Thermomechanical Devulcanisation of Ethylene Propylene Diene Monomer (EPDM) Rubber and Its Subsequent Reintegration into Virgin Rubber
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Rubber Vulcanisates
2.3. Devulcanisation
2.4. Revulcanisation
2.5. Testing
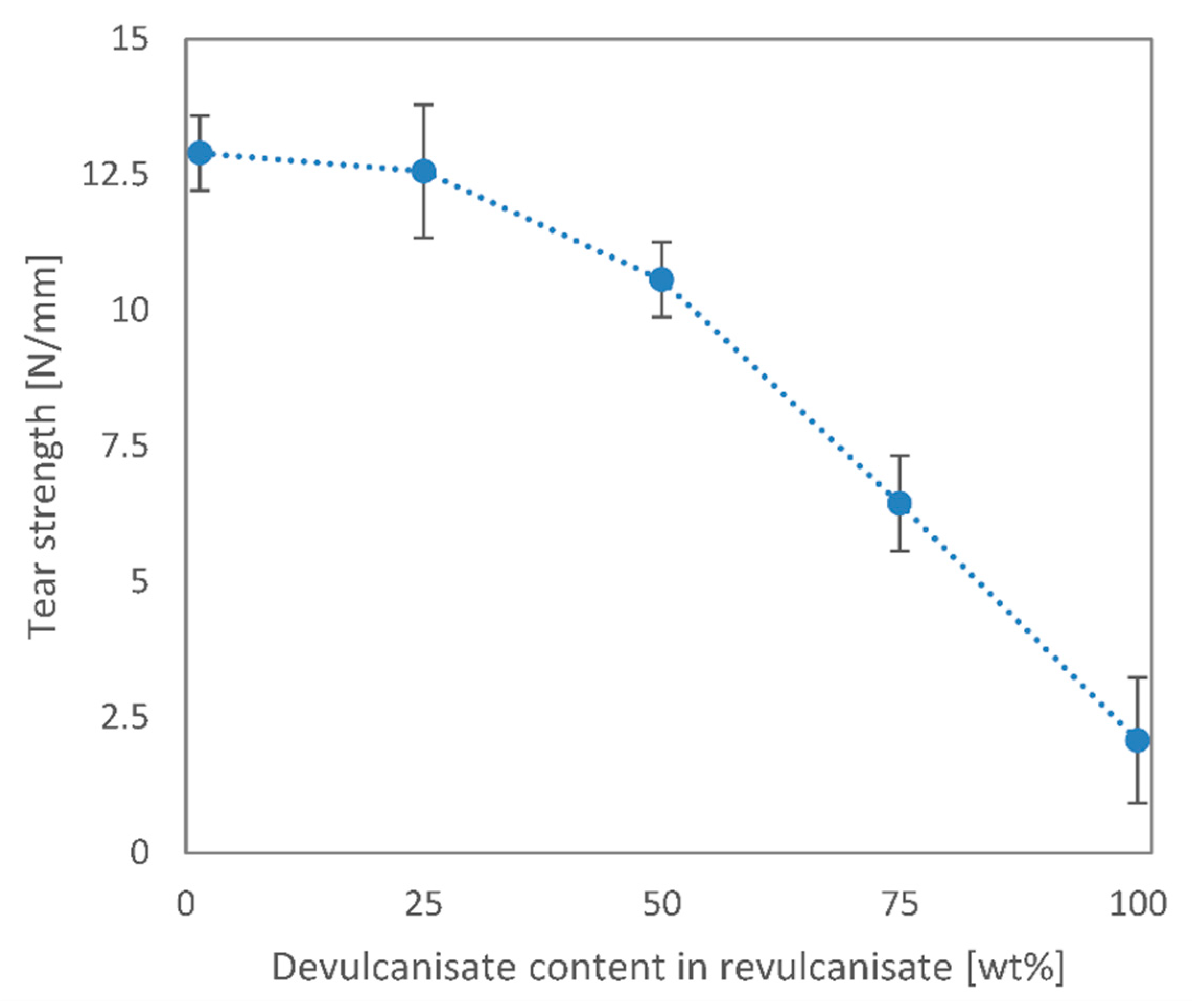
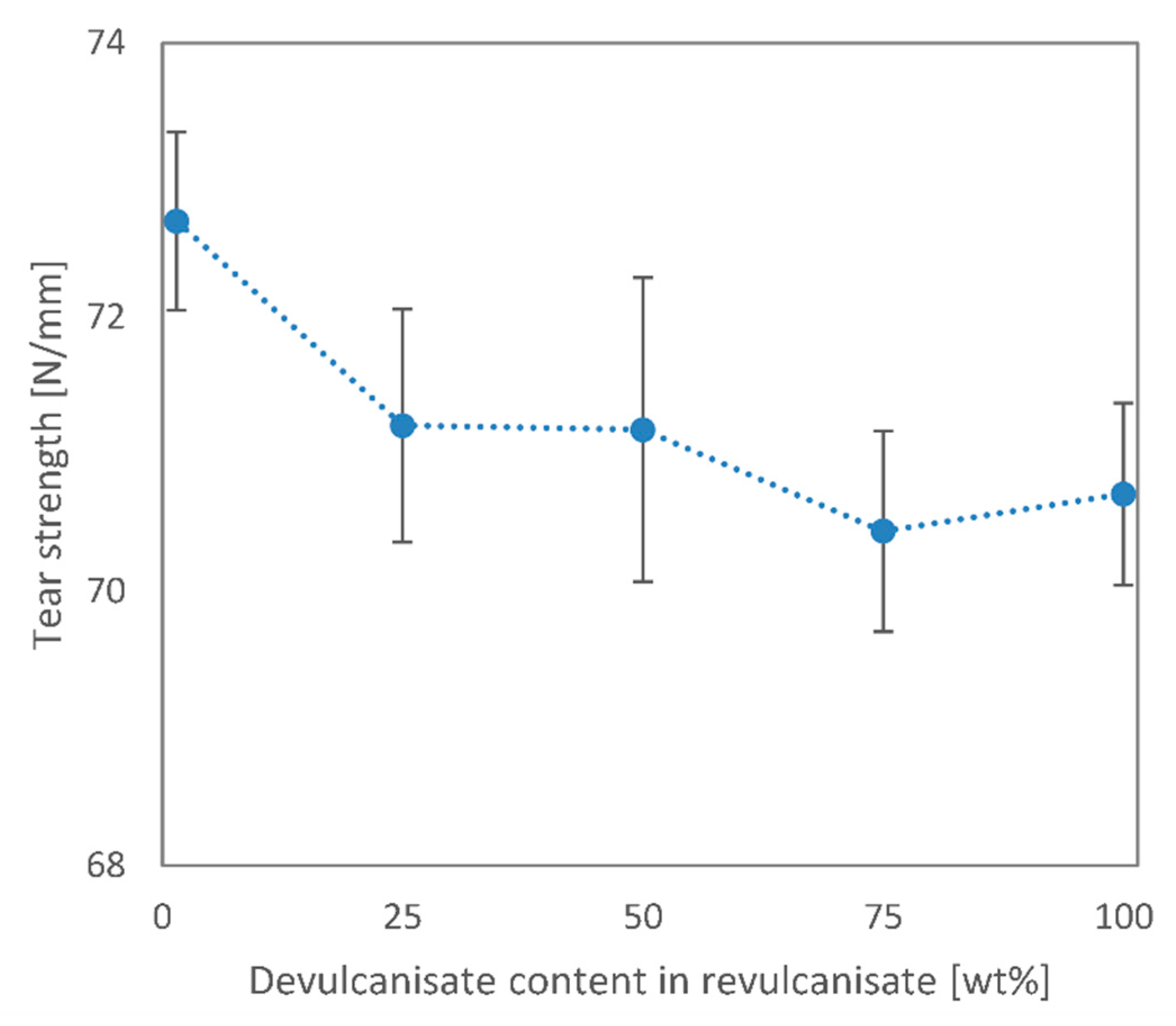
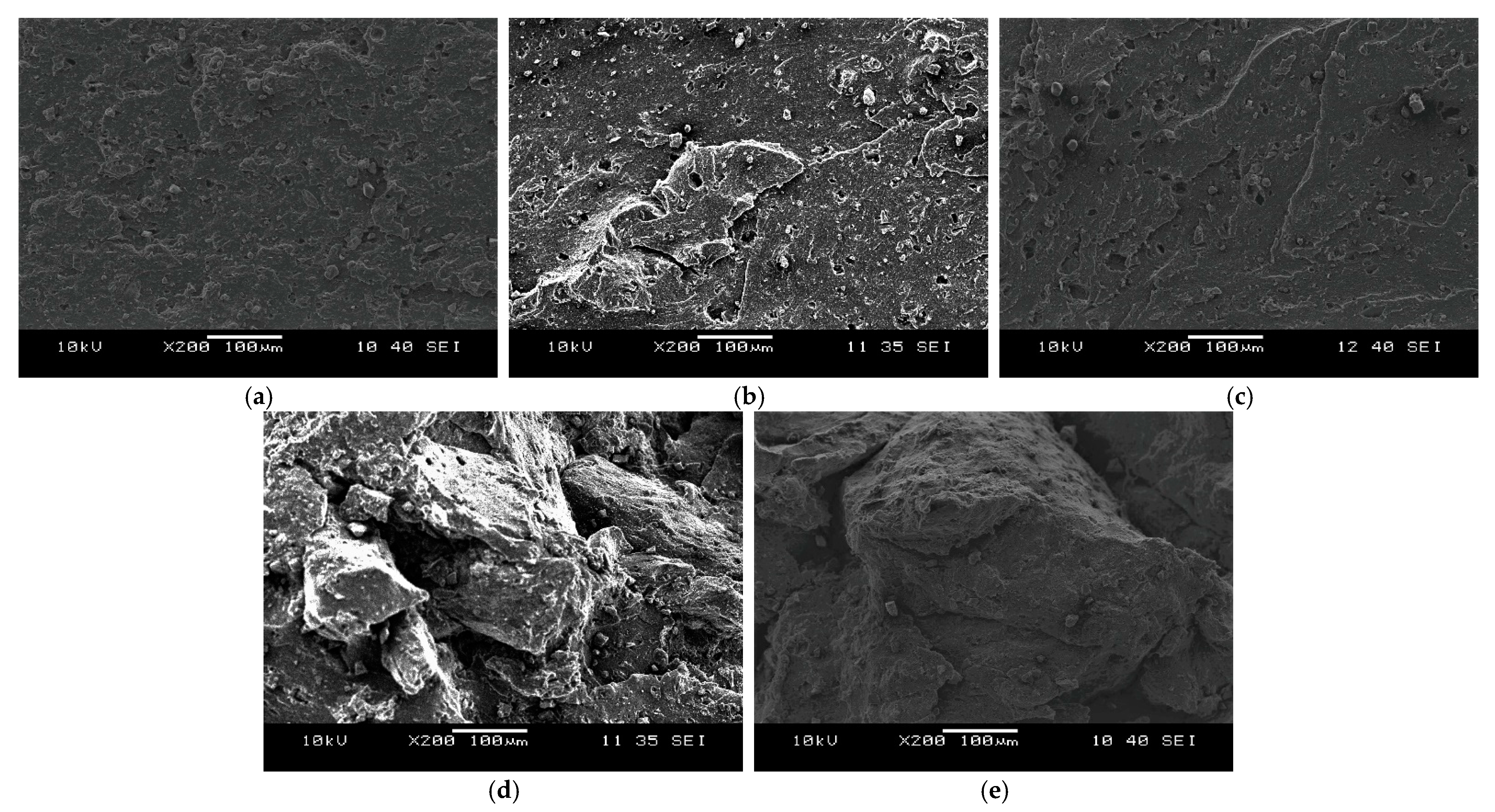
3. Results and Discussion
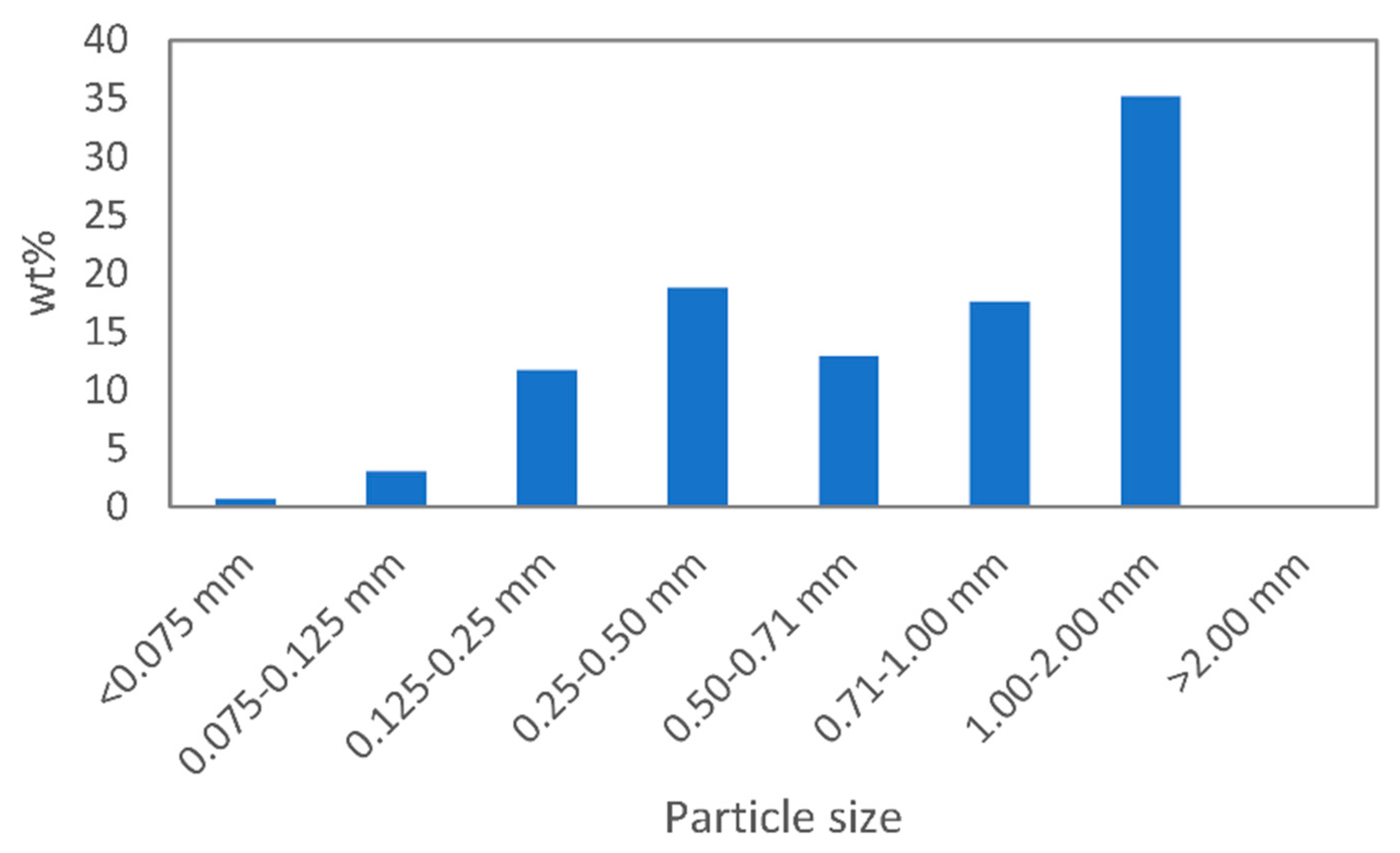
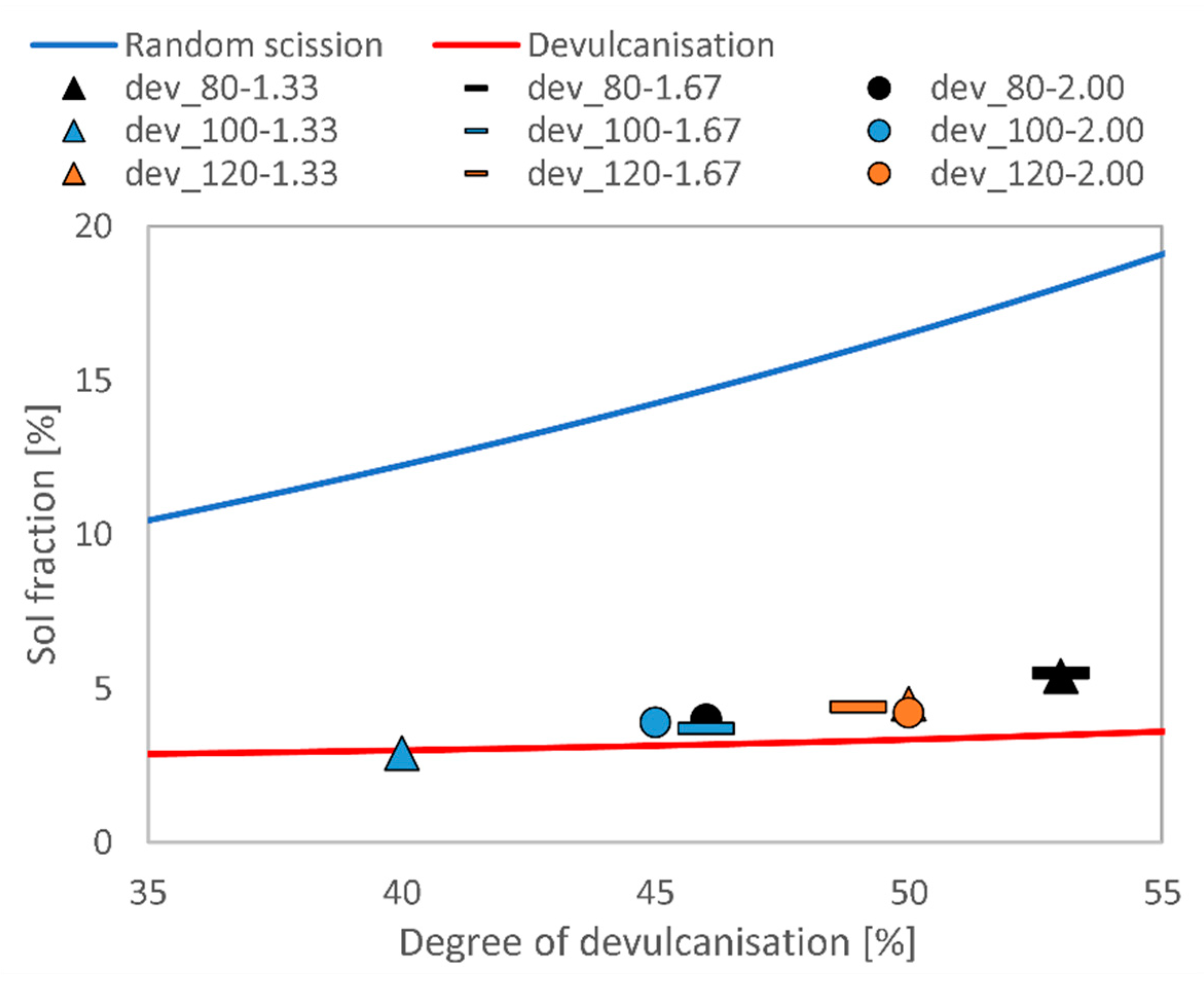
3.1. Devulcanisation of EPDM Rubber
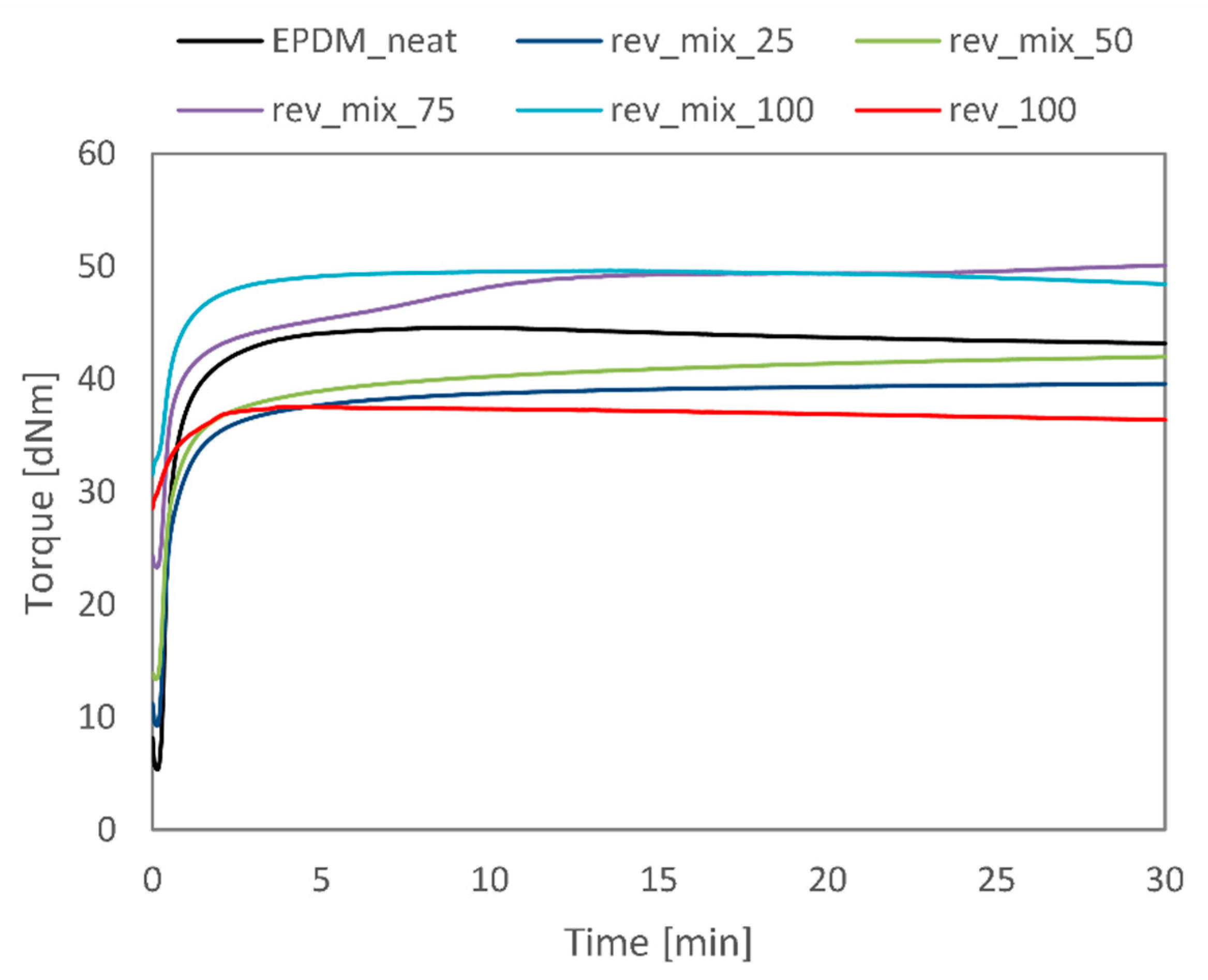
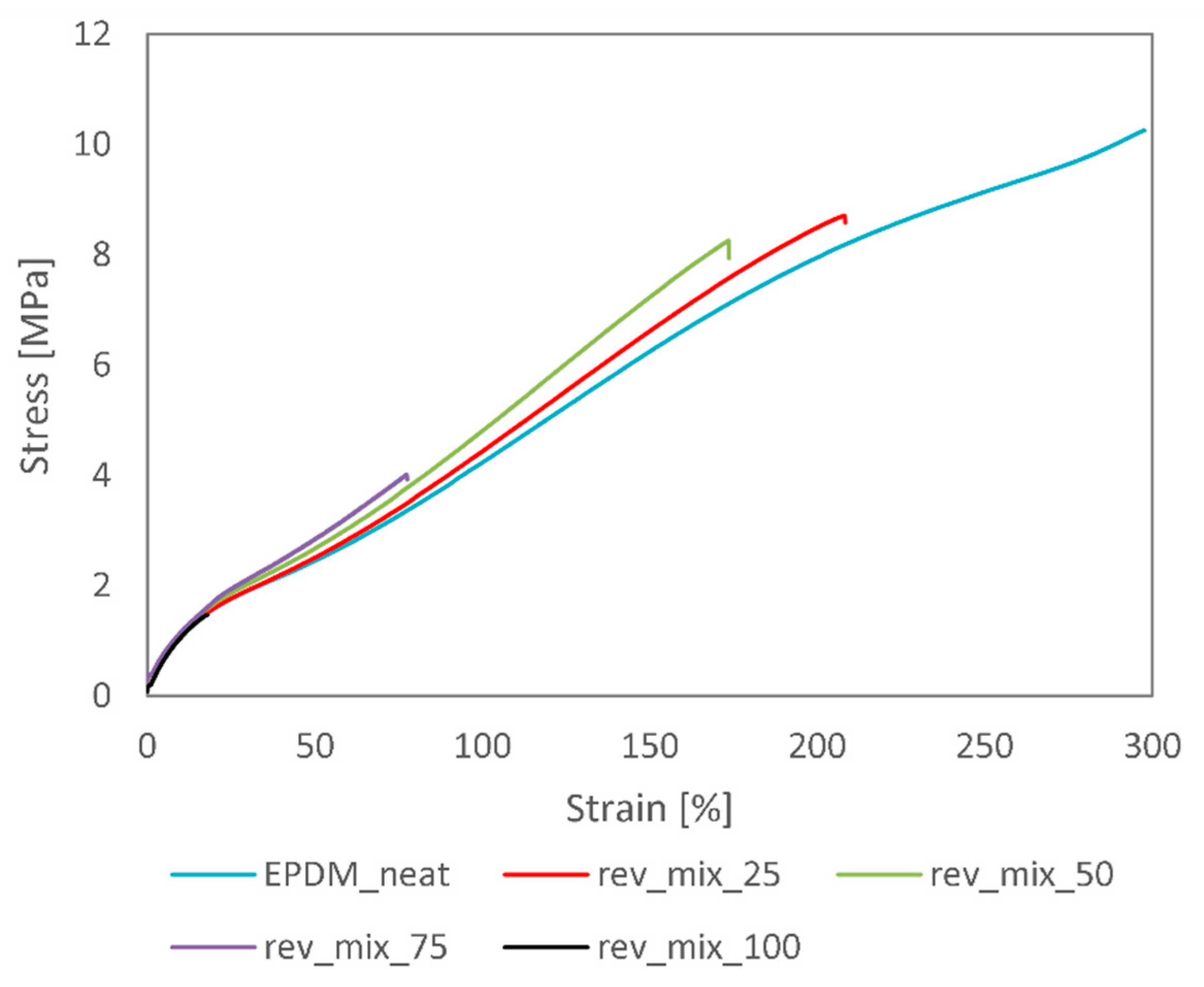
3.2. Revulcanisation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beroe. Styrene Butadiene Rubber (SBR) Commodity Report; Beroe: Raleigh, NC, USA, 2019. [Google Scholar]
- Plastics Europe. Plastics—The Facts 2017; An Analysis of European Plastics Production, Demand and Waste Data; Plastics Europe–Association of Plastics Manufacturers: Brussels, Belgium, 2018. [Google Scholar]
- International Rubber Study Group. Statistical Bulletin, July–September 2019 ed.; International Rubber Study Group: Singapore, 2019. [Google Scholar]
- Simon, D.Á.; Pirityi, D.; Tamás-Bényei, P.; Bárány, T. Microwave devulcanization of ground tire rubber and applicability in SBR compounds. J. Appl. Polym. Sci. 2020, 137, 48351. [Google Scholar] [CrossRef]
