Influence of Elongation of Paclitaxel-Eluting Electrospun-Produced Stent Coating on Paclitaxel Release and Transport through the Arterial Wall after Stenting
Abstract
:1. Introduction
2. Materials and Methods
2.1. Production of 3H-Labeled Paclitaxel
2.2. Fabrication of the Matrices by Electrospinning
2.3. Fabrication of PTX-Eluting Stents
2.4. SEM Analysis
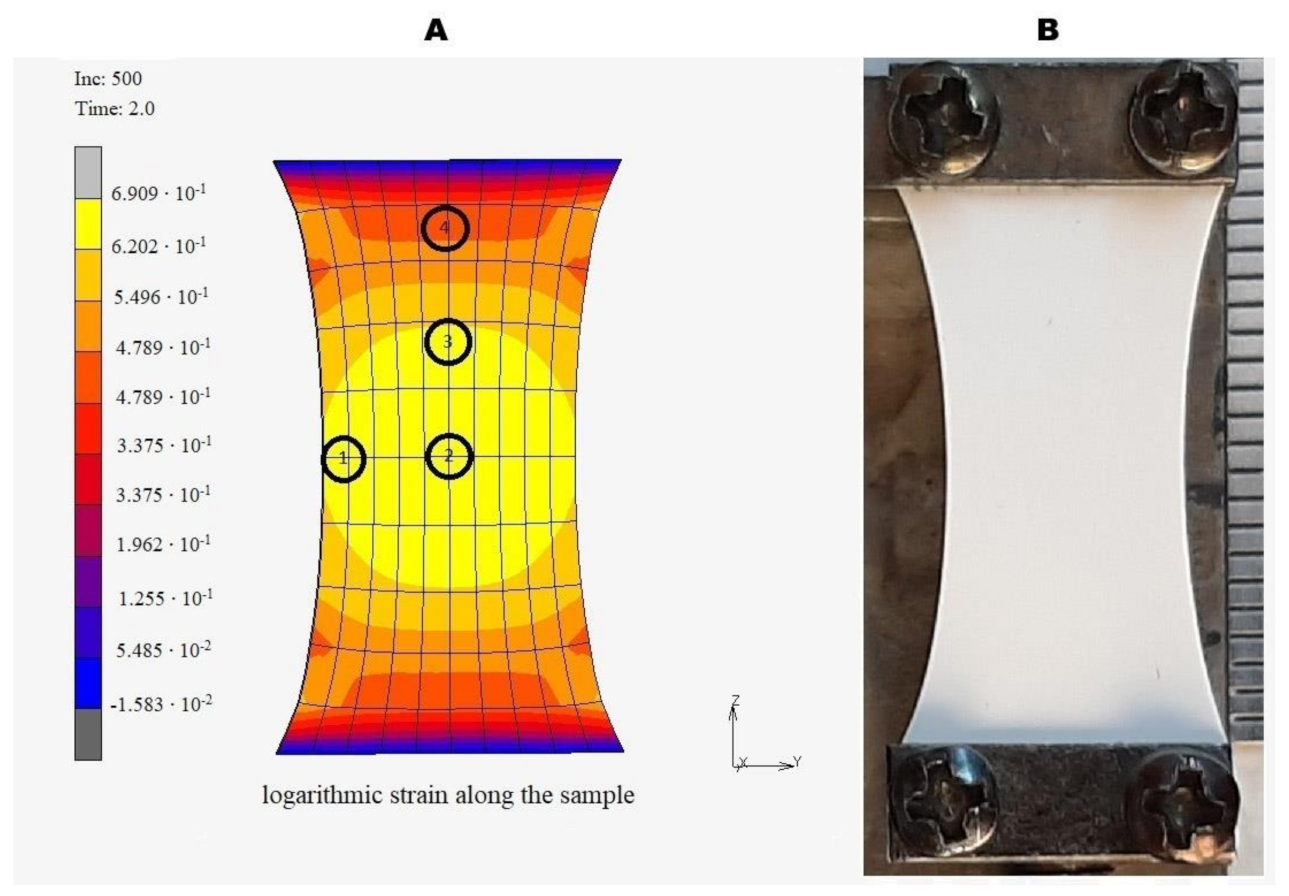
2.5. Modeling of Uniaxial Deformation of the Matrices
2.6. PTX Release Kinetics from Intact and Twofold Elongated Matrices
2.7. PTX Release from Stent through Arterial Wall
2.8. Diffusion Flow and Effective Diffusivity of PTX through the Arterial Wall
2.9. Statistical Processing of Data
3. Results
3.1. Preparation and Characterization of the PTX Matrices
3.2. The Influence of Matrix Elongation on the Fiber Structure
3.3. Influence of Matrix Elongation on PTX Release
3.4. The Effect of the Arterial Wall on the Rate of PTX Release
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Kuznetsov, K.A.; Stepanova, A.O.; Kvon, R.I.; Douglas, T.E.L.; Kuznetsov, N.A.; Chernonosova, V.S.; Zaporozhchenko, I.A.; Kharkova, M.V.; Romanova, I.V.; Karpenko, A.A.; et al. Electrospun produced 3D matrices for covering of vascular stents: Paclitaxel release depending on fiber structure and composition of the external environment. Materials 2018, 11, 2176. [Google Scholar] [CrossRef] [Green Version]
- Kuznetsov, K.A.; Murashov, I.S.; Chernonosova, V.S.; Chelobanov, B.P.; Stepanova, A.O.; Sergeevichev, D.S.; Karpenko, A.A.; Laktionov, P.P. Vascular Stents Coated with Electrospun Drug-Eluting Material: Functioning in Rabbit Iliac Artery. Polymers 2020, 12, 1741. [Google Scholar] [CrossRef] [PubMed]
- Puranik, A.S.; Dawson, E.R.; Peppas, N.A. Recent advances in drug eluting stents. Int. J. Pharm. 2013, 441, 665–679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manfredi, J.J.; Parness, J.; Horwitz, S.B. Taxol binds to cellular microtubules. J. Cell Biol. 1982, 94, 688–696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Axel, D.I.; Kunert, W.; Goggelmann, C.; Oberhoff, M.; Herdeg, C.; Kuttner, A.; Wild, D.H.; Brehm, B.R.; Riessen, R.G.; Koveker, K.R.K. Paclitaxel inhibits arterial smooth muscle cell proliferation and migration in vitro and in vivo using local drug delivery. Circulation 1997, 96, 636–645. [Google Scholar] [CrossRef] [PubMed]
