Impact Modifiers and Compatibilizers for Versatile Epoxy-Based Adhesive Films with Curing and Deoxidizing Capabilities
Abstract
1. Introduction
2. Experiment
2.1. Materials
2.1.1. Polymer
2.1.2. Compatibilizer
2.1.3. Curing Agent and Solvent
2.1.4. Metal Solder and Al Substrate
2.2. Fabrication of Hybrid Adhesive Films
2.3. Adhesive Film Bonding Procedure
2.4. Characterization
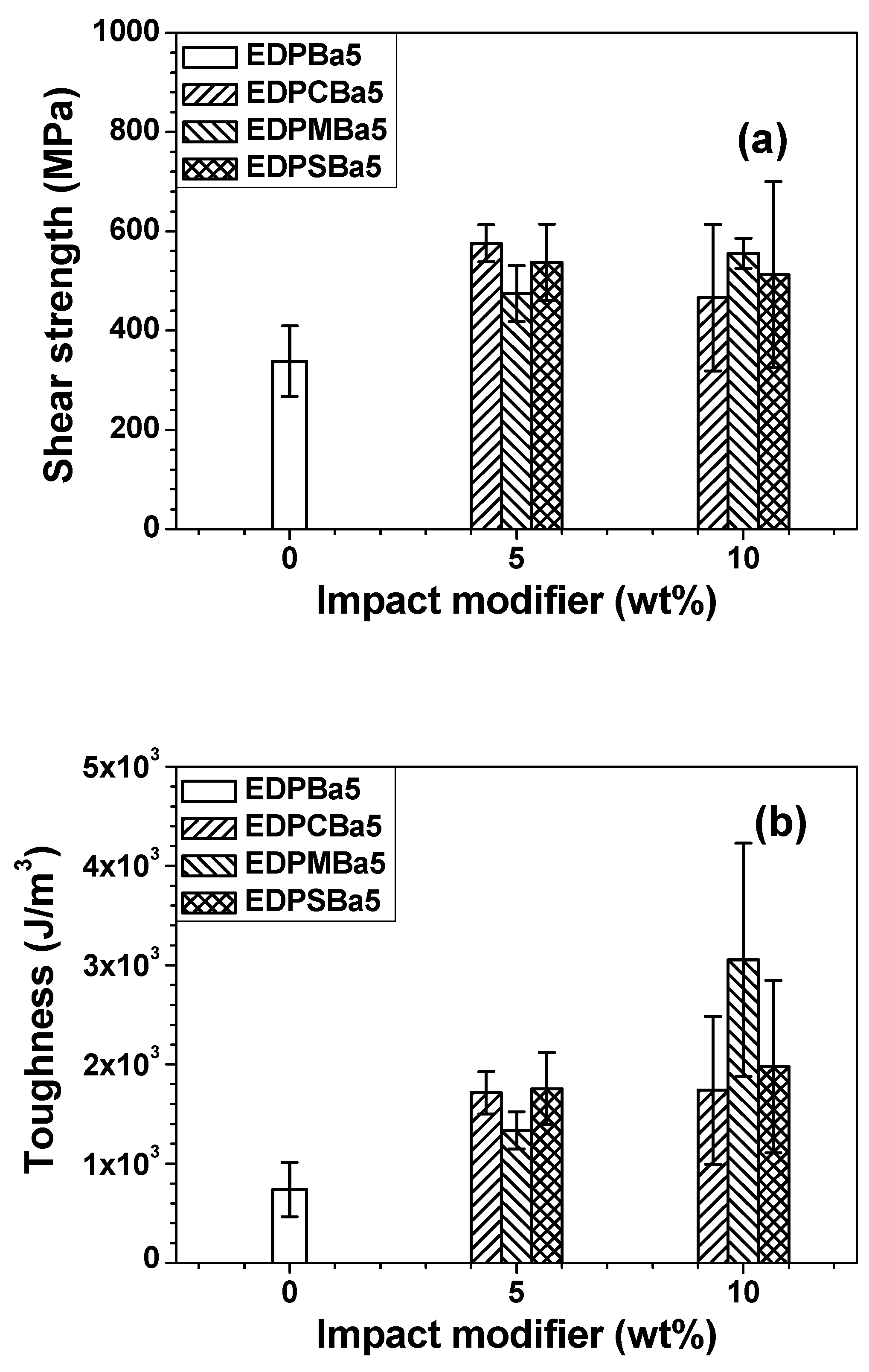
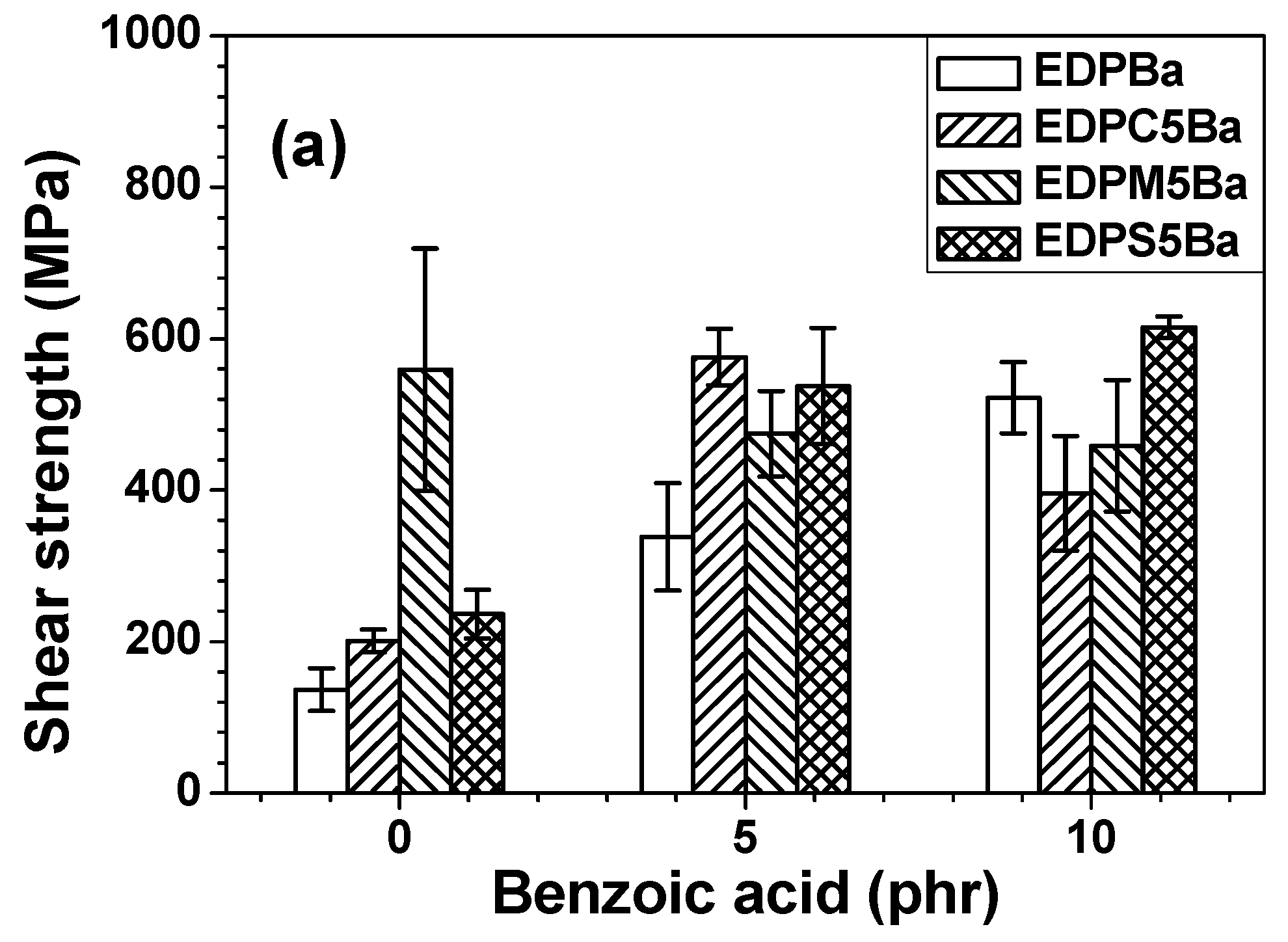
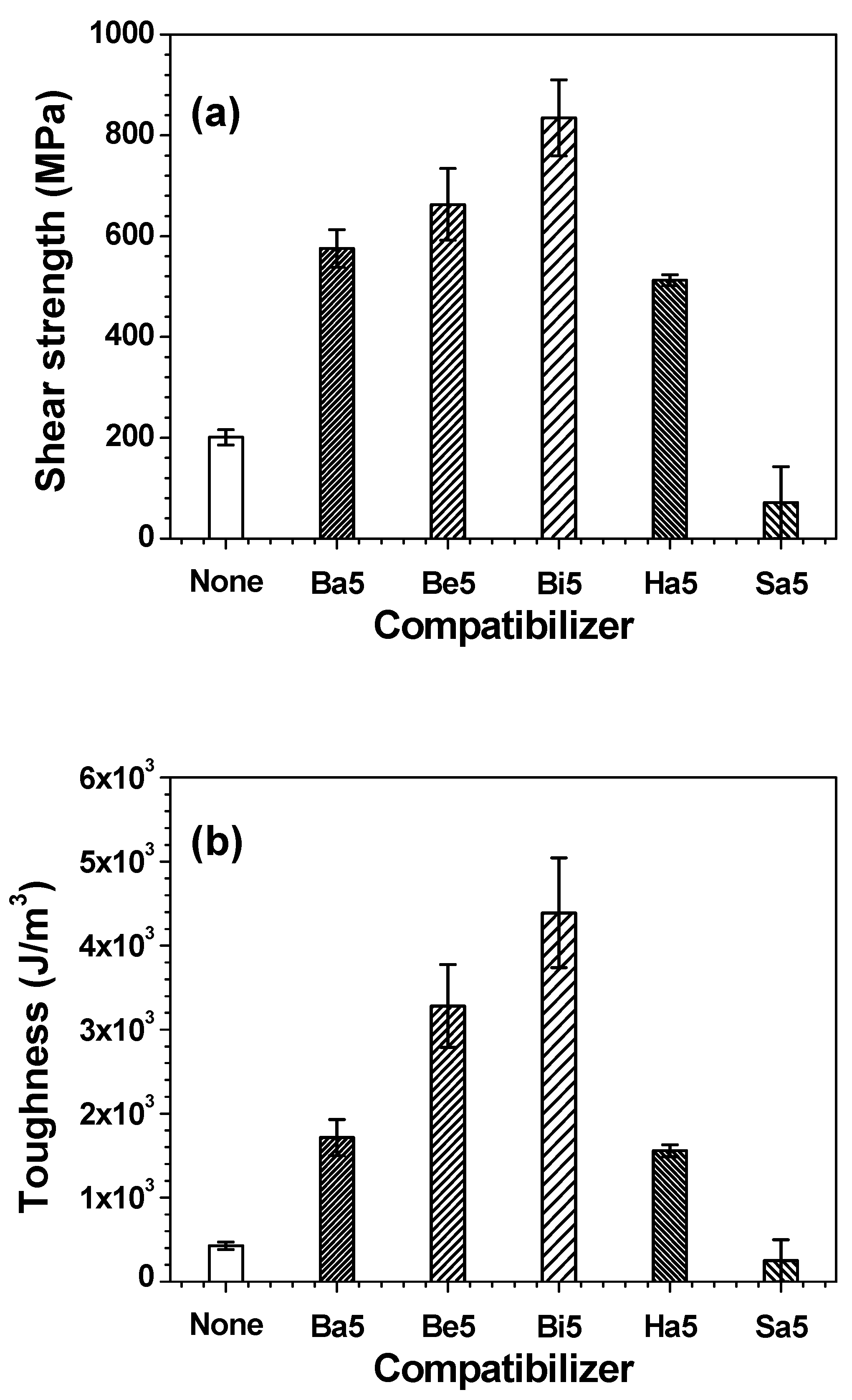
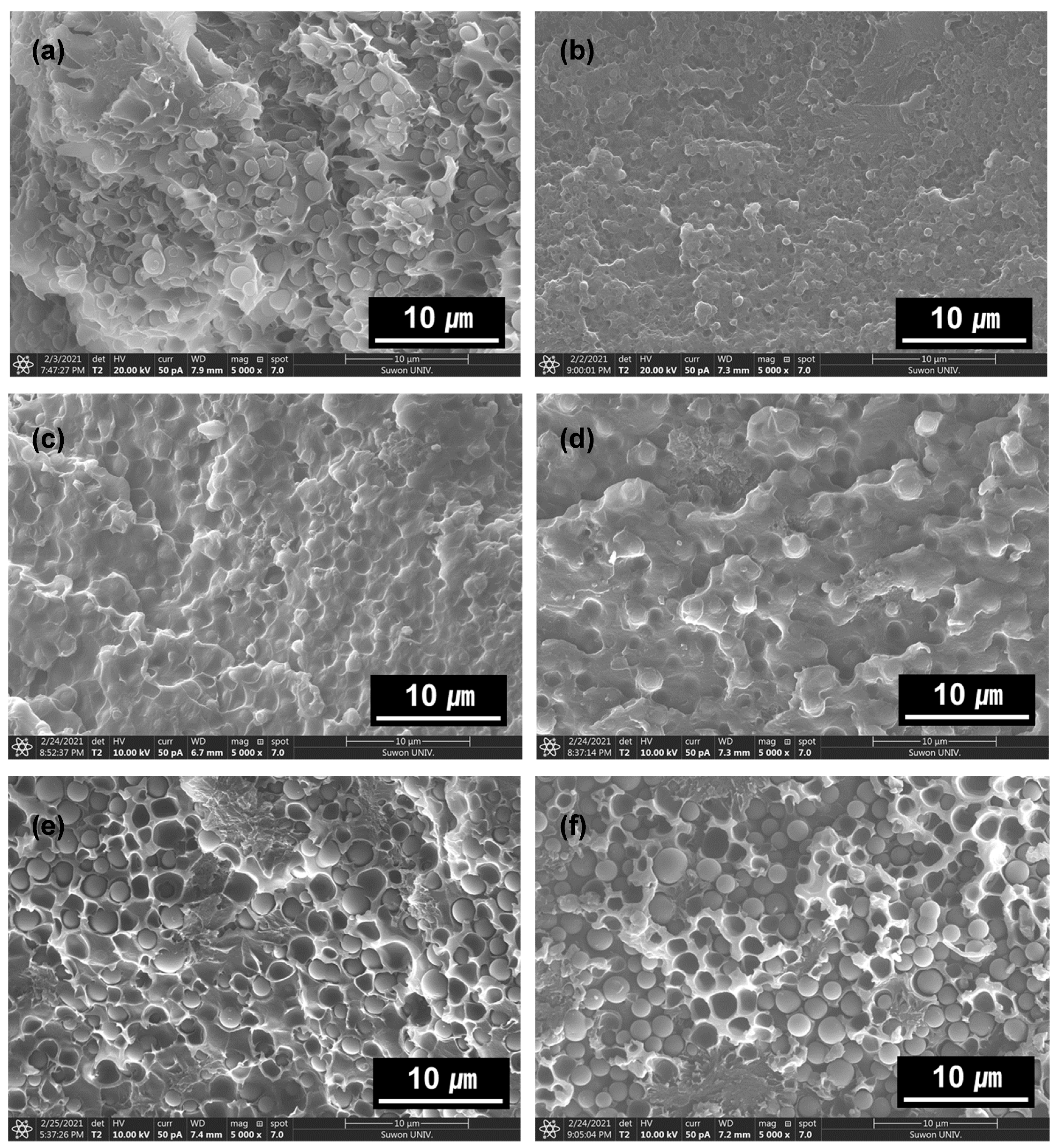
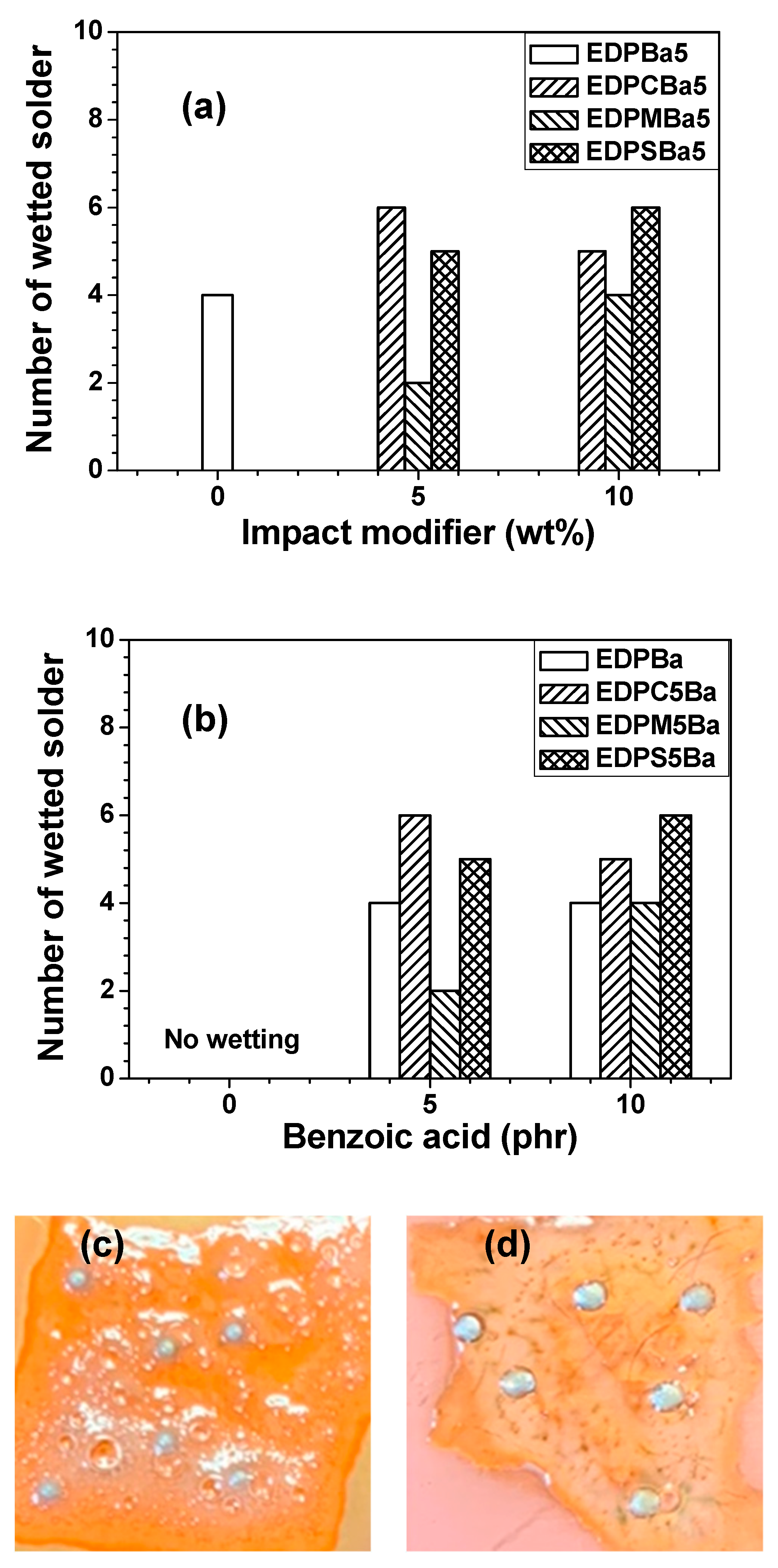
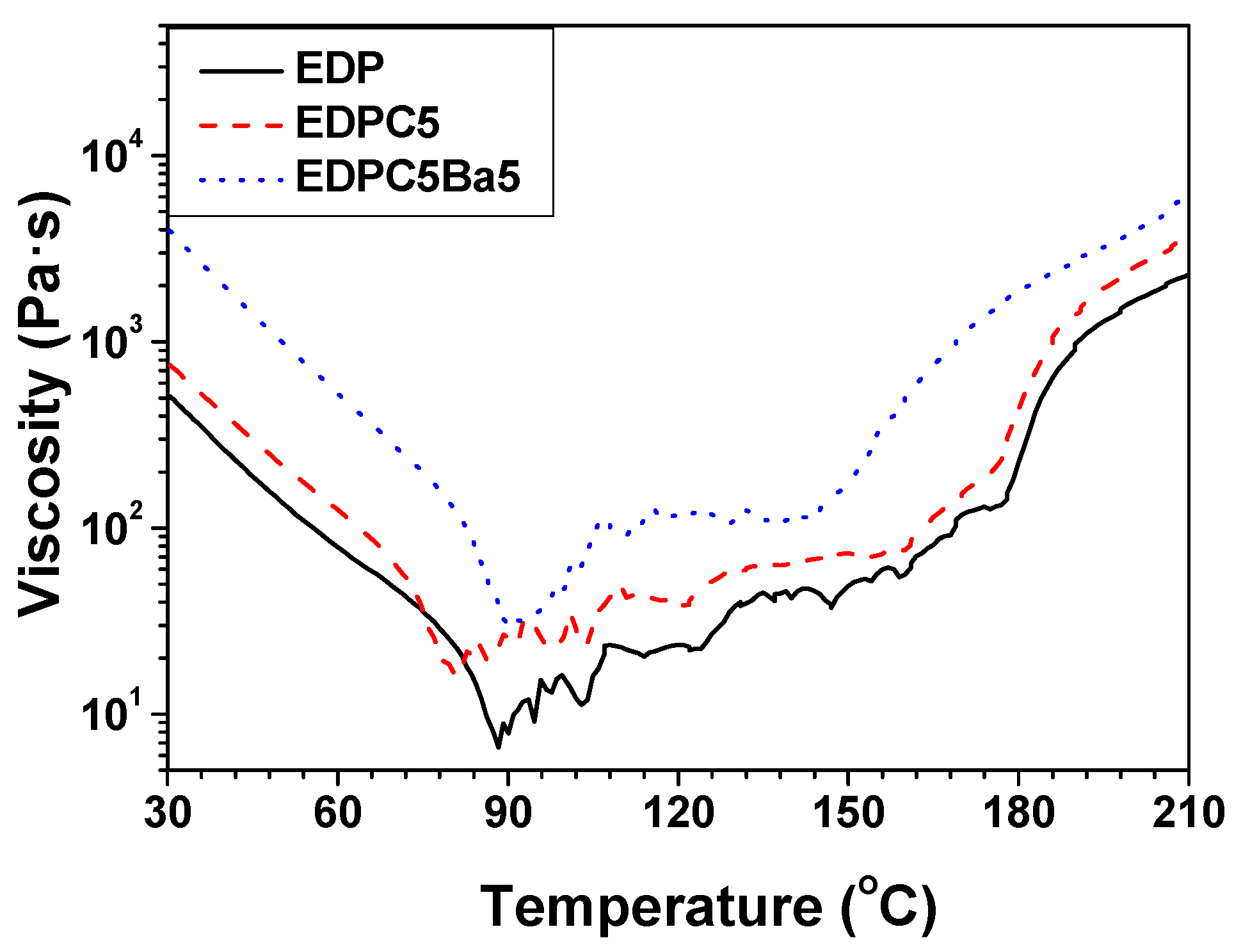
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee Sanchez, W.A.; Huang, C.-Y.; Chen, J.-X.; Soong, Y.-C.; Chan, Y.-N.; Chiou, K.-C.; Lee, T.-M.; Cheng, C.-C.; Chiu, C.-W. Enhanced Thermal Conductivity of Epoxy Composites Filled with Al2O3/Boron Nitride Hybrids for Underfill Encapsulation Materials. Polymers 2021, 13, 147. