Leflunomide Loaded Chitosan Nanoparticles for the Preparation of Aliphatic Polyester Based Skin Patches
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of NPs
2.3. Characterization of NPs
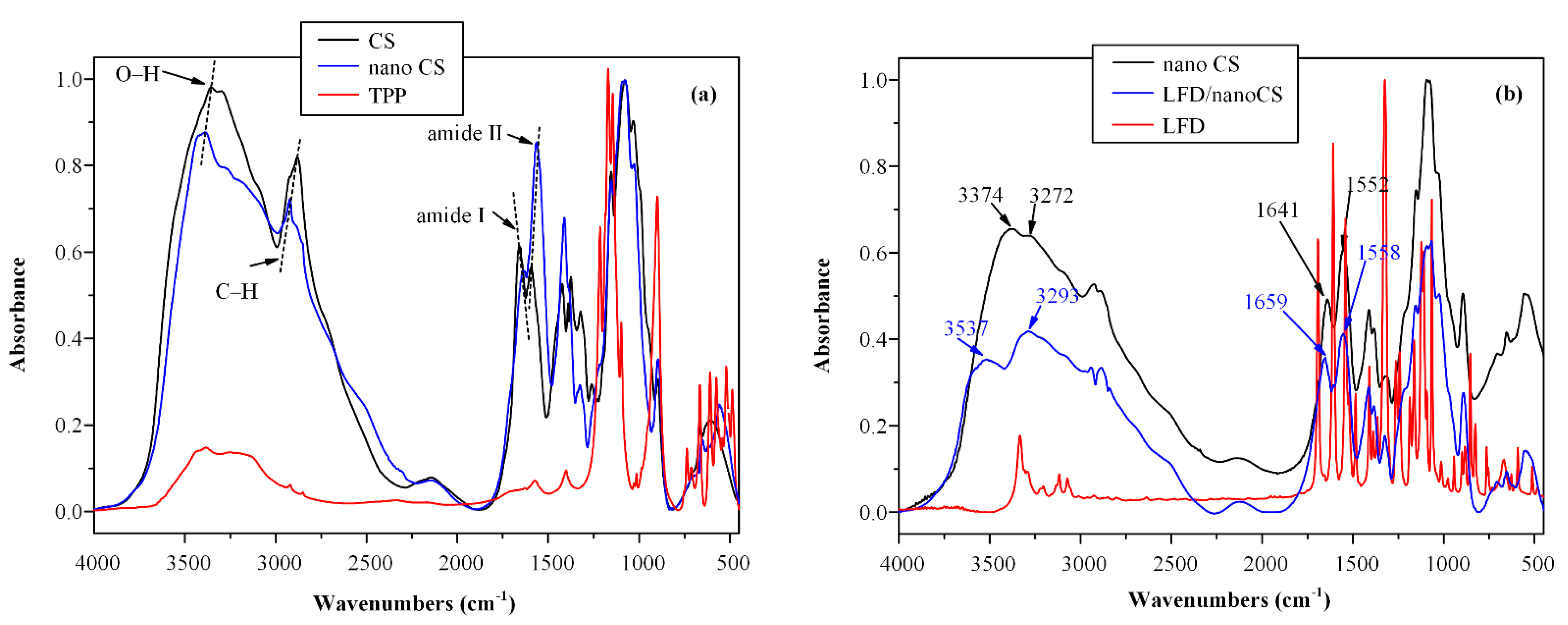
2.3.1. Fourier-Transformed Infrared Spectroscopy (FTIR)
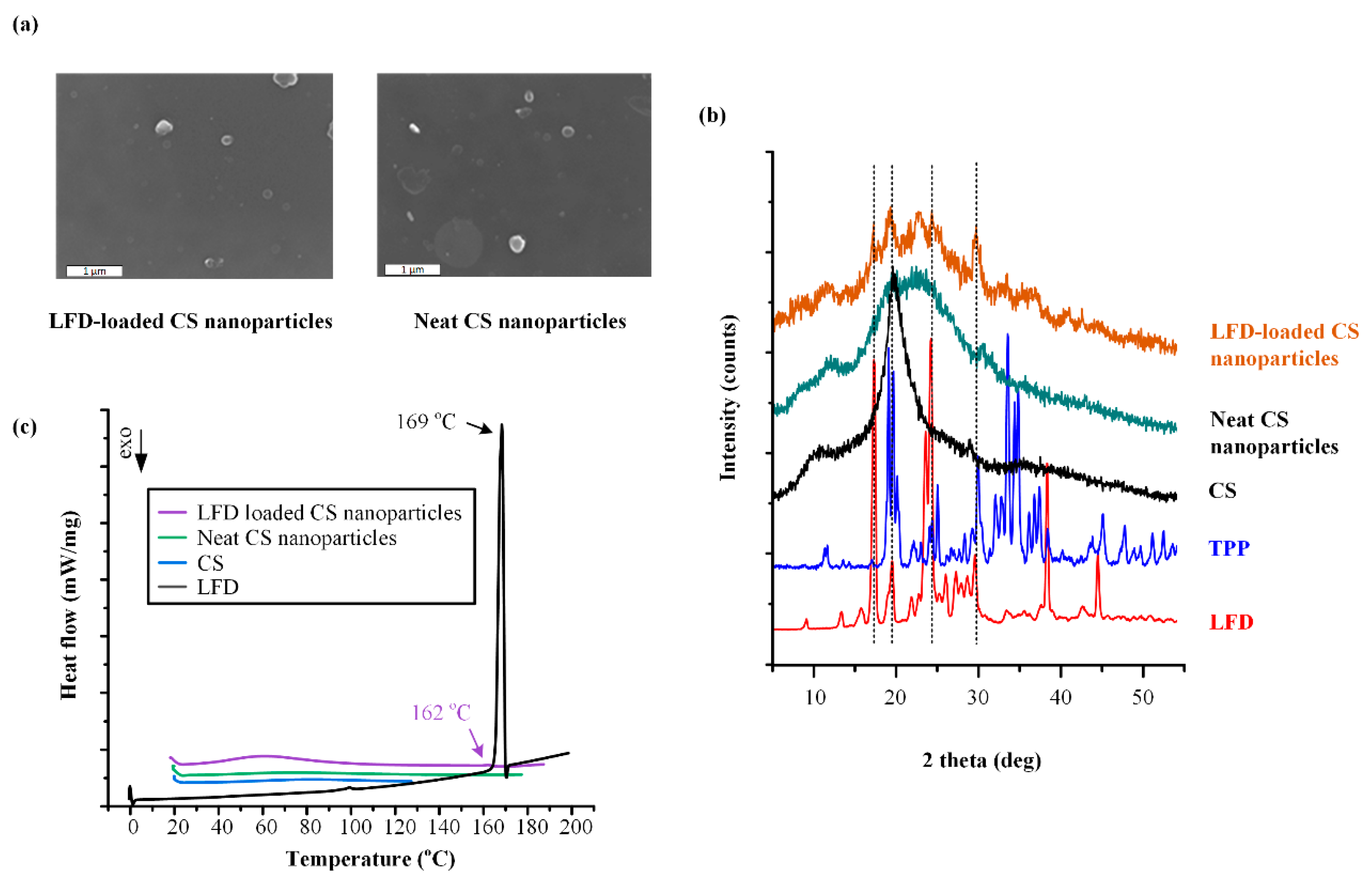
2.3.2. Wide-Angle X-ray Scattering (XRD)
2.3.3. Differential Scanning Calorimetry (DSC)
2.3.4. Field Emission Scanning Electron Microscopy (FESEM)
2.3.5. High-Pressure Liquid Chromatography (HPLC)
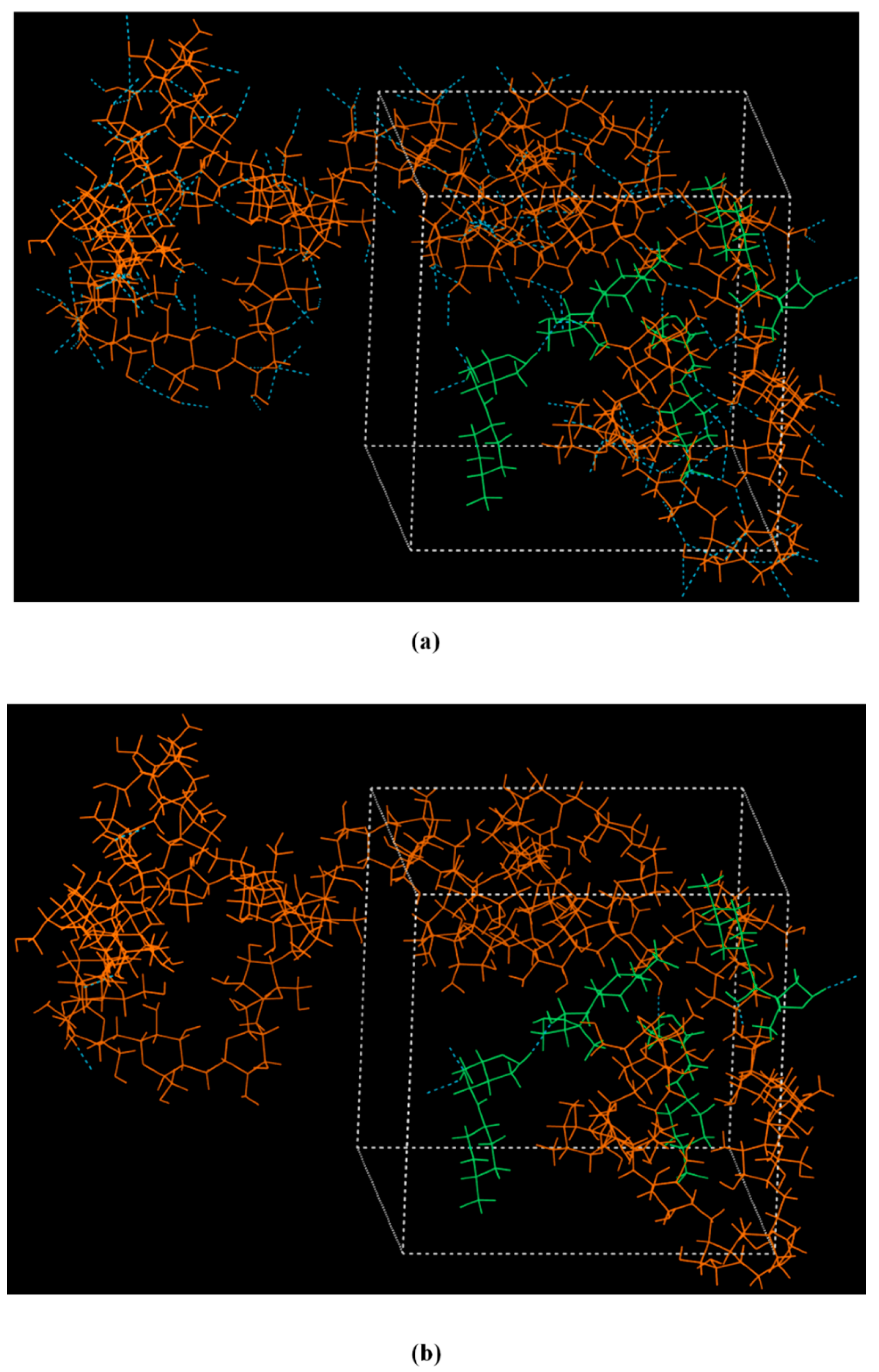
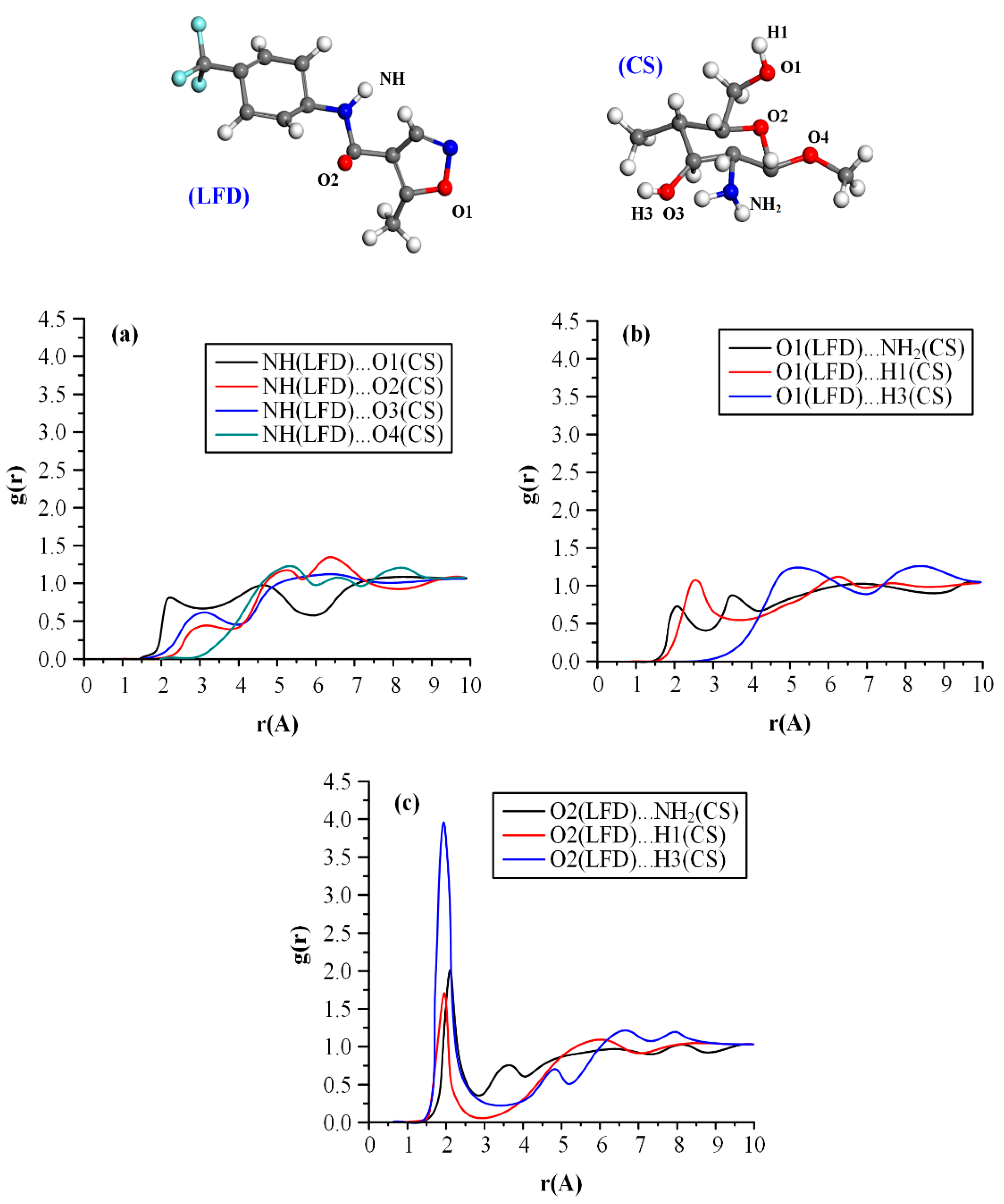
2.3.6. Molecular Dynamics (MD) Simulations
2.4. Preparation of Thin-Film Patches via Spin-Coating
2.5. Characterization of Patches
In Vitro Dissolution Studies
2.6. Statistical Analysis
3. Results and Discussion
3.1. LFD-CS NPs
3.2. PLLA and PLGA Thin-Film Patches
3.2.1. Optimization of Spin-Coating Process
3.2.2. LFD-loaded Thin-Film Patches
3.2.3. LFD-Loaded CS NPs Emended on Thin-Film Patches
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Alfaro-Lara, R.; Espinosa-Ortega, H.F.; Arce-Salinas, C.A. Systematic review and meta-analysis of the efficacy and safety of leflunomide and methotrexate in the treatment of rheumatoid arthritis. Reumatol. Clin. 2019, 15, 133–139. [Google Scholar] [CrossRef]
- Bullock, J.; Rizvi, S.A.A.; Saleh, A.M.; Ahmed, S.S.; Do, D.P.; Ansari, R.A.; Ahmed, J. Rheumatoid Arthritis: A Brief Overview of the Treatment. Med. Princ. Pract. 2018, 27, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Bar-Or, A.; Pachner, A.; Menguy-Vacheron, F.; Kaplan, J.; Wiendl, H. Teriflunomide and its mechanism of action in multiple sclerosis. Drugs 2014, 74, 659–674. [Google Scholar] [CrossRef]
- O’Connor, P.; Wolinsky, J.S.; Confavreux, C.; Comi, G.; Kappos, L.; Olsson, T.P.; Benzerdjeb, H.; Truffinet, P.; Wang, L.; Miller, A.; et al. Randomized trial of oral teriflunomide for relapsing multiple sclerosis. N. Engl. J. Med. 2011, 365, 1293–1303. [Google Scholar] [CrossRef] [PubMed]
