Functionalization of Single-Walled Carbon Nanotubes with End-Capped Polystyrene via a Single-Step Diels–Alder Cycloaddition
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Physical and Analytical Methods
2.3. Synthesis of Diphenylethylene-Cyclobutene End-Capped Polystyrene, 1.
2.4. Synthesis of SWNTs-g-PS
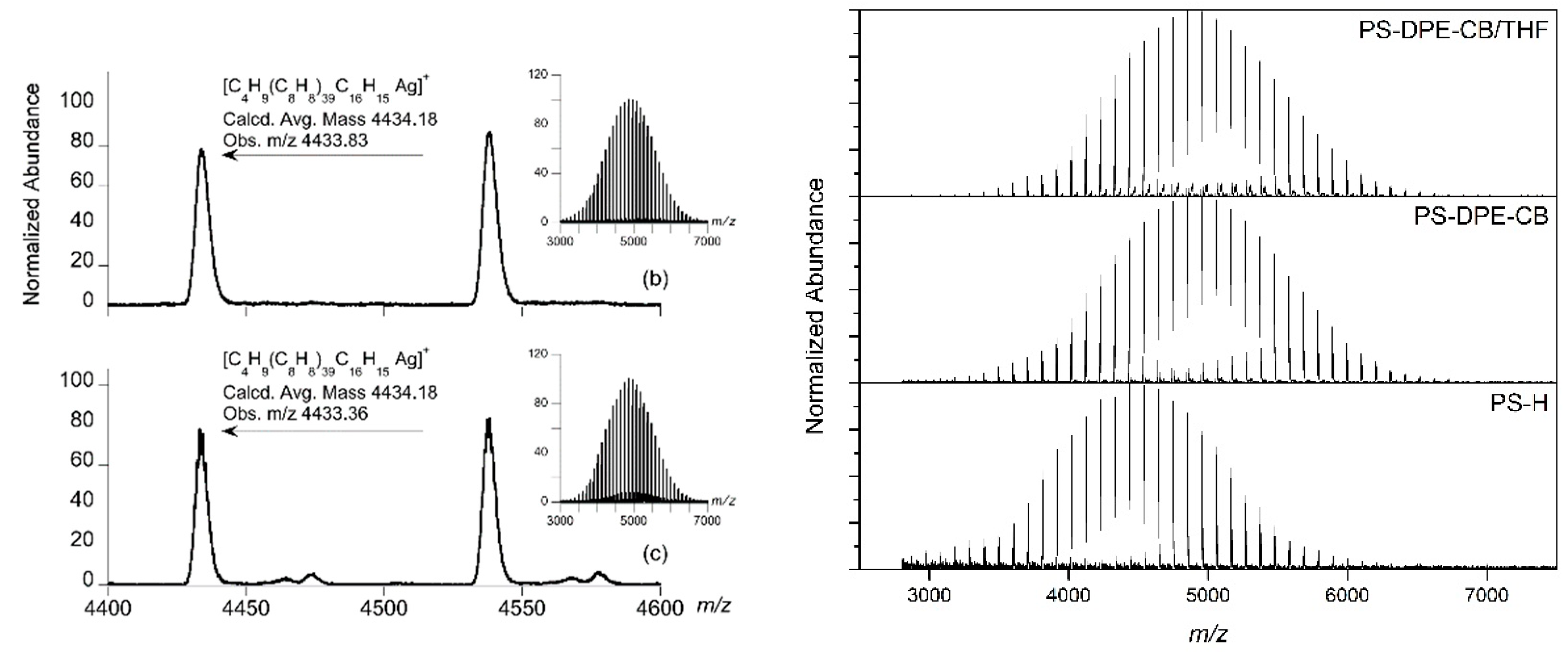
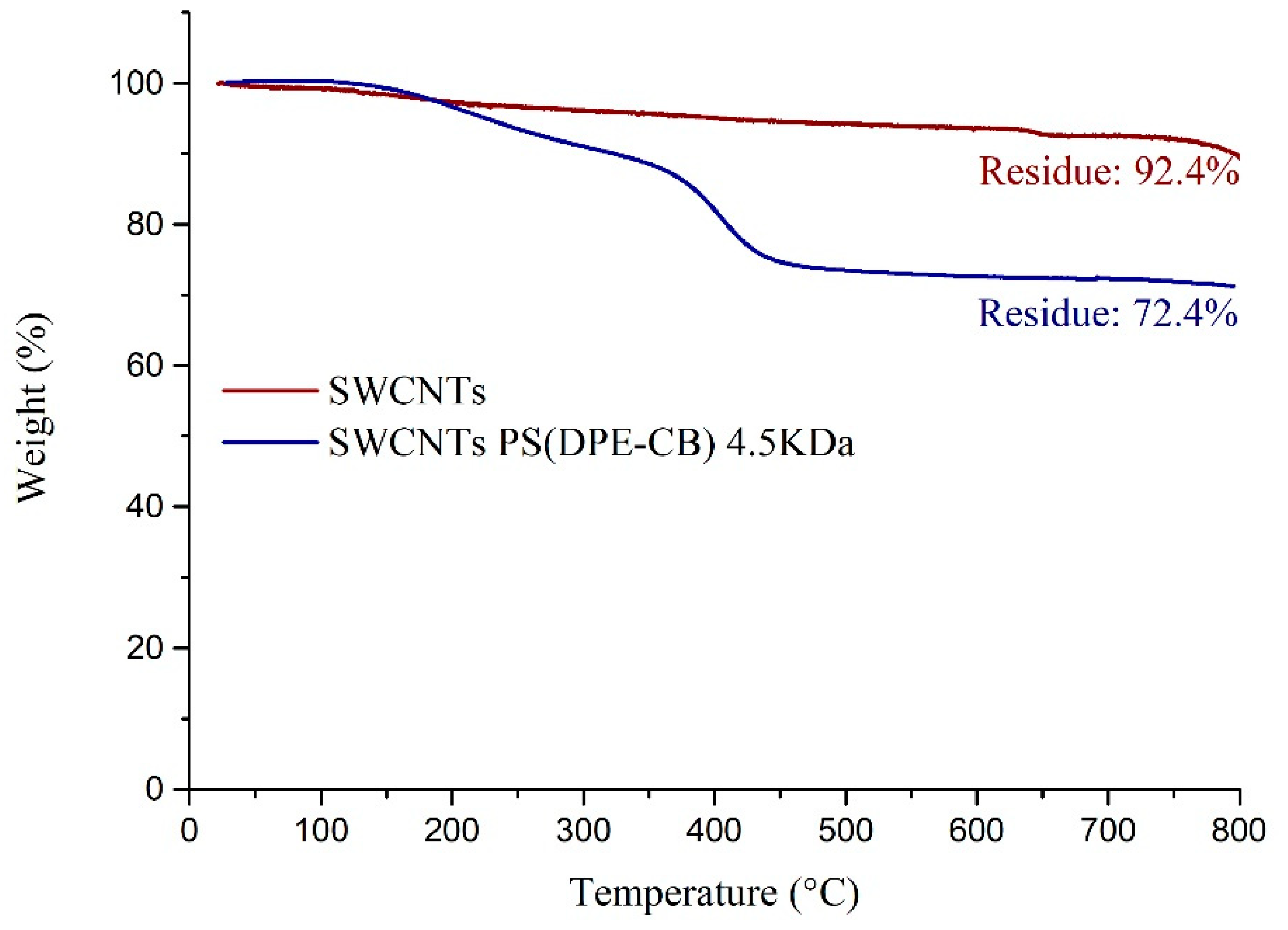
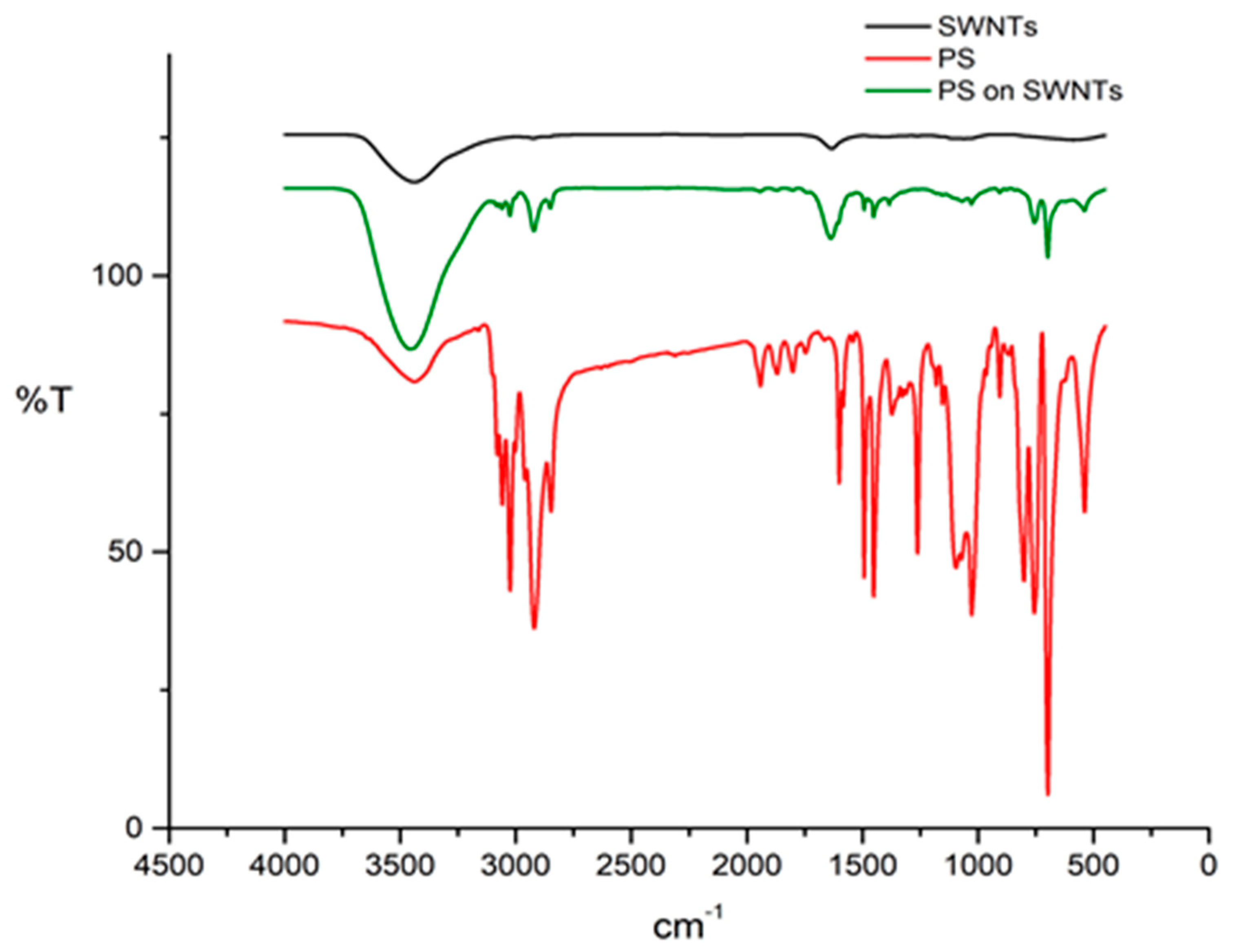
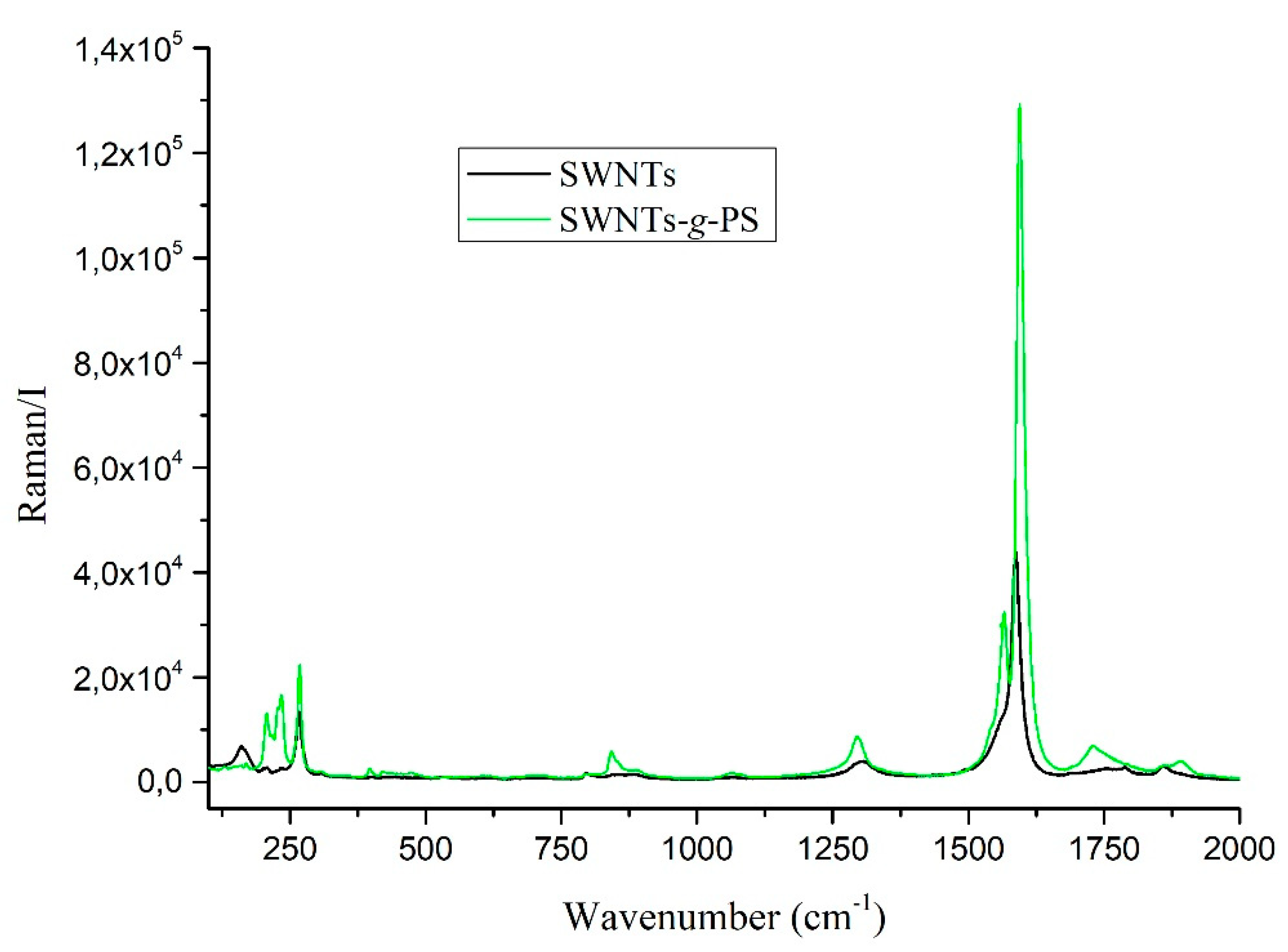
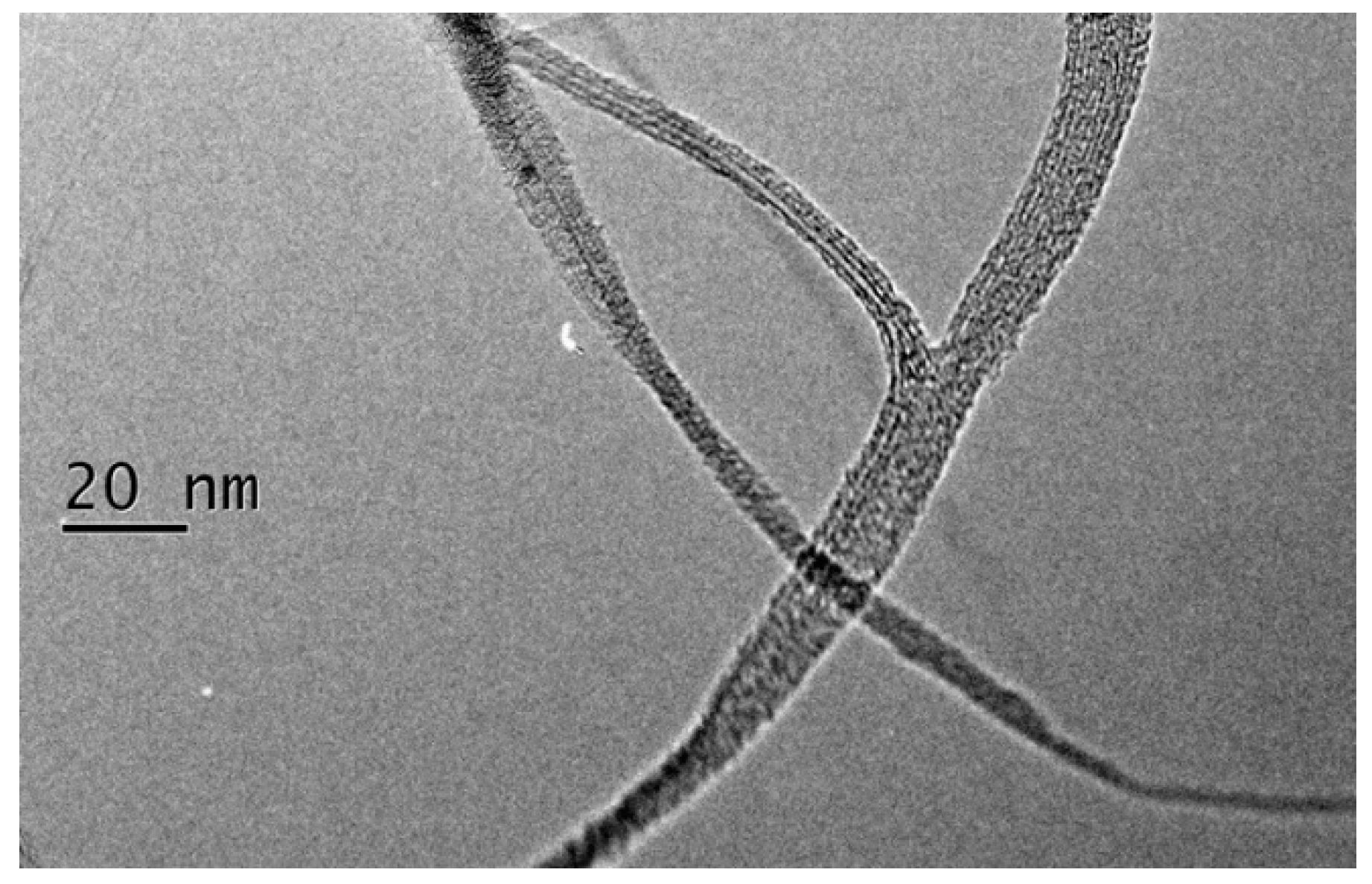
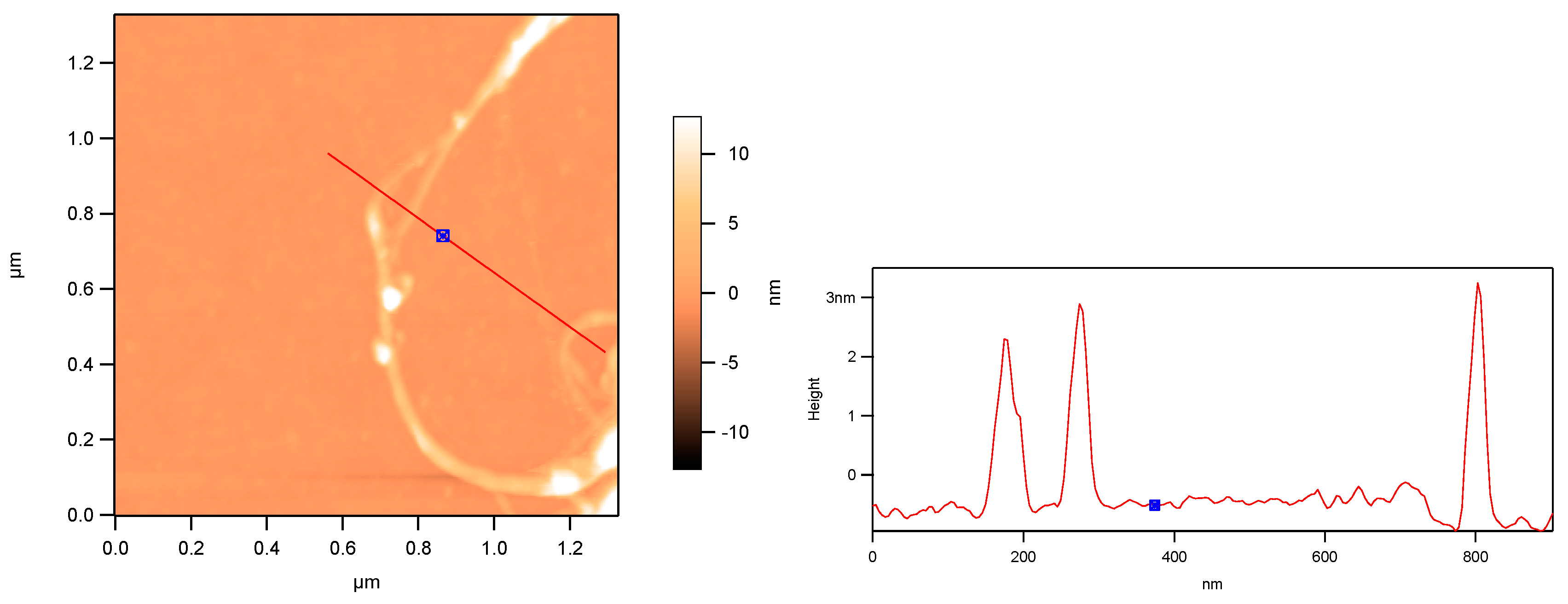
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gao, G.; Cagin, T.; Goddard, W.A. Energetics, structure, mechanical and vibrational properties of single-walled carbon nanotubes. Nanotechnology 1998, 9, 184–191. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Terentjev, E.M. Tailoring the electrical properties of carbon nanotube-polymer composites. Adv. Funct. Mater. 2010, 23, 4062–4068. [Google Scholar] [CrossRef]
- Murakami, H.; Nomura, T.; Nakashima, N. Noncovalent porphyrin-functionalized single-walled carbon nanotubes in solution and the formation of porphyrin–nanotube nanocomposites. Chem. Phys. Lett. 2003, 378, 481–486. [Google Scholar]
- Liu, I.-C.; Huang, H.-M.; Chang, C.-Y.; Tsai, H.-C.; Hsu, C.-H.; Tsiang, R.C.-C. Preparing a styrenic polymer composite containing well-dispersed carbon nanotubes: anionic polymerization of a nanotube-bound p-methylstyrene. Macromolecules 2004, 37, 283–287. [Google Scholar] [CrossRef]
- Lu, X.; Hiremath, N.; Hong, K.; Evora, M.C.; Ranson, V.H.; Naskar, A.K.; Bhat, G.S.; Kang, N.G.; Mays, J.W. Improving mechanical properties of carbon nanotube fibers through simultaneous solid-state cycloaddition and crosslinking. Nanotechnology 2017, 28, 145603. [Google Scholar] [CrossRef]