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Xue, Y.; Li, X.; Wen, Y.; Liu, J.; Xue, Z.; Shi, D.; Zhou, X.; Xie, X.; Mai, Y.-W. High-Performance Epoxy/Binary Spherical Alumina Composite as Underfill Material for Electronic Packaging. Compos. Part. A Appl. Sci. Manuf. 2019, 118, 67–74. [Google Scholar] [CrossRef]
- Li, G.; He, Y.; Zhu, P.; Zhao, T.; Sun, R.; Lu, D.; Wong, C. Tailored Surface Chemistry of SiO2 Particles with Improved Rheological, Thermal-Mechanical and Adhesive Properties of Epoxy Based Composites for Underfill Applications. Polymer 2018, 156, 111–120. [Google Scholar] [CrossRef]
- Zhao, Y.; Drummer, D. Influence of Filler Content and Filler Size on the Curing Kinetics of an Epoxy Resin. Polymers 2019, 11, 1797. [Google Scholar] [CrossRef]
- Duo, L.; Zhang, Z.; Zheng, K.; Wang, D.; Xu, C.; Xia, Y. Perhydropolysilazane Derived SiON Interfacial Layer for Cu/Epoxy Molding Compound Composite. Surf. Coat. Technol. 2020, 391, 125703. [Google Scholar] [CrossRef]
