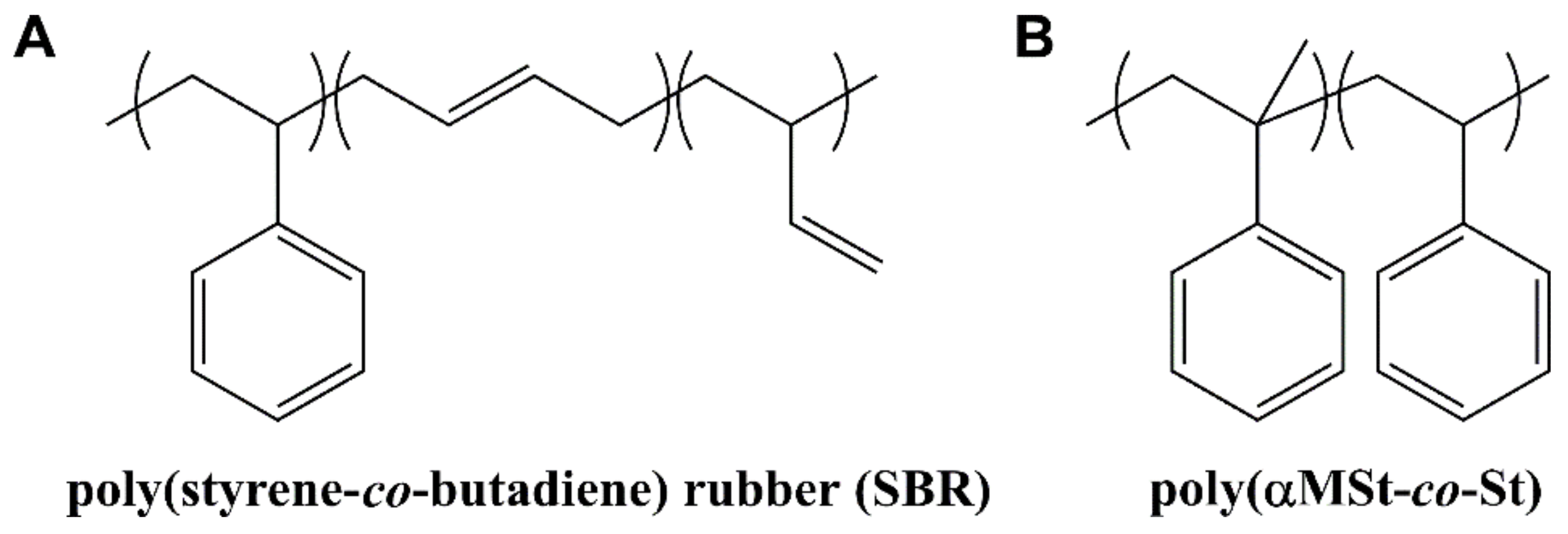
An Investigation on the Thermally Induced Compatibilization of SBR and α-Methylstyrene/Styrene Resin
Abstract
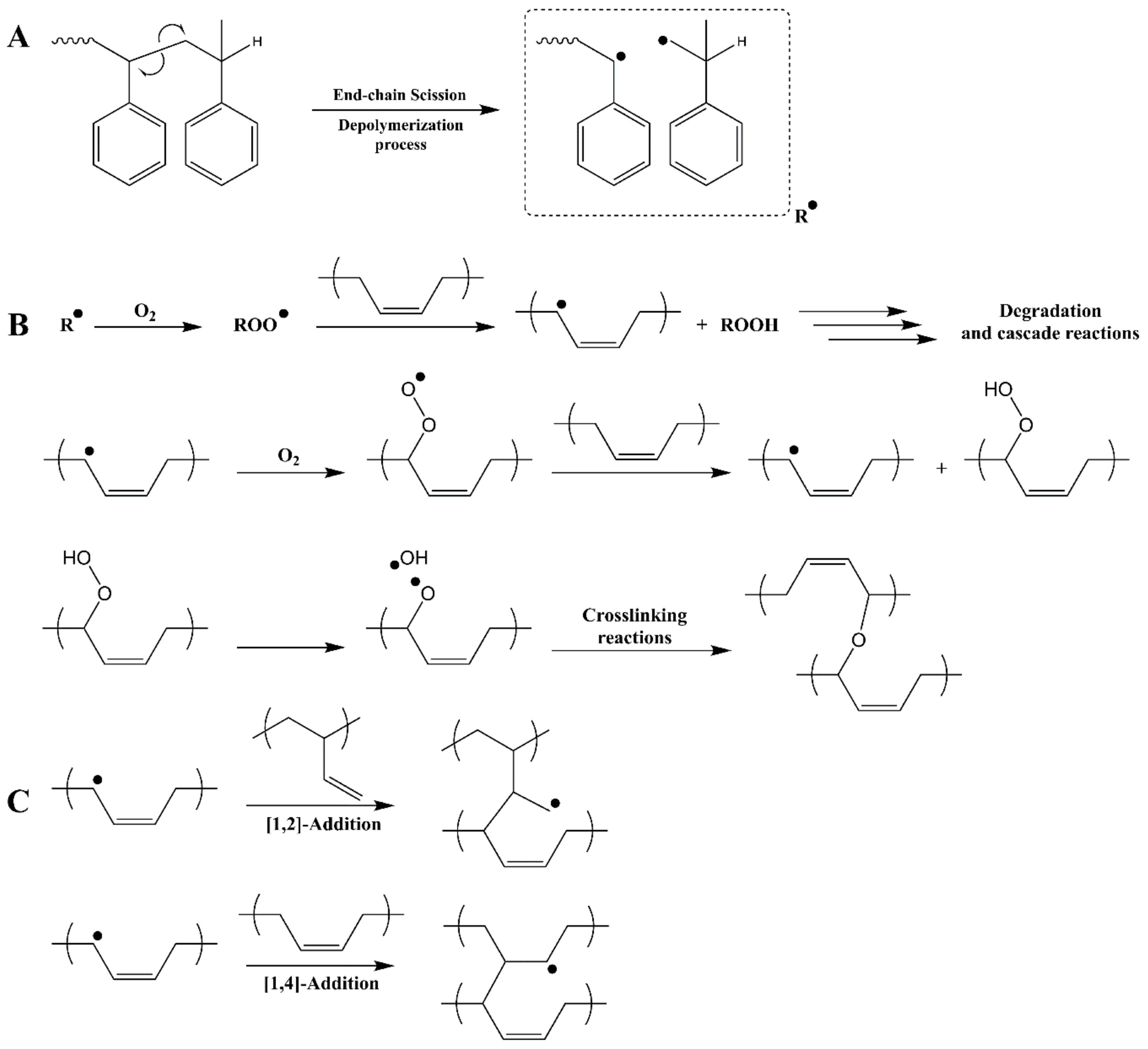
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Poly(αMSt-co-St) Resin
2.3. SBR/Poly(αMSt-co-St) Blending Procedure
2.4. Rheological Mesurements
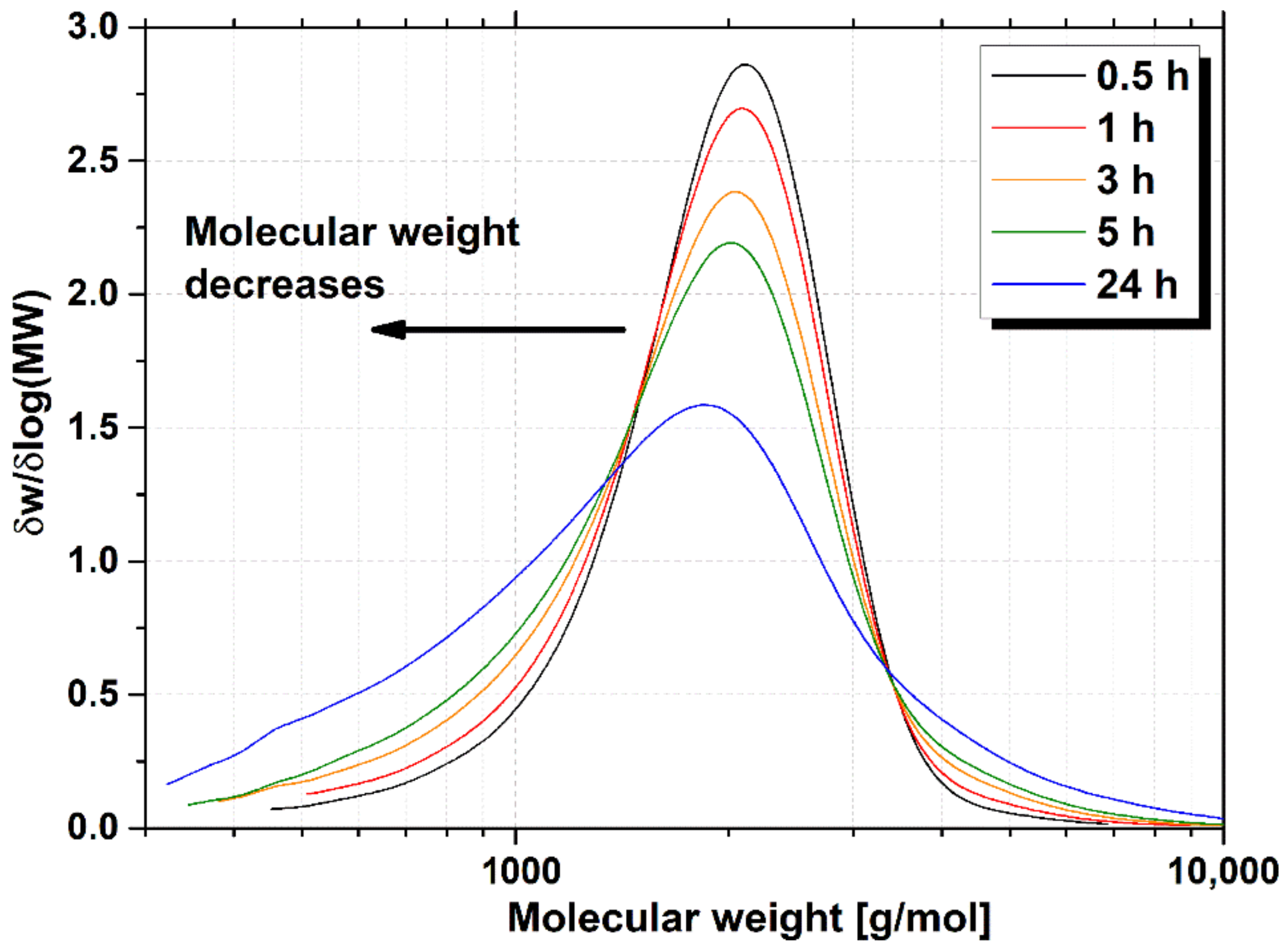
2.5. Gel Permeation Chromatography Measurements
2.6. Atomic Force Microscopy Measurements
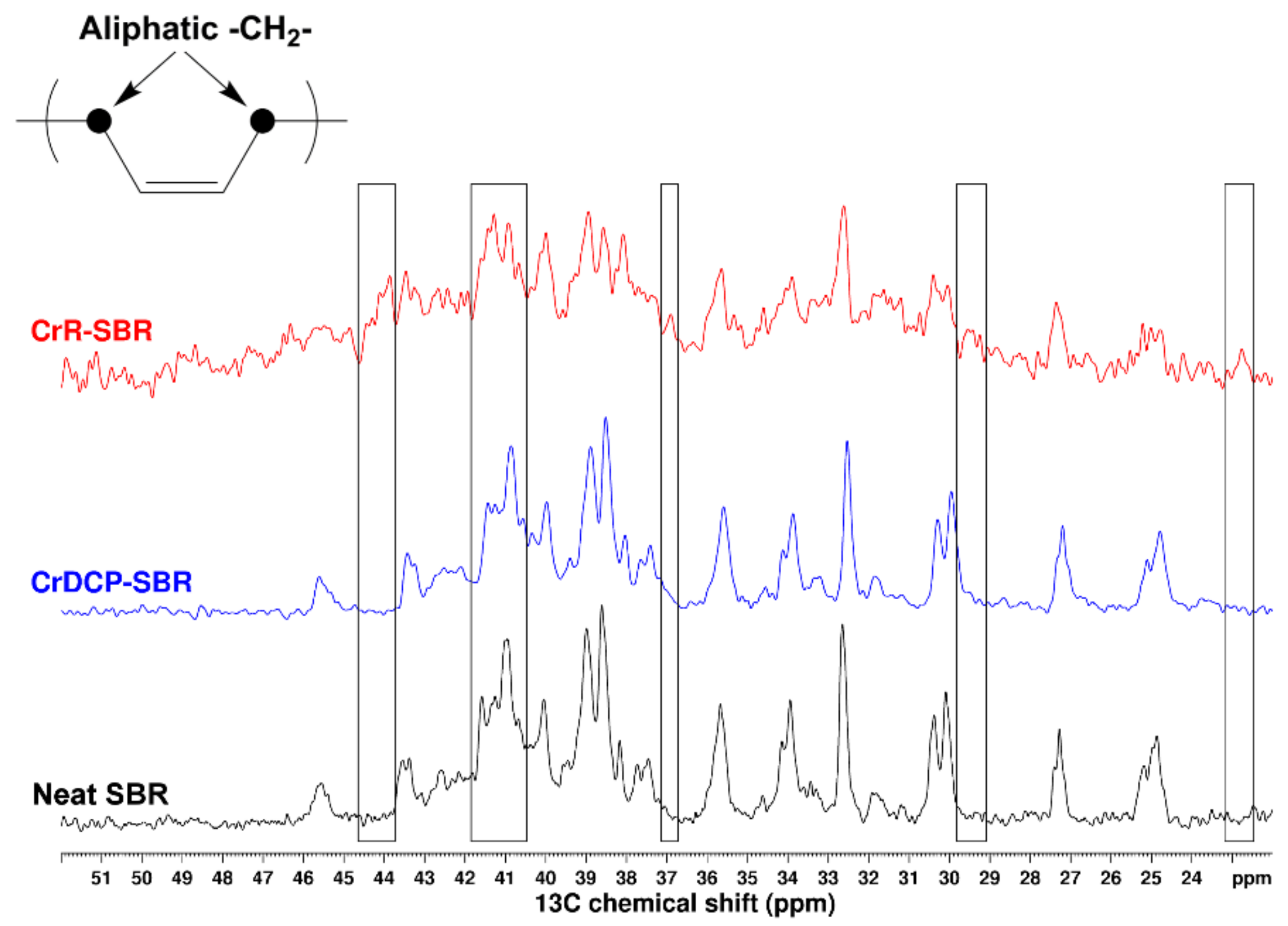
2.7. Nuclear Magnetic Resonance Measurements