- Bilalis, P.; Katsigiannopoulos, D.; Avgeropoulos, A.; Sakellariou, G. Non-covalent functionalization of carbon nanotubes with polymers. RSC Adv. 2014, 4, 2911–2934. [Google Scholar] [CrossRef]
- Baskaran, D.; Sakellariou, G.; Mays, J.W.; Bratcher, M.S. Grafting reactions of living macroanions with multi-walled carbon nanotubes. J. Nanosci. Nanotechnol. 2007, 7, 1560–1567. [Google Scholar] [CrossRef]
- Li, H.; Cheng, F.; Duft, A.M.; Adronov, A. Functionalization of single-walled carbon nanotubes with well-defined polystyrene by “click” coupling. J. Am. Chem. Soc. 2005, 127, 14518–14524. [Google Scholar] [CrossRef]
- Liu, Y.; Yao, Z.; Adronov, A. Functionalization of single-walled carbon nanotubes with well-defined polymers by radical coupling. Macromolecules 2005, 38, 1172–1179. [Google Scholar] [CrossRef]
- Baskaran, D.; Dunlap, J.R.; Mays, J.W.; Bratcher, M.S. Grafting efficiency of hydroxy-terminated poly(methyl methacrylate) with multiwalled carbon nanotubes. Macromol. Rapid Commun. 2005, 26, 481–486. [Google Scholar] [CrossRef]
- Sakellariou, G.; Priftis, D.; Baskaran, D. Surface-initiated polymerization from carbon nanotubes: Strategies and perspectives. Chem. Soc. Rev. 2013, 42, 677–704. [Google Scholar] [CrossRef] [PubMed]
- Barbey, R.; Lavanant, L.; Paripovic, D.; Schuwer, N.; Sugnaux, C.; Tugulu, S.; Klok, H.-A. Polymer brushes via surface-initiated controlled radical polymerization: Synthesis, characterization, properties, and applications. Chem. Rev. 2009, 109, 5437–5527. [Google Scholar] [CrossRef]
- Zoppe, J.O.; Ataman, N.C.; Mocny, P.; Wang, J.; Moraes, J.; Klok, H.-A. Surface-initiated controlled radical polymerization: State-of-the-art, opportunities, and challenges in surface and interface engineering with polymer brushes. Chem. Rev. 2017, 117, 1105–1318. [Google Scholar] [CrossRef]