- Su, L.; Yu, X.; Li, K.; Pecht, M. Defect Inspection of Flip Chip Solder Joints Based on Non-Destructive Methods: A Review. Microelectron. Reliab. 2020, 110, 113657. [Google Scholar] [CrossRef]
- Wan, Y.-J.; Li, G.; Yao, Y.-M.; Zeng, X.-L.; Zhu, P.-L.; Sun, R. Recent Advances in Polymer-Based Electronic Packaging Materials. Compos. Commun. 2020, 19, 154–167. [Google Scholar] [CrossRef]
- Ma, D.; Wang, W.D.; Lahiri, S.K. Scallop Formation and Dissolution of Cu–Sn Intermetallic Compound during Solder Reflow. J. Appl. Phys. 2002, 91, 3312–3317. [Google Scholar] [CrossRef]
- Chen, Y.; Chan, Y.; Chen, C. Wettability and Interfacial Reactions between the Molten Sn–3.0 wt% Ag–0.5 wt% Cu Solder (SAC305) and Ni–Co Alloys. J. Alloy. Compd. 2010, 507, 419–424. [Google Scholar] [CrossRef]
- Shnawah, D.A.; Sabri, M.F.M.; Badruddin, I.A. A Review on Thermal Cycling and Drop Impact Reliability of SAC Solder Joint in Portable Electronic Products. Microelectron. Reliab. 2012, 52, 90–99. [Google Scholar] [CrossRef]
