Efficient Surface Immobilization of Chemically Modified Hyaluronans for Enhanced Bioactivity and Survival of In Vitro-Cultured Embryonic Salivary Gland Mesenchymal Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Amine-Functionalized HA (HA–Amine; HA–EDA)
2.3. Synthesis of Thiolated HA (HA–Thiol; HA–SH)
2.4. Synthesis of CA-Functionalized HA (HA–Catechol; HA–CA)
2.5. HA Derivative Coating on Polycarbonate Membrane
2.6. Characterization of Surfaces Coated with HA Derivatives
2.7. Isolation and In Vitro Culture of Mouse eSMG
2.8. mRNA Isolation and Quantitative Real-Time RT-PCR (qRT-PCR)
2.9. Separation of Mesenchyme and Formation of Mesenchymal Feeder Cell Layer
2.10. Immunofluorescence Staining
2.11. Imaging of In Vitro-Cultured Mouse eSMGs
2.12. Data Analysis
3. Results and Discussion
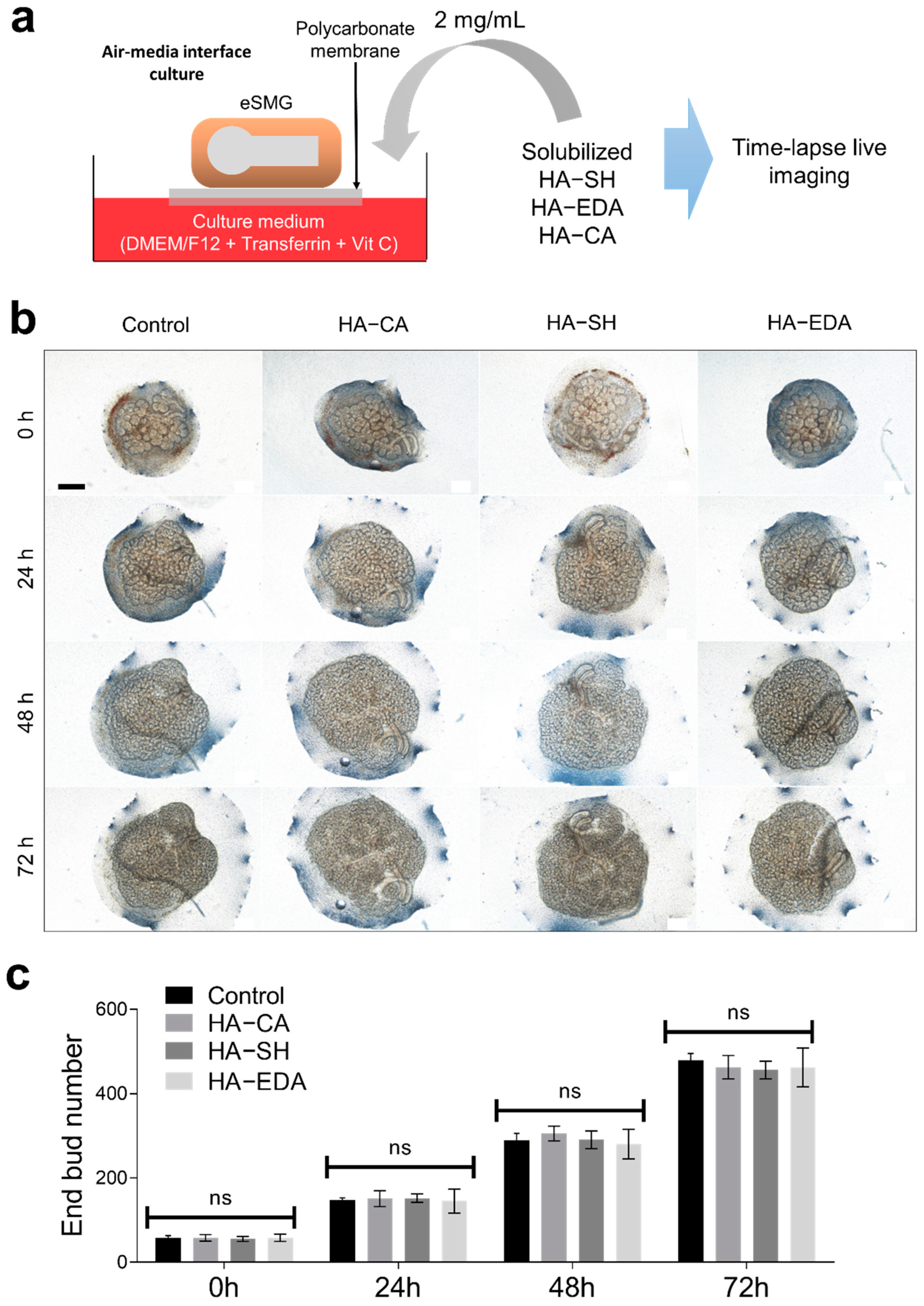
3.1. Synthesis of HA Derivatives and Their Coating Application
3.2. Biocompatibility of HA Derivatives for Embryonic Salivary Gland Culture
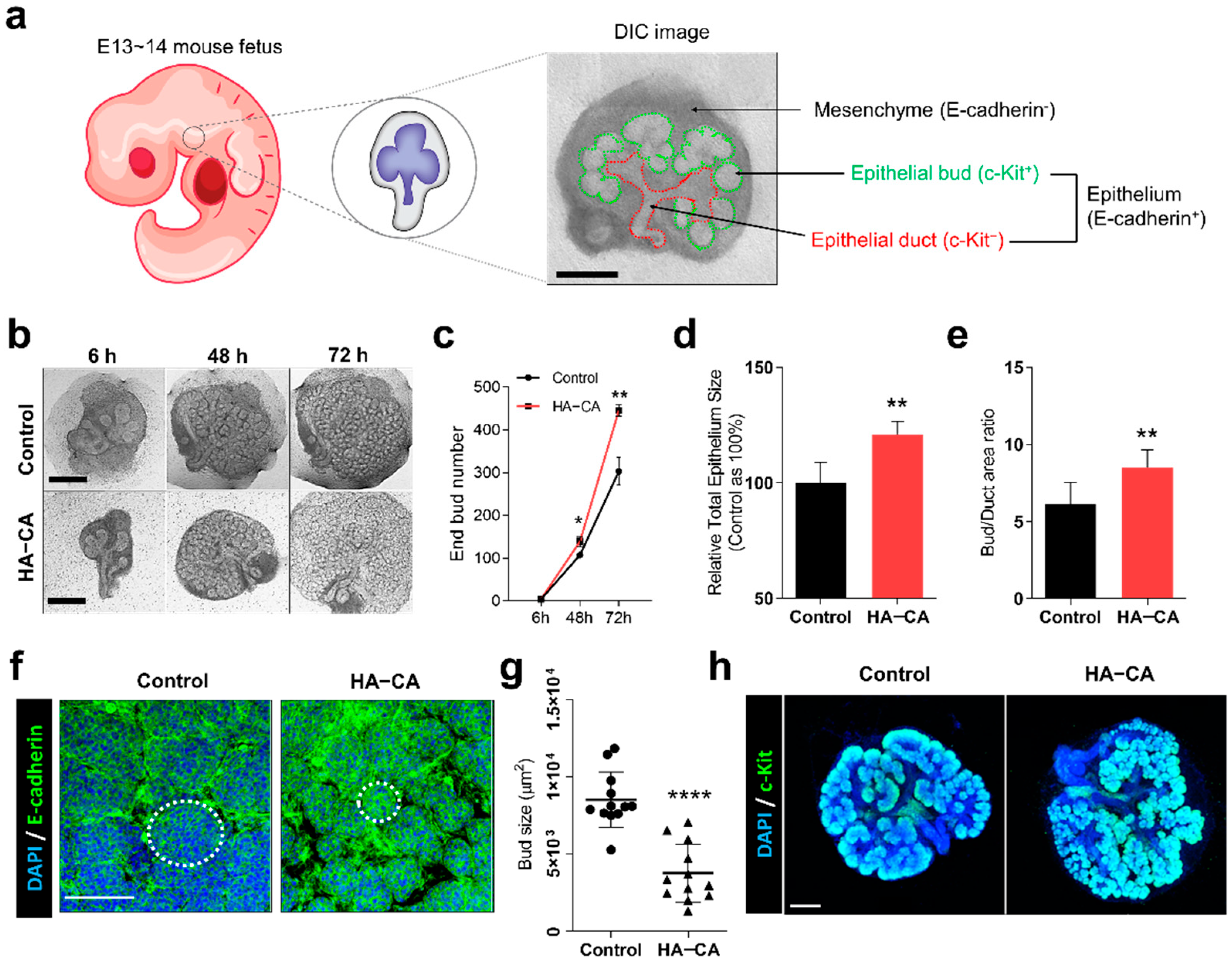
3.3. HA–CA Coating Specifically Enhances Proliferation of Epithelial Buds, But Not Ducts
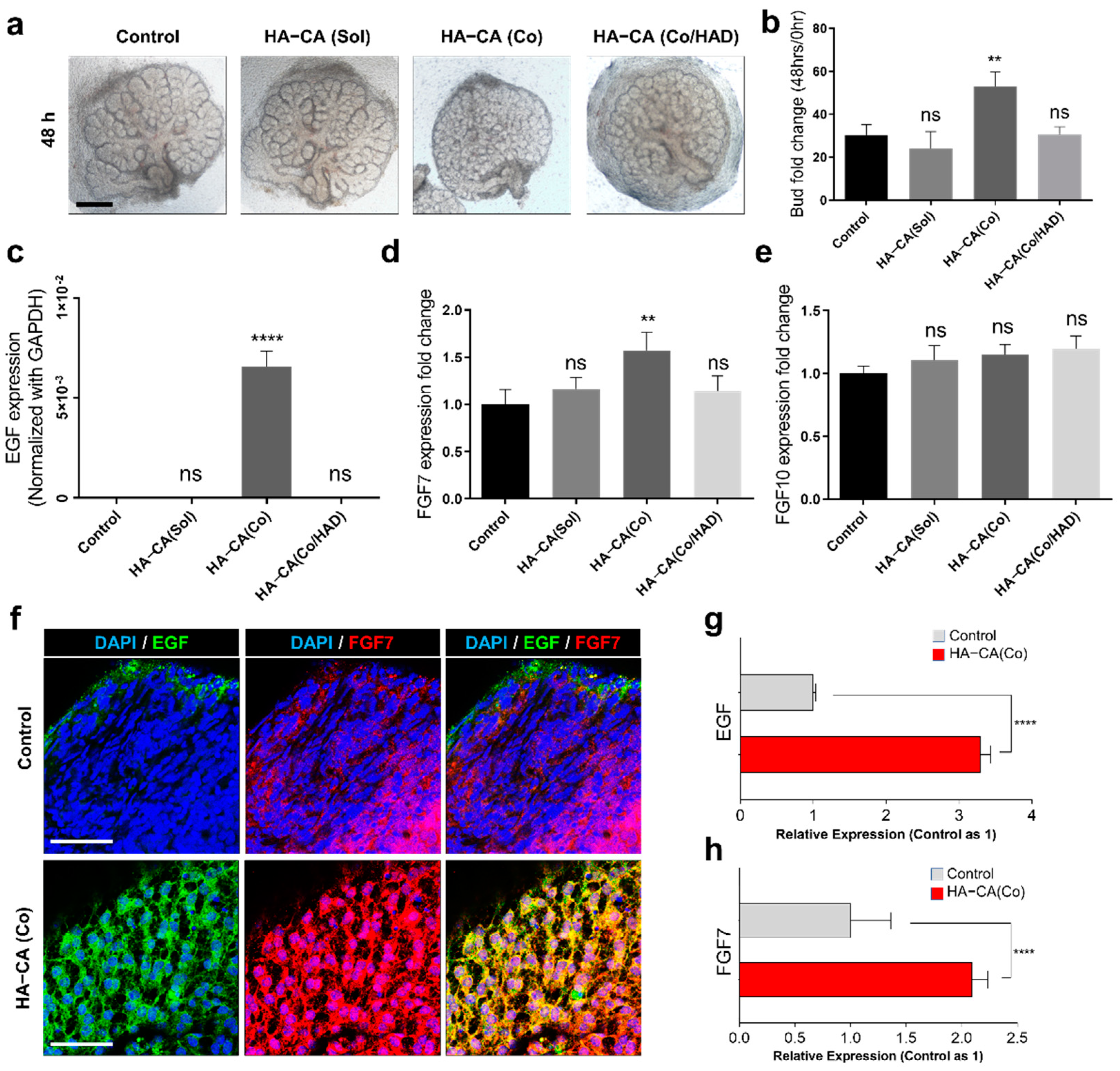
3.4. Immobilized HA–CA, Not Solubilized Form, Boosts EGF and FGF7 Production from eSMG Mesenchyme
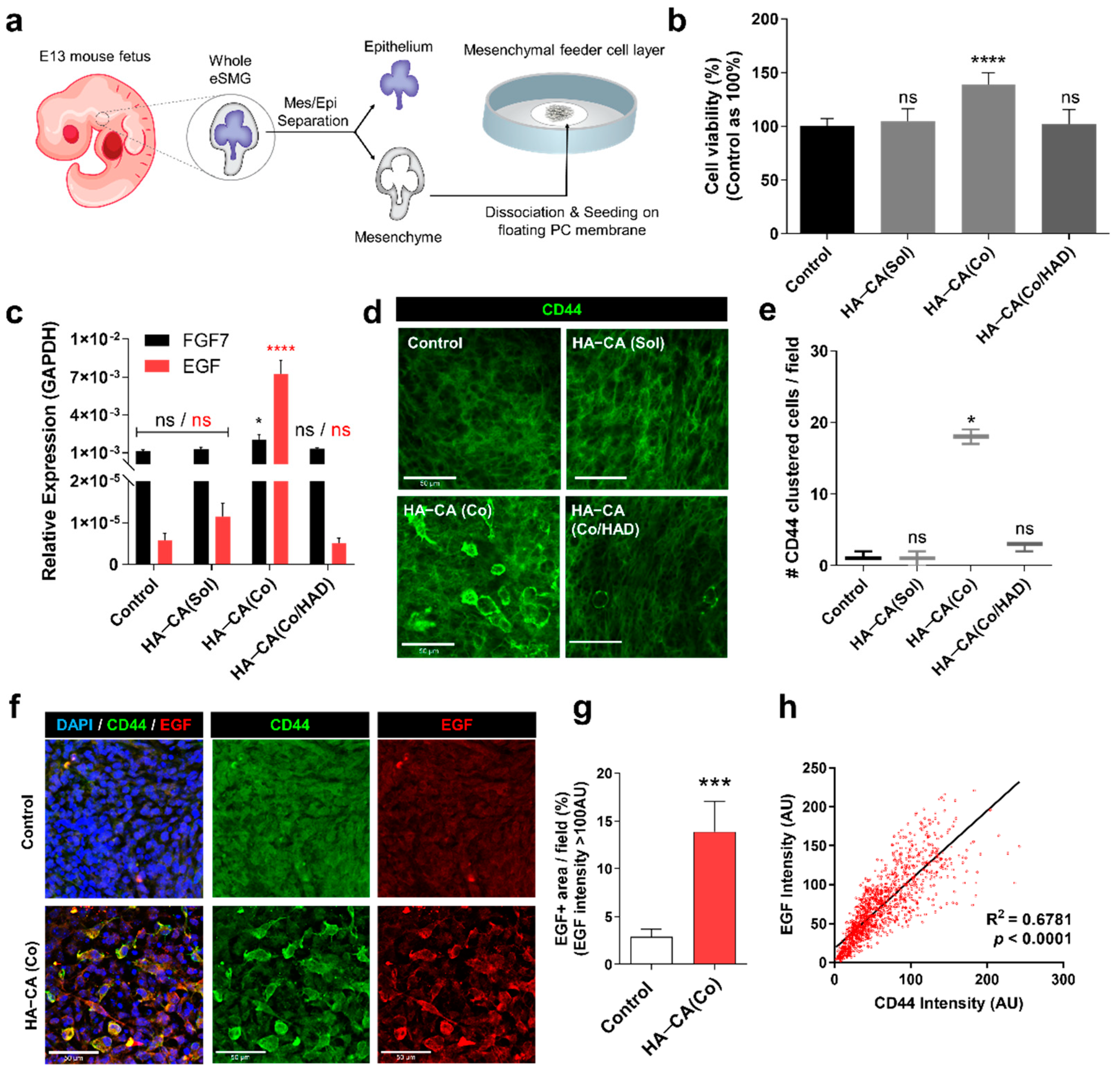
3.5. HA–CA-Coated Surface Supports Viability and Bioactivity of Isolated eSMG Mesenchyme by inducing CD44 Clustering
