Lignin Extraction from Waste Pine Sawdust Using a Biomass Derived Binary Solvent System
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Biomass Fractionation
2.3. Statistical Analysis
2.4. Determination of Lignin Content
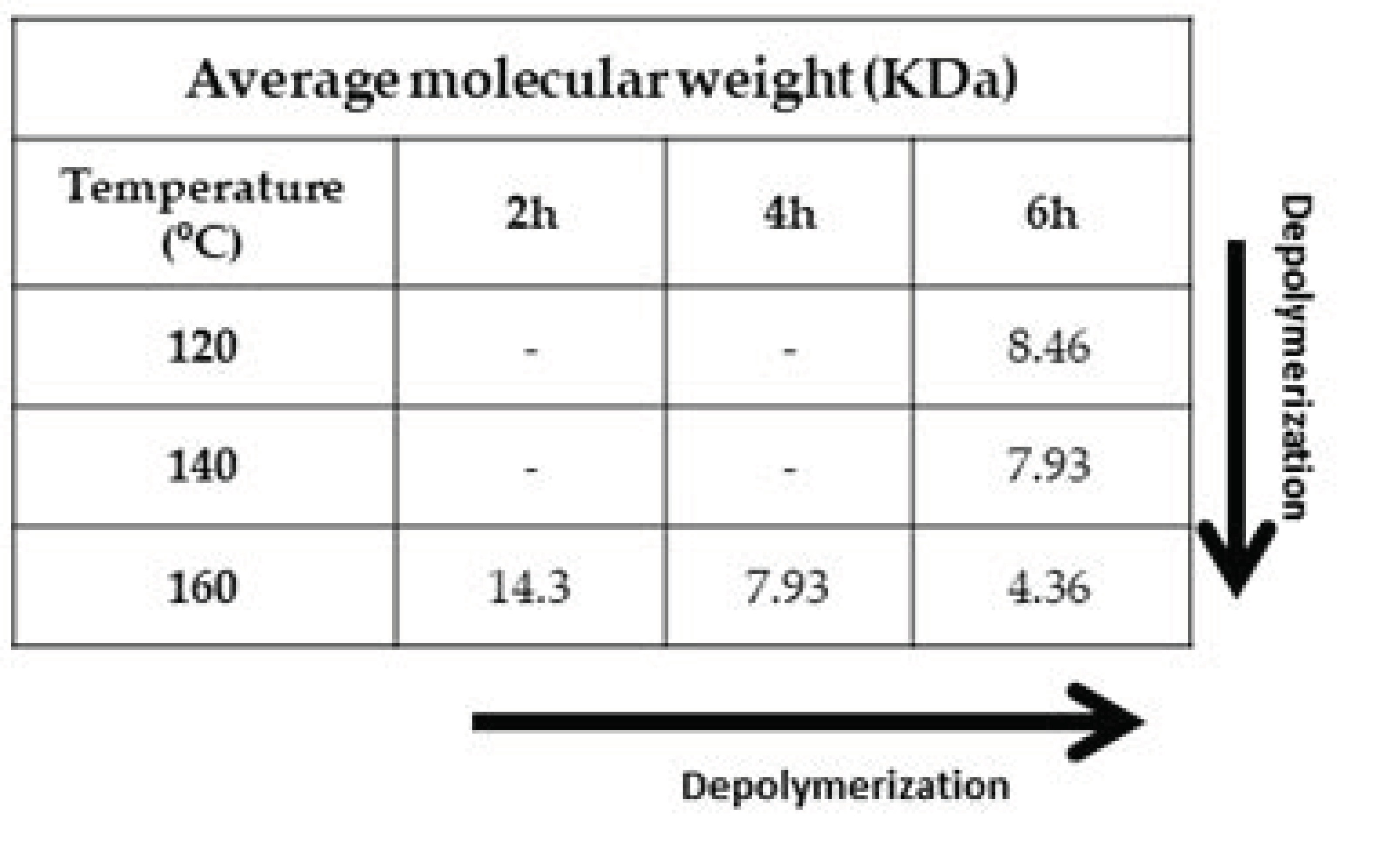
2.5. Viscosity Average Molecular Weight of Lignin
