid Interface Sci. 2019, 536, 372–380. [Google Scholar] [CrossRef]
- Banerjee, I.; Mishra, D.; Das, T.; Maiti, T.K. Wound PH-Responsive Sustained Release of Therapeutics from a Poly(NIPAAm-Co-AAc) Hydrogel. J. Biomater. Sci. Polym. Ed. 2012, 23, 111–132. [Google Scholar] [CrossRef] [PubMed]
- Koehler, J.; Wallmeyer, L.; Hedtrich, S.; Goepferich, A.M.; Brandl, F.P. PH-Modulating Poly(Ethylene Glycol)/Alginate Hydrogel Dressings for the Treatment of Chronic Wounds. Macromol. Biosci. 2017, 17, 1600369. [Google Scholar] [CrossRef]
- Zhu, J.; Han, H.; Ye, T.-T.; Li, F.-X.; Wang, X.-L.; Yu, J.-Y.; Wu, D.-Q. Biodegradable and PH Sensitive Peptide Based Hydrogel as Controlled Release System for Antibacterial Wound Dressing Application. Molecules 2018, 23, 3383. [Google Scholar] [CrossRef] [PubMed]
- Sahle, F.F.; Balzus, B.; Gerecke, C.; Kleuser, B.; Bodmeier, R. Formulation and in Vitro Evaluation of Polymeric Enteric Nanoparticles as Dermal Carriers with PH-Dependent Targeting Potential. Eur. J. Pharm. Sci. 2016, 92, 98–109. [Google Scholar] [CrossRef]
- Mavuso, S.; Marimuthu, T.; Kumar, P.; Kondiah, P.P.D.; du Toit, L.C.; Choonara, Y.E.; Pillay, V. In Vitro, Ex Vivo, and In Vivo Evaluation of a Dual PH/Redox Responsive Nanoliposomal Sludge for Transdermal Drug Delivery. J. Pharm. Sci. 2018, 107, 1028–1036. [Google Scholar] [CrossRef]
- Yamazaki, N.; Sugimoto, T.; Fukushima, M.; Teranishi, R.; Kotaka, A.; Shinde, C.; Kumei, T.; Sumida, Y.; Munekata, Y.; Maruyama, K.; et al. Dual-Stimuli Responsive Liposomes Using PH- and Temperature-Sensitive Polymers for Controlled Transdermal Delivery. Polym. Chem. 2017, 8, 1507–1518. [Google Scholar] [CrossRef]
- Lopez, V.C.; Hadgraft, J.; Snowden, M.J. The Use of Colloidal Microgels as a (Trans)Dermal Drug Delivery System. Int. J. Pharm. 2005, 292, 137–147. [Google Scholar] [CrossRef]
- Lee, E.; Kim, B. Smart Delivery System for Cosmetic Ingredients Using PH-Sensitive Polymer Hydrogel Particles. Korean J. Chem. Eng. 2011, 28, 1347–1350. [Google Scholar] [CrossRef]
- Şen, M.; Uzun, C.; Güven, O. Controlled Release of Terbinafine Hydrochloride from PH Sensitive Poly(Acrylamide/Maleic Acid) Hydrogels. Int. J. Pharm. 2000, 203, 149–157. [Google Scholar] [CrossRef]
- Vuković, J.S.; Perić-Grujić, A.A.; Mitić-Ćulafić, D.S.; Božić Nedeljković, B.D.; Tomić, S.L. Antibacterial Activity of PH-Sensitive Silver(I)/Poly(2-Hydroxyethyl Acrylate/Itaconic Acid) Hydrogels. Macromol. Res. 2020, 28, 382–389. [Google Scholar] [CrossRef]
- Dong, P.; Sahle, F.F.; Lohan, S.B.; Saeidpour, S.; Albrecht, S.; Teutloff, C.; Bodmeier, R.; Unbehauen, M.; Wolff, C.; Haag, R.; et al. PH-Sensitive Eudragit® L 100 Nanoparticles Promote Cutaneous Penetration and Drug Release on the Skin. J. Control Release 2019, 295, 214–222. [Google Scholar] [CrossRef]
- Ibarra-Montaño, E.L.; Rodríguez-Laguna, N.; Aníbal Sánchez-Hernández, A.; Rojas-Hernández, A. Determination of PKa Values for Acrylic, Methacrylic and Itaconic Acids by 1H and 13C NMR in Deuterated Water. J. Appl. Sol. Chem. Model. 2015, 4, 7–18. [Google Scholar] [CrossRef]
- Rizi, K.; Green, R.J.; Donaldson, M.X.; Williams, A.C. Using PH Abnormalities in Diseased Skin to Trigger and Target Topical Therapy. Pharm. Res. 2011, 28, 2589–2598. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, Q. Recent Development of Chitosan-Based Polyelectrolyte Complexes with Natural Polysaccharides for Drug Delivery. Int. J. Biol. Macromol. 2014, 64, 353–367. [Google Scholar] [CrossRef]