- Forrest, M. Overview of the world rubber recycling market. In Recycling and Reuse of Waste Rubber, 1st ed.; Potter, D., Ed.; Smithers Rapra: Shawbury, UK, 2014; Volume 1, pp. 17–30. [Google Scholar]
- Isayev, A.I. Recycling of rubbers. In Science and Technology of Rubber, 4th ed.; Mark, J.E., Erman, B., Roland, C.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 1, pp. 697–764. [Google Scholar]
- Sutanto, P.; Picchioni, F.; Janssen, L.P.B.M. Modelling a continuous devulcanization in an extruder. Chem. Eng. Sci. 2006, 61, 7077–7086. [Google Scholar] [CrossRef]
- Fukumori, K.; Matsushita, M. Material Recycling Technology of Crosslinked Rubber Waste. R D Rev. Toyota CRDL 2003, 38, 39–47. [Google Scholar]
- Fukumori, K.; Mouri, M.; Sato, N.; Okamoto, H.; Matsushita, M. Continuous recycling of vulcanisates. Int. Polym. Sci. Technol. 2001, 28, 5–11. [Google Scholar] [CrossRef]
- Verbruggen, M.A.L.; Does, L.V.D.; Noordermeer, J.W.M.; Duin, M.V.; Manuel, H.J. Mechanisms involved in the recycling of NR and EPDM. Rubber Chem. Technol. 1999, 72, 731–740. [Google Scholar] [CrossRef]
- Kaewpetch, B.; Prasongsuk, S.; Poompradub, S. Devulcanization of natural rubber vulcanizates by Bacillus cereus TISTR 2651. Express Polym. Lett. 2019, 13, 877–888. [Google Scholar] [CrossRef]
- Tatangelo, V.; Mangili, I.; Caracino, P.; Anzano, M.; Najmi, Z.; Bestetti, G.; Collina, E.; Franzetti, A.; Lasagni, M. Biological devulcanisation of ground natural rubber by Gordonia desulfuricans DSM 44462T strain. Appl. Microbiol. Biotechnol. 2016, 100, 8931–8942. [Google Scholar] [CrossRef]