- Grube, E.; Silber, S.; Hauptmann, K.E.; Mueller, R.; Buellesfeld, L.; Gerckens, U.; Russell, M.E. TAXUS I: Six- and twelve-month results from a randomized, double-blind trial on a slow-release paclitaxel-eluting stent for de novo coronary lesions. Circulation 2003, 107, 38–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuno, T.; Ueyama, H.; Mikami, T.; Takagi, H.; Numasawa, Y.; Anzai, H.; Bangalore, S. Mortality in patients undergoing revascularization with paclitaxel eluting devices for infrainguinal peripheral artery disease: Insights from a network meta-analysis of randomized trials. Catheter. Cardiovasc. Interv. 2020, 96, E467–E478. [Google Scholar] [CrossRef] [PubMed]
- Heldman, A.W.; Cheng, L.; Jenkins, G.M.; Heller, P.F.; Kim, D.W.; Ware, M., Jr.; Nater, C.; Hruban, R.H.; Rezai, B.; Abella, B.S.; et al. Paclitaxel stent coating inhibits neointimal hyperplasia at 4 weeks in a porcine model of coronary restenosis. Circulation 2001, 103, 2289–2295. [Google Scholar] [CrossRef] [Green Version]
- Bisdas, T.; Beropoulis, E.; Argyriou, A.; Torsello, G.; Stavroulakis, K. 1-Year all-comers analysis of the Eluvia drug-eluting stent for long femoropopliteal lesions after suboptimal angioplasty. JACC Cardiovasc. Interv. 2018, 11, 957–966. [Google Scholar] [CrossRef]
- Creel, C.J.; Lovich, M.A.; Edelman, E.R. Arterial paclitaxel distribution and deposition. Circ. Res. 2000, 86, 879–884. [Google Scholar] [CrossRef] [Green Version]
- Que, C.; Gao, Y.; Raina, S.A.; Zhang, G.G.Z.; Taylor, L.S. Paclitaxel crystal seeds with different intrinsic properties and their impact on dissolution of paclitaxel-HPMCAS amorphous solid dispersions. Cryst. Growth Des. 2018, 18, 1548–1559. [Google Scholar] [CrossRef]
- Tzafriri, A.R.; Parikh, S.A.; Edelman, E.R. Taking paclitaxel coated balloons to a higher level: Predicting coating dissolution kinetics, tissue retention and dosing dynamics. J. Control Release 2019, 28, 94–102. [Google Scholar] [CrossRef]
- Levin, A.d.; Vukmirovic, N.; Hwang, C.-W.; Edelman, E.R. Specific binding to intracellular proteins determines arterial transport properties for rapamycin and paclitaxel. Proc. Natl Acad. Sci. USA 2004, 101, 9463–9467. [Google Scholar] [CrossRef] [Green Version]
- Livingston, M.; Tan, A. Coating techniques and release kinetics of drug-eluting stents. J. Med. Devices 2019, 10, 010801. [Google Scholar] [CrossRef]
- Bae, I.H.; Lim, K.S.; Park, D.S.; Shim, J.W.; Lee, S.Y.; Jang, E.J.; Park, J.K.; Kim, J.H.; Jeong, M.H. Sirolimus coating on heparinized stents prevents restenosis and thrombosis. J. Biomater. Appl. 2017, 31, 1337–1345. [Google Scholar] [CrossRef]