3. Results
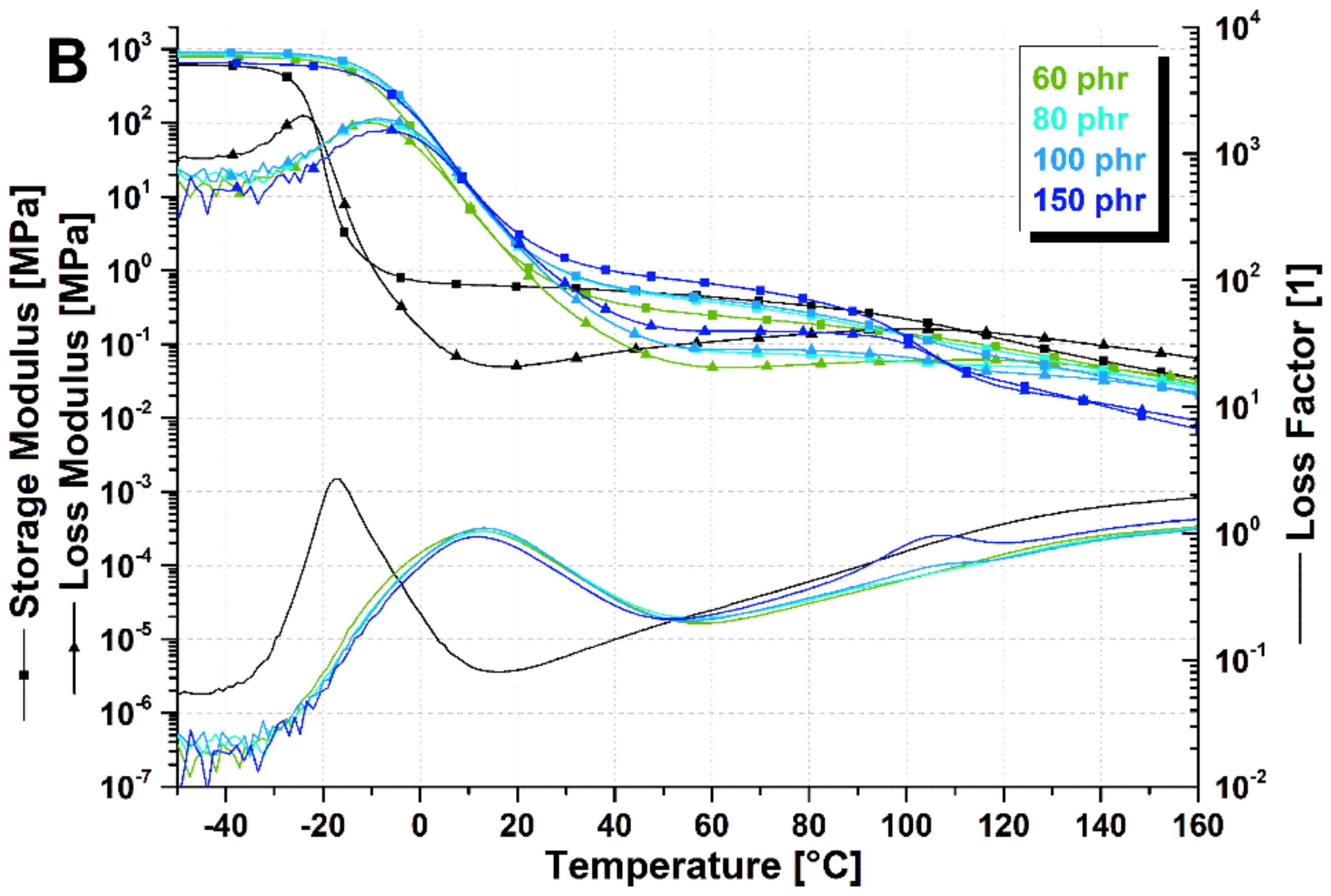
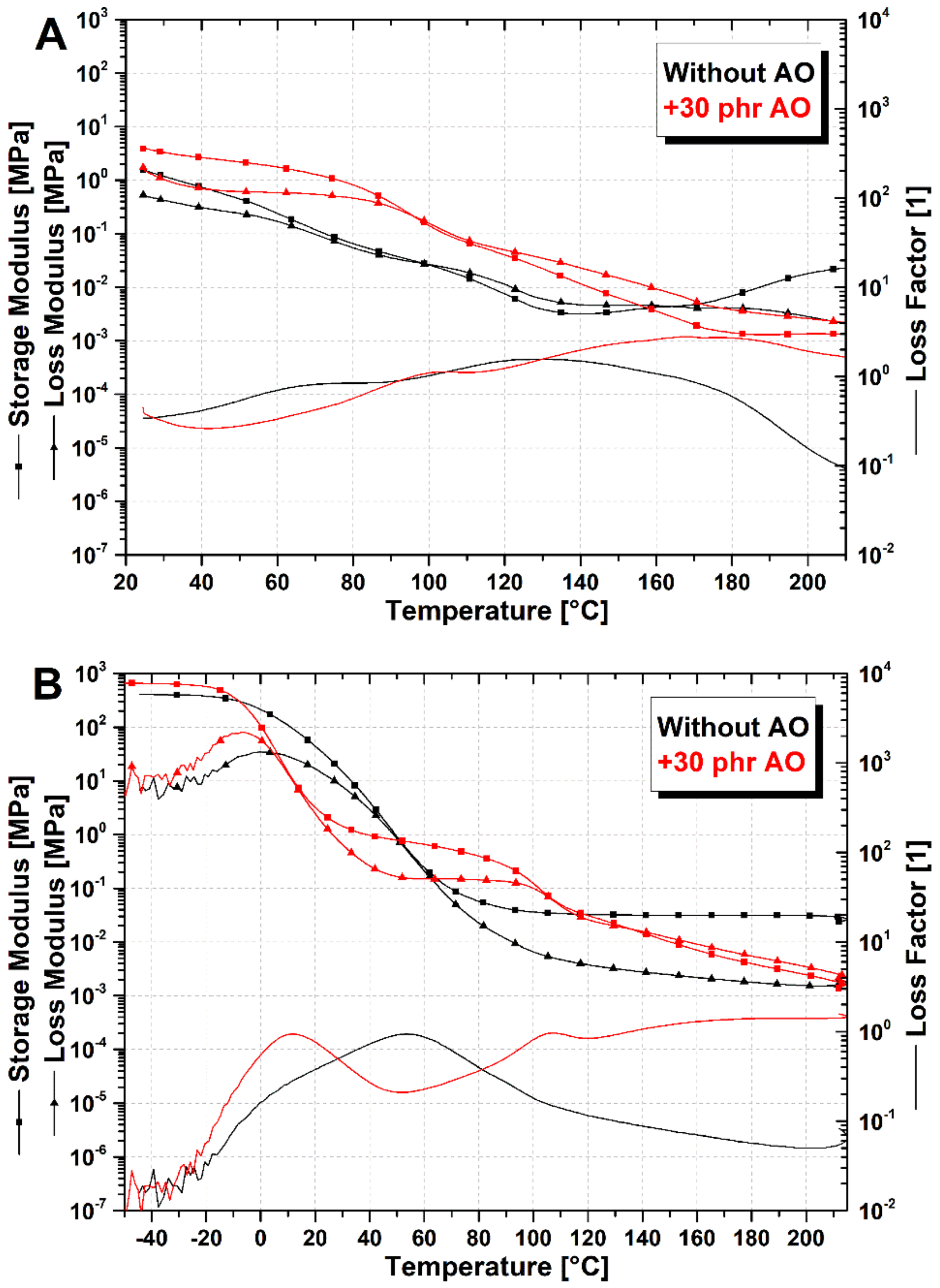
3.1. Viscoelastic Characterization of SBR/Poly(αMSt-co-St) Blends at Different Resin Concentrations
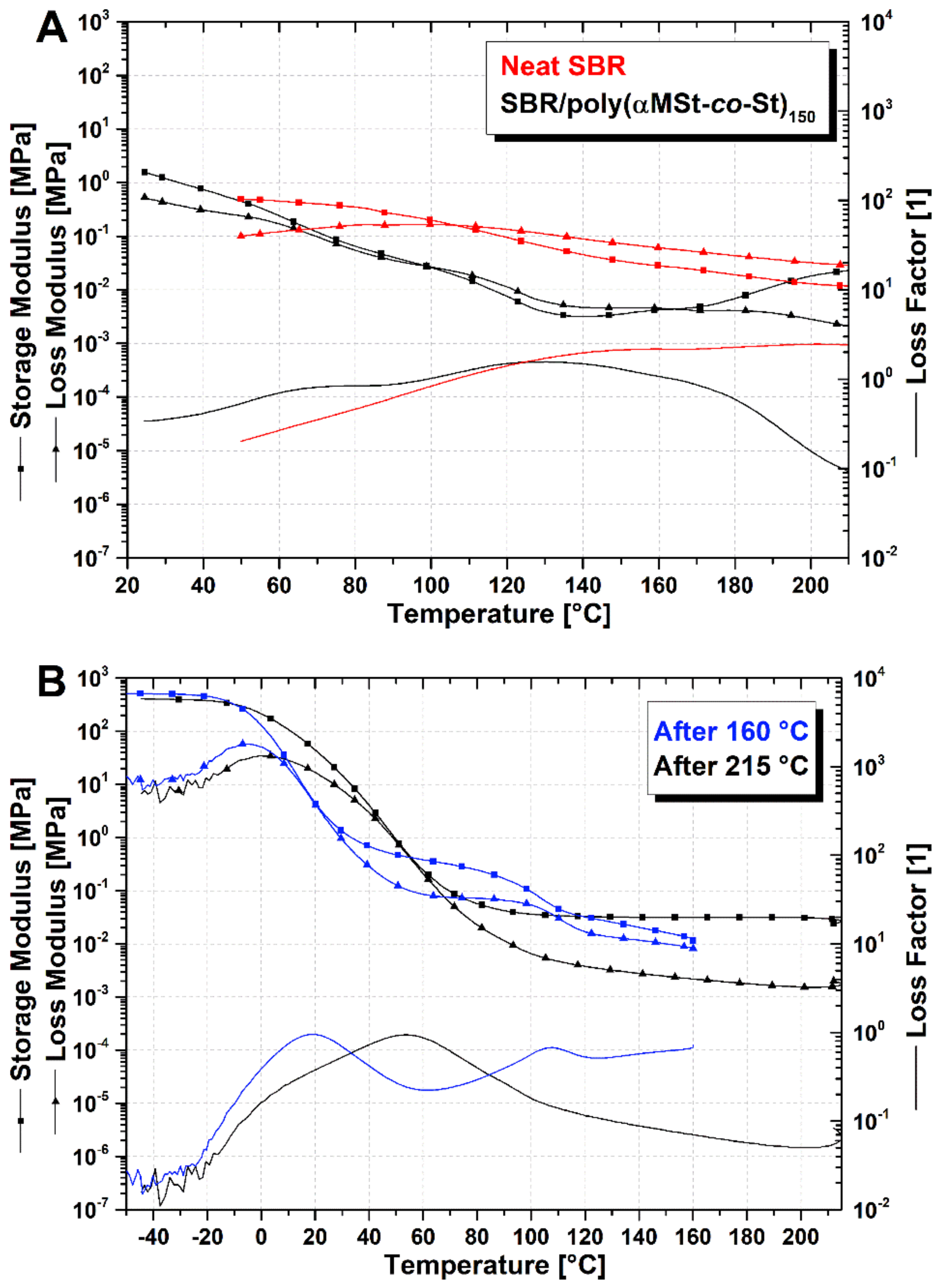
3.2. Evidence of SBR/Poly(αMSt-co-St)150 Blends Crosslinking and Enhanced Compatibility
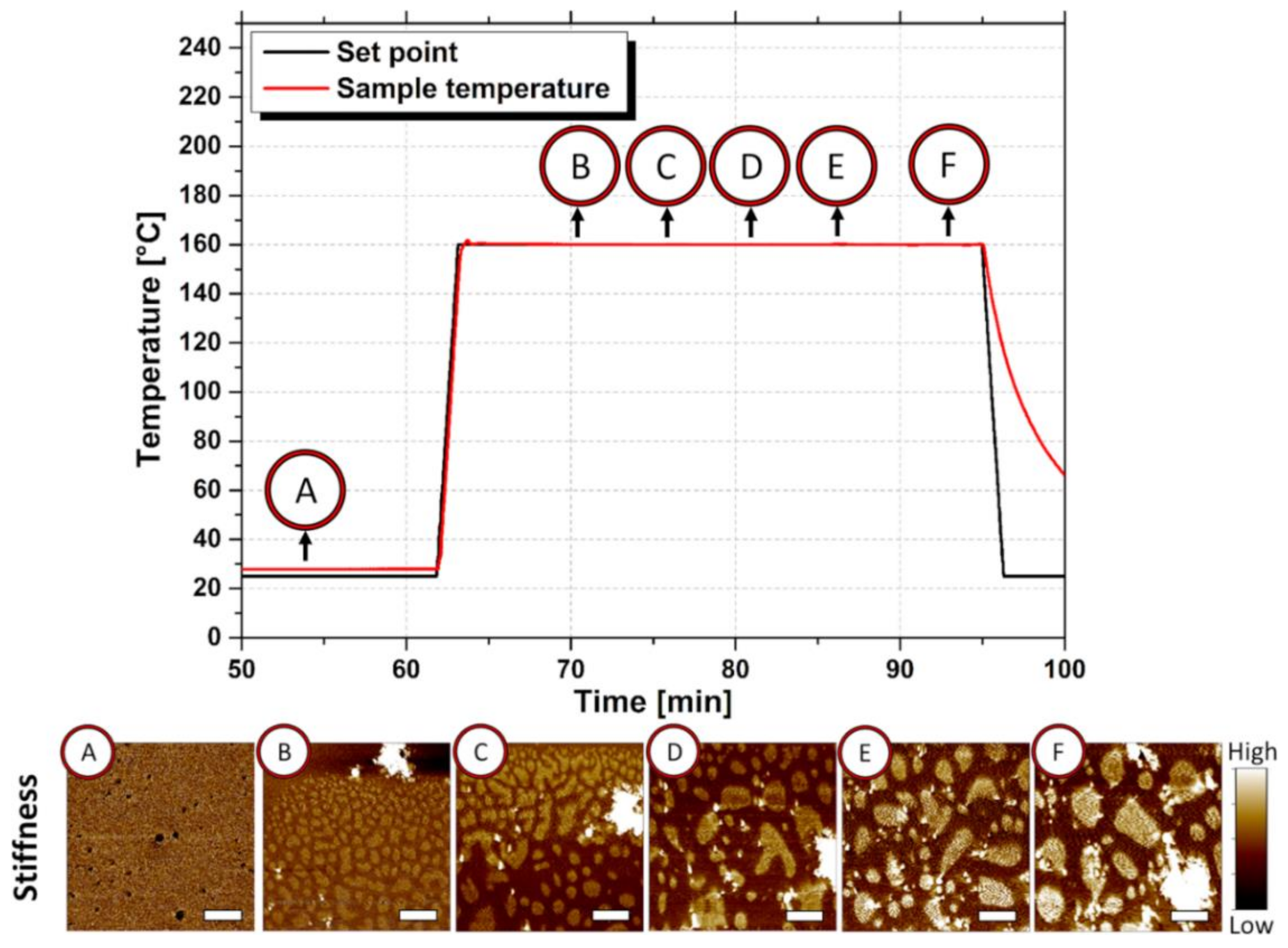
3.3. Atomic Force Microscopy Measurements of SBR/Poly(αMSt-co-St)150
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Paul, D.; Barlow, J. Polymer blends. J. Macromol. Sci. Macromol. Chem. 1980, 18, 109–168. [Google Scholar] [CrossRef]
