Latex-Based Polystyrene Nanocomposites with Non-Covalently Modified Carbon Nanotubes
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of PS Microparticles
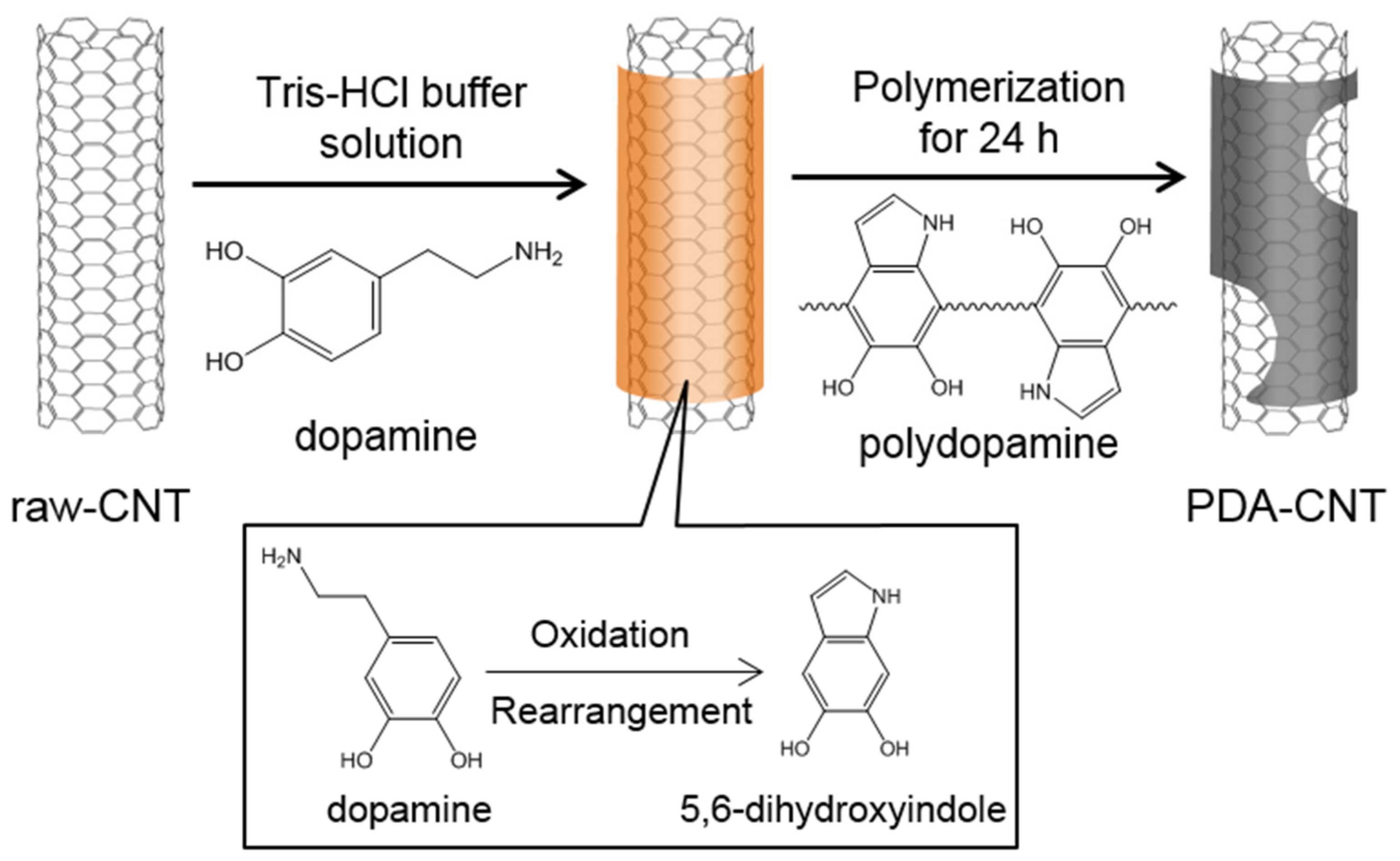
2.3. Preparation of PDA-CNTs
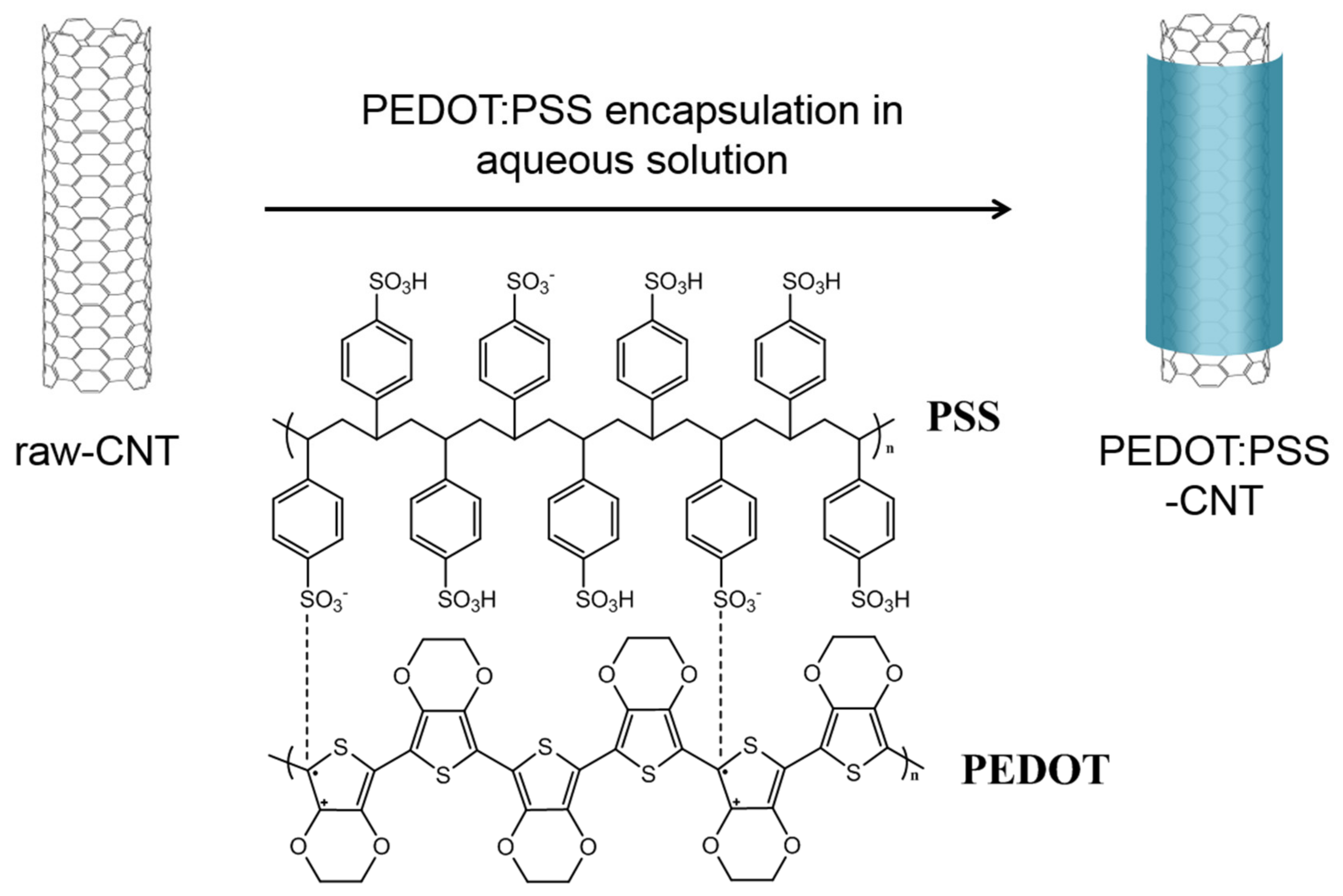
2.4. Preparation of PEDOT:PSS-CNTs
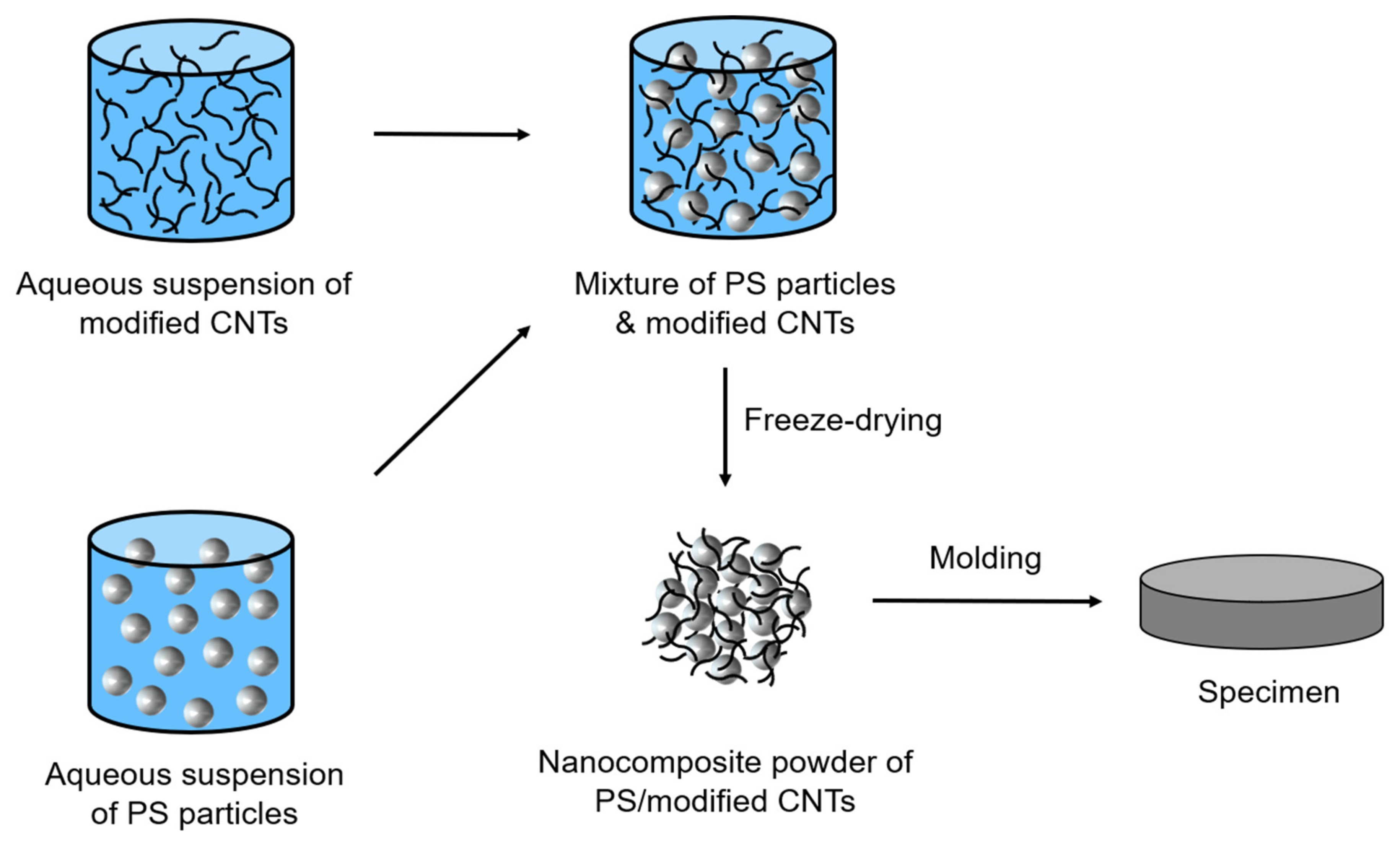
2.5. Preparation of PS/Polymer-Modified CNT Nanocomposites
2.6. Characterization
3. Results and Discussion
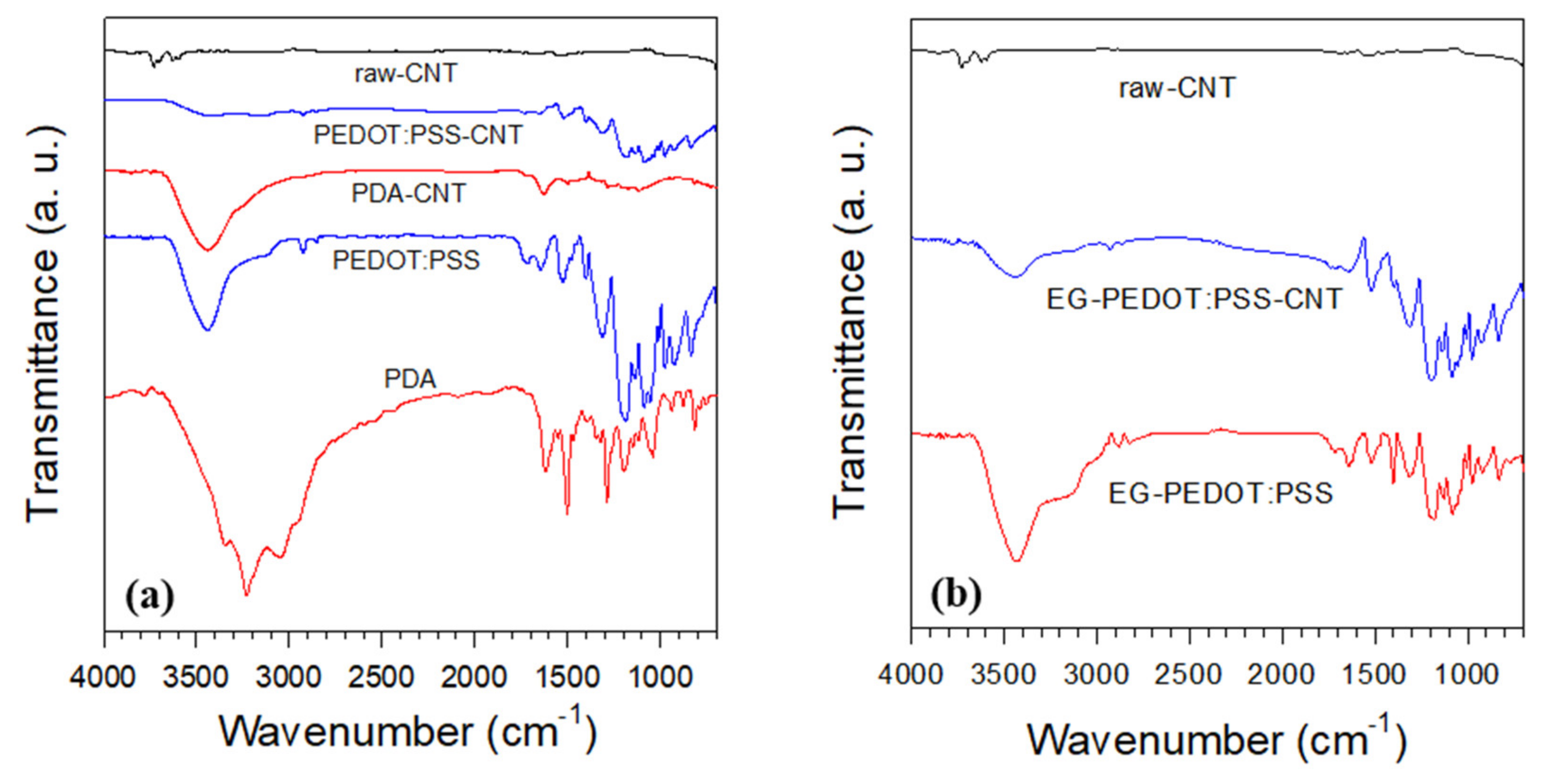
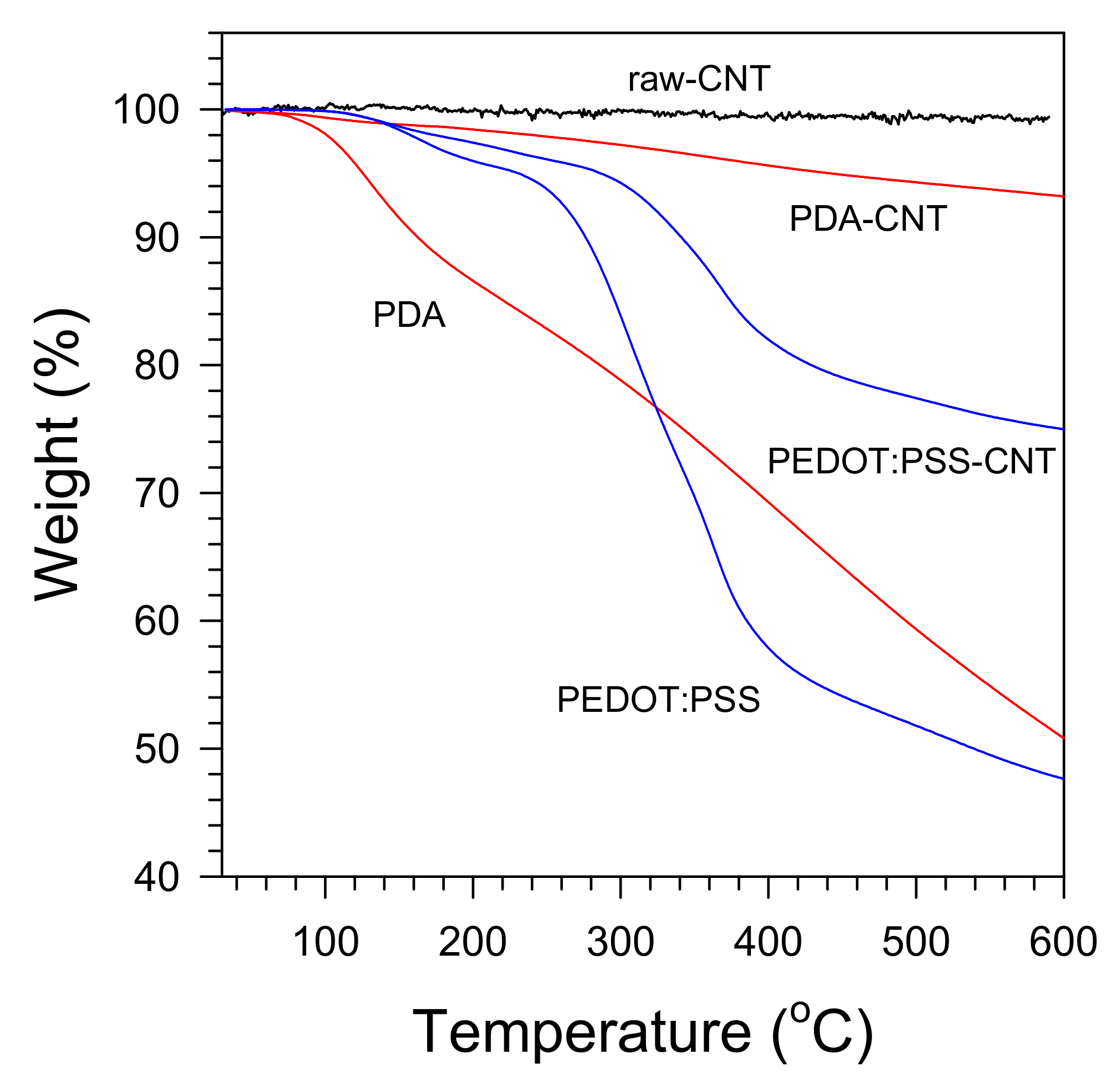
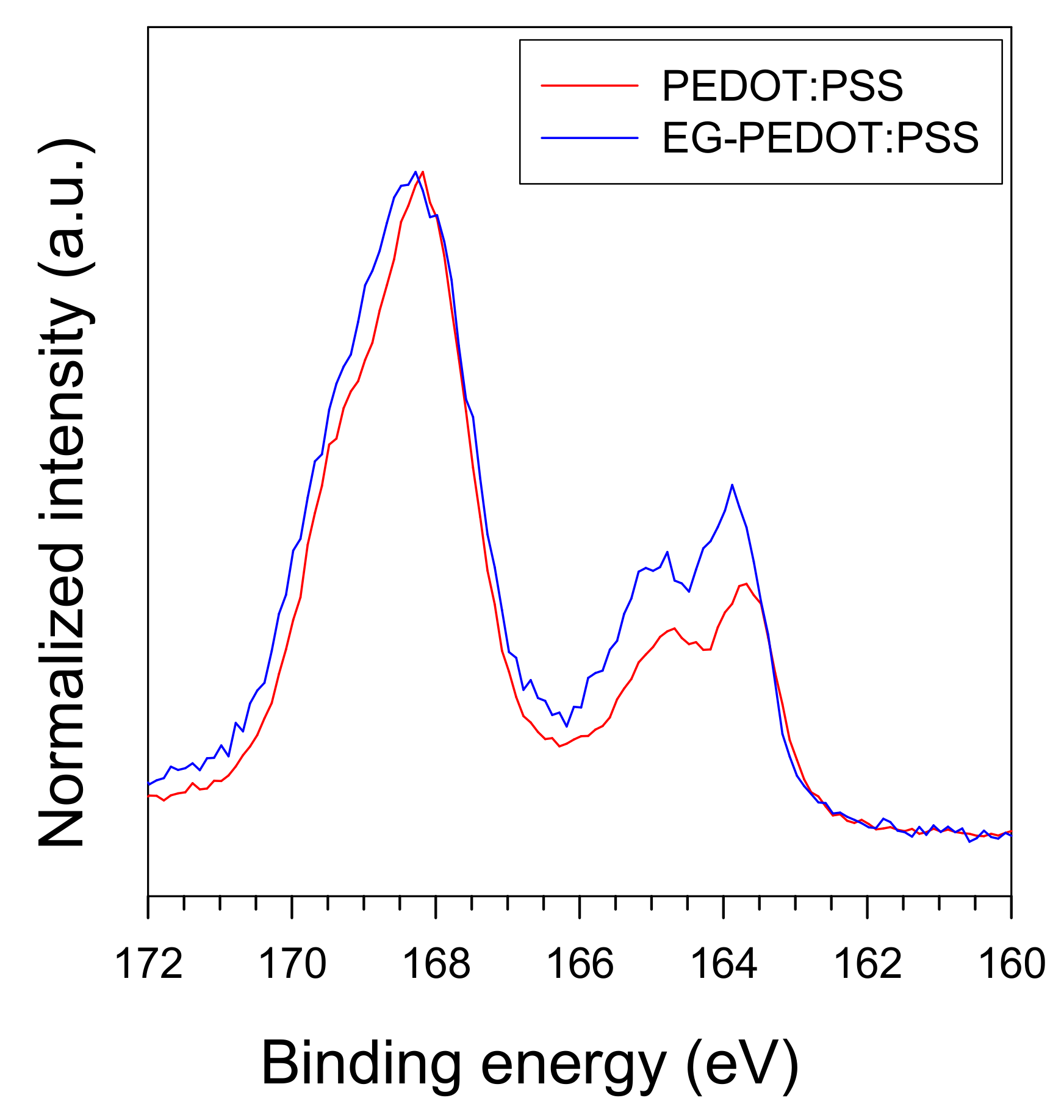
3.1. Characterization of Materials
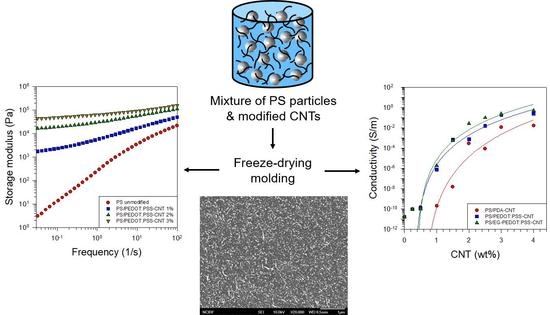
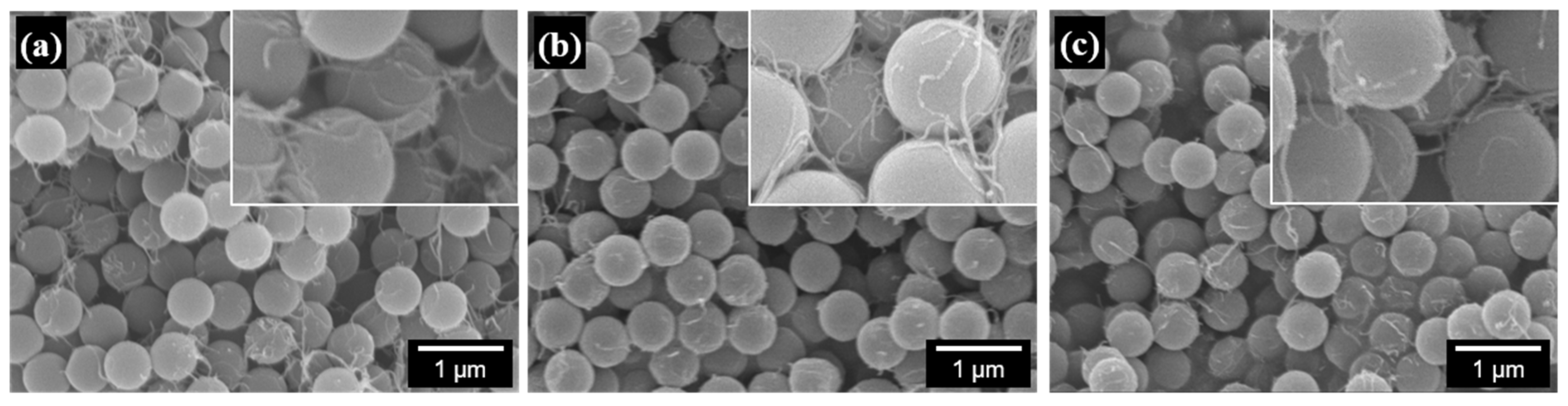
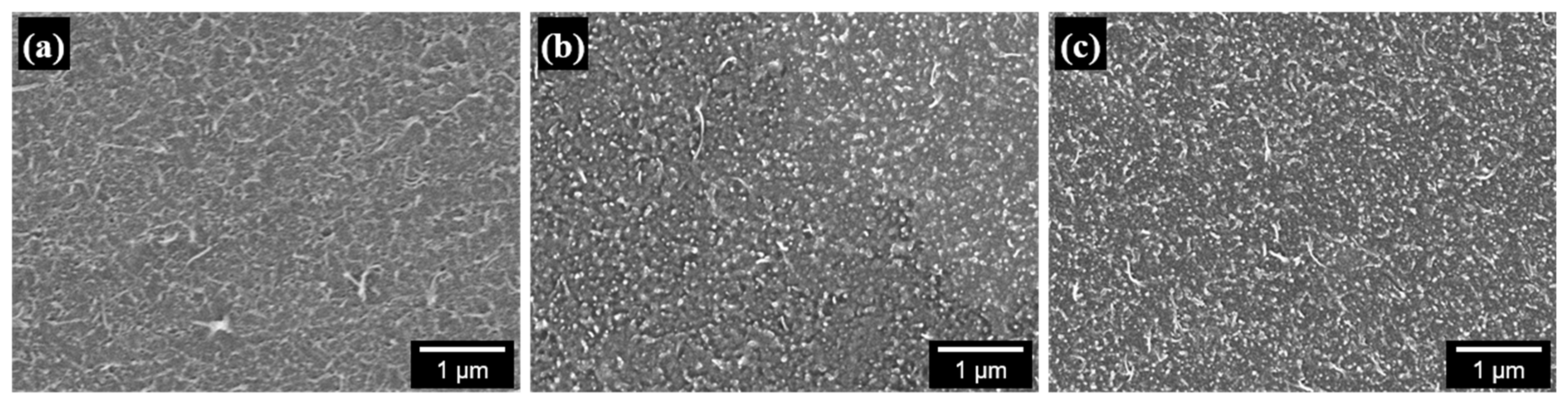
3.2. Morphology of PS/CNT Nanocomposites
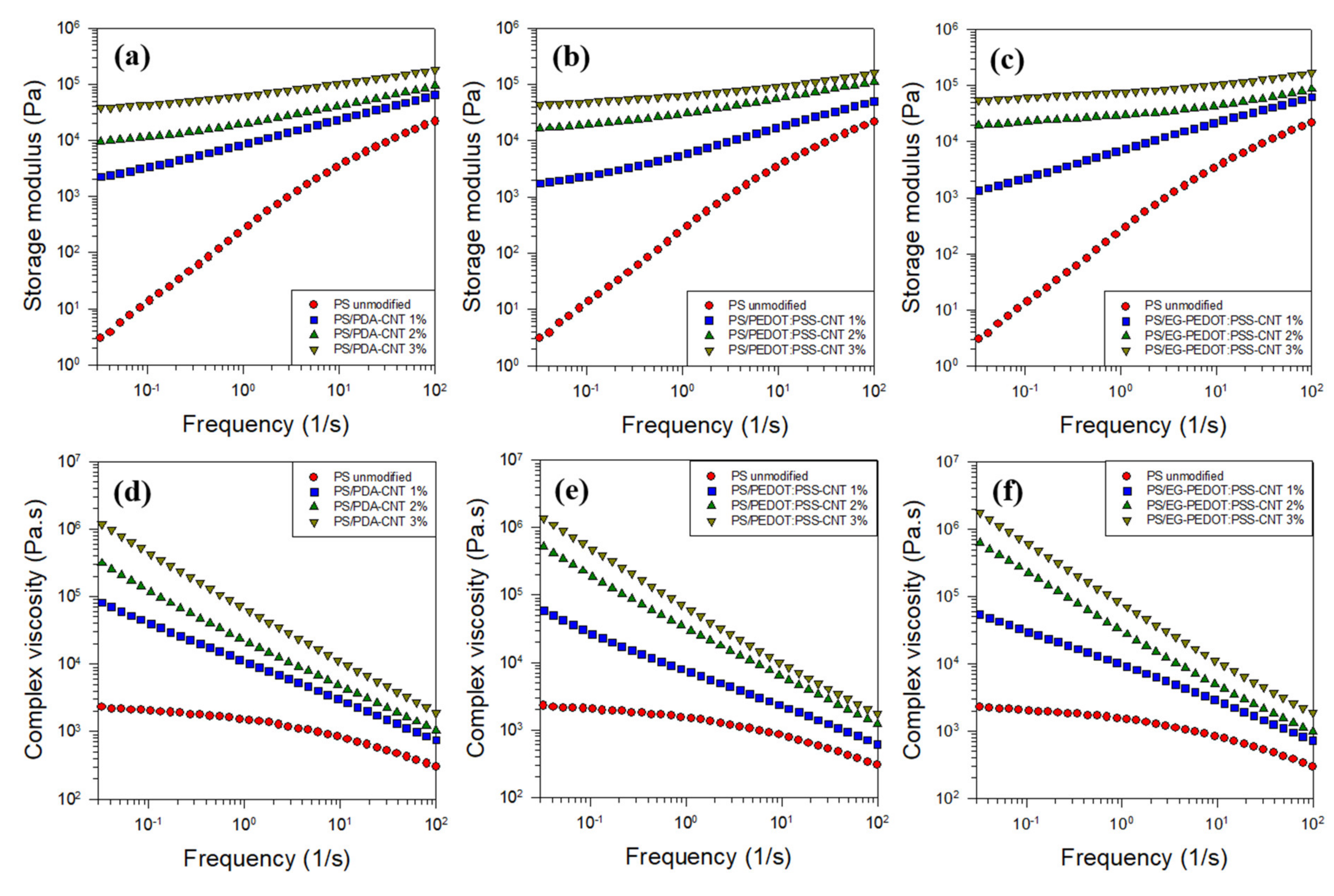
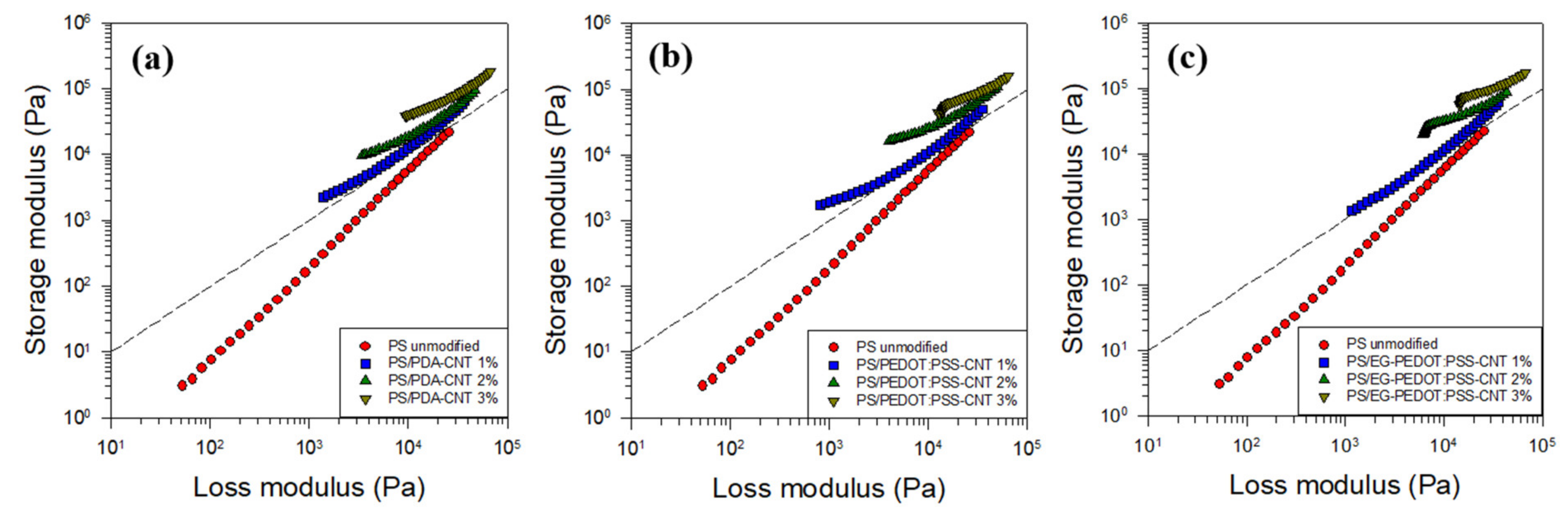
3.3. Rheological Properties of PS/CNT Nanocomposites
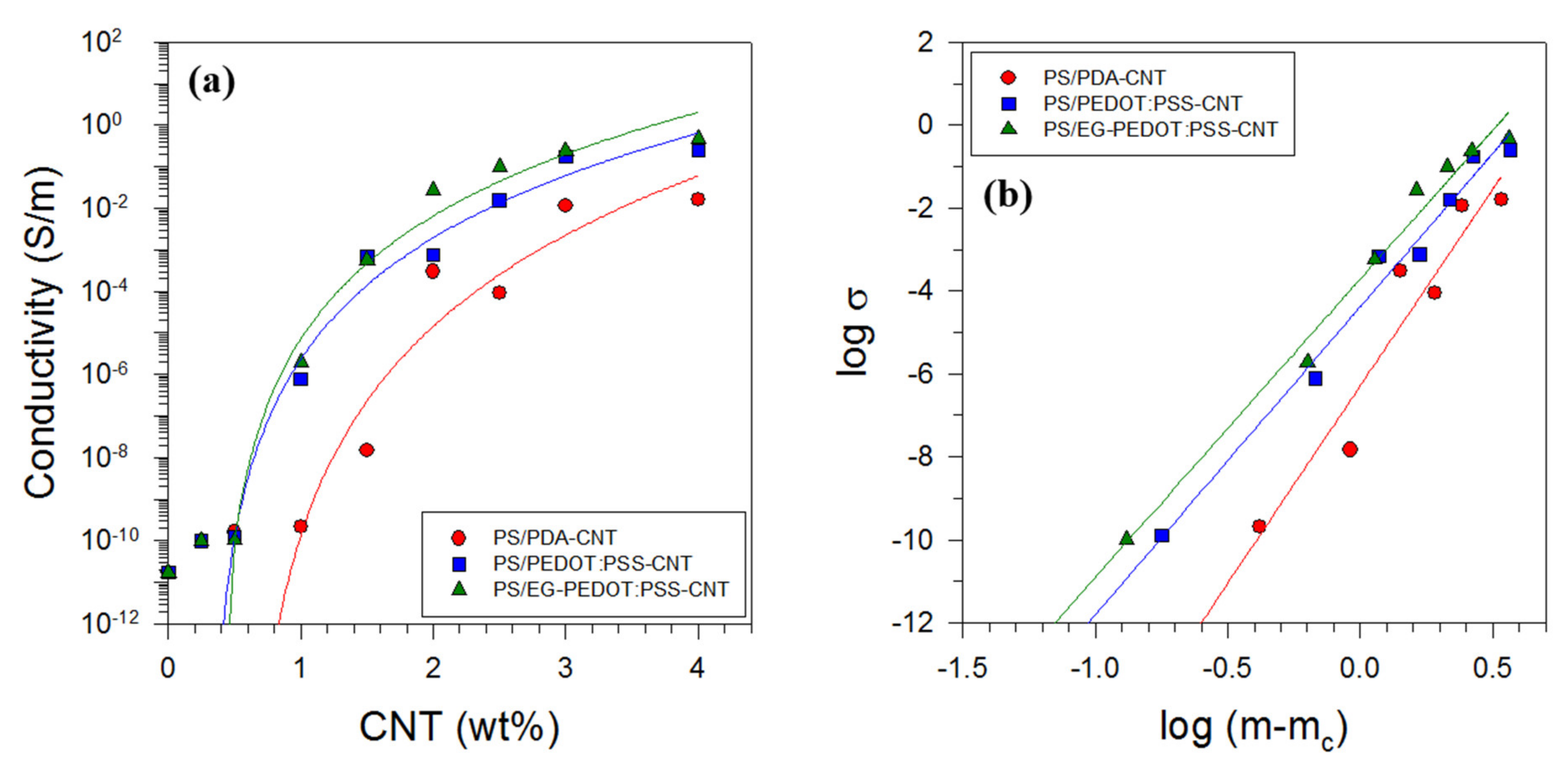
3.4. Electrical Properties of PS/CNT Nanocomposites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Balazs, A.C.; Emrick, T.; Russell, T.P. Nanoparticle polymer composites: Where two small worlds meet. Science 2006, 314, 1107–1110. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Zhang, W.; Wang, C.; Wen, T.C.; Wei, Y. One-dimensional conducting polymer nanocomposites: Synthesis, properties and applications. Prog. Polym. Sci. 2011, 36, 671–712. [Google Scholar] [CrossRef]
- Xia, Y.; Yang, P.; Sun, Y.; Wu, Y.; Mayers, B.; Gates, B.; Yin, Y.; Kim, F.; Yan, H. One-dimensional nanostructures: Synthesis, characterization, and applications. Adv. Mater. 2003, 15, 353–389. [Google Scholar] [CrossRef]
- Hu, J.; Odom, T.W.; Lieber, C.M. Chemistry and physics in one dimension: Synthesis and properties of nanowires and nanotubes. Acc. Chem. Res. 1999, 32, 435–445. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphite carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Dror, Y.; Pyckhout-Hintzen, W.; Cohen, Y. Conformation of polymers dispersing single-walled carbon nanotubes in water: A small-angle neutron scattering study. Macromolecules 2005, 38, 7828–7836. [Google Scholar] [CrossRef]