- Scott, L.J. Teriflunomide: A Review in Relapsing-Remitting Multiple Sclerosis. Drugs 2019, 79, 875–886. [Google Scholar] [CrossRef]
- Cherwinski, H.M.; Cohn, R.G.; Cheung, P.; Webster, D.J.; Xu, Y.Z.; Caulfield, J.P.; Young, J.M.; Nakano, G.; Ransom, J.T. The immunosuppressant leflunomide inhibits lymphocyte proliferation by inhibiting pyrimidine biosynthesis. J. Pharmacol. Exp. Ther. 1995, 275, 1043–1049. [Google Scholar]
- Kremer, J.M.; Cannon, G.W. Benefit/risk of leflunomide in rheumatoid arthritis. Clin. Exp. Rheumatol. 2004, 22, S95–S100. [Google Scholar]
- Li, E.K.; Tam, L.S.; Tomlinson, B. Leflunomide in the treatment of rheumatoid arthritis. Clin. Ther. 2004, 26, 447–459. [Google Scholar] [CrossRef]
- Prakash, A.; Jarvis, B. Leflunomide: A review of its use in active rheumatoid arthritis. Drugs 1999, 58, 1137–1164. [Google Scholar] [CrossRef]
- Siafaka, P.I.; Barmbalexis, P.; Bikiaris, D.N. Novel electrospun nanofibrous matrices prepared from poly(lactic acid)/poly(butylene adipate) blends for controlled release formulations of an anti-rheumatoid agent. Eur. J. Pharm. Sci. 2016, 88, 12–25. [Google Scholar] [CrossRef]
- Xi, H.; Cun, D.; Xiang, R.; Guan, Y.; Zhang, Y.; Li, Y.; Fang, L. Intra-articular drug delivery from an optimized topical patch containing teriflunomide and lornoxicam for rheumatoid arthritis treatment: Does the topical patch really enhance a local treatment? J. Control. Release 2013, 169, 73–81. [Google Scholar] [CrossRef]
- Bae, J.; Park, J.W. Topical delivery of leflunomide for rheumatoid arthritis treatment: Evaluation of local tissue deposition of teriflunomide and its anti-inflammatory effects in an arthritis rat model. Drug Dev. Ind. Pharm. 2016, 42, 254–262. [Google Scholar] [CrossRef]
- Pund, S.; Pawar, S.; Gangurde, S.; Divate, D. Transcutaneous delivery of leflunomide nanoemulgel: Mechanistic investigation into physicomechanical characteristics, in vitro anti-psoriatic and anti-melanoma activity. Int. J. Pharm. 2015, 487, 148–156. [Google Scholar] [CrossRef]