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Knopf-Marques, H.; Pravda, M.; Wolfova, L.; Velebny, V.; Schaaf, P.; Vrana, N.E.; Lavalle, P. Hyaluronic acid and its derivatives in coating and delivery systems: Applications in tissue engineering, regenerative medicine and immunomodulation. Adv. Healthc. Mater. 2016, 5, 2841–2855. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.Z.; Liu, Y.; Luo, Y.; Roberts, M.C.; Prestwich, G.D. Disulfide cross-linked hyaluronan hydrogels. Biomacromolecules 2002, 3, 1304–1311. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Burdick, J.A.; Kobler, J.; Clifton, R.J.; Rosowski, J.J.; Zeitels, S.M.; Langer, R. Synthesis and characterization of in situ cross-linkable hyaluronic acid-based hydrogels with potential application for vocal fold regeneration. Macromolecules 2004, 37, 3239–3248. [Google Scholar] [CrossRef]
- Almeida, P.V.; Shahbazi, M.-A.; Mäkilä, E.; Kaasalainen, M.; Salonen, J.; Hirvonen, J.; Santos, H.A. Amine-modified hyaluronic acid-functionalized porous silicon nanoparticles for targeting breast cancer tumors. Nanoscale 2014, 6, 10377–10387. [Google Scholar] [CrossRef]
- Park, Y.D.; Tirelli, N.; Hubbell, J.A. Photopolymerized hyaluronic acid-based hydrogels and interpenetrating networks. Biomaterials 2003, 24, 893–900. [Google Scholar] [CrossRef]
- Hong, S.; Yang, K.; Kang, B.; Lee, C.; Song, I.T.; Byun, E.; Park, K.I.; Cho, S.W.; Lee, H. Hyaluronic acid catechol: A biopolymer exhibiting a pH-dependent adhesive or cohesive property for human neural stem cell engineering. Adv. Funct. Mater. 2013, 23, 1774–1780. [Google Scholar] [CrossRef]