- Holzinger, M.; Abraham, J.; Whelan, P.; Graupner, R.; Ley, L.; Hennrich, F.; Kappes, M.; Hirsch, A. Functionalization of single-walled carbon nanotubes with (R-)oxycarbonyl nitrenes. J. Am. Chem. Soc. 2003, 125, 8566–8580. [Google Scholar] [CrossRef] [PubMed]
- Bahr, J.L.; Yang, J.; Kosynkin, D.V.; Bronikowski, M.J.; Smalley, R.E.; Tour, J.M. Functionalization of carbon nanotubes by electrochemical reduction of aryl diazonium salts: A bucky paper electrode. J. Am. Chem. Soc. 2001, 123, 6536–6542. [Google Scholar] [CrossRef] [PubMed]
- Holzinger, M.; Vostrowsky, O.; Hirsch, A.; Hennrich, F.; Kappes, M.; Weiss, R.; Jellen, F. Sidewall functionalization of carbon nanotubes. Angew. Chem. Int. Ed. 2001, 40, 4002–4005. [Google Scholar] [CrossRef]
- Georgakilas, V.; Kordatos, K.; Prato, M.; Guldi, D.M.; Holzinger, M.; Hirsch, A. Organic functionalization of carbon nanotubes. J. Am. Chem. Soc. 2002, 124, 760–761. [Google Scholar] [CrossRef] [PubMed]
- Umek, P.; Seo, J.W.; Hernadi, K.; Mrzel, A.; Pechy, P.; Mihailovic, D.D.; Forro, L. Addition of carbon radicals generated from organic peroxides to single wall carbon nanotubes. Chem. Mater. 2003, 15, 4751–4755. [Google Scholar] [CrossRef]
- Mickelson, E.T.; Huffman, C.B.; Rinzler, A.; Smalley, R.E.; Hauge, R.H.; Margrave, J.L. Fluorination of single-wall carbon nanotubes. Chem. Phys. Lett. 1998, 296, 188–194. [Google Scholar] [CrossRef]
- Brunetti, F.; Ferrrero, M.; Munoz, J.; Meneghetti, M.; Prato, M.; Vasquez, E. Microwave-induced multiple functionalization of carbon nanotubes. J. Am. Chem. Soc. 2008, 130, 8094–8100. [Google Scholar] [CrossRef] [PubMed]