- Ahmed, T.A.; Aljaeid, B.M. Preparation, characterization, and potential application of chitosan, chitosan derivatives, and chitosan metal nanoparticles in pharmaceutical drug delivery. Drug Des. Dev. Ther. 2016, 10, 483–507. [Google Scholar] [CrossRef] [PubMed]
- Pestov, A.; Bratskaya, S. Chitosan and Its Derivatives as Highly Efficient Polymer Ligands. Molecules 2016, 21, 330. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cai, C.; Li, J.; Li, J.; Li, J.; Sun, T.; Wang, L.; Wu, H.; Yu, G. Chitosan-Based Nanomaterials for Drug Delivery. Molecules 2018, 23, 2661. [Google Scholar] [CrossRef] [PubMed]
- Ta, Q.; Ting, J.; Harwood, S.; Browning, N.; Simm, A.; Ross, K.; Olier, I.; Al-Kassas, R. Chitosan nanoparticles for enhancing drugs and cosmetic components penetration through the skin. Eur. J. Pharm. Sci. 2021, 160, 105765. [Google Scholar] [CrossRef]
- Kenry, L.B. Recent Advances in Biodegradable Conducting Polymers and Their Biomedical Applications. Biomacromolecules 2018, 19, 1783–1803. [Google Scholar] [CrossRef] [PubMed]
- Khan, W.; Muthupandian, S.; Farah, S.; Kumar, N.; Domb, A.J. Biodegradable polymers derived from amino acids. Macromol. Biosci. 2011, 11, 1625–1636. [Google Scholar] [CrossRef]
- Pişkin, E. Biodegradable polymers as biomaterials. J. Biomater. Sci. Polym. Ed. 1995, 6, 775–795. [Google Scholar] [CrossRef] [PubMed]
- Washington, K.E.; Kularatne, R.N.; Karmegam, V.; Biewer, M.C.; Stefan, M.C. Recent advances in aliphatic polyesters for drug delivery applications. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, e1446. [Google Scholar] [CrossRef]
- d’Arcy, R.; Burke, J.; Tirelli, N. Branched polyesters: Preparative strategies and applications. Adv. Drug Deliv. Rev. 2016, 107, 60–81. [Google Scholar] [CrossRef] [PubMed]
- Attia, M.F.; Brummel, B.R.; Lex, T.R.; Van Horn, B.A.; Whitehead, D.C.; Alexis, F. Recent Advances in Polyesters for Biomedical Imaging. Adv. Healthc. Mater. 2018, 7, e1800798. [Google Scholar] [CrossRef]
- Contreras-Cáceres, R.; Cabeza, L.; Perazzoli, G.; Díaz, A.; López-Romero, J.M.; Melguizo, C.; Prados, J. Electrospun Nanofibers: Recent Applications in Drug Delivery and Cancer Therapy. Nanomaterials 2019, 9, 656. [Google Scholar] [CrossRef]