- Simon, D.Á.; Halász, I.Z.; Karger-Kocsis, J.; Bárány, T. Microwave devulcanized crumb rubbers in polypropylene based thermoplastic dynamic vulcanizates. Polymers 2018, 10, 767. [Google Scholar] [CrossRef]
- Formela, K.; Hejna, A.; Zedler, Ł.; Colom, X.; Cañavate, J. Microwave treatment in waste rubber recycling—Recent advances and limitations. Express Polym. Lett. 2019, 13, 565–588. [Google Scholar] [CrossRef]
- Garcia, P.S.; Gouveia, R.F.; Maia, J.M.; Scuracchio, C.H.; Cruz, S.A. 2D and 3D imaging of the deformation behavior of partially devulcanized rubber/polypropylene blends. Express Polym. Lett. 2018, 12, 1047–1060. [Google Scholar] [CrossRef]
- Barbosa, R.; Ambrósio, J.D. Devulcanization of natural rubber compounds by extrusion using thermoplastics and characterization of revulcanized compounds. J. Polym. Res. 2019, 26, 160. [Google Scholar] [CrossRef]
- Diaz, R.; Colomines, G.; Peuvrel-Disdier, E.; Deterre, R. Thermo-mechanical recycling of rubber: Relationship between material properties and specific mechanical energy. J. Mater. Process. Technol. 2018, 252, 454–468. [Google Scholar] [CrossRef]
- Zhang, X.; Saha, P.; Cao, L.; Li, H.; Kim, J. Devulcanization of waste rubber powder using thiobisphenols as novel reclaiming agent. Waste Manag. 2018, 78, 980–991. [Google Scholar] [CrossRef]