- Bilalis, P.; Katsigiannopoulos, D.; Avgeropoulos, A.; Sakellariou, G. Non-covalent functionalization of carbon nanotubes with polymers. RSC Adv. 2014, 4, 2911–2934. [Google Scholar] [CrossRef]
- Tummala, N.R.; Striolo, A. SDS surfactants on carbon nanotubes: Aggregate morphology. ACS Nano 2009, 3, 595–602. [Google Scholar] [CrossRef]
- Haggenmueller, R.; Rahatekar, S.S.; Fagan, J.A.; Chun, J.; Becker, M.L.; Naik, R.R.; Krauss, T.; Carlson, L.; Kadla, J.F.; Trulove, P.C.; et al. Comparison of the quality of aqueous dispersions of single wall carbon nanotubes using surfactants and biomolecules. Langmuir 2008, 24, 5070–5078. [Google Scholar] [CrossRef]
- Tsai, Y.C.; Chiu, C.C.; Tsai, M.C.; Wu, J.Y.; Tseng, T.F.; Wu, T.M.; Hsu, S.F. Dispersion of carbon nanotubes in low pH aqueous solutions by means of alumina-coated silica nanoparticles. Carbon 2007, 45, 2823–2827. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef]
- Hu, H.; Yu, B.; Ye, Q.; Gu, Y.; Zhou, F. Modification of carbon nanotubes with a nanothin polydopamine layer and polydimethylamino-ethyl methacrylate brushes. Carbon 2010, 48, 2347–2353. [Google Scholar] [CrossRef]
- Hao, M.; Tang, M.; Wang, W.; Tian, M.; Zhang, L.; Lu, Y. Silver-nanoparticle-decorated multiwalled carbon nanotubes prepared by poly(dopamine) functionalization and ultraviolet irradiation. Compos. Part B Eng. 2016, 95, 395–403. [Google Scholar] [CrossRef]
- Fei, B.; Qian, B.; Yang, Z.; Wang, R.; Liu, W.C.; Mak, C.L.; Xin, J.H. Coating carbon nanotubes by spontaneous oxidative polymerization of dopamine. Carbon 2008, 46, 1792–1797. [Google Scholar] [CrossRef]