- Park, H.-J.; Jin, Y.; Shin, J.; Yang, K.; Lee, C.; Yang, H.S.; Cho, S.-W. Catechol-functionalized hyaluronic acid hydrogels enhance angiogenesis and osteogenesis of human adipose-derived stem cells in critical tissue defects. Biomacromolecules 2016, 17, 1939–1948. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Neoh, K.-G.; Shi, Z.; Kang, E.-T.; Poh, C.; Wang, W. An in vitro assessment of titanium functionalized with polysaccharides conjugated with vascular endothelial growth factor for enhanced osseointegration and inhibition of bacterial adhesion. Biomaterials 2010, 31, 8854–8863. [Google Scholar] [CrossRef]
- Lee, S.-W.; Ryu, J.H.; Do, M.J.; Namkoong, E.; Lee, H.; Park, K. NiCHE platform: Nature-inspired catechol-conjugated hyaluronic acid environment platform for salivary gland tissue engineering. ACS Appl. Mater. Interfaces 2020, 12, 4285–4294. [Google Scholar] [CrossRef]
- Peach, R.J.; Hollenbaugh, D.; Stamenkovic, I.; Aruffo, A. Identification of hyaluronic acid binding sites in the extracellular domain of CD44. J. Cell Biol. 1993, 122, 257–264. [Google Scholar] [CrossRef]
- Orian-Rousseau, V.; Sleeman, J. CD44 is a multidomain signaling platform that integrates extracellular matrix cues with growth factor and cytokine signals. Adv. Cancer Res. 2014, 123, 231–254. [Google Scholar] [PubMed]
- Yang, C.; Cao, M.; Liu, H.; He, Y.; Xu, J.; Du, Y.; Liu, Y.; Wang, W.; Cui, L.; Hu, J. The high and low molecular weight forms of hyaluronan have distinct effects on CD44 clustering. J. Biol. Chem. 2012, 287, 43094–43107. [Google Scholar] [CrossRef] [PubMed]
- Lombaert, I.; Movahednia, M.M.; Adine, C.; Ferreira, J.N. Concise review: Salivary gland regeneration: Therapeutic approaches from stem cells to tissue organoids. Stem Cells 2017, 35, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Harunaga, J.; Hsu, J.; Yamada, K. Dynamics of salivary gland morphogenesis. J. Dent. Res. 2011, 90, 1070–1077. [Google Scholar] [CrossRef] [PubMed]