- Paul, D.R.; Newman, S. Polymer Blends; Academic Press: New York, NY, USA, 1978; Volume 2, Available online: http://site.ebrary.com/id/10700820 (accessed on 22 April 2020).
- Painter, P.C.; Coleman, M.M. Essentials of Polymer Science and Engineering; DEStech Publications: Lancaster, PA, USA, 2009. [Google Scholar]
- Wilkie, C.A. Polymer Blends Handbook, 2nd ed.; Springer: Dordrecht, The Netherlands, 2014. [Google Scholar]
- Kawahara, S.; Akiyama, S.; Kano, Y. Miscibility and pressure-sensitive adhesive properties of poly (vinylethylene-co-1,4-butadiene)/terpene resin blends. Polymer 1991, 32, 1681–1687. [Google Scholar] [CrossRef]
- Fujita, M.; Kajiyama, M.; Takemura, A.; Ono, H.; Mizumachi, H.; Hayashi, S. Effects of miscibility on probe tack of natural-rubber-based pressure-sensitive adhesives. J. Appl. Polym. Sci. 1998, 70, 771–776. [Google Scholar] [CrossRef]
- Vleugels, N.; Pille-Wolf, W.; Dierkes, W.K.; Noordermeer, J.W.M. Understanding the Influence of Oligomeric Resins on Traction and Rolling Resistance of Silica-Reinforced Tire Treads. Rubber Chem. Technol. 2015, 88, 65–79. [Google Scholar] [CrossRef]
- Barlow, F.W. Rubber Compounding: Principles, Materials, and Techniques; Dekker, M., Ed.; EBSCO Information Services: New York, NY, USA, 1993; Available online: http://search.ebscohost.com/login.aspx?direct=true&scope=site&db=nlebk&db=nlabk&AN=47068 (accessed on 21 April 2020).
- Rodgers, B. Rubber Compounding: Chemistry and Applications; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Rodgers, B.; Waddell, W. Tire engineering. In The Science and Technology of Rubber; Elsevier: Amsterdam, The Netherlands, 2013; pp. 653–695. Available online: http://linkinghub.elsevier.com/retrieve/pii/B9780123945846000145 (accessed on 16 October 2017).
- Heinrich, G.; Vilgis, T. Why silica technology needs S-SBR in high performance tires? Kautsch. Gummi Kunstst. 2008, 61, 368–376. [Google Scholar]
- Hays, D.; Browne, A.L. The Physics of Tire Traction: Theory and Experiment; Springer: New York, NY, USA, 2013; Available online: https://public.ebookcentral.proquest.com/choice/publicfullrecord.aspx?p=5575702 (accessed on 9 April 2020).