- Basavaraj, K.H.; Johnsy, G.; Navya, M.A.; Rashmi, R.; Siddaramaiah. Biopolymers as transdermal drug delivery systems in dermatology therapy. Crit. Rev. Ther. Drug Carr. Syst. 2010, 27, 155–185. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Guo, Q. Silk fibroin hydrogel scaffolds incorporated with chitosan nanoparticles repair articular cartilage defects by regulating TGF-β1 and BMP-2. Arthritis Res. Ther. 2021, 23, 50. [Google Scholar] [CrossRef]
- Shafique, M.; Sohail, M.; Minhas, M.U.; Khaliq, T.; Kousar, M.; Khan, S.; Hussain, Z.; Mahmood, A.; Abbasi, M.; Aziz, H.C.; et al. Bio-functional hydrogel membranes loaded with chitosan nanoparticles for accelerated wound healing. Int. J. Biol. Macromol. 2021, 170, 207–221. [Google Scholar] [CrossRef] [PubMed]
- Tuğcu-Demiröz, F.; Saar, S.; Kara, A.A.; Yıldız, A.; Tunçel, E.; Acartürk, F. Development and characterization of chitosan nanoparticles loaded nanofiber hybrid system for vaginal controlled release of benzydamine. Eur. J. Pharm. Sci. 2021, 161, 105801. [Google Scholar] [CrossRef] [PubMed]
- Bernkop-Schnürch, A.; Guggi, D.; Pinter, Y. Thiolated chitosans: Development and in vitro evaluation of a mucoadhesive, permeation enhancing oral drug delivery system. J. Control. Release 2004, 94, 177–186. [Google Scholar] [CrossRef]
- Bernkop-Schnürch, A.; Hornof, M.; Guggi, D. Thiolated chitosans. Eur. J. Pharm. Biopharm. 2004, 57, 9–17. [Google Scholar] [CrossRef]
- Bernkop-Schnürch, A.; Scholler, S.; Biebel, R.G. Development of controlled drug release systems based on thiolated polymers. J. Control. Release 2000, 66, 39–48. [Google Scholar] [CrossRef]
- Yoksan, R.; Jirawutthiwongchai, J.; Arpo, K. Encapsulation of ascorbyl palmitate in chitosan nanoparticles by oil-in-water emulsion and ionic gelation processes. Colloids Surf. B 2010, 76, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Pedretti, A.; Villa, L.; Vistoli, G. VEGA—An open platform to develop chemo-bio-informatics applications, using plug-in