- Jelonek, K.; Jaworska, J.; Pastusiak, M.; Sobota, M.; Włodarczyk, J.; Karpeta-Jarzabek, P.; Kaczmarczyk, B.; Kasperczyk, J.; Dobrzyński, P. Effect of vascular scaffold composition on release of sirolimus. Eur. J. Pharm. Biopharm. 2018, 132, 41–49. [Google Scholar] [CrossRef]
- Raval, A.; Parikh, J.; Engineer, C. Mechanism and in vitro release kinetic study of sirolimus from a biodegradable polymeric matrix coated cardiovascular stent. Ind. Eng. Chem. Res. 2011, 50, 9539–9549. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Xiao, J.; Fang, T.; Hu, N.; Li, M.; Deng, L.; Cheng, Y.; Zhu, Y.; Cui, W. An electrospun fiber-covered stent with programmable dual drug release for endothelialization acceleration and lumen stenosis prevention. Acta Biomater. 2019, 94, 295–305. [Google Scholar] [CrossRef]
- Rychter, M.; Milanowski, B.; Grzeskowiak, B.F.; Jarek, M.; Kempinski, M.; Coy, E.L.; Borysiak, S.; Baranowska-Korczyc, A.; Lulek, J. Cilostazol-loaded electrospun three-dimensional systems for potential cardiovascular application: Effect of fibers hydrophilization on drug release, and cytocompatibility. J. Colloid Interface Sci. 2019, 15, 310–327. [Google Scholar] [CrossRef]
- Mirbagheri, M.S.; Mohebbi-Kalhori, D.; Jirofti, N. Evaluation of mechanical properties and medical applications of polycaprolactone small diameter artificial blood vessels. Int. J. Basic Sci. Med. 2017, 2, 58–70. [Google Scholar] [CrossRef] [Green Version]
- Chernonosova, V.S.; Kvon, R.I.; Stepanova, A.O.; Larichev, Y.V.; Karpenko, A.A.; Chelobanov, B.P.; Kiseleva, E.V.; Laktionov, P.P. Human serum albumin in electrospun PCL fibers: Structure, release, and exposure on fibersurface. Polym. Adv. Technol. 2017, 28, 819–827. [Google Scholar] [CrossRef]
- Sidorov, V.N.; Polak, Y.V.; Laktionov, P.P.; Roshcke, V.V.; Kist, A.G. Method of Production of Tritium Labeled Organic Compounds and the Device for Its Implementation. SU Patent 1823961 A3, 18 January 1991. [Google Scholar]
- Nazarkina, Z.K.; Chelobanov, B.P.; Chernonosova, V.S.; Romanova, I.V.; Karpenko, A.A.; Laktionov, P.P. Sirolimus-Eluting Electrospun-Produced Matrices as Coatings for Vascular Stents: Dependence of Drug Release on Matrix Structure and Composition of the External Environment. Materials 2020, 13, 2692. [Google Scholar] [CrossRef] [PubMed]
- Simo, J.C.; Miehe, C. Associative coupled thermoplasticity at finite strains: Formulation, numerical analysis and implementation. Comput. Methods Appl. Mech. Eng. 1992, 98, 41–104. [Google Scholar] [CrossRef]