- Dijkhuis, K.A.J.; Babu, I.; Lopulissa, J.S.; Noordermeer, J.W.M.; Dierkes, W.K. A Mechanistic Approach to EPDM Devulcanization. Rubber Chem. Technol. 2008, 81, 190–208. [Google Scholar] [CrossRef]
- Yun, J.; Isayev, A.I. Superior mechanical properties of ultrasonically recycled EPDM rubber. Rubber Chem. Technol. 2003, 76, 253–270. [Google Scholar] [CrossRef]
- Yun, J.; Yashin, V.V.; Isayev, A.I. Ultrasonic Devulcanization of Carbon Black-Filled Ethylene Propylene Diene Monomer Rubber. J. Appl. Polym. Sci. 2003, 91, 1646–1656. [Google Scholar] [CrossRef]
- Warner, W.C. Methods of devulcanization. Rubber Chem. Technol. 1994, 67, 559–566. [Google Scholar] [CrossRef]
- Myhre, M.; Saiwari, S.; Dierker, W.; Noordermeer, J. Rubber recycling: Chemistry, processing, and applications. Rubber Chem. Technol. 2012, 85, 408–449. [Google Scholar] [CrossRef]
- Movahed, S.O.; Ansarifar, A.; Estagy, S. A review of the reclaiming of rubber waste and recent work on the recycling of ethylene-propylene-diene rubber waste. Rubber Chem. Technol. 2016, 89, 54–78. [Google Scholar] [CrossRef]
- Macsiniuc, A.; Rochette, A.; Rodrigue, D. Understanding the regeneration of EPDM rubber crumbs from used tyres. Prog. Rubber Plast. Recycl. Technol. 2010, 26, 51–81. [Google Scholar] [CrossRef]
- Horikx, M.M. Chain scissions in a polymer network. J. Polym. Sci. 1956, 19, 445–454. [Google Scholar] [CrossRef]
- Flory, P.J. Thermodynamics of high polymer solutions. J. Chem. Phys. 1942, 10, 51–61. [Google Scholar] [CrossRef]