- Liu, T.; Kim, K.C.; Lee, B.; Chen, Z.; Noda, S.; Jang, S.S.; Lee, S.W. Self-polymerized dopamine as an organic cathode for Li- and Na-ion batteries. Energy Environ. Sci. 2017, 10, 205–215. [Google Scholar] [CrossRef]
- Zhou, J.; Lubineau, G. Improving electrical conductivity in polycarbonate nanocomposites using highly conductive PEDOT/PSS coated MWCNTs. ACS Appl. Mater. Interfaces 2013, 5, 6189–6200. [Google Scholar] [CrossRef]
- Zhou, J.; Ventura, I.A.; Lubineau, G. Probing the role of poly(3,4-ethylenedioxythiophene)/poly(styrenesulfonate)-coated multiwalled carbon nanotubes in the thermal and mechanical properties of polycarbonate nanocomposites. Ind. Eng. Chem. Res. 2014, 53, 3539–3549. [Google Scholar] [CrossRef]
- Huang, J.H.; Kekuda, D.; Chu, C.W.; Ho, K.C. Electrochemical characterization of the solvent-enhanced conductivity of poly(3,4-ethylenedioxythiophene) and its application in polymer solar cells. J. Mater. Chem. 2009, 19, 3704–3712. [Google Scholar] [CrossRef]
- Ouyang, J.; Xu, Q.; Chu, C.W.; Yang, Y.; Li, G.; Shinar, J. On the mechanism of conductivity enhancement in poly(3,4-ethylenedioxythiophene):poly(styrene sulfonate) film through solvent treatment. Polymer 2004, 45, 8443–8450. [Google Scholar] [CrossRef]
- Okuzaki, H.; Harashina, Y.; Yan, H. Highly conductive PEDOT/PSS microfibers fabricated by wet-spinning and dip-treatment in ethylene glycol. Eur. Polym. J. 2009, 45, 256–261. [Google Scholar] [CrossRef]
- Kim, Y.H.; Sachse, C.; Machala, M.L.; May, C.; Müller-Meskamp, L.; Leo, K. Highly conductive PEDOT/PSS electrode with optimized solvent and thermal post-treatment for ITO-free organic solar cells. Adv. Funct. Mater. 2011, 21, 1076–1081. [Google Scholar] [CrossRef]