- Shutov, A.V. Efficient implicit integration for finite-strain viscoplasticity with a nested multiplicative split. Comput. Methods Appl. Mech. Eng. 2016, 306, 151–174. [Google Scholar] [CrossRef] [Green Version]
- Rozemond, H. Laboratory animal protection: The European Convention and the Dutch. Act. Vet. Q. 1986, 8, 346–349. [Google Scholar] [CrossRef] [Green Version]
- Dill, K.; Bromberg, S. Molecular Driving Forces: Statistical Thermodynamics in Chemistry and Biology; Garland Science: New York, NY, USA, 2003. [Google Scholar]
- Shah, V.P.; Tsong, Y.; Sathe, P.; Williams, R.L. Dissolution Profile Comparison Using Similarity Factor, f2. Dissolution Technol. 1999, 6, 15. [Google Scholar] [CrossRef]
- Purcell, M.; Neault, J.F.; Tajmir-Riahi, H.A. Interaction of taxol with human serum albumin. Biochim. Biophys. Acta 2000, 1478, 61–68. [Google Scholar] [CrossRef]
- Weaving, G.; Batstone, G.F.; Jones, R.G. Age and sex variation in serum albumin concentration: An observational study. Ann. Clin. Biochem. 2016, 53, 106–111. [Google Scholar] [CrossRef] [Green Version]
- Cherniy, V.I. The role and place of albumin in modern infusion-transfusion therapy. Emerg. Med. 2017, 1, 23–31. [Google Scholar] [CrossRef] [Green Version]
- Nikol, S.; Huehns, T.Y.; Höfling, B. Molecular biology and post-angioplasty restenosis. Atherosclerosis 1996, 123, 17–31. [Google Scholar] [CrossRef]
- Konijn, L.C.D.; Wakkie, T.; Spreen, M.I.; de Jong, P.A.; van Dijk, L.C.; Wever, J.J.; Veger, H.T.C.; Statius van Eps, R.G.; Mali, W.P.T.M.; van Overhagen, H. 10-year paclitaxel dose-related outcomes of drug-eluting stents treated below the knee in patients with chronic limb-threatening ischemia (the PADI trial). Cardiovasc. Interv. Radiol. 2020, 43, 1881–1888. [Google Scholar] [CrossRef]
- Tzafriri, A.R.; Levin, A.D.; Edelman, E.R. Diffusion-limited binding explains binary dose response for local arterial and tumour drug delivery. Cell Prolif. 2009, 42, 348–363. [Google Scholar] [CrossRef] [Green Version]
- Lovich, M.A.; Creel, C.; Hong, K.; Hwang, C.W.; Edelman, E.R. Carrier proteins determine local pharmacokinetics and arterial distribution of paclitaxel. J. Pharm Sci. 2001, 90, 1324–1335. [Google Scholar] [CrossRef]




| Elasticity Modulus | Poisson’s Ratio | Initial Uniaxial Yield Stress | Linear Isotropic Hardening Coefficient |
|---|---|---|---|
| 1 [dimension of stress] | 0.45 [-] | 0.05 [dimension of stress] | 0.225 [dimension of stress] |
| Point Number | ||||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Stretch in the axial direction | 1.883 | 1.996 | 1.851 | 1.594 |