- Borghese, E. Explantation experiments on the influence of the connective tissue capsule on the development of the epithelial part of the submandibular gland of Mus musculus. J. Anat. 1950, 84, 303. [Google Scholar]
- Patel, V.N.; Rebustini, I.T.; Hoffman, M.P. Salivary gland branching morphogenesis. Differentiation 2006, 74, 349–364. [Google Scholar] [CrossRef]
- Hosseini, Z.F.; Nelson, D.A.; Moskwa, N.; Sfakis, L.M.; Castracane, J.; Larsen, M. FGF2-dependent mesenchyme and laminin-111 are niche factors in salivary gland organoids. J. Cell Sci. 2018, 131, jcs208728. [Google Scholar] [CrossRef]
- Tatullo, M.; Marrelli, M.; Falisi, G.; Rastelli, C.; Palmieri, F.; Gargari, M.; Zavan, B.; Paduano, F.; Benagiano, V. Mechanical influence of tissue culture plates and extracellular matrix on mesenchymal stem cell behavior: A topical review. Int. J. Immunopathol. Pharmacol. 2016, 29, 3–8. [Google Scholar] [CrossRef]
- Marrelli, M.; Codispoti, B.; Shelton, R.M.; Scheven, B.A.; Cooper, P.R.; Tatullo, M.; Paduano, F. Dental pulp stem cell mechanoresponsiveness: Effects of mechanical stimuli on dental pulp stem cell behavior. Front. Physiol. 2018, 9, 1685. [Google Scholar] [CrossRef]
- Thierry, B.; Kujawa, P.; Tkaczyk, C.; Winnik, F.M.; Bilodeau, L.; Tabrizian, M. Delivery platform for hydrophobic drugs: Prodrug approach combined with self-assembled multilayers. J. Am. Chem. Soc. 2005, 127, 1626–1627. [Google Scholar] [CrossRef]