- Kelly, R. Keratins in wound healing. In Wound Healing Biomaterials; Elsevier: Oxford, UK, 2016; pp. 353–365. ISBN 978-1-78242-456-7. [Google Scholar]
- Peralta Ramos, M.L.; González, J.A.; Fabian, L.; Pérez, C.J.; Villanueva, M.E.; Copello, G.J. Sustainable and Smart Keratin Hydrogel with PH-Sensitive Swelling and Enhanced Mechanical Properties. Mater. Sci. Eng. C 2017, 78, 619–626. [Google Scholar] [CrossRef]
- Gulfam, M.; Sahle, F.F.; Lowe, T.L. Design Strategies for Chemical-Stimuli-Responsive Programmable Nanotherapeutics. Drug Discov. Today 2019, 24, 129–147. [Google Scholar] [CrossRef]
- Chen, W.; Meng, F.; Cheng, R.; Zhong, Z. PH-Sensitive Degradable Polymersomes for Triggered Release of Anticancer Drugs: A Comparative Study with Micelles. J. Control Release 2010, 142, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Liu, Y.; Zhou, M.; Wang, W.; Shi, M.; Xing, M.; Liao, W. Theranostic PH-Sensitive Nanoparticles for Highly Efficient Targeted Delivery of Doxorubicin for Breast Tumor Treatment. IJN 2018, 13, 1119–1137. [Google Scholar] [CrossRef]
- Quattrone, A.; Czajka, A.; Sibilla, S. Thermosensitive Hydrogel Mask Significantly Improves Skin Moisture and Skin Tone; Bilateral Clinical Trial. Cosmetics 2017, 4, 17. [Google Scholar] [CrossRef]
- Rozman, B.; Zvonar, A.; Falson, F.; Gasperlin, M. Temperature-Sensitive Microemulsion Gel: An Effective Topical Delivery System for Simultaneous Delivery of Vitamins C and E. AAPS Pharmscitech 2009, 10, 54–61. [Google Scholar] [CrossRef]
- Giulbudagian, M.; Hönzke, S.; Bergueiro, J.; Işık, D.; Schumacher, F.; Saeidpour, S.; Lohan, S.B.; Meinke, M.C.; Teutloff, C.; Schäfer-Korting, M.; et al. Enhanced Topical Delivery of Dexamethasone by β-Cyclodextrin Decorated Thermoresponsive Nanogels. Nanoscale 2018, 10, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.-H.; Chon, J.; Kim, Y.-I.; Lee, H.-J.; Oh, D.-W.; Lee, H.-G.; Han, C.-S.; Kim, D.-W.; Park, C.-W. Preparation and Evaluation of Tacrolimus-Loaded Thermosensitive Solid Lipid Nanoparticles for Improved Dermal Distribution. IJN 2019, 14, 5381–5396. [Google Scholar] [CrossRef] [PubMed]
- Witting, M.; Molina, M.; Obst, K.; Plank, R.; Eckl, K.M.; Hennies, H.C.; Calderón, M.; Frieß, W.; Hedtrich, S. Thermosensitive Dendritic Polyglycerol-Based Nanogels for Cutaneous Delivery of Biomacromolecules. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1179–1187. [Google Scholar] [CrossRef]
- Asadian-Birjand, M.; Bergueiro, J.; Rancan, F.; Cuggino, J.C.; Mutihac, R.-C.; Achazi, K.; Derneddle, J.; Blume-Peytayi, U.; Vogt, A.; Calderón, M. Engineering Thermoresponsive Polyether-Based Nanogels for Temperature Dependent Skin Penetration. Polym. Chem. 2015, 6, 5827–5831. [Google Scholar] [CrossRef]
- Klouda, L.; Mikos, A.G. Thermoresponsive Hydrogels in Biomedical Applications. Eur. J. Pharm. Biopharm. 2008, 68, 34–45. [Google Scholar] [CrossRef]
- Brugués, A.P.; Naveros, B.C.; Calpena Campmany, A.C.; Pastor, P.H.; Saladrigas, R.F.; Lizandra, C.R. Developing Cutaneous Applications of Paromomycin Entrapped in Stimuli-Sensitive Block Copolymer Nanogel Dispersions. Nanomedicine 2015, 10, 227–240. [Google Scholar] [CrossRef] [PubMed]
- Heilmann, S.; Küchler, S.; Wischke, C.; Lendlein, A.; Stein, C.; Schäfer-Korting, M. A Thermosensitive Morphine-Containing Hydrogel for the Treatment of Large-Scale Skin Wounds. Int. J. Pharm. 2013, 444, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Zhang, H.; Cheng, S.; Zhai, G.; Shen, C. Development of Curcumin Loaded Nanostructured Lipid Carrier Based Thermosensitive in Situ Gel for Dermal Delivery. Colloids Surf. A Physicochem. Eng. Asp. 2016, 506, 356–362. [Google Scholar] [CrossRef]