2.6. Solvatochromic Kamlet–Taft Measurements
2.7. Scanning Electron Microscopy
2.8. Fourier Transform Infrared (FTIR) Spectroscopy
3. Results
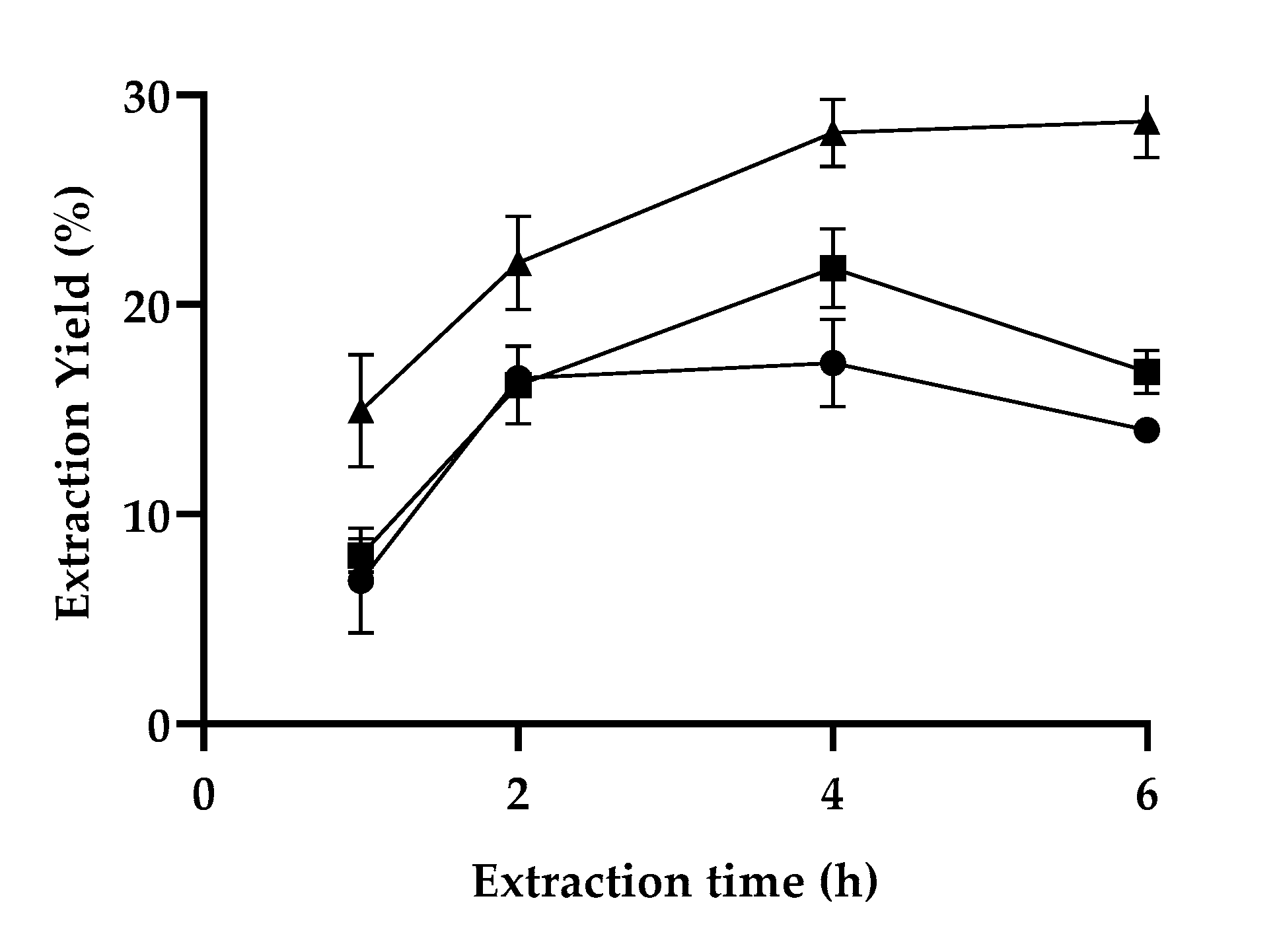
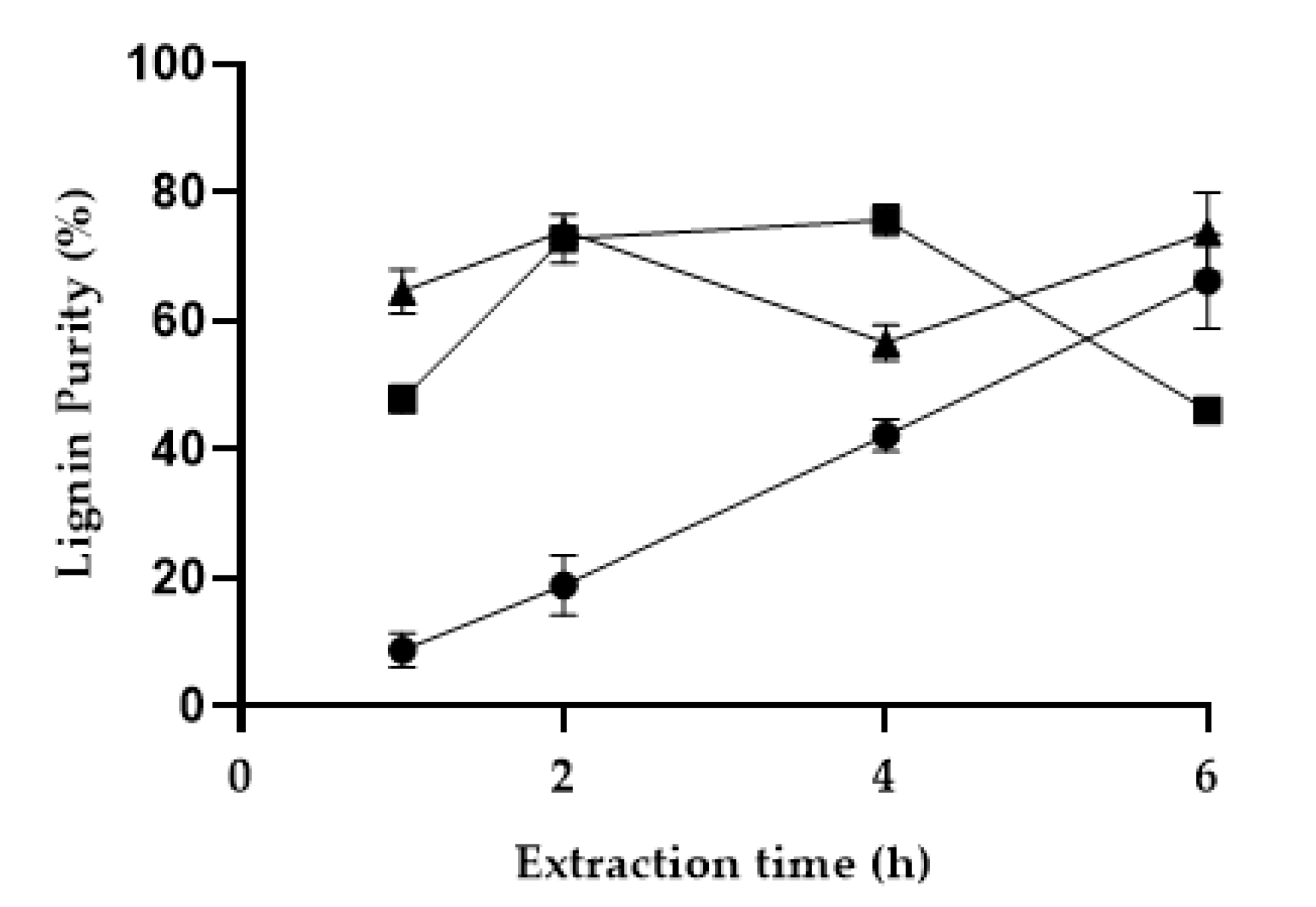
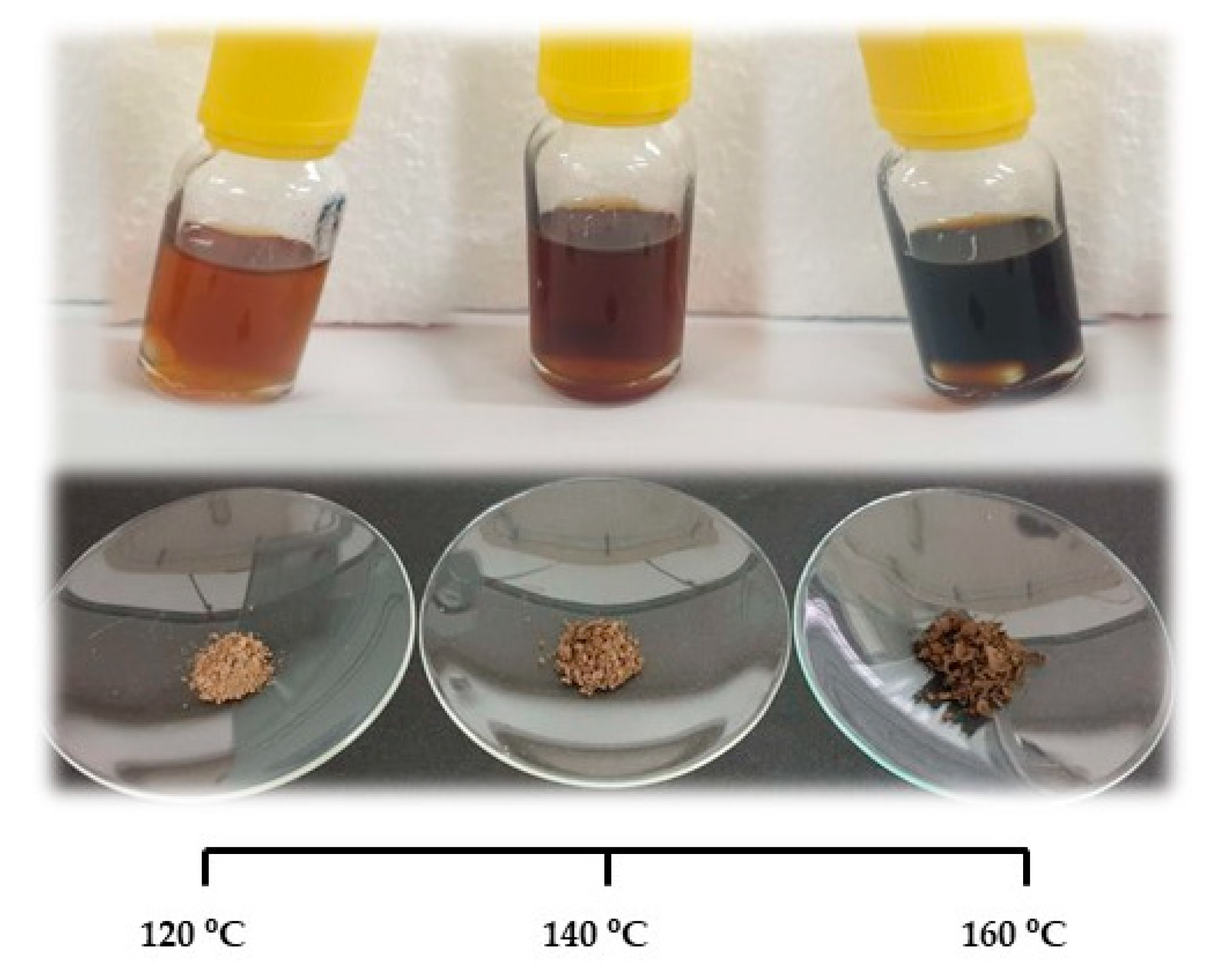
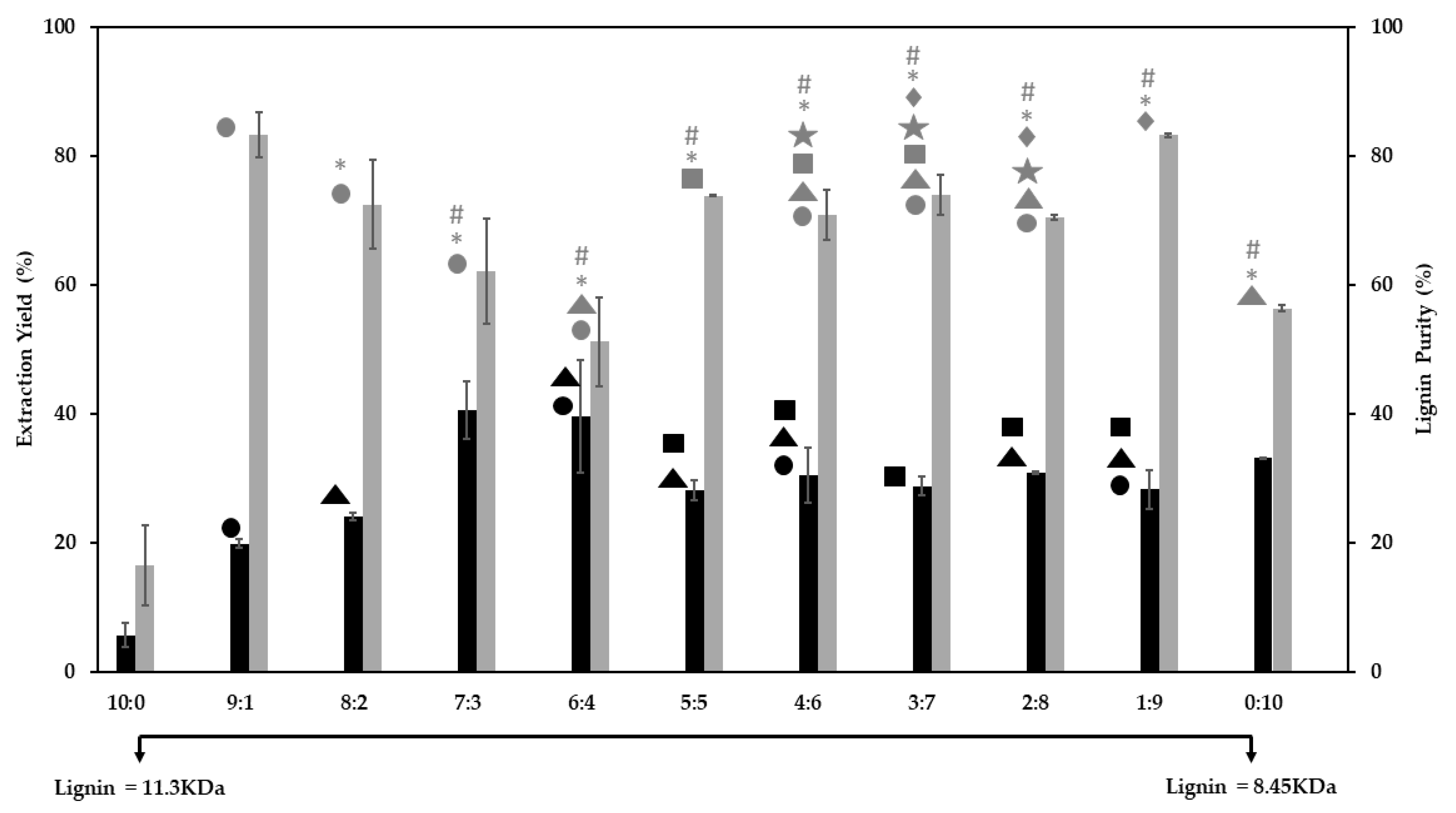