architecture and script programming. J. Comput. Aided Mol. Des. 2004, 18, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.V.; Hashemianzadeh, S.M. Molecular dynamics approach for behavior assessment of chitosan nanoparticles in carrying of donepezil and rivastigmine drug molecules. Mater. Res. Express 2019, 6, 045069. [Google Scholar] [CrossRef]
- Moradi, S.; Hosseini, E.; Abdoli, M.; Khani, S.; Shahlaei, M. Comparative molecular dynamic simulation study on the use of chitosan for temperature stabilization of interferon αII. Carbohydr. Polym. 2019, 203, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Deepa, G.; Sivakumar, K.C.; Sajeevan, T.P. Molecular simulation and in vitro evaluation of chitosan nanoparticles as drug delivery systems for the controlled release of anticancer drug cytarabine against solid tumours. 3 Biotech 2018, 8, 493. [Google Scholar] [CrossRef]
- Vega, D.; Petragalli, A.; Fernández, D.; Ellena, J.A. Polymorphism on leflunomide: Stability and crystal structures. J. Pharm. Sci. 2006, 95, 1075–1083. [Google Scholar] [CrossRef]
- Sun, H.; Mumby, S.J.; Maple, J.R.; Hagler, A.T. An ab Initio CFF93 All-Atom Force Field for Polycarbonates. J. Am. Chem. Soc. 1994, 116, 2978–2987. [Google Scholar] [CrossRef]
- Li, D.; Panchal, K.; Mafi, R.; Xi, L. An Atomistic Evaluation of the Compatibility and Plasticization Efficacy of Phthalates in Poly(vinyl chloride). Macromolecules 2018, 51, 6997–7012. [Google Scholar] [CrossRef]
- Gunsteren, W.F.V.; Mark, A.E. Validation of molecular dynamics simulation. J. Chem. Phys. 1998, 108, 6109–6116. [Google Scholar] [CrossRef]
- Kim, Y.; Beck-Broichsitter, M.; Banga, A.K. Design and Evaluation of a Poly(Lactide-co-Glycolide)-Based In Situ Film-Forming System for Topical Delivery of Trolamine Salicylate. Pharmaceutics 2019, 11, 409. [Google Scholar] [CrossRef] [PubMed]
- Kontogiannopoulos, K.N.; Assimopoulou, A.N.; Tsivintzelis, I.; Panayiotou, C.; Papageorgiou, V.P. Electrospun fiber mats containing shikonin and derivatives with potential biomedical applications. Int. J. Pharm. 2011, 409, 216–228. [Google Scholar] [CrossRef] [PubMed]