- Ferreira, N.N.; Ferreira, L.M.B.; Cardoso, V.M.O.; Boni, F.I.; Souza, A.L.R.; Gremião, M.P.D. Recent Advances in Smart Hydrogels for Biomedical Applications: From Self-Assembly to Functional Approaches. Eur. Polym. J. 2018, 99, 117–133. [Google Scholar] [CrossRef]
- Gandhi, A.; Paul, A.; Sen, S.O.; Sen, K.K. Studies on Thermoresponsive Polymers: Phase Behaviour, Drug Delivery and Biomedical Applications. Asian J. Pharm. Sci. 2015, 10, 99–107. [Google Scholar] [CrossRef]
- Ghosh Dastidar, D.; Chakrabarti, G. Thermoresponsive Drug Delivery Systems, Characterization and Application. In Applications of Targeted Nano Drugs and Delivery Systems; Elsevier: Oxford, UK, 2019; pp. 133–155. ISBN 978-0-12-814029-1. [Google Scholar]
- Spěváček, J. NMR Investigations of Phase Transition in Aqueous Polymer Solutions and Gels. Curr. Opin. Colloid Interface Sci. 2009, 14, 184–191. [Google Scholar] [CrossRef]
- Spěváček, J.; Konefał, R.; Dybal, J.; Čadová, E.; Kovářová, J. Thermoresponsive Behavior of Block Copolymers of PEO and PNIPAm with Different Architecture in Aqueous Solutions: A Study by NMR, FTIR, DSC and Quantum-Chemical Calculations. Eur. Polym. J. 2017, 94, 471–483. [Google Scholar] [CrossRef]
- Jadhav, S.A.; Scalarone, D.; Brunella, V.; Ugazio, E.; Sapino, S.; Berlier, G. Thermoresponsive Copolymer-Grafted SBA-15 Porous Silica Particles for Temperature-Triggered Topical Delivery Systems. Express Polym. Lett. 2017, 11, 96–105. [Google Scholar] [CrossRef]
- Parhi, R. Development and Optimization of Pluronic® F127 and HPMC Based Thermosensitive Gel for the Skin Delivery of Metoprolol Succinate. J. Drug Deliv. Sci. Technol. 2016, 36, 23–33. [Google Scholar] [CrossRef]
- Wang, W.; Wat, E.; Hui, P.C.L.; Chan, B.; Ng, F.S.F.; Kan, C.-W.; Wang, X.; Hu, H.; Wong, E.C.W.; Lau, C.B.S.; et al. Dual-Functional Transdermal Drug Delivery System with Controllable Drug Loading Based on Thermosensitive Poloxamer Hydrogel for Atopic Dermatitis Treatment. Sci. Rep. 2016, 6, 24112. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-Y.; Hui, P.; Wat, E.; Ng, F.; Kan, C.-W.; Lau, C.; Leung, P.-C. Enhanced Transdermal Permeability via Constructing the Porous Structure of Poloxamer-Based Hydrogel. Polymers 2016, 8, 406. [Google Scholar] [CrossRef]
- Pelegrino, M.T.; de Araújo, D.R.; Seabra, A.B. S-Nitrosoglutathione-Containing Chitosan Nanoparticles Dispersed in Pluronic F-127 Hydrogel: Potential Uses in Topical Applications. J. Drug Deliv. Sci. Technol. 2018, 43, 211–220. [Google Scholar] [CrossRef]
- Arafa, M.G.; El-Kased, R.F.; Elmazar, M.M. Thermoresponsive Gels Containing Gold Nanoparticles as Smart Antibacterial and Wound Healing Agents. Sci. Rep. 2018, 8, 13674. [Google Scholar] [CrossRef] [PubMed]
- Pham, L.; Dang, L.H.; Truong, M.D.; Nguyen, T.H.; Le, L.; Le, V.T.; Nam, N.D.; Bach, L.G.; Nguyen, V.T.; Tran, N.Q. A Dual Synergistic of Curcumin and Gelatin on Thermal-Responsive Hydrogel Based on Chitosan-P123 in Wound Healing Application. Biomed. Pharmacother. 2019, 117, 109183. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-C.; Fang, C.-L.; Al-Suwayeh, S.A.; Leu, Y.-L.; Fang, J.-Y. Transdermal Delivery of Selegiline from Alginate–Pluronic Composite Thermogels. Int. J. Pharm. 2011, 415, 119–128. [Google Scholar] [CrossRef]