- Kim, J.Y.; Jung, J.H.; Lee, D.E.; Joo, J. Enhancement of electrical conductivity of poly(3,4-ethylenedioxythiophene)/poly(4-styrenesulfonate) by a change of solvents. Synth. Met. 2002, 126, 311–316. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, L.; Sun, J.; Liu, Y.; Wang, Y.; Wang, J. Incorporation of single-walled carbon nanotubes with PEDOT/PSS in DMSO for the production of transparent conducting films. Diam. Relat. Mater. 2012, 22, 82–87. [Google Scholar] [CrossRef]
- Xia, Y.; Ouyang, J. PEDOT/PSS films with significantly enhanced conductivities induced by preferential solvation with cosolvents and their application in polymer photovoltaic cells. J. Mater. Chem. 2011, 21, 4927–4936. [Google Scholar] [CrossRef]
- Crispin, X.; Jakobsson, F.L.E.; Crispin, A.; Grim, P.C.M.; Andersson, P.; Volodin, A.; van Haesendonck, C.; van der Auweraer, M.; Salaneck, W.R.; Berggren, M. The origin of the high conductivity of poly(3,4-ethylenedioxythiophene)—Poly(styrenesulfonate) (PEDOT-PSS) plastic electrodes. Chem. Mater. 2006, 18, 4354–4360. [Google Scholar] [CrossRef]
- Park, S.; Tark, S.J.; Kim, D. Effect of sorbitol doping in PEDOT/PSS on the electrical performance of organic photovoltaic devices. Curr. Appl. Phys. 2011, 11, 1299–1301. [Google Scholar] [CrossRef]
- He, H.; Zhang, L.; Guan, X.; Cheng, H.; Liu, X.; Yu, S.; Wei, J.; Ouyang, J. Biocompatible conductive polymers with high conductivity and high stretchability. ACS Appl. Mater. Interfaces 2019, 11, 26185–26193. [Google Scholar] [CrossRef] [PubMed]
- Fan, B.; Mei, X.; Ouyang, J. Significant conductivity enhancement of conductive poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) films by adding anionic surfactants into polymer solution. Macromolecules 2008, 41, 5971–5973. [Google Scholar] [CrossRef]
- Badre, C.; Marquant, L.; Alsayed, A.M.; Hough, L.A. Highly conductive poly(3,4-ethylenedioxythiophene): Poly(styrenesulfonate) films using 1-ethylimidazolium tetracyanoborate ionic liquid. Adv. Funct. Mater. 2012, 22, 2723–2727. [Google Scholar] [CrossRef]