- Jang, K.-S.; Eom, Y.-S.; Choi, K.-S.; Bae, H.-C. Crosslinkable Deoxidizing Hybrid Adhesive of Epoxy–Diacid for Electrical Interconnections in Semiconductor Packaging. Polym. Int. 2018, 67, 1241–1247. [Google Scholar] [CrossRef]
- de Kluizenaar, E.E. Surface Oxidation of Molten Soft Solder: An Auger Study. J. Vac. Sci. Technol. A 1983, 1, 1480–1485. [Google Scholar] [CrossRef]
- Dudek, M.A.; Chawla, N. Oxidation Behavior of Rare-Earth-Containing Pb-Free Solders. J. Elec Mater. 2009, 38, 210–220. [Google Scholar] [CrossRef]
- Jiang, J.; Lee, J.-E.; Kim, K.-S.; Suganuma, K. Oxidation Behavior of Sn–Zn Solders under High-Temperature and High-Humidity Conditions. J. Alloy. Compd. 2008, 462, 244–251. [Google Scholar] [CrossRef]
- Jang, K.-S.; Eom, Y.-S.; Choi, K.-S.; Bae, H.-C. Synchronous Curable Deoxidizing Capability of Epoxy–Anhydride Adhesive: Deoxidation Quantification via Spectroscopic Analysis. J. Appl. Polym. Sci. 2018, 135, 46639. [Google Scholar] [CrossRef]