- Huggins, M.L. Thermodynamic properties of solutions of long-chain compounds. Ann. N. Y. Acad. Sci. 1942, 43, 1–32. [Google Scholar] [CrossRef]
- Verbruggen, M.A.L.; Does, L.V.D.; Dierkes, W.K.; Noordermeer, J.W.M. Experimental validation of the Charlesby and Horikx models applied to devulcanisation of sulfur and peroxide vulcanisates of NR and EPDM. Rubber Chem. Technol. 2016, 89, 671–688. [Google Scholar] [CrossRef]
- Forrest, M. Characterisation of devulcanised rubber and products containing devulcanised rubber. In Recycling and Reuse of Waste Rubber, 1st ed.; Potter, D., Ed.; Smithers Rapra: Shawbury, UK, 2014; Volume 1, pp. 105–134. [Google Scholar]
- Hajba, S.; Tábi, T. Cross effect of natural rubber and annealing on the properties of poly(lactic acid). Period. Polytech. Mech. Eng. 2019, 63, 270–277. [Google Scholar] [CrossRef]
- Hassan, M.M.; Aly, R.O.; Aal, S.E.A.; El-Masry, A.M.; Fathy, E.S. Mechanochemical devulcanization and gamma irradiation of devulcanized waste rubber/high density polyethylene thermoplastic elastomer. J. Ind. Eng. Chem. 2013, 19, 1722–1729. [Google Scholar] [CrossRef]
- Jacob, C.; De, P.P.; Bhowmick, A.K.; De, S.K. Recycling of EPDM waste. II. Replacement of virgin rubber by ground EPDM vulcanizate in EPDM/PP thermoplastic elastomeric composition. J. Appl. Polym. Sci. 2001, 82, 3304–3312. [Google Scholar] [CrossRef]