- Umeki, N.; Sato, T.; Harada, M.; Takeda, J.; Saito, S.; Iwao, Y.; Itai, S. Preparation and evaluation of biodegradable films containing the potent osteogenic compound BFB0261 for localized delivery. Int. J. Pharm. 2011, 404, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.; Sousa Lobo, J.M. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- Siafaka, P.I.; Titopoulou, A.; Koukaras, E.N.; Kostoglou, M.; Koutris, E.; Karavas, E.; Bikiaris, D.N. Chitosan derivatives as effective nanocarriers for ocular release of timolol drug. Int. J. Pharm. 2015, 495, 249–264. [Google Scholar] [CrossRef]
- Ntohogian, S.; Gavriliadou, V.; Christodoulou, E.; Nanaki, S.; Lykidou, S.; Naidis, P.; Mischopoulou, L.; Barmpalexis, P.; Nikolaidis, N.; Bikiaris, D.N. Chitosan Nanoparticles with Encapsulated Natural and UF-Purified Annatto and Saffron for the Preparation of UV Protective Cosmetic Emulsions. Molecules 2018, 23, 2107. [Google Scholar] [CrossRef]
- Nanaki, S.; Tseklima, M.; Christodoulou, E.; Triantafyllidis, K.; Kostoglou, M.; Bikiaris, D.N. Thiolated Chitosan Masked Polymeric Microspheres with Incorporated Mesocellular Silica Foam (MCF) for Intranasal Delivery of Paliperidone. Polymers 2017, 9, 617. [Google Scholar] [CrossRef]
- Michailidou, G.; Ainali, N.M.; Xanthopoulou, E.; Nanaki, S.; Kostoglou, M.; Koukaras, E.N.; Bikiaris, D.N. Effect of Poly(vinyl alcohol) on Nanoencapsulation of Budesonide in Chitosan Nanoparticles via Ionic Gelation and Its Improved Bioavailability. Polymers 2020, 12, 1101. [Google Scholar] [CrossRef]
- Koukaras, E.N.; Papadimitriou, S.A.; Bikiaris, D.N.; Froudakis, G.E. Insight on the formation of chitosan nanoparticles through ionotropic gelation with tripolyphosphate. Mol. Pharm. 2012, 9, 2856–2862. [Google Scholar] [CrossRef]
- Karava, A.; Lazaridou, M.; Nanaki, S.; Michailidou, G.; Christodoulou, E.; Kostoglou, M.; Iatrou, H.; Bikiaris, D.N. Chitosan Derivatives with Mucoadhesive and Antimicrobial Properties for Simultaneous Nanoencapsulation and Extended Ocular Release Formulations of Dexamethasone and Chloramphenicol Drugs. Pharmaceutics 2020, 12, 594. [Google Scholar] [CrossRef]