- Shi, S.H.; Wong, C.P. Study of the Fluxing Agent Effects on the Properties of No-Flow Underfill Materials for Flip-Chip Applications. IEEE Trans. Compon. Packag. Technol. 1999, 22, 141–151. [Google Scholar] [CrossRef]
- Zhang, F.; Ming, L.; Chen, W.T.; Chian, K.S. An Investigation into the Effects of Flux Residues on Properties of Underfill Materials for Flip Chip Packages. IEEE Trans. Compon. Packag. Technol. 2003, 26, 233–238. [Google Scholar] [CrossRef]
- Jang, K.-S.; Eom, Y.-S.; Choi, K.-S.; Bae, H.-C. Versatile Epoxy/Phenoxy/Anhydride-Based Hybrid Adhesive Films for Deoxidization and Electrical Interconnection. Ind. Eng. Chem. Res. 2018, 57, 7181–7187. [Google Scholar] [CrossRef]
- Eom, Y.-S.; Jang, K.-S.; Moon, J.-T.; Nam, J.-D. Electrical Interconnection with a Smart ACA Composed of Fluxing Polymer and Solder Powder. ETRI J. 2010, 32, 414–421. [Google Scholar] [CrossRef]
- Eom, Y.-S.; Jang, K.; Moon, J.-T.; Nam, J.-D.; Kim, J.-M. Electrical and Mechanical Characterization of an Anisotropic Conductive Adhesive with a Low Melting Point Solder. Microelectron. Eng. 2008, 85, 2202–2206. [Google Scholar] [CrossRef]