- Coleman, K.S.; Bailey, S.R.; Fogden, S.; Green, M.L.H. Functionalization of single-walled carbon nanotubes via the Bingel reaction. J. Am. Chem. Soc. 2003, 125, 8722–8723. [Google Scholar] [CrossRef]
- Tchoul, M.N.; Ford, W.T.; Lolli, G.; Resasco, D.E.; Arepalli, S. Effect of mild nitric acid oxidation on dispersability, size, and structure of single-walled carbon nanotubes. Chem. Mater. 2007, 19, 5765–5772. [Google Scholar] [CrossRef]
- Datsyuk, V.; Kalyva, M.; Papagelis, K.; Parthenios, J.; Tasis, D.; Siokou, A.; Kallitsis, I.; Galiotis, C. Chemical oxidation of multiwalled carbon nanotubes. Carbon 2008, 46, 833–840. [Google Scholar] [CrossRef]
- Chang, C.M.; Liu, Y.L. Functionalization of multi-walled carbon nanotubes with furan and maleimide compounds through Diels-Alder cycloaddition. Carbon 2009, 47, 3041–3049. [Google Scholar] [CrossRef]
- Delgado, J.D.; De La Cruz, P.; Langa, F.; Urbina, A.; Casado, J.; Lόpez Navarrete, J.T. Microwave-assisted sidewall functionalization of single-wall carbon nanotubes by Diels-Alder cycloaddition. Chem. Commun. 2004, 10, 1734–1735. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, J.; Edwards, C.L.; Alemany, L.B.; Khabashesku, V.N.; Barron, A.R. Diels-Alder addition to fluorinated single walled carbón nanotubes. Chem. Commun. 2005, 26, 3265–3267. [Google Scholar] [CrossRef] [PubMed]
- Ménard-Moyon, C.; Dumas, F.; Doris, E.; Mioskowski, C. Functionalization of single-wall carbon nanotubes by tandem high-pressure/Cr(CO)6 activation of Diels-Alder cycloaddition. J. Am. Chem. Soc. 2006, 128, 14764–14765. [Google Scholar] [CrossRef] [PubMed]