3.1. Optimization of the Lignin Extraction Conditions
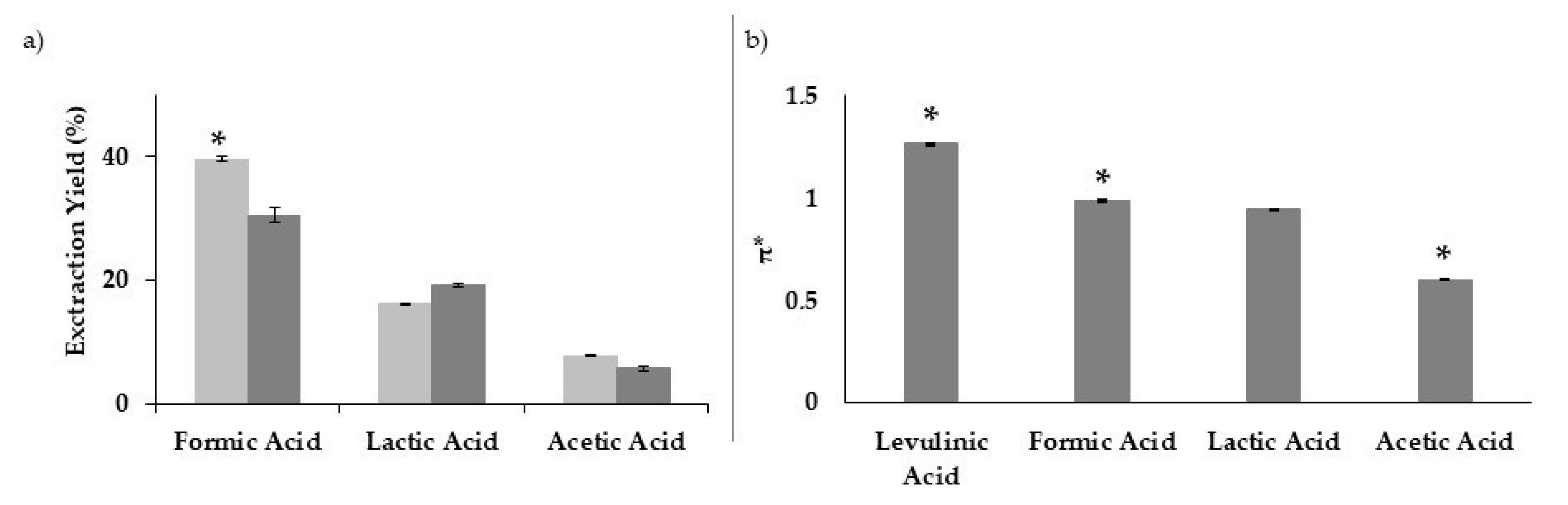
3.2. Extraction Efficiency and Solvent Polarizability