- Sutanto, P.; Picchioni, F.; Janssen, L.P.B.M.; Dijkhuis, K.A.J.; Dierkes, W.K.; Noordermeer, J.W.M. EPDM rubber reclaim from devulcanized EPDM. J. Appl. Polym. Sci. 2006, 102, 5948–5957. [Google Scholar] [CrossRef]
- Movahed, S.O.; Ansarifar, A.; Zohuri, G.; Ghaneie, N.; Kermany, Y. Devulcanization of ethylene-propylene-diene waste rubber by microwaves and chemical agents. J. Elastomers Plast. 2014, 48, 122–144. [Google Scholar] [CrossRef]
- Jiang, K.; Shi, J.; Ge, Y.; Zou, R.; Yao, P.; Li, X.; Zhang, L. Complete devulcanization of sulfur-cured butyl rubber by using supercritical carbon dioxide. J. Appl. Polym. Sci. 2012, 127, 2397–2406. [Google Scholar] [CrossRef]
- de Sousa, F.D.B.; Zanchet, A.; Scuracchio, C.H. From devulcanization to revulcanization: Challenges in getting recycled tire rubber for technical applications. ACS Sustain. Chem. Eng. 2019, 7, 8755–8765. [Google Scholar] [CrossRef]
- Jacob, C.; De, P.P.; Bhowmick, A.K.; De, S.K. Recycling of EPDM waste. I. Effect of ground EPDM vulcanizate on properties of EPDM rubber. J. Appl. Polym. Sci. 2001, 82, 3293–3303. [Google Scholar] [CrossRef]
- Seghar, S.; Asaro, L.; Rolland-Monnet, M.; Hocine, N.A. Thermo-mechanical devulcanization and recycling of rubber industry waste. Resour. Conserv. Recycl. 2019, 144, 180–186. [Google Scholar] [CrossRef]

| Component | Concentration [phr] |
|---|---|
| Dutral TER 4047 | 100 |
| Zinc oxide | 4 |
| Zinc stearate | 1 |