- Ouyang, J. Solution-processed PEDOT/PSS films with conductivities as indium tin oxide through a treatment with mild and weak organic acids. ACS Appl. Mater. Interfaces 2013, 5, 13082–13088. [Google Scholar] [CrossRef]
- Mengistie, D.A.; Ibrahem, M.A.; Wang, P.C.; Chu, C.W. Highly conductive PEDOT/PSS treated with formic acid for ITO-free polymer solar cells. ACS Appl. Mater. Interfaces 2014, 6, 2292–2299. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, J.; Chen, P.; Zhang, B.; He, J.; Hu, G.H. Enhanced interactions between multi-walled carbon nanotubes and polystyrene induced by melt mixing. Carbon 2006, 44, 692–698. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Winey, K.I. Polymer nanocomposites containing carbon nanotubes. Macromolecules 2006, 39, 5194–5205. [Google Scholar] [CrossRef]
- Barraza, H.J.; Pompeo, F.; O’Rear, E.A.; Resasco, D.E. SWNT-filled thermoplastic and elastomeric composites prepared by miniemulsion polymerization. Nano Lett. 2002, 2, 797–802. [Google Scholar] [CrossRef]
- Regev, O.; ElKati, P.N.B.; Loos, J.; Koning, C.E. Preparation of conductive nanotube-polymer composites using latex technology. Adv. Mater. 2004, 16, 248–251. [Google Scholar] [CrossRef]
- Grossiord, N.; Loos, J.; Regev, O.; Koning, C.E. Toolbox for dispersing carbon nanotubes into polymers to get conductive nanocomposites. Chem. Mater. 2006, 18, 1089–1099. [Google Scholar] [CrossRef]
- Park, J.S.; An, J.H.; Jang, K.S.; Lee, S.J. Rheological and electrical properties of polystyrene nanocomposites via incorporation of polymer-wrapped carbon nanotubes. Korea Aust. Rheol. J. 2019, 31, 111–118. [Google Scholar] [CrossRef]
- Sureshkumar, M.; Na, H.Y.; Ahn, K.H.; Lee, S.J. Conductive nanocomposites based on polystyrene microspheres and silver nanowires by latex blending. ACS Appl. Mater. Interfaces 2015, 7, 756–764. [Google Scholar] [CrossRef]
- Kim, J.M.; Jang, K.S.; Lee, S.J. Electrically conductive polystyrene nanocomposites incorporated with aspect ratio-controlled silver nanowires. J. Appl. Polym. Sci. 2019, 136, 47927. [Google Scholar] [CrossRef]