- Osorio-Blanco, E.R.; Rancan, F.; Klossek, A.; Nissen, J.H.; Hoffmann, L.; Bergueiro, J.; Riedel, S.; Vogt, A.; Rühl, E.; Calderón, M. Polyglycerol-Based Thermoresponsive Nanocapsules Induce Skin Hydration and Serve as a Skin Penetration Enhancer. ACS Appl. Mater. Interfaces 2020, 12, 30136–30144. [Google Scholar] [CrossRef]
- Samah, N.H.A.; Heard, C.M. Enhanced in Vitro Transdermal Delivery of Caffeine Using a Temperature- and PH-Sensitive Nanogel, Poly(NIPAM-Co-AAc). Int. J. Pharm. 2013, 453, 630–640. [Google Scholar] [CrossRef]
- Ugazio, E.; Gastaldi, L.; Brunella, V.; Scalarone, D.; Jadhav, S.A.; Oliaro-Bosso, S.; Zonari, D.; Berlier, G.; Miletto, I.; Sapino, S. Thermoresponsive Mesoporous Silica Nanoparticles as a Carrier for Skin Delivery of Quercetin. Int. J. Pharm. 2016, 511, 446–454. [Google Scholar] [CrossRef]
- Gerecke, C.; Edlich, A.; Giulbudagian, M.; Schumacher, F.; Zhang, N.; Said, A.; Yealland, G.; Lohan, S.B.; Neumann, F.; Meinke, M.C.; et al. Biocompatibility and Characterization of Polyglycerol-Based Thermoresponsive Nanogels Designed as Novel Drug-Delivery Systems and Their Intracellular Localization in Keratinocytes. Nanotoxicology 2017, 11, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-Y.; Chen, K.-S.; Run-Chu, L. Design and Evaluation of Drug-Loaded Wound Dressing Having Thermoresponsive, Adhesive, Absorptive and Easy Peeling Properties. Biomaterials 2001, 22, 2999–3004. [Google Scholar] [CrossRef]
- Lopez, V.C.; Raghavan, S.L.; Snowden, M.J. Colloidal Microgels as Transdermal Delivery Systems. React. Funct. Polym. 2004, 58, 175–185. [Google Scholar] [CrossRef]
- Haddow, P.; McAuley, W.J.; Kirton, S.B.; Cook, M.T. Poly(N-Isopropyl Acrylamide)–Poly(Ethylene Glycol)–Poly(N-Isopropyl Acrylamide) as a Thermoreversible Gelator for Topical Administration. Mater. Adv. 2020, 1, 371–386. [Google Scholar] [CrossRef]
- op ‘t Veld, R.C.; van den Boomen, O.I.; Lundvig, D.M.S.; Bronkhorst, E.M.; Kouwer, P.H.J.; Jansen, J.A.; Middelkoop, E.; Von den Hoff, J.W.; Rowan, A.E.; Wagener, F.A.D.T.G. Thermosensitive Biomimetic Polyisocyanopeptide Hydrogels May Facilitate Wound Repair. Biomaterials 2018, 181, 392–401. [Google Scholar] [CrossRef]
- Indulekha, S.; Arunkumar, P.; Bahadur, D.; Srivastava, R. Thermoresponsive Polymeric Gel as an On-Demand Transdermal Drug Delivery System for Pain Management. Mater. Sci. Eng. C 2016, 62, 113–122. [Google Scholar] [CrossRef]
- Zavgorodnya, O.; Carmona-Moran, C.A.; Kozlovskaya, V.; Liu, F.; Wick, T.M.; Kharlampieva, E. Temperature-Responsive Nanogel Multilayers of Poly(N-Vinylcaprolactam) for Topical Drug Delivery. J. Colloid Interface Sci. 2017, 506, 589–602. [Google Scholar] [CrossRef]
- Liu, M.; Chen, W.; Zhang, X.; Su, P.; Yue, F.; Zeng, S.; Du, S. Improved Surface Adhesion and Wound Healing Effect of Madecassoside Liposomes Modified by Temperature-Responsive PEG-PCL-PEG Copolymers. Eur. J. Pharm. Sci. 2020, 151, 105373. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hu, H.; Yang, Z.; He, L.; Kong, Y.; Fei, B.; Xin, J.H. Smart Hydrogel-Functionalized Textile System with Moisture Management Property for Skin Application. Smart Mater. Struct. 2014, 23, 125027. [Google Scholar] [CrossRef]
- Asadian-Birjand, M.; Sousa-Herves, A.; Steinhilber, D.; Cuggino, J.C.; Calderon, M. Functional Nanogels for Biomedical Applications. CMC 2012, 19, 5029–5043. [Google Scholar] [CrossRef]