| UltraLube UL160 | 3 |
| PEG 4000 1 | 1 |
| Dolomite B | 30 |
| N550 carbon black | 45 |
| N772 carbon black | 40 |
| DK 350 oil | 15 |
| TBTD 2 | 0.8 |
| MBT 3 | 1.5 |
| ZDBC 4 | 0.8 |
| Sulphur | 1 |
| Total | 243.1 |
| Speed of Rolls [rpm] | ||||
| roll 1: 3 | roll 1: 3 | roll 1: 3 | ||
| roll 2: 4 | roll 2: 5 | roll 2: 6 | ||
| Friction [–] | ||||
| 1.33 | 1.67 | 2.00 | ||
| Roll temperature [°C] | 80 | dev_80-1.33 | dev_80-1.67 | dev_80-2.00 |
| 100 | dev_100-1.33 | dev_100-1.67 | dev_100-2.00 | |
| 120 | dev_120-1.33 | dev_120-1.67 | dev_120-2.00 | |
| Component | Concentration [phr] |
|---|---|
| dev_80-1.33 | 243.1 |
| Zinc oxide | 4 |
| Zinc stearate | 1 |
| TBTD | 0.8 |
| MBT | 1.5 |
| ZDBC | 0.8 |
| Sulphur | 1 |
| Total | 252.2 |
| Sample Name | dev_80-1.33_mix Content [wt%] | Virgin EPDM Content [wt%] | dev_80-1.33 Content [wt%] |
|---|---|---|---|
| EPDM_neat | 0 | 100 | 0 |
| rev_mix_25 | 25 | 75 | 0 |
| rev_mix_50 | 50 | 50 | 0 |
| rev_mix_75 | 75 | 25 | 0 |
| rev_mix_100 | 100 | 0 | 0 |
| rev_100 | 0 | 0 | 100 |
| Sample Name | Degree of Devulcanisation [%] | Sol Content [wt%] |
|---|---|---|
| dev_80-1.33 | 53 ± 4.2 | 5.4 ± 0.1 |
| dev_80-1.67 | 53 ± 4.4 | 5.5 ± 0.5 |
| dev_80-2.00 | 46 ± 5.3 | 4.0 ± 0.1 |
| dev_100-1.33 | 40 ± 4.3 | 2.9 ± 0.5 |
| dev_100-1.67 | 46 ± 6.0 | 3.7 ± 1.2 |
| dev_100-2.00 | 45 ± 5.1 | 3.9 ± 0.4 |
| dev_120-1.33 | 50 ± 8.0 | 4.5 ± 0.6 |