- Ahn, S.J.; Ahn, K.H.; Lee, S.J. Film squeezing process for generating oblate spheroidal particles with high yield and uniform sizes. Colloid Polym. Sci. 2016, 294, 859–867. [Google Scholar] [CrossRef]
- Perloff, D.S. Four-point sheet resistance correction factors for thin rectangular samples. Solid State Electron. 1977, 20, 681–687. [Google Scholar] [CrossRef]
- Sun, D.; Jin, L.; Chen, Y.; Zhang, J.R.; Zhu, J.J. Microwave-assisted in situ synthesis of graphene/PEDOT hybrid and its application in supercapacitors. ChemPlusChem 2013, 78, 227–234. [Google Scholar] [CrossRef]
- Zhu, Z.; Liu, C.; Shi, H.; Jiang, Q.; Xu, J.; Jiang, F.; Xiong, J.; Liu, E. An effective approach to enhanced thermoelectric properties of PEDOT/PSS films by a DES post-treatment. J. Polym. Sci. Part B Polym. Phys. 2015, 53, 885–892. [Google Scholar] [CrossRef]
- Jiang, Y.; Lu, Y.; Zhang, L.; Liu, L.; Dai, Y.; Wang, W. Preparation and characterization of silver nanoparticles immobilized on multi-walled carbon nanotubes by poly(dopamine) functionalization. J. Nanopart. Res. 2012, 14, 938. [Google Scholar] [CrossRef]
- Kim, H.; Ahn, K.H.; Lee, S.J. Conductive poly(high internal phase emulsion) foams incorporated with polydopamine-coated carbon nanotubes. Polymer 2017, 110, 187–195. [Google Scholar] [CrossRef]
- Laurent, C.; Flahaut, E.; Peigney, A. The weight and density of carbon nanotubes versus the number of walls and diameter. Carbon 2010, 48, 2994–2996. [Google Scholar] [CrossRef]
- Antiohos, D.; Folkes, G.; Sherrell, P.; Ashraf, S.; Wallace, G.G.; Aitchison, P.; Harris, A.H.; Chen, J.; Minett, A.I. Compositional effects of PEDOT/PSS/single walled carbon nanotube films on supercapacitor device performance. J. Mater. Chem. 2011, 21, 15987–15994. [Google Scholar] [CrossRef]