- Zydziak, N.; Hübner, C.; Bruns, M.; Barner-Kowollik, C. One-step functionalization of single-walled carbon nanotubes with cyclopentadienyl-capped macromolecules via Diels-Alder chemistry. Macromolecules 2011, 44, 3374–3380. [Google Scholar] [CrossRef]
- Bernal, M.; Liras, M.; Verdejo, R.; Lopez-Manchado, M.A.; Quijada-Garrido, I.; Paris, R. Modification of carbon nanotubes with well-controlled fluorescent styrene-based polymers using the Diels-Alder reaction. Polymer 2011, 52, 5739–5745. [Google Scholar] [CrossRef]
- Munirasu, S.; Albueme, J.; Boschetti-de-Fierro, A.; Abetz, V. Functionalization of carbon materials using the Diels-Alder reaction. Macromol. Rapid Comm. 2010, 31, 574–579. [Google Scholar] [CrossRef]
- Zydziak, N.; Preuss, C.M.; Winkler, V.; Bruns, M.; Hübner, C.; Barner-Kowollik, C. Hetero Diels-Alder chemistry for the functionalization of single-walled carbon nanotubes with cyclopentadienyl end-capped polymer strands. Macromol. Rapid Commun. 2013, 34, 672–680. [Google Scholar] [CrossRef]
- Lu, X.; Tian, F.; Wang, N.; Zhang, Q. Organic functionalization of the sidewalls of carbon nanotubes by Diels-Alder reactions: A theoretical prediction. Org. Lett. 2002, 4, 4313–4315. [Google Scholar] [CrossRef]
- Zydziak, N.; Yameen, B.; Barner-Kowollik, C. Diels-Alder reactions for carbon material synthesis and surface functionalization. Polym. Chem. 2013, 4, 4072–4086. [Google Scholar] [CrossRef]
- Sakellariou, G.; Ji, H.; Mays, J.W.; Hadjichristidis, N.; Baskaran, D. Controlled covalent functionalization of multiwalled carbon nanotubes using [4+2] cycloaddition of benzocyclobutenes. Chem. Mater. 2007, 19, 6370–6372. [Google Scholar] [CrossRef]
- Sakellariou, G.; Ji, H.; Mays, J.W.; Baskaran, D. Enhanced polymer grafting from multiwalled carbon nanotubes through living anionic surface-initiated polymerization. Chem. Mater. 2008, 20, 6217–6230. [Google Scholar] [CrossRef]