- Ling, X.; Zhao, C.; Huang, L.; Wang, Q.; Tu, J.; Shen, Y.; Sun, C. Synthesis and characterization of hyaluronic acid–platinum (iv) nanoconjugate with enhanced antitumor response and reduced adverse effects. RSC Adv. 2015, 5, 81668–81681. [Google Scholar] [CrossRef]
- Bian, S.; He, M.; Sui, J.; Cai, H.; Sun, Y.; Liang, J.; Fan, Y.; Zhang, X. The self-crosslinking smart hyaluronic acid hydrogels as injectable three-dimensional scaffolds for cells culture. Colloids Surf. B Biointerfaces 2016, 140, 392–402. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, C.; Ryu, J.H. Adhesive Catechol-Conjugated Hyaluronic Acid for Biomedical Applications: A Mini Review. Appl. Sci. 2021, 11, 21. [Google Scholar] [CrossRef]
- Ozdemir, T.; Fowler, E.W.; Liu, S.; Harrington, D.A.; Witt, R.L.; Farach-Carson, M.C.; Pradhan-Bhatt, S.; Jia, X. Tuning hydrogel properties to promote the assembly of salivary gland spheroids in 3D. ACS Biomater. Sci. Eng. 2016, 2, 2217–2230. [Google Scholar] [CrossRef] [PubMed]
- Pradhan-Bhatt, S.; Harrington, D.A.; Duncan, R.L.; Farach-Carson, M.C.; Jia, X.; Witt, R.L. A novel in vivo model for evaluating functional restoration of a tissue-engineered salivary gland. Laryngoscope 2014, 124, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Fiorica, C.; Pitarresi, G.; Palumbo, F.S.; Di Stefano, M.; Calascibetta, F.; Giammona, G. A new hyaluronic acid pH sensitive derivative obtained by ATRP for potential oral administration of proteins. Int. J. Pharm. 2013, 457, 150–157. [Google Scholar] [CrossRef]
- Palumbo, F.S.; Pitarresi, G.; Fiorica, C.; Rigogliuso, S.; Ghersi, G.; Giammona, G. Chemical hydrogels based on a hyaluronic acid-graft-α-elastin derivative as potential scaffolds for tissue engineering. Mater. Sci. Eng. C 2013, 33, 2541–2549. [Google Scholar] [CrossRef]