- Liu, Y.; Weng, B.; Razal, J.M.; Xu, Q.; Zhao, C.; Hou, Y.; Seyedin, S.; Jalili, R.; Wallace, G.G.; Chen, J. High-performance flexible all-solid-state supercapacitor from large free-standing graphene-PEDOT/PSS films. Sci. Rep. 2015, 5, 17045. [Google Scholar] [CrossRef]
- Gupta, B.; Mehta, M.; Melvin, A.; Kamalakannan, R.; Dash, S.; Kamruddin, M.; Tyagi, A.K. Poly (3,4-ethylenedioxythiophene) (PEDOT) and poly (3,4-ethylenedioxythiophene)-few walled carbon nanotube (PEDOT-FWCNT) nanocomposite based thin films for Schottky diode application. Mater. Chem. Phys. 2014, 147, 867–877. [Google Scholar] [CrossRef]
- Mengistie, D.A.; Wang, P.C.; Chu, C.W. Effect of molecular weight of additives on the conductivity of PEDOT/PSS and efficiency for ITO-free organic solar cells. J. Mater. Chem. A 2013, 1, 9907–9915. [Google Scholar] [CrossRef]
- Zhao, L.; Li, Y.; Liu, Z.; Shimizu, H. Carbon nanotube-conducting polymer core-shell hybrid using an imidazolium-salt-based ionic liquid as a linker: Designed as a potential platinum electrode alternative material for large-scale solution processing. Chem. Mater. 2010, 22, 5949–5956. [Google Scholar] [CrossRef]
- Ouyang, J.; Chu, C.W.; Chen, F.C.; Xu, Q.; Yang, Y. High-conductivity poly(3,4-ethylenedioxythiophene):Poly(styrene sulfonate) film and its application in polymer optoelectronic devices. Adv. Funct. Mater. 2005, 15, 203–208. [Google Scholar] [CrossRef]
- Ouyang, J. “Secondary doping” methods to significantly enhance the conductivity of PEDOT/PSS for its application as transparent electrode of optoelectronic devices. Displays 2013, 34, 423–436. [Google Scholar] [CrossRef]
- Du, F.; Scogna, R.C.; Zhou, W.; Brand, S.; Fischer, J.E.; Winey, K.I. Nanotube networks in polymer nanocomposites: Rheology and electrical conductivity. Macromolecules 2004, 37, 9048–9055. [Google Scholar] [CrossRef]
- Lee, K.M.; Han, C.D. Rheology of organoclay nanocomposites: Effects of polymer matrix/organoclay compatibility and the gallery distance of organoclay. Macromolecules 2003, 36, 7165–7178. [Google Scholar] [CrossRef]