- Bikiaris, N.D.; Michailidou, G.; Lazaridou, M.; Christodoulou, E.; Gounari, E.; Ofrydopoulou, A.; Lambropoulou, D.A.; Vergkizi-Nikolakaki, S.; Lykidou, S.; Nikolaidis, N. Innovative Skin Product Emulsions with Enhanced Antioxidant, Antimicrobial and UV Protection Properties Containing Nanoparticles of Pure and Modified Chitosan with Encapsulated Fresh Pomegranate Juice. Polymers 2020, 12, 1542. [Google Scholar] [CrossRef] [PubMed]
- Shafiei, M.; Jafarizadeh-Malmiri, H.; Rezaei, M. Biological activities of chitosan and prepared chitosan-tripolyphosphate nanoparticles using ionic gelation method against various pathogenic bacteria and fungi strains. Biologia 2019, 74, 1561–1568. [Google Scholar] [CrossRef]
- Newman, A.; Zografi, G. Commentary: Considerations in the Measurement of Glass Transition Temperatures of Pharmaceutical Amorphous Solids. AAPS Pharmscitech 2019, 21, 26. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, G.Z.; Bikiaris, D.N. Biodegradable poly(alkylene succinate) blends: Thermal behavior and miscibility study. J. Polym. Sci. Part B Polym. Phys. 2006, 44, 584–597. [Google Scholar] [CrossRef]
- Eguiazabal, J.I.; Iruin, J.J.; Cortazar, M.; Guzmán, G.M. Glass transition temperatures in blends of poly(N-vinyl-2-pyrrolidone) with a copolymer of bisphenol A and epichlorohydrin or with poly(vinyl butyral). Die Makromol. Chem. 1984, 185, 1761–1766. [Google Scholar] [CrossRef]
- Papageorgiou, G.Z.; Achilias, D.S.; Nanaki, S.; Beslikas, T.; Bikiaris, D. PLA nanocomposites: Effect of filler type on non-isothermal crystallization. Thermochim. Acta 2010, 511, 129–139. [Google Scholar] [CrossRef]


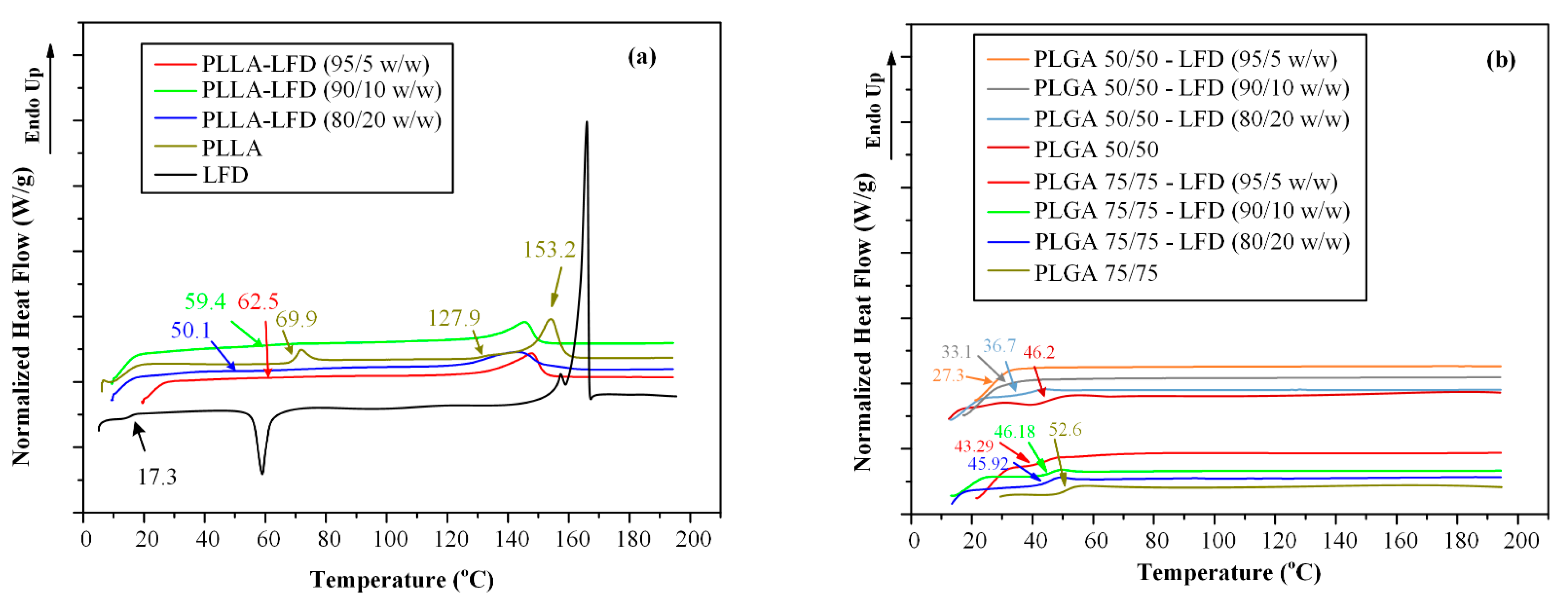

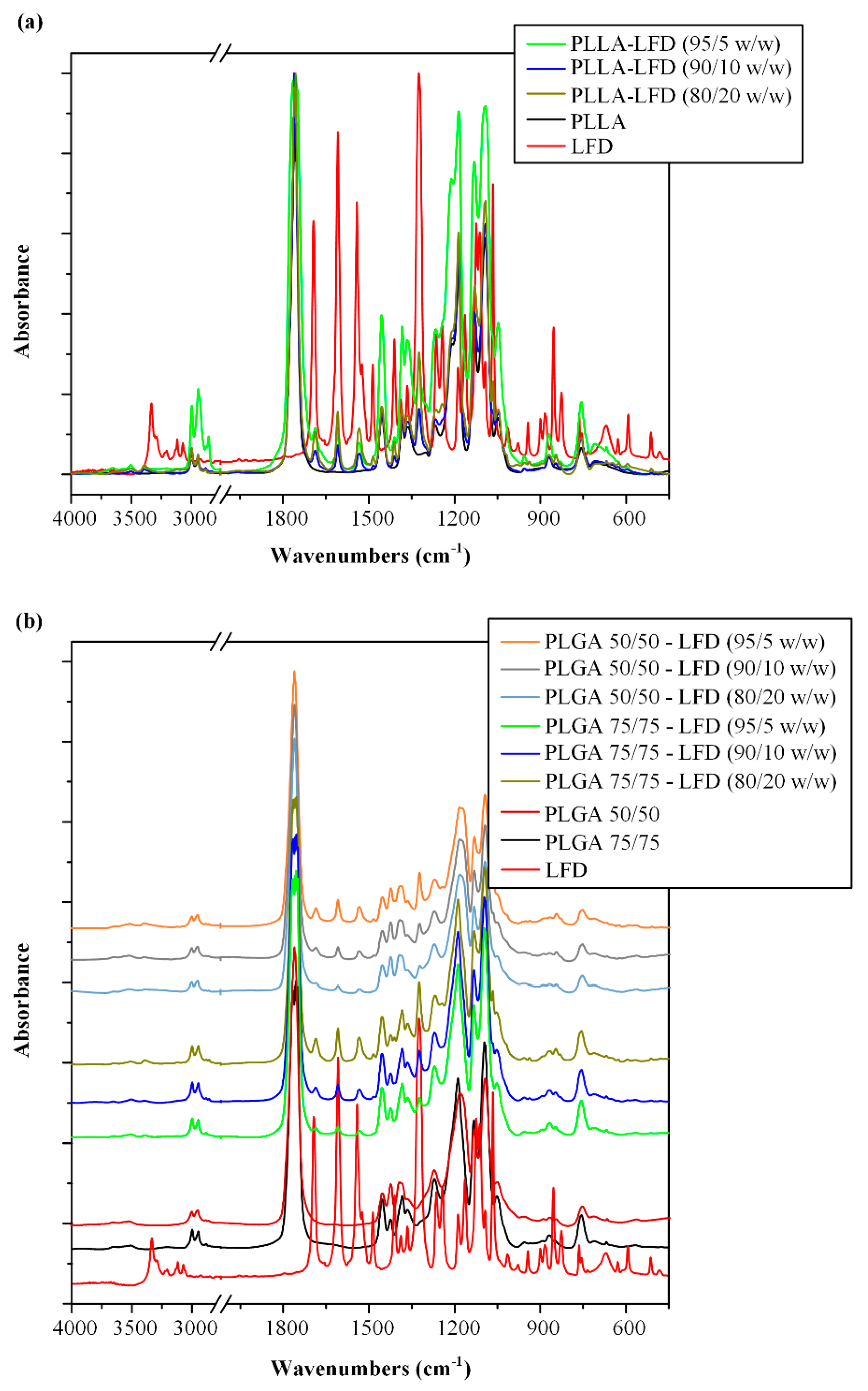
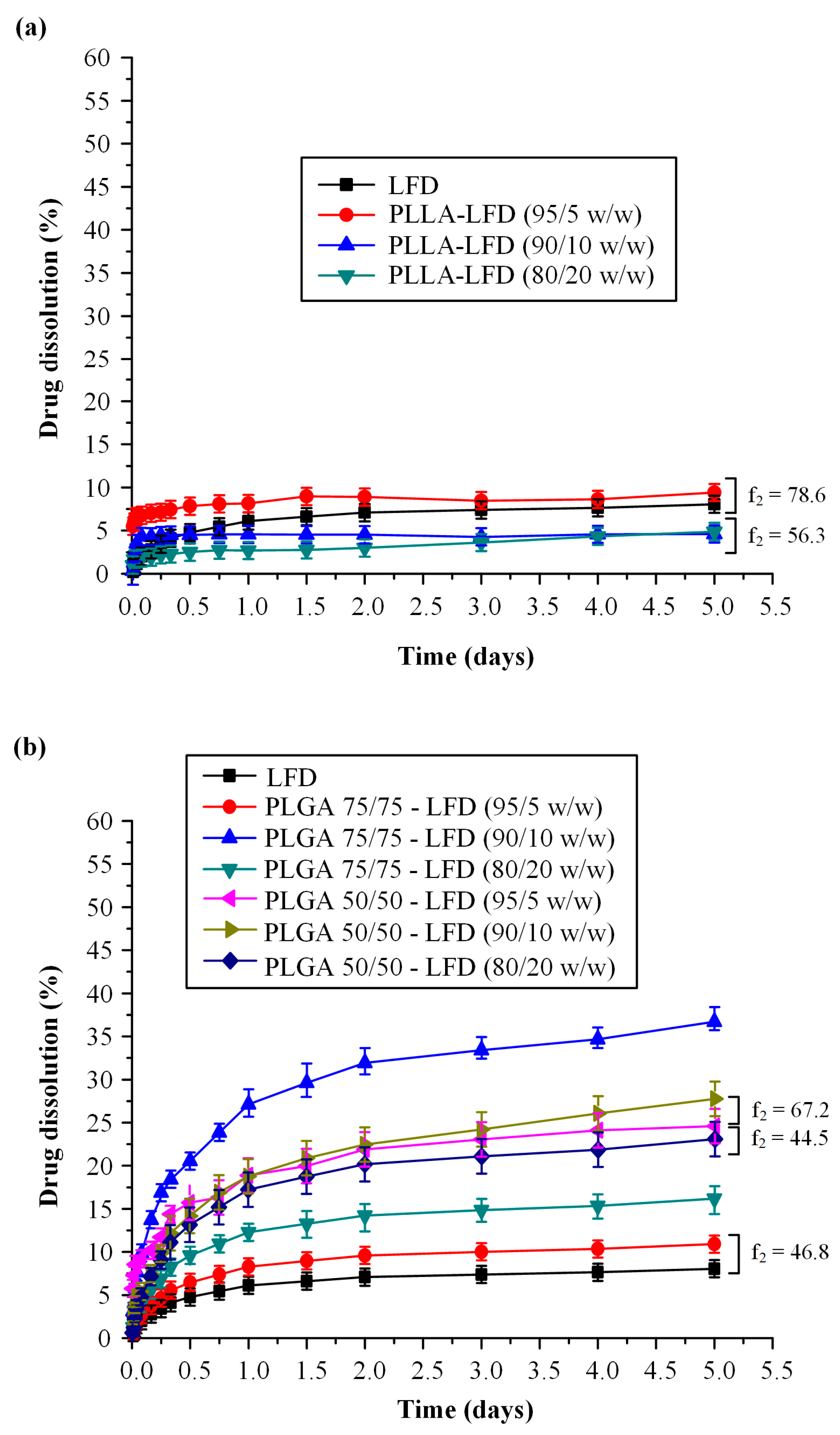
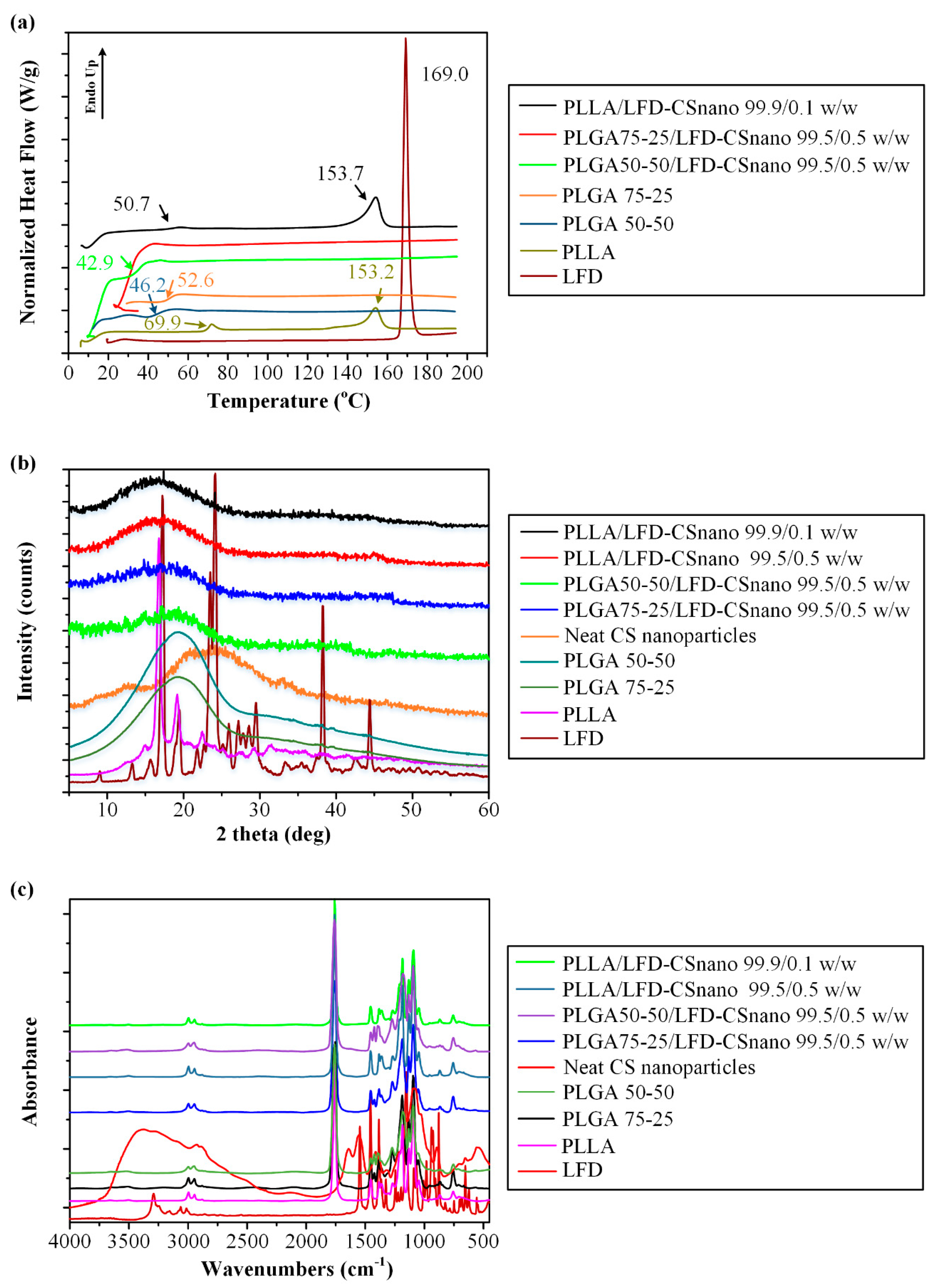

| Code | PLLA * (%wt.) | PLGA # (%wt.) | LFD (%wt.) | |
|---|---|---|---|---|
| 75/25 | 50/50 | |||
| S1 | 95.0 | - | - | 5.0 |