- Scott, J.; Quintarelli, G.; Dellovo, M. The chemical and histochemical properties of Alcian blue. Histochemie 1964, 4, 73–85. [Google Scholar] [CrossRef]
- Lee, S.-W.; Kim, J.; Do, M.; Namkoong, E.; Lee, H.; Ryu, J.H.; Park, K. Developmental role of hyaluronic acid and its application in salivary gland tissue engineering. Acta Biomater. 2020, 115, 275–287. [Google Scholar] [CrossRef]
- Lombaert, I.M.; Abrams, S.R.; Li, L.; Eswarakumar, V.P.; Sethi, A.J.; Witt, R.L.; Hoffman, M.P. Combined KIT and FGFR2b signaling regulates epithelial progenitor expansion during organogenesis. Stem Cell Rep. 2013, 1, 604–619. [Google Scholar] [CrossRef]
- Häärä, O.; Koivisto, T.; Miettinen, P.J. EGF-receptor regulates salivary gland branching morphogenesis by supporting proliferation and maturation of epithelial cells and survival of mesenchymal cells. Differentiation 2009, 77, 298–306. [Google Scholar] [CrossRef]
- Goetz, R.; Mohammadi, M. Exploring mechanisms of FGF signalling through the lens of structural biology. Nat. Rev. Mol. Cell Biol. 2013, 14, 166–180. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, Z.; Myers, C.; Heim, V.M.; Lathrop, C.A.; Rebustini, I.T.; Stewart, J.S.; Larsen, M.; Hoffman, M.P. FGFR2b signaling regulates ex vivo submandibular gland epithelial cell proliferation and branching morphogenesis. Development 2005, 132, 1223–1234. [Google Scholar] [CrossRef] [PubMed]
- Spagnuolo, G.; Codispoti, B.; Marrelli, M.; Rengo, C.; Rengo, S.; Tatullo, M. Commitment of oral-derived stem cells in dental and maxillofacial applications. Dent. J. 2018, 6, 72. [Google Scholar] [CrossRef] [PubMed]