- Calderon, M.; Quadir, M.A.; Sharma, S.K.; Haag, R. Dendritic Polyglycerols for Biomedical Applications. Adv. Mater. 2010, 22, 190–218. [Google Scholar] [CrossRef]
- Wadajkar, A.S.; Koppolu, B.; Rahimi, M.; Nguyen, K.T. Cytotoxic Evaluation of N-Isopropylacrylamide Monomers and Temperature-Sensitive Poly(N-Isopropylacrylamide) Nanoparticles. J. Nanopart Res. 2009, 11, 1375–1382. [Google Scholar] [CrossRef]
- Lutz, J.-F.; Akdemir, Ö.; Hoth, A. Point by Point Comparison of Two Thermosensitive Polymers Exhibiting a Similar LCST: Is the Age of Poly(NIPAM) Over? J. Am. Chem. Soc. 2006, 128, 13046–13047. [Google Scholar] [CrossRef]
- Lutz, J.-F.; Hoth, A. Preparation of Ideal PEG Analogues with a Tunable Thermosensitivity by Controlled Radical Copolymerization of 2-(2-Methoxyethoxy)Ethyl Methacrylate and Oligo(Ethylene Glycol) Methacrylate. Macromolecules 2006, 39, 893–896. [Google Scholar] [CrossRef]
- Thomas, A.; Müller, S.S.; Frey, H. Beyond Poly(Ethylene Glycol): Linear Polyglycerol as a Multifunctional Polyether for Biomedical and Pharmaceutical Applications. Biomacromolecules 2014, 15, 1935–1954. [Google Scholar] [CrossRef] [PubMed]
- Giulbudagian, M.; Yealland, G.; Hönzke, S.; Edlich, A.; Geisendörfer, B.; Kleuser, B.; Hedtrich, S.; Calderón, M. Breaking the Barrier—Potent Anti-Inflammatory Activity Following Efficient Topical Delivery of Etanercept Using Thermoresponsive Nanogels. Theranostics 2018, 8, 450–463. [Google Scholar] [CrossRef] [PubMed]
- Giulbudagian, M.; Rancan, F.; Klossek, A.; Yamamoto, K.; Jurisch, J.; Neto, V.C.; Schrade, P.; Bachmann, S.; Rühl, E.; Blume-Peytavi, U.; et al. Correlation between the Chemical Composition of Thermoresponsive Nanogels and Their Interaction with the Skin Barrier. J. Control Release 2016, 243, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Kozlovskaya, V.; Kharlampieva, E. Self-Assemblies of Thermoresponsive Poly( N-Vinylcaprolactam) Polymers for Applications in Biomedical Field. ACS Appl. Polym. Mater. 2020, 2, 26–39. [Google Scholar] [CrossRef]
- Vihola, H.; Laukkanen, A.; Valtola, L.; Tenhu, H.; Hirvonen, J. Cytotoxicity of Thermosensitive Polymers Poly(N-Isopropylacrylamide), Poly(N-Vinylcaprolactam) and Amphiphilically Modified Poly(N-Vinylcaprolactam). Biomaterials 2005, 26, 3055–3064. [Google Scholar] [CrossRef] [PubMed]
- Alexandridis, P.; Alan Hatton, T. Poly(Ethylene Oxide)-Poly(Propylene Oxide)-Poly(Ethylene Oxide) Block Copolymer Surfactants in Aqueous Solutions and at Interfaces: Thermodynamics, Structure, Dynamics, and Modeling. Colloids Surf. A Physicochem. Eng. Asp. 1995, 96, 1–46. [Google Scholar] [CrossRef]
- Bej, R.; Achazi, K.; Haag, R.; Ghosh, S. Polymersome Formation by Amphiphilic Polyglycerol-b-Polydisulfide-b-Polyglycerol and Glutathione-Triggered Intracellular Drug Delivery. Biomacromolecules 2020, 21, 3353–3363. [Google Scholar] [CrossRef]
- Jiang, Z.; Liu, H.; He, H.; Ribbe, A.E.; Thayumanavan, S. Blended Assemblies of Amphiphilic Random and Block Copolymers for Tunable Encapsulation and Release of Hydrophobic Guest Molecules. Macromolecules 2020, 53, 2713–2723. [Google Scholar] [CrossRef]
- Jiang, Z.; Thayumanavan, S. Disulfide--Containing Macromolecules for Therapeutic Delivery. Isr. J. Chem. 2020, 60, 132–139. [Google Scholar] [CrossRef]
- Kuppusamy, P.; Li, H.; Ilangovan, G.; Cardounel, A.J.; Zweier, J.L.; Yamada, K.; Krishna, M.C.; Mitchell, J.B. Noninvasive Imaging of Tumor Redox Status and Its Modification by Tissue Glutathione Levels. Cancer Res. 2002, 62, 307–312. [Google Scholar]