- Priftis, D.; Petzetakis, N.; Sakellariou, G.; Pitsikalis, M.; Baskaran, D.; Mays, J.W.; Hadjichristidis, N. Surface-initiated titanium-mediated coordination polymerization from catalyst-functionalized single and multiwalled carbón nanotubes. Macromolecules 2009, 42, 3340–3346. [Google Scholar] [CrossRef]
- Priftis, D.; Sakellariou, G.; Baskaran, D.; Mays, J.W.; Hadjichristidis, N. Polymer grafted Janus multi-walled carbón nanotubes. Soft Matter 2009, 5, 4272–4278. [Google Scholar] [CrossRef]
- Priftis, D.; Sakellariou, G.; Hadjichristidis, N.; Penott, E.K.; Lorenzo, A.T.; Muller, A.J. Surface modification of multiwalled carbón nanotubes with biocompatible polymers via ring opening and living anionic surface initiated polymerization. Kinetics and crystallization behavior. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 4379–4390. [Google Scholar] [CrossRef]
- Gkikas, M.; Das, B.P.; Tsianou, M.; Iatrou, H.; Sakellariou, G. Surface initiated ring-opening polymerization of L-proline N-carboxy anhydride from single and multi walled carbon nanotubes. Eur. Polym. J. 2013, 49, 3095–3103. [Google Scholar] [CrossRef]
- Priftis, D.; Sakellariou, G.; Mays, J.W.; Hadjichristidis, N. Novel diblock copolymer-grafted multiwalled carbon nanotubes via a combination of living and controlled/living surface polymerizations. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 1104–1112. [Google Scholar] [CrossRef]
- Harth, E.; Van Horn, B.; Lee, V.Y.; Germack, D.S.; Gonzales, C.P.; Miller, R.D.; Hawker, C.J. A facile approach to architectually defined nanoparticles via intramolecular chain collapse. J. Am. Chem. Soc. 2002, 124, 8656–8660. [Google Scholar] [CrossRef] [PubMed]