- Ballini, A.; Boccaccio, A.; Saini, R.; Van Pham, P.; Tatullo, M. Dental-derived stem cells and their secretome and interactions with bioscaffolds/biomaterials in regenerative medicine: From the in vitro research to translational applications. Stem Cells Int. 2017, 2017, e6975251. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.-w.; Kim, J.; Cong, X.; Yu, G.-Y.; Ryu, J.H.; Park, K. Efficient Surface Immobilization of Chemically Modified Hyaluronans for Enhanced Bioactivity and Survival of In Vitro-Cultured Embryonic Salivary Gland Mesenchymal Cells. Polymers 2021, 13, 1216. https://doi.org/10.3390/polym13081216
Lee S-w, Kim J, Cong X, Yu G-Y, Ryu JH, Park K. Efficient Surface Immobilization of Chemically Modified Hyaluronans for Enhanced Bioactivity and Survival of In Vitro-Cultured Embryonic Salivary Gland Mesenchymal Cells. Polymers. 2021; 13(8):1216. https://doi.org/10.3390/polym13081216
Chicago/Turabian StyleLee, Sang-woo, Junchul Kim, Xin Cong, Guang-Yan Yu, Ji Hyun Ryu, and Kyungpyo Park. 2021. "Efficient Surface Immobilization of Chemically Modified Hyaluronans for Enhanced Bioactivity and Survival of In Vitro-Cultured Embryonic Salivary Gland Mesenchymal Cells" Polymers 13, no. 8: 1216. https://doi.org/10.3390/polym13081216
APA StyleLee, S.-w., Kim, J., Cong, X., Yu, G.-Y., Ryu, J. H., & Park, K. (2021). Efficient Surface Immobilization of Chemically Modified Hyaluronans for Enhanced Bioactivity and Survival of In Vitro-Cultured Embryonic Salivary Gland Mesenchymal Cells. Polymers, 13(8), 1216. https://doi.org/10.3390/polym13081216