- Rao, N.V.; Ko, H.; Lee, J.; Park, J.H. Recent Progress and Advances in Stimuli-Responsive Polymers for Cancer Therapy. Front. Bioeng. Biotechnol. 2018, 6, 110. [Google Scholar] [CrossRef] [PubMed]
- Wen, H. Redox Sensitive Nanoparticles with Disulfide Bond Linked Sheddable Shell for Intracellular Drug Delivery. Med. Chem. 2014, 4. [Google Scholar] [CrossRef]
- Van Gheluwe, L.; Buchy, E.; Chourpa, I.; Munnier, E. Three-Step Synthesis of a Redox-Responsive Blend of PEG–Block–PLA and PLA and Application to the Nanoencapsulation of Retinol. Polymers 2020, 12, 2350. [Google Scholar] [CrossRef]
- Kim, S.-E.; Lee, P.W.; Pokorski, J.K. Biologically Triggered Delivery of EGF from Polymer Fiber Patches. ACS Macro Lett. 2017, 6, 593–597. [Google Scholar] [CrossRef]
- Bawa, P.; Pillay, V.; Choonara, Y.E.; du Toit, L.C. Stimuli-Responsive Polymers and Their Applications in Drug Delivery. Biomed. Mater. 2009, 4, 022001. [Google Scholar] [CrossRef] [PubMed]
- Im, J.S.; Bai, B.C.; Lee, Y.-S. The Effect of Carbon Nanotubes on Drug Delivery in an Electro-Sensitive Transdermal Drug Delivery System. Biomaterials 2010, 31, 1414–1419. [Google Scholar] [CrossRef]
- Oktay, S.; Alemdar, N. Electrically Controlled Release of 5-Fluorouracil from Conductive Gelatin Methacryloyl-Based Hydrogels: Electrically Controlled Release of 5-Fluorouracil from Conductive Gelatin Methacryloyl-Based Hydrogels. J. Appl. Polym. Sci. 2019, 136, 46914. [Google Scholar] [CrossRef]
- Hu, W.; Qiu, L.; Cheng, L.; Hu, Q.; Liu, Y.; Hu, Z.; Chen, D.; Cheng, L. Redox and PH Dual Responsive Poly(Amidoamine) Dendrimer-Poly(Ethylene Glycol) Conjugates for Intracellular Delivery of Doxorubicin. Acta Biomater. 2016, 36, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Lurie, D.; Mader, K. Monitoring Drug Delivery Processes by EPR and Related Techniques—Principles and Applications. Adv. Drug Deliv. Rev. 2005, 57, 1171–1190. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wang, X.; Chen, S.; Li, S.; Liu, X. Acute Skin Barrier Disruption with Repeated Tape Stripping: An in Vivo Model for Damage Skin Barrier. Skin Res. Technol. 2013, 19, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Larese Filon, F.; Mauro, M.; Adami, G.; Bovenzi, M.; Crosera, M. Nanoparticles Skin Absorption: New Aspects for a Safety Profile Evaluation. Regul. Toxicol. Pharmacol. 2015, 72, 310–322. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.; Mohammed, Y.; Pastore, M.; Namjoshi, S.; Yousef, S.; Alinaghi, A.; Haridass, I.; Abd, E.; Leite-Silva, V.; Benson, H.; et al. Topical and Cutaneous Delivery Using Nanosystems. J. Control Release 2017, 247, 86–105. [Google Scholar] [CrossRef] [PubMed]
- Ramanunny, A.K.; Wadhwa, S.; Gulati, M.; Singh, S.K.; Kapoor, B.; Dureja, H.; Chellappan, D.K.; Anand, K.; Dua, K.; Khursheed, R.; et al. Nanocarriers for Treatment of Dermatological Diseases: Principle, Perspective and Practices. Eur. J. Pharmacol. 2021, 890, 173691. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.; Chen, J.; Yang, L.; Shi, L.; Tao, Q.; Hui, B.; Li, J. The Effect of PH on the LCST of Poly(N-Isopropylacrylamide) and Poly(N-Isopropylacrylamide-Co-Acrylic Acid). J. Biomater. Sci. Polym. Ed. 2004, 15, 585–594. [Google Scholar] [CrossRef]
- Dancik, Y.; Kichou, H.; Eklouh-Molinier, C.; Soucé, M.; Munnier, E.; Chourpa, I.; Bonnier, F. Freezing Weakens the Barrier Function of Reconstructed Human Epidermis as Evidenced by Raman Spectroscopy and Percutaneous Permeation. Pharmaceutics 2020, 12, 1041. [Google Scholar] [CrossRef]