| S2 | 90.0 | - | - | 10.0 |
| S3 | 80.0 | - | - | 20.0 |
| S4 | - | 95.0 | - | 5.0 |
| S5 | - | 90.0 | - | 10.0 |
| S6 | - | 80.0 | - | 20.0 |
| S7 | - | - | 95.0 | 5.0 |
| S8 | - | - | 90.0 | 10.0 |
| S9 | - | - | 80.0 | 20.0 |
| Sample-ID | Drug Loading (%) | p-Value * | EE (%) | p-Value * |
|---|---|---|---|---|
| PLLA-LFD (95/5 w/w) | 4.98 ± 0.14 | - | 99.6 ± 0.31 | - |
| PLLA-LFD (90/10 w/w) | 9.35 ± 0.27 | - | 93.5 ± 0.82 | - |
| PLLA-LFD (80/20 w/w) | 19.20 ± 0.36 | - | 96.0 ± 1.06 | - |
| PLGA 75/25-LFD (95/5 w/w) | 4.54 ± 0.12 | >0.05 | 90.8 ± 0.52 | <0.05 |
| PLGA 75/25-LFD (90/10 w/w) | 8.36 ± 0.24 | <0.05 | 83.6 ± 1.12 | <0.05 |
| PLGA 75/25-LFD (80/20 w/w) | 18.33 ± 0.38 | >0.05 | 91.7 ± 1.29 | <0.05 |
| PLGA 50/50-LFD (95/5 w/w) | 3.33 ± 0.15 | <0.05 | 66.6 ± 0.94 | <0.05 |
| PLGA 50/50-LFD (90/10 w/w) | 7.39 ± 0.26 | <0.05 | 73.9 ± 1.23 | <0.05 |
| PLGA 50/50-LFD (80/20 w/w) | 17.23 ± 0.35 | <0.05 | 86.2 ± 1.58 | <0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nanaki, S.G.; Andrianidou, S.; Barmpalexis, P.; Christodoulou, E.; Bikiaris, D.N. Leflunomide Loaded Chitosan Nanoparticles for the Preparation of Aliphatic Polyester Based Skin Patches. Polymers 2021, 13, 1539. https://doi.org/10.3390/polym13101539
Nanaki SG, Andrianidou S, Barmpalexis P, Christodoulou E, Bikiaris DN. Leflunomide Loaded Chitosan Nanoparticles for the Preparation of Aliphatic Polyester Based Skin Patches. Polymers. 2021; 13(10):1539. https://doi.org/10.3390/polym13101539
Chicago/Turabian StyleNanaki, Stavroula G., Sophia Andrianidou, Panagiotis Barmpalexis, Evi Christodoulou, and Dimitrios N. Bikiaris. 2021. "Leflunomide Loaded Chitosan Nanoparticles for the Preparation of Aliphatic Polyester Based Skin Patches" Polymers 13, no. 10: 1539. https://doi.org/10.3390/polym13101539
APA StyleNanaki, S. G., Andrianidou, S., Barmpalexis, P., Christodoulou, E., & Bikiaris, D. N. (2021). Leflunomide Loaded Chitosan Nanoparticles for the Preparation of Aliphatic Polyester Based Skin Patches. Polymers, 13(10), 1539. https://doi.org/10.3390/polym13101539