| Stretch in the transverse direction | 0.729 | 0.720 | 0.766 | 0.872 |
| Change of area [×times] | 1.373 | 1.437 | 1.417 | 1.390 |
| Matrices Incubated in PBS without Any Medium Replacement | |||
| Matrix Type | 5%PCL/PTX Elongated | 5%PCL/10%HSA/PTX Elongated | 5%PCL/10%HSA/3%DMSO/PTX Elongated |
| 5%PCL/PTX | f1 = 11.9 f2 = 64.7 | - | - |
| 5%PCL/10%HSA/PTX | - | f1 = 9.6 f2 = 67.6 | - |
| 5%PCL/10%HSA/3%DMSO/PTX | - | - | f1 = 7.31 f2 = 73.8 |
| Matrices Incubated in BP without Any Medium Replacement | |||
| Matrix Type | 5%PCL/PTX Elongated | 5%PCL/10%HSA/PTX Elongated | 5%PCL/10%HSA/3%DMSO/PTX Elongated |
| 5%PCL/PTX | f1 = 6.8 f2 = 65.2 | - | - |
| 5%PCL/10%HSA/PTX | - | f1 = 8.2 f2 = 60.1 | - |
| 5%PCL/10%HSA/3%DMSO/PTX | - | - | f1 = 4.2 f2 = 75.0 |
| Matrices Incubated in BP with Medium Replacement | |||
| Matrix Type | 5%PCL/PTX Elongated | 5%PCL/10%HSA/PTX Elongated | 5%PCL/10%HSA/3%DMSO/PTX Elongated |
| 5%PCL/PTX | f1 = 6.5 f2 = 64.7 | - | - |
| 5%PCL/10%HSA/PTX | - | f1 = 6.4 f2 = 65.7 | - |
| 5%PCL/10%HSA/3%DMSO/PTX | - | - | f1 = 5.4 f2 = 68.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nazarkina, Z.K.; Chelobanov, B.P.; Kuznetsov, K.A.; Shutov, A.V.; Romanova, I.V.; Karpenko, A.A.; Laktionov, P.P. Influence of Elongation of Paclitaxel-Eluting Electrospun-Produced Stent Coating on Paclitaxel Release and Transport through the Arterial Wall after Stenting. Polymers 2021, 13, 1165. https://doi.org/10.3390/polym13071165
Nazarkina ZK, Chelobanov BP, Kuznetsov KA, Shutov AV, Romanova IV, Karpenko AA, Laktionov PP. Influence of Elongation of Paclitaxel-Eluting Electrospun-Produced Stent Coating on Paclitaxel Release and Transport through the Arterial Wall after Stenting. Polymers. 2021; 13(7):1165. https://doi.org/10.3390/polym13071165
Chicago/Turabian StyleNazarkina, Zhanna K., Boris P. Chelobanov, Konstantin A. Kuznetsov, Alexey V. Shutov, Irina V. Romanova, Andrey A. Karpenko, and Pavel P. Laktionov. 2021. "Influence of Elongation of Paclitaxel-Eluting Electrospun-Produced Stent Coating on Paclitaxel Release and Transport through the Arterial Wall after Stenting" Polymers 13, no. 7: 1165. https://doi.org/10.3390/polym13071165
APA StyleNazarkina, Z. K., Chelobanov, B. P., Kuznetsov, K. A., Shutov, A. V., Romanova, I. V., Karpenko, A. A., & Laktionov, P. P. (2021). Influence of Elongation of Paclitaxel-Eluting Electrospun-Produced Stent Coating on Paclitaxel Release and Transport through the Arterial Wall after Stenting. Polymers, 13(7), 1165. https://doi.org/10.3390/polym13071165