- Eom, Y.-S.; Son, J.-H.; Jang, K.-S.; Lee, H.-S.; Bae, H.-C.; Choi, K.-S.; Choi, H.-S. Characterization of Fluxing and Hybrid Underfills with Micro-Encapsulated Catalyst for Long Pot Life. ETRI J. 2014, 36, 343–351. [Google Scholar] [CrossRef]
- Jang, K.-S. Compatibilizing Effects for Semicrystalline Polymer-Infiltrated Amorphous Polymer Matrix: Mechanical (Toughness), Thermal, and Morphological Behavior of Compatibilized Polypropylene/Polycarbonate Blends. J. Appl. Polym. Sci. 2019, 136, 47684. [Google Scholar] [CrossRef]
- Jang, K.-S. Probing Toughness of Polycarbonate (PC: Ductile and Brittle)/Polypropylene (PP) Blends and Talc-Triggered PC/PP Brittle Composites with Diverse Impact Fracture Parameters. J. Appl. Polym. Sci. 2019, 136, 47110. [Google Scholar] [CrossRef]
- Minale, M.; Moldenaers, P.; Mewis, J. Effect of Shear History on the Morphology of Immiscible Polymer Blends. Macromolecules 1997, 30, 5470–5475. [Google Scholar] [CrossRef]