- Kim, S.W.; Lee, G.H.; Heo, G.S. Identification of Tackifying Resins and Reinforcing Resins in Cured Rubber. Rubber Chem. Technol. 1999, 72, 181–198. [Google Scholar] [CrossRef]
- Powers, P.O. Resins Used in Rubber. Rubber Chem. Technol. 1963, 36, 1542–1570. [Google Scholar] [CrossRef]
- Class, J.B.; Chu, S.G. The viscoelastic properties of rubber–resin blends. I. The effect of resin structure. J. Appl. Polym. Sci. 1985, 30, 805–814. [Google Scholar] [CrossRef]
- Class, J.B.; Chu, S.G. The viscoelastic properties of rubber–resin blends. II. The effect of resin molecular weight. J. Appl. Polym. Sci. 1985, 30, 815–824. [Google Scholar] [CrossRef]
- Class, J.B.; Chu, S.G. The viscoelastic properties of rubber–resin blends. III. The effect of resin concentration. J. Appl. Polym. Sci. 1985, 30, 825–842. [Google Scholar] [CrossRef]
- Akiyama, S. Phase behavior and pressure sensitive adhesive properties in blends of poly (styrene-b-isoprene-b-styrene) with tackifier resin. Polymer 2000, 41, 4021–4027. [Google Scholar] [CrossRef]
- Aubrey, D.W.; Sherriff, M. Viscoelasticity of rubber-resin mixtures. J. Polym. Sci. Polym. Chem. Ed. 1978, 16, 2631–2643. [Google Scholar] [CrossRef]
- Aubrey, D.W.; Sherriff, M. Peel adhesion and viscoelasticity of rubber–resin blends. J. Polym. Sci. Polym. Chem. Ed. 1980, 18, 2597–2608. [Google Scholar] [CrossRef]
- Copley, B.C. Tackification Studies of Natural Rubber/Styrene-Butadiene Rubber Blends. Rubber Chem. Technol. 1982, 55, 416–427. [Google Scholar] [CrossRef]
- L’Heveder, S.; Sportelli, F.; Isitman, N.A. Investigation of solubility in plasticised rubber systems for tire applications. Plast. Rubber Compos. 2016, 45, 1–7. [Google Scholar] [CrossRef]
- Flory, P.J. Principles of Polymer Chemistry, 3rd ed.; Springer: New York, NY, USA, 1953. [Google Scholar]
- Rubinstein, M.; Colby, R.H. Polymer Physics; Oxford University Press: Oxford, UK; New York, NY, USA, 2003. [Google Scholar]
- Teraoka, I. Polymer solutions: An introduction to Physical Properties; Wiley: New York, NY, USA, 2002. [Google Scholar]
- Wolf, A.; Desport, J.S.; Dieden, R.; Frache, G.; Weydert, M.; Poorters, L.; Schmidt, D.F.; Verge, P. Sequence-Controlled α-Methylstyrene/Styrene Copolymers: Syntheses and Sequence Distribution Resolution. Macromolecules 2020, 53, 8032–8040. [Google Scholar] [CrossRef]
- Chen, W.; Kobayashi, S.; Inoue, T.; Ohnaga, T.; Ougizawa, T. Polymerization-induced spinodal decomposition of poly(ethylene-co-vinyl acetate)methyl methacrylate mixture and the influence of incorporating poly(vinyl acetate) macromonomer. Polymer 1994, 35, 4015–4021. [Google Scholar] [CrossRef]
- Yamanaka, K.; Takagi, Y.; Inoue, T. Reaction-induced phase separation in rubber-modified epoxy resins. Polymer 1989, 30, 1839–1844. [Google Scholar] [CrossRef]
- Yamanaka, K.; Inoue, T. Phase separation mechanism of rubber-modified epoxy. J. Mater. Sci. 1990, 25, 241–245. [Google Scholar] [CrossRef]
- Yamanaka, K.; Inoue, T. Structure development in epoxy resin modified with poly(ether sulphone). Polymer 1989, 30, 662–667. [Google Scholar] [CrossRef]
- Visconti, S.; Marchessault, R.H. Small Angle Light Scattering by Elastomer-Reinforced Epoxy Resins. Macromolecules 1974, 7, 913–917. [Google Scholar] [CrossRef]
- Okada, M.; Fujimoto, K.; Nose, T. Phase Separation Induced by Polymerization of 2-Chlorostyrene in a Polystyrene/Dibutyl Phthalate Mixture. Macromolecules 1995, 28, 1795–1800. [Google Scholar] [CrossRef]
- Kojima, T.; Ohnaga, T.; Inoue, T. Two-phases structure and mechanical properties of poly(methyl methacrylate)/poly(ethylene)-co-vinylacetate) alloys by polymerization-induced phase decomposition. Polymer 1995, 36, 2197–2201. [Google Scholar] [CrossRef]
- Manzione, L.T.; Gillham, J.K.; McPherson, C.A. Rubber-modified epoxies. I. Transitions and morphology. J. Appl. Polym. Sci. 1981, 26, 889–905. [Google Scholar] [CrossRef]
- Williams, M.L.; Landel, R.F.; Ferry, J.D. The Temperature Dependence of Relaxation Mechanisms in Amorphous Polymers and Other Glass-forming Liquids. J. Am. Chem. Soc. 1955, 77, 3701–3707. [Google Scholar] [CrossRef]
- Ferry, J.D. Viscoelastic Properties of Polymers, 3rd ed.; Wiley: New York, NY, USA, 1980. [Google Scholar]
- Mark, J.E. (Ed.) Polymer Data Handbook; Oxford University Press: New York, NY, USA, 1999. [Google Scholar]
- Bamford, C.H.; Tipper, C.F.H. Degradation of Polymers; Elsevier Scientific Publishing Co: Amsterdam, The Netherlands; New York, NY, USA, 1975. [Google Scholar]
- Guaita, M.; Chiantore, O. Molecular mass changes in the thermal degradation of poly-α-methylstyrene. Polym. Degrad. Stab. 1985, 11, 167–179. [Google Scholar] [CrossRef]