- Neupane, R.; Boddu, S.H.S.; Renukuntla, J.; Babu, R.J.; Tiwari, A.K. Alternatives to Biological Skin in Permeation Studies: Current Trends and Possibilities. Pharmaceutics 2020, 12, 152. [Google Scholar] [CrossRef] [PubMed]
- Kazem, S.; Linssen, E.C.; Gibbs, S. Skin Metabolism Phase I and Phase II Enzymes in Native and Reconstructed Human Skin: A Short Review. Drug Discov. Today 2019, 24, 1899–1910. [Google Scholar] [CrossRef] [PubMed]
- Huet, F.; Severino-Freire, M.; Chéret, J.; Gouin, O.; Praneuf, J.; Pierre, O.; Misery, L.; Le Gall-Ianotto, C. Reconstructed Human Epidermis for in Vitro Studies on Atopic Dermatitis: A Review. J. Dermatol. Sci. 2018, 89, 213–218. [Google Scholar] [CrossRef] [PubMed]


| pH-Sensitive Polymers | Structure of the Monomer | Type of SDDS | Application | Ref. | |
|---|---|---|---|---|---|
| Cationic System | Chitosan (≅ chitin with % degree of de-acetylation) |  DA: degree of acetylation | Nanosystems | Topical therapy of skin cancers | [34,35,36,37,38,39] |
| Treatment for atopic dermatitis | [40] | ||||
| Hydrogels | Textile-based transdermal therapy | [41] | |||
| Wound dressings | [42] | ||||
| Dimethylaminoethyl-functional methacrylate Eudragit E100 |  | Microsystems | Skin care | [43] | |
| Anionic System | Carboxymethyl Chitosan |  | Hydrogels | Transdermal drug delivery system | [44] |
| Hyaluronic acid (HA) | 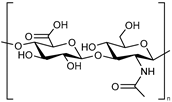 | Hydrogels | Transdermal delivery systems for skin lesions | [45] | |
| Transdermal delivery system for psoriasis skin relief | [46] | ||||
| Textile-based transdermal therapy | [41] | ||||
| Wound healing to treat skin burn lesions | [47] | ||||
| Carboxymethyl cellulose (CMC) | 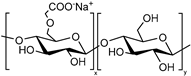 | Hydrogels | Potential treatment of atopic dermatitis | [48] | |
| Cellulose acetate phthalate |  | Nanosystems | Dermal carriers | [49] | |
| Hydroxypropyl methyl cellulose phthalate (HPMCP) |  | Nanosystems | Dermal carriers | [49] | |
| Carboxylated agarose | 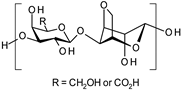 | Hydrogels | Wound dressings | [50] | |
| Alginate | 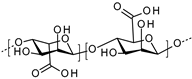 | Microsystems | Wound healing | [51] | |
| Keratin | - | Hydrogels | Antimicrobial wound dressing | [52] | |
| Anionic System | Poly(acrylic acid) | 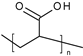 | Hydrogels | Wound healing | [53] |
| Treatment of chronic wounds | [54] | ||||
| Antibacterial wound dressing application | [55] | ||||
| Poly(methacrylic acid) | 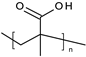 | Nanosystems | Dermal carriers | [49,56] | |
| Transdermal delivery system | [57] | ||||
| Delivering cosmetic agents to melanocytes | [58] | ||||
| Microgel | (Trans)dermal drug delivery system | [59] | |||
| Hydrogel | Smart delivery system for cosmetic ingredients | [60] | |||
| Poly(maleic acid) |  | Hydrogels | Local therapeutic transdermal delivery applications | [61] | |
| Poly(itaconic acid) | 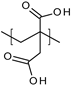 | Hydrogels | Treatment of bacterial infections | [62] | |
| Thermoresponsive Polymers | Monomer Structure | Type of SDDS | T°C Phase Transition | Application | Ref. |
|---|---|---|---|---|---|
| Poloxamers (or Pluronics®) | 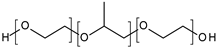 | Nanosystems | ~25 °C | Treatment of cutaneous Leishmanias. | [79] |
| - | Delivery drugs into deep skin layers | [75] | |||