- Seyler, R.J. Assignment of the Glass Transition; ASTM International: West Conshohocken, PA, USA, 1994; ISBN 978-0-8031-1995-6. [Google Scholar]
- Supanchaiyamat, N.; Shuttleworth, P.S.; Hunt, A.J.; Clark, J.H.; Matharu, A.S. Thermosetting Resin Based on Epoxidised Linseed Oil and Bio-Derived Crosslinker. Green Chem. 2012, 14, 1759–1765. [Google Scholar] [CrossRef]
- Chmielewska, D.; Sterzyński, T.; Dudziec, B. Epoxy Compositions Cured with Aluminosilsesquioxanes: Thermomechanical Properties. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
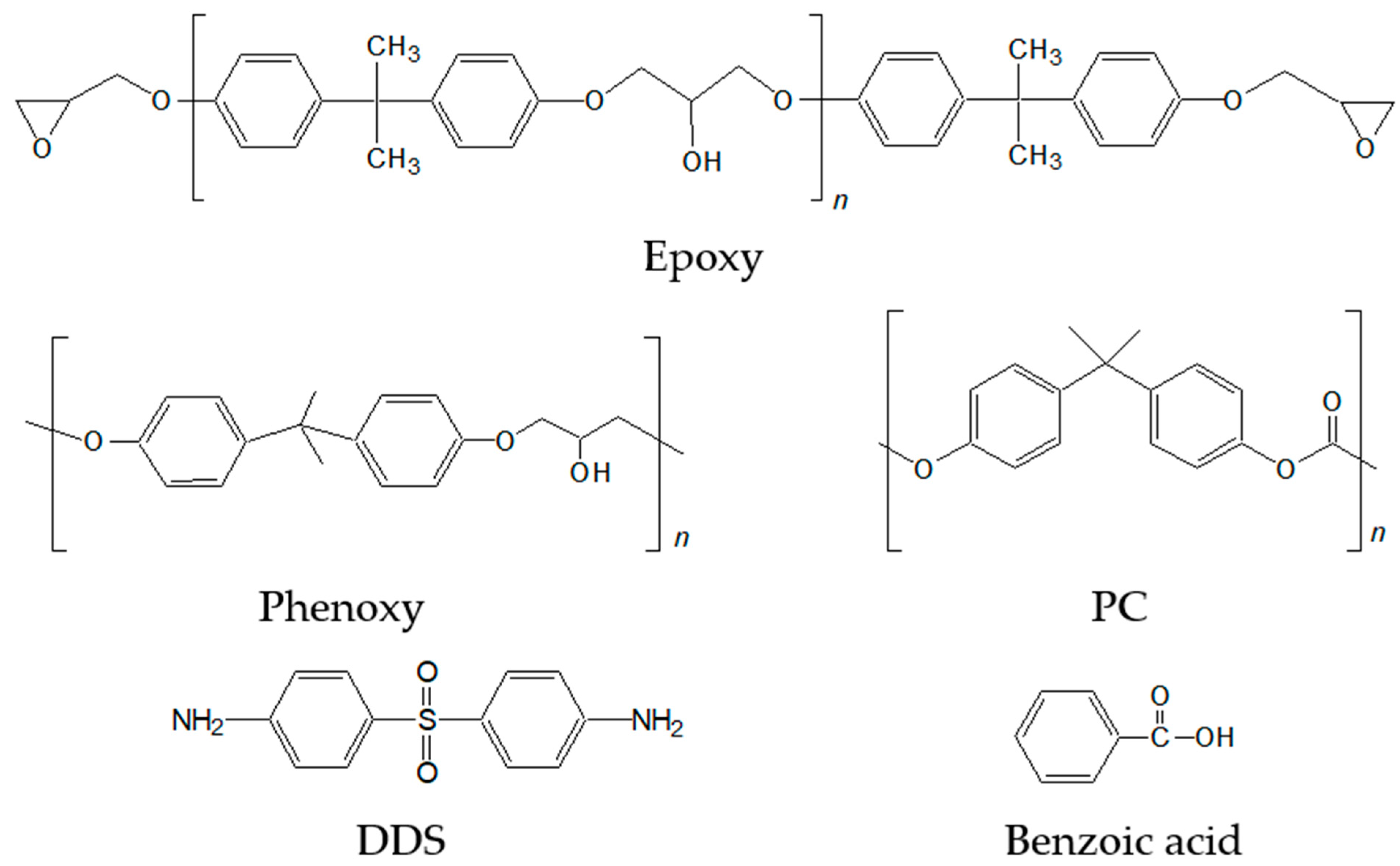
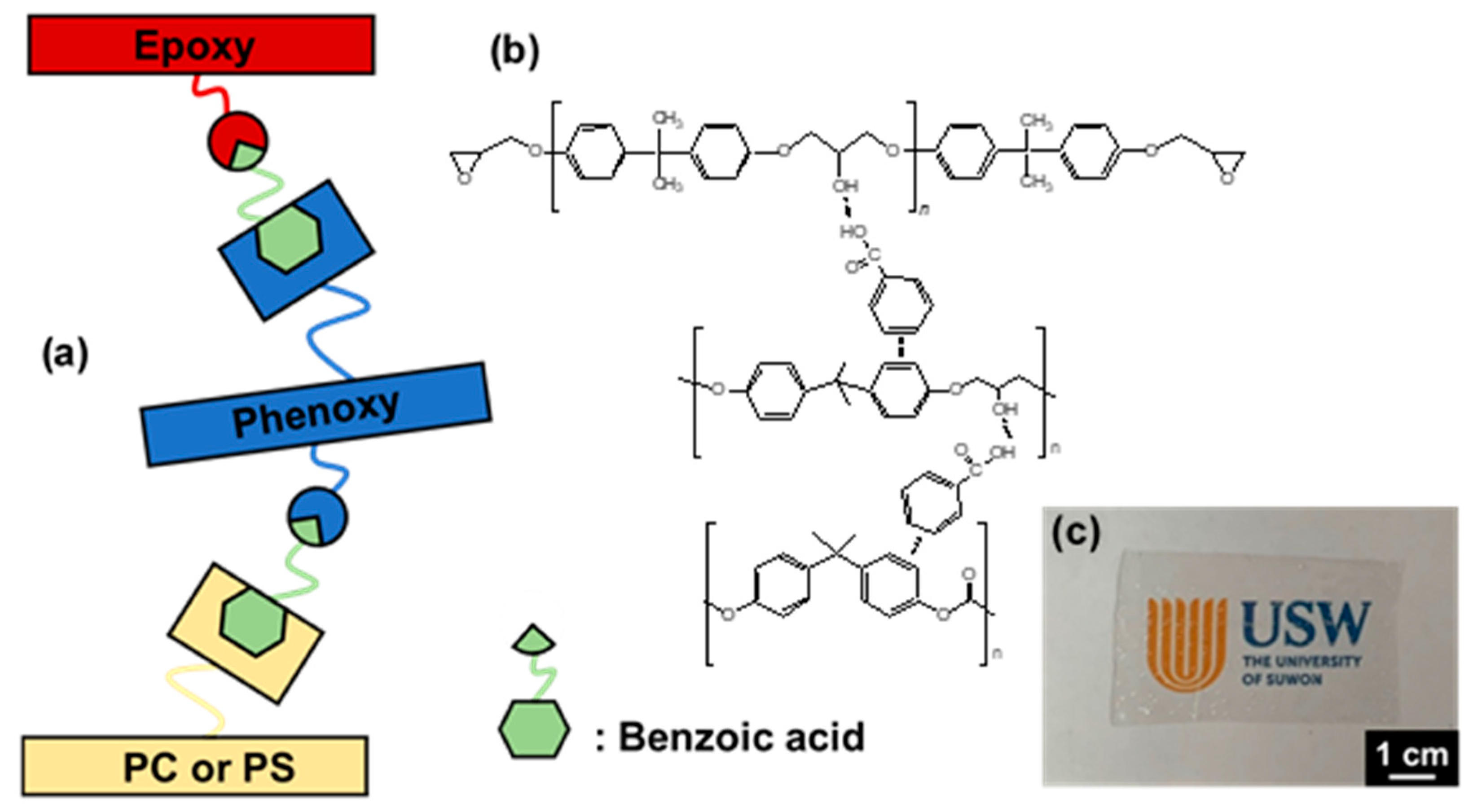



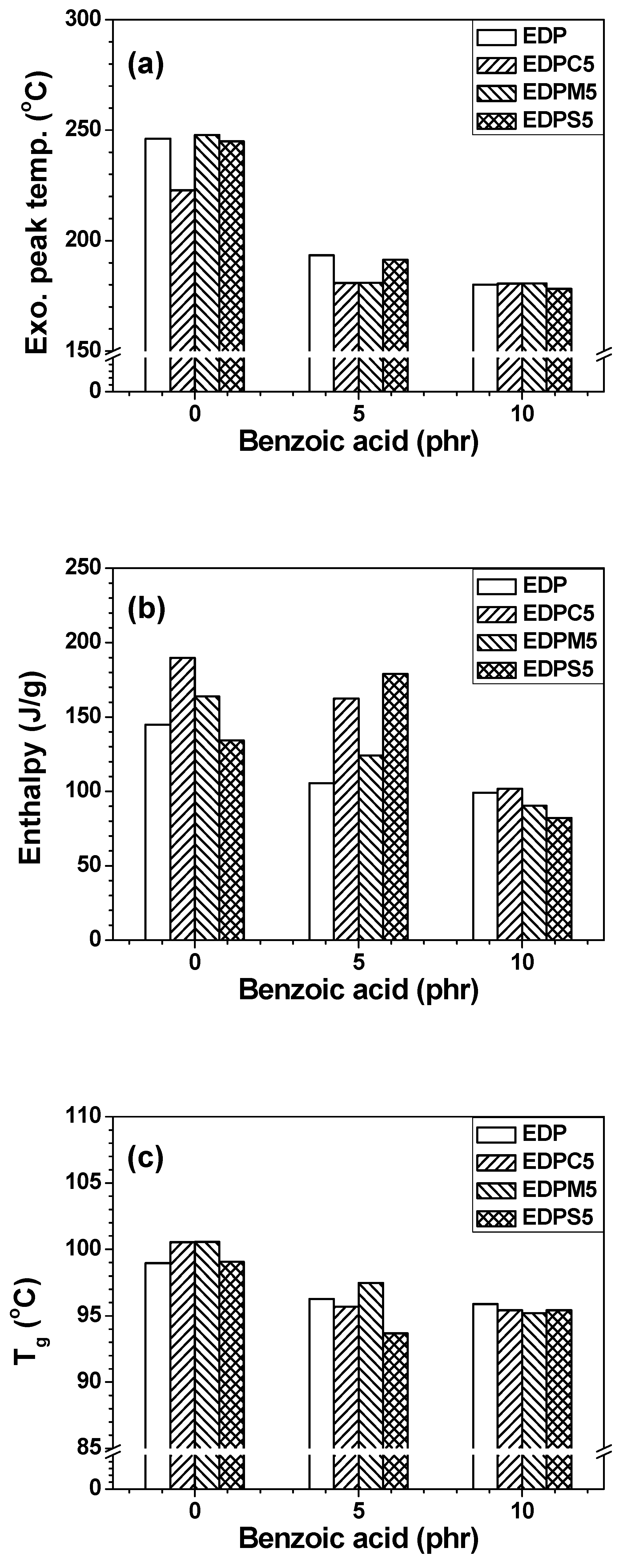

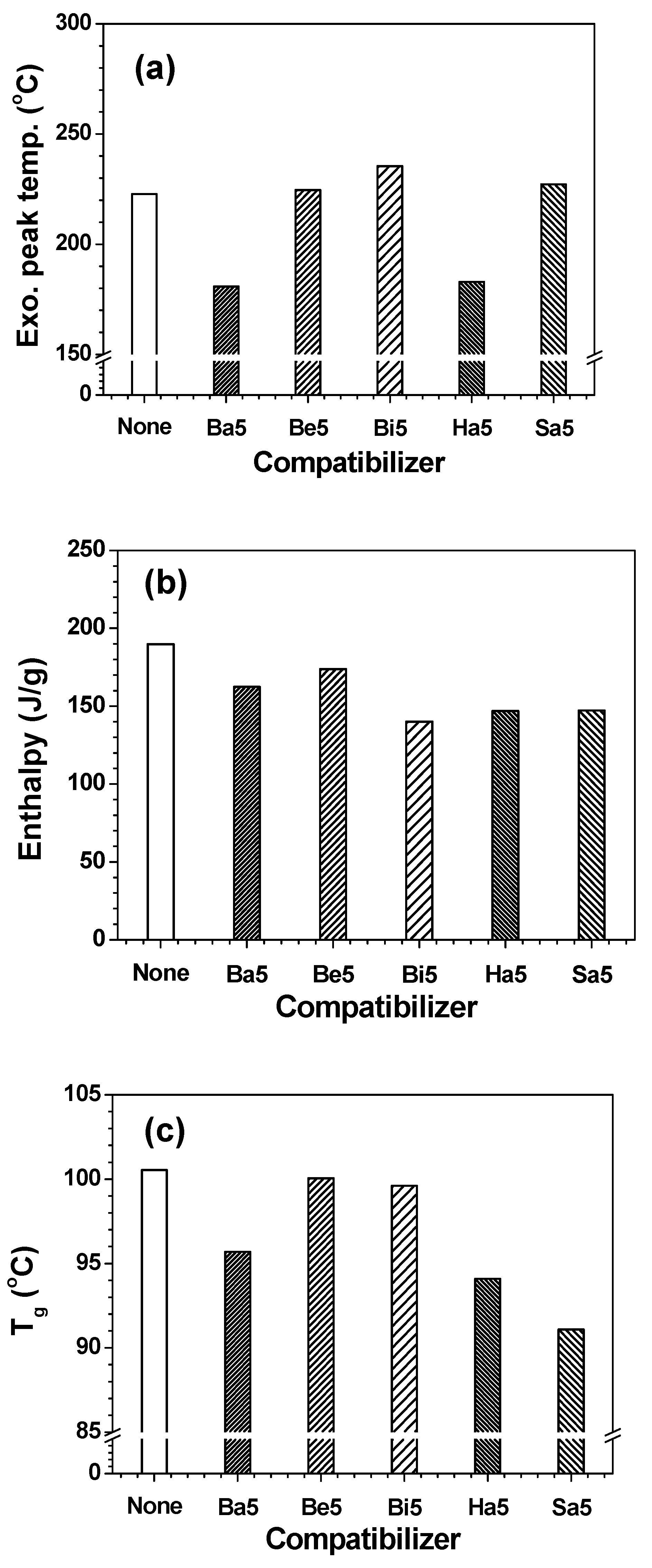
| Name | Epoxy | DDS | Phenoxy | PC | PMMA | PS |
|---|---|---|---|---|---|---|
| Sample name | E | D | P | C | M | S |
| Name | Benzoic acid | Benzene | Biphneyl | Hexanoic acid | Stearic acid | |