- Klonos, P.; Patelis, N.; Glynos, E.; Sakellariou, G.; Kyritsis, A. Molecular dynamics in polystyrene single-chain nanoparticles. Macromolecules 2019, 52, 9334–9340. [Google Scholar] [CrossRef]
- Sakellariou, G.; Avgeropoulos, A.; Hadjichristidis, N.; Mays, J.W.; Baskaran, D. Functionalized organic nanoparticles from core-crosslinked poly(4-vinylbenzocyclobutene-b-butadiene) diblock copolymer micelles. Polymer 2009, 50, 6202–6211. [Google Scholar] [CrossRef]
- Chiag, I.W.; Brinson, B.E.; Huang, A.Y.; Willis, P.A.; Bronikowski, M.J.; Margrave, J.L.; Smalley, R.E.; Huage, R.H. Purification and characterization of single-wall carbon nanotubes (SWNTs) obtained from the gas-phase decomposition of CO (HiPco Process). J. Phys. Chem. B 2001, 105, 8297–8301. [Google Scholar] [CrossRef]
- Hadjichristidis, N.; Iatrou, H.; Pispas, S.; Pitsikalis, M. Anionic polymerization: High vacuum techniques. J. Polym. Sci. Part A Polym. Chem. 2000, 38, 3211–3234. [Google Scholar] [CrossRef]
- Uhrig, D.; Mays, J.W. Experimental techniques in high-vacuum anionic polymerization. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 6179–6222. [Google Scholar] [CrossRef]
- Nega, A.D.; Pefkianakis, E.K.; Vougioukalakis, G.C.; Sakellariou, G. Synthesis of P3HT-b-PS donor-acceptor diblock copolymer carrying pendant fullerenes at precise positions along the PS block. Eur. Polym. J. 2016, 83, 148–160. [Google Scholar] [CrossRef]
- Segura, J.L.; Martin, N. o-Quinodimethanes: Efficient intermediates in organic synthesis. Chem. Rev. 1999, 99, 3199–3246. [Google Scholar] [CrossRef]
- Peigney, A.; Laurent, C.; Flahaut, E.; Bacsa, R.R.; Rousset, A. Specific Surface area of carbon nanotubes and bundles of carbon nanotubes. Carbon 2001, 39, 507–514. [Google Scholar] [CrossRef]