| Hydrogels | - | Skin delivery against hypertension | [88] | ||
| 32 °C | Dermal delivery system | [81] | |||
| 37 °C | Atopic dermatitis treatment | [89,90] | |||
| ~20 °C | Topical formulations | [91] | |||
| 37 °C | Skin inflammation and wound healing | [92] | |||
| 36.7 °C | Wound healing application | [93] | |||
| ~24°C or 30.4 °C | Topical therapeutic formulation | [94] | |||
| 32 °C | Wound healing to treat skin burn lesions | [95] | |||
| 30 °C | Textile-based transdermal therapy | [41] | |||
| Poly(N-isopropylacrylamide)(PNIPAM) |  | Nanosystems | 31 to 37 °C | Topical drug delivery carrier | [96] |
| 35 °C | Topical delivery systems for biomacromolecules | [76] | |||
| ~33 °C | Dermal delivery | [97] | |||
| ~34 °C | Cutaneous drug delivery | [98] | |||
| 41.2 °C | Topical delivery systems | [87] | |||
| ~41 °C | Skin penetration enhancer | [95] | |||
| Microsystems | 30–34 °C | Develop a novel unique wound dressing | [99] | ||
| 34 °C | Transdermal delivery systems | [100] | |||
| Hydrogels | 35–36 °C | Wound healing | [53] | ||
| ~32 °C | Treatment for psoriasis skin relief | [46] | |||
| 36 °C | Topical administration (vaginal drug delivery) | [101] | |||
| Poly(N-isopropylmethacryl amide) (PNIPMAM) | 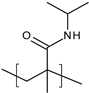 | Nanosystems | 34 °C | Skin penetration enhancer | [95] |
| Poly(ethyl glycidyl ether-co-methyl glycidyl ether) | 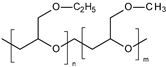 | Nanosystems | 34 °C | Treatment of severe skin diseases | [98] |
| ~34 °C | Treatment of inflammatory skin diseases | [10] | |||
| ~32 °C | Topical delivery into barrier-deficient skin | [74] | |||
| Poly(ethylene glycol) methacrylate | 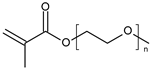 | Nanosystems | 36 °C | Applications in dermatotherapy and transdermal drug delivery | [77] |
| >35 °C | Delivering cosmetic agents to melanocytes | [58] | |||
| oligo(-ethylene glycol)-decorated polyisocyanopeptide (PIC) | 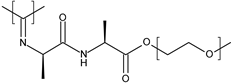 | Hydrogels | RT | Facilitate wound repair | [102] |
| Poly(N-vinyl caprolactam) (PVCL) |  | Hydrogels | 35 °C | Transdermal drug delivery system for pain management | [103] |
| ~32 °C | Develop skin-sensitive materials for topical drug delivery | [104] | |||
| PEG-PCL-PEG PEG: Poly(ethylene glycol) PCL: Poly(e-caprolactone) |  | Nanosystems | ~37 °C | Improve liposomes adhesion for wound healing | [105] |
| Hydrogels | 34 °C | Applications in skin care and wound treatment | [106] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van Gheluwe, L.; Chourpa, I.; Gaigne, C.; Munnier, E. Polymer-Based Smart Drug Delivery Systems for Skin Application and Demonstration of Stimuli-Responsiveness. Polymers 2021, 13, 1285. https://doi.org/10.3390/polym13081285
Van Gheluwe L, Chourpa I, Gaigne C, Munnier E. Polymer-Based Smart Drug Delivery Systems for Skin Application and Demonstration of Stimuli-Responsiveness. Polymers. 2021; 13(8):1285. https://doi.org/10.3390/polym13081285
Chicago/Turabian StyleVan Gheluwe, Louise, Igor Chourpa, Coline Gaigne, and Emilie Munnier. 2021. "Polymer-Based Smart Drug Delivery Systems for Skin Application and Demonstration of Stimuli-Responsiveness" Polymers 13, no. 8: 1285. https://doi.org/10.3390/polym13081285
APA StyleVan Gheluwe, L., Chourpa, I., Gaigne, C., & Munnier, E. (2021). Polymer-Based Smart Drug Delivery Systems for Skin Application and Demonstration of Stimuli-Responsiveness. Polymers, 13(8), 1285. https://doi.org/10.3390/polym13081285