| Sample name | Ba | Be | Bi | Ha | Sa | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, B.-Y.; Lee, D.-H.; Jang, K.-S. Impact Modifiers and Compatibilizers for Versatile Epoxy-Based Adhesive Films with Curing and Deoxidizing Capabilities. Polymers 2021, 13, 1129. https://doi.org/10.3390/polym13071129
Lee B-Y, Lee D-H, Jang K-S. Impact Modifiers and Compatibilizers for Versatile Epoxy-Based Adhesive Films with Curing and Deoxidizing Capabilities. Polymers. 2021; 13(7):1129. https://doi.org/10.3390/polym13071129
Chicago/Turabian StyleLee, Bo-Young, Dae-Hyeon Lee, and Keon-Soo Jang. 2021. "Impact Modifiers and Compatibilizers for Versatile Epoxy-Based Adhesive Films with Curing and Deoxidizing Capabilities" Polymers 13, no. 7: 1129. https://doi.org/10.3390/polym13071129
APA StyleLee, B.-Y., Lee, D.-H., & Jang, K.-S. (2021). Impact Modifiers and Compatibilizers for Versatile Epoxy-Based Adhesive Films with Curing and Deoxidizing Capabilities. Polymers, 13(7), 1129. https://doi.org/10.3390/polym13071129