- Murakata, T.; Saito, Y.; Yosikawa, T.; Suzuki, T.; Sato, S. Solvent effect on thermal degradation of polystyrene and poly-α-methylstyrene. Polymer 1993, 34, 1436–1439. [Google Scholar] [CrossRef]
- Kruželák, J.; Sýkora, R.; Hudec, I. Vulcanization of Rubber Compounds with Peroxide Curing Systems. Rubber Chem. Technol. 2017, 90, 60–88. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, T.; Liu, Y.; Zhang, A.; Yuan, C.; Zhang, W. Cross-linking process of cis-polybutadiene rubber with peroxides studied by two-dimensional infrared correlation spectroscopy: A detailed tracking. RSC Adv. 2015, 5, 10231–10242. [Google Scholar] [CrossRef]
- Sasuga, T.; Takehisa, M. Effect of high pressure on radiation-induced cross-linking of synthetic rubbers. J. Macromol. Sci. Part B 1975, 11, 389–401. [Google Scholar] [CrossRef]
- Xing, W.; Li, H.; Huang, G.; Cai, L.-H.; Wu, J. Graphene oxide induced crosslinking and reinforcement of elastomers. Compos. Sci. Technol. 2017, 144, 223–229. [Google Scholar] [CrossRef]
- Valentin, J.L.; Rodriguez, A.; Marcos-Fernandez, A.; González, L. Dicumyl peroxide cross-linking of nitrile rubbers with different content in acrylonitrile. J. Appl. Polym. Sci. 2005, 96, 1–5. [Google Scholar] [CrossRef]
- Sperling, L.H. Interpenetrating polymer networks: An overview. In Interpenetrating Polymer Networks; Klempner, D., Sperling, L.H., Utracki, L.A., Eds.; American Chemical Society: Washington, DC, USA, 1994; pp. 3–38. [Google Scholar] [CrossRef]
- Sperling, L.H. Interpenetrating Polymer Networks and Related Materials, 1st ed.; Springer: Berlin/Heidelberg, Germany, 1981. [Google Scholar]
- Kim, S.C.; Sperling, L.H. (Eds.) IPNs around the World: Science and Engineering; John Wiley: Chichester, UK; New York, NY, USA, 1997. [Google Scholar]
- Wu, X.; He, G.; Yan, X.; Li, X.; Xiao, W.; Jiang, X. Miscibility, morphology, and phase behavior of IPNs. In Micro- and Nano-Structured Interpenetrating Polymer Networks; Thomas, S., Grande, D., Cvelbar, U., Raju, K.V.S.N., Narayan, R., Thomas, S.P., Akhina, H., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 29–68. [Google Scholar] [CrossRef]
- Lipatov, I.S.; Alekseeva, T. Phase-Separated Interpenetrating Polymer Networks; Springer: Berlin, Germany; New York, NY, USA, 2007. [Google Scholar]
- Rigoussen, A.; Verge, P.; Raquez, J.-M.; Dubois, P. Natural Phenolic Antioxidants As a Source of Biocompatibilizers for Immiscible Polymer Blends. ACS Sustain. Chem. Eng. 2018, 6, 13349–13357. [Google Scholar] [CrossRef]
- Rigoussen, A.; Verge, P.; Raquez, J.-M.; Habibi, Y.; Dubois, P. In-depth investigation on the effect and role of cardanol in the compatibilization of PLA/ABS immiscible blends by reactive extrusion. Eur. Polym. J. 2017, 93, 272–283. [Google Scholar] [CrossRef]



| Sample + Heat Treatment 1 | State | m Swell (g) | m Dry (g) | m Toluene (g) | %swelling | Ve × 104 (mol/cm3) 2 |
|---|---|---|---|---|---|---|
| Neat SBR after 215 °C | Soluble | - | - | - | - | - |
| SBR/poly(αMSt-co-St)150 after 160 °C | Soluble | - | - | - | - | - |
| SBR/poly(αMSt-co-St)150 after 215 °C | Swollen | 0.4062 | 0.0191 | 0.3871 | 2027 | 2.18 |
| Sample + Heat Treatment | Modulus (GPa) | Morphology | |
|---|---|---|---|
| SBR | AMS | ||
| Poly(αMSt-co-St) | - | 3.6 ± 0.4 | Single phase |
| Neat SBR | 0.80 ± 0.05 | - | Single phase |
| SBR/poly(αMSt-co-St)150 | 1.15 ± 0.20 | - | Single phase |
| SBR/poly(αMSt-co-St)150 after 160 °C | 1.80 ± 0.20 | 2.8 ± 0.4 | Dual phase |
| SBR/poly(αMSt-co-St)150 after 215 °C | 2.00 ± 0.35 | - | Single phase |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wolf, A.; Fernandes, J.P.C.; Yan, C.; Dieden, R.; Poorters, L.; Weydert, M.; Verge, P. An Investigation on the Thermally Induced Compatibilization of SBR and α-Methylstyrene/Styrene Resin. Polymers 2021, 13, 1267. https://doi.org/10.3390/polym13081267
Wolf A, Fernandes JPC, Yan C, Dieden R, Poorters L, Weydert M, Verge P. An Investigation on the Thermally Induced Compatibilization of SBR and α-Methylstyrene/Styrene Resin. Polymers. 2021; 13(8):1267. https://doi.org/10.3390/polym13081267
Chicago/Turabian StyleWolf, Arnaud, João Paulo Cosas Fernandes, Chuanyu Yan, Reiner Dieden, Laurent Poorters, Marc Weydert, and Pierre Verge. 2021. "An Investigation on the Thermally Induced Compatibilization of SBR and α-Methylstyrene/Styrene Resin" Polymers 13, no. 8: 1267. https://doi.org/10.3390/polym13081267
APA StyleWolf, A., Fernandes, J. P. C., Yan, C., Dieden, R., Poorters, L., Weydert, M., & Verge, P. (2021). An Investigation on the Thermally Induced Compatibilization of SBR and α-Methylstyrene/Styrene Resin. Polymers, 13(8), 1267. https://doi.org/10.3390/polym13081267