- Hu, G.; Zhao, C.; Zhang, S.; Yang, M.; Wang, Z. Low percolation thresholds of electrical conductivity and rheology in poly(ethylene terephthalate) through the networks of multi-walled carbon nanotubes. Polymer 2006, 47, 480–488. [Google Scholar] [CrossRef]
- Gelves, G.A.; Al-Saleh, M.H.; Sundararaj, U. Highly electrically conductive and high performance EMI shielding nanowire/polymer nanocomposites by miscible mixing and precipitation. J. Mater. Chem. 2011, 21, 829–836. [Google Scholar] [CrossRef]
- Weber, M.; Kamal, M.R. Estimation of the volume resistivity of electrically conductive composites. Polym. Compos. 1997, 18, 711–725. [Google Scholar] [CrossRef]
| Film Type | EG Content (wt%) | Coating Speed (rpm) | Sheet Resistance (Ω sq−1) |
|---|---|---|---|
| PEDOT:PSS | 0 | 600 | 10,845 |
| 2000 | 18,588 | ||
| EG-PEDOT:PSS | 2 | 600 | 360 |
| 2000 | 1188 | ||
| EG-PEDOT:PSS | 5 | 600 | 240 |
| 2000 | 801 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, J.P.; Choi, S.H.; Chung, D.-W.; Lee, S.J. Latex-Based Polystyrene Nanocomposites with Non-Covalently Modified Carbon Nanotubes. Polymers 2021, 13, 1168. https://doi.org/10.3390/polym13071168
Song JP, Choi SH, Chung D-W, Lee SJ. Latex-Based Polystyrene Nanocomposites with Non-Covalently Modified Carbon Nanotubes. Polymers. 2021; 13(7):1168. https://doi.org/10.3390/polym13071168
Chicago/Turabian StyleSong, Jae Phil, Sung Ho Choi, Dae-Won Chung, and Seong Jae Lee. 2021. "Latex-Based Polystyrene Nanocomposites with Non-Covalently Modified Carbon Nanotubes" Polymers 13, no. 7: 1168. https://doi.org/10.3390/polym13071168
APA StyleSong, J. P., Choi, S. H., Chung, D.-W., & Lee, S. J. (2021). Latex-Based Polystyrene Nanocomposites with Non-Covalently Modified Carbon Nanotubes. Polymers, 13(7), 1168. https://doi.org/10.3390/polym13071168