| dev_120-1.67 | 49 ± 6.5 | 4.4 ± 0.1 |
| dev_120-2.00 | 50 ± 4.0 | 4.2 ± 0.4 |
| EPDM_neat | rev_100 | rev_mix_25 | rev_mix_50 | rev_mix_75 | rev_mix_100 | |
|---|---|---|---|---|---|---|
| Scorch Time [s] | 14 | – | 13 | 13 | 15 | 16 |
| t90 [min] | 1.72 | 1.97 | 2.92 | 5.45 | 8.68 | 2.41 |
| Smin [dNm] | 5.36 | 28.55 | 9.26 | 13.39 | 23.30 | 28.55 |
| Smax [dNm] | 44.56 | 37.54 | 39.58 | 41.99 | 50.09 | 37.54 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pirityi, D.Z.; Pölöskei, K. Thermomechanical Devulcanisation of Ethylene Propylene Diene Monomer (EPDM) Rubber and Its Subsequent Reintegration into Virgin Rubber. Polymers 2021, 13, 1116. https://doi.org/10.3390/polym13071116
Pirityi DZ, Pölöskei K. Thermomechanical Devulcanisation of Ethylene Propylene Diene Monomer (EPDM) Rubber and Its Subsequent Reintegration into Virgin Rubber. Polymers. 2021; 13(7):1116. https://doi.org/10.3390/polym13071116
Chicago/Turabian StylePirityi, Dávid Zoltán, and Kornél Pölöskei. 2021. "Thermomechanical Devulcanisation of Ethylene Propylene Diene Monomer (EPDM) Rubber and Its Subsequent Reintegration into Virgin Rubber" Polymers 13, no. 7: 1116. https://doi.org/10.3390/polym13071116
APA StylePirityi, D. Z., & Pölöskei, K. (2021). Thermomechanical Devulcanisation of Ethylene Propylene Diene Monomer (EPDM) Rubber and Its Subsequent Reintegration into Virgin Rubber. Polymers, 13(7), 1116. https://doi.org/10.3390/polym13071116