- Dresselhaus, M.D.; Dresselhaus, G.; Saito, R.; Jorio, A. Raman spectroscopy of carbon nanotubes. Phys. Rep. 2005, 409, 47–99. [Google Scholar] [CrossRef]
- Thomsen, C.; Reich, S. Double resonant Raman scattering in graphite. Phys. Rev. Lett. 2000, 85, 5214–5217. [Google Scholar] [CrossRef] [PubMed]
- Hennrich, F.; Krupke, R.; Lebedkin, S.; Arnold, K.; Fischer, R.; Resasco, D.E.; Kappes, M.M. Raman spectroscopy of individual single-walled carbon nanotubes from various sources J. Phys. Chem. B 2005, 109, 10567–10573. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stathouraki, M.-M.; Pantazidis, C.; Mygiakis, E.; Avgeropoulos, A.; Sakellariou, G. Functionalization of Single-Walled Carbon Nanotubes with End-Capped Polystyrene via a Single-Step Diels–Alder Cycloaddition. Polymers 2021, 13, 1169. https://doi.org/10.3390/polym13071169
Stathouraki M-M, Pantazidis C, Mygiakis E, Avgeropoulos A, Sakellariou G. Functionalization of Single-Walled Carbon Nanotubes with End-Capped Polystyrene via a Single-Step Diels–Alder Cycloaddition. Polymers. 2021; 13(7):1169. https://doi.org/10.3390/polym13071169
Chicago/Turabian StyleStathouraki, Maria-Malvina, Christos Pantazidis, Emmanouil Mygiakis, Apostolos Avgeropoulos, and Georgios Sakellariou. 2021. "Functionalization of Single-Walled Carbon Nanotubes with End-Capped Polystyrene via a Single-Step Diels–Alder Cycloaddition" Polymers 13, no. 7: 1169. https://doi.org/10.3390/polym13071169
APA StyleStathouraki, M.-M., Pantazidis, C., Mygiakis, E., Avgeropoulos, A., & Sakellariou, G. (2021). Functionalization of Single-Walled Carbon Nanotubes with End-Capped Polystyrene via a Single-Step Diels–Alder Cycloaddition. Polymers, 13(7), 1169. https://doi.org/10.3390/polym13071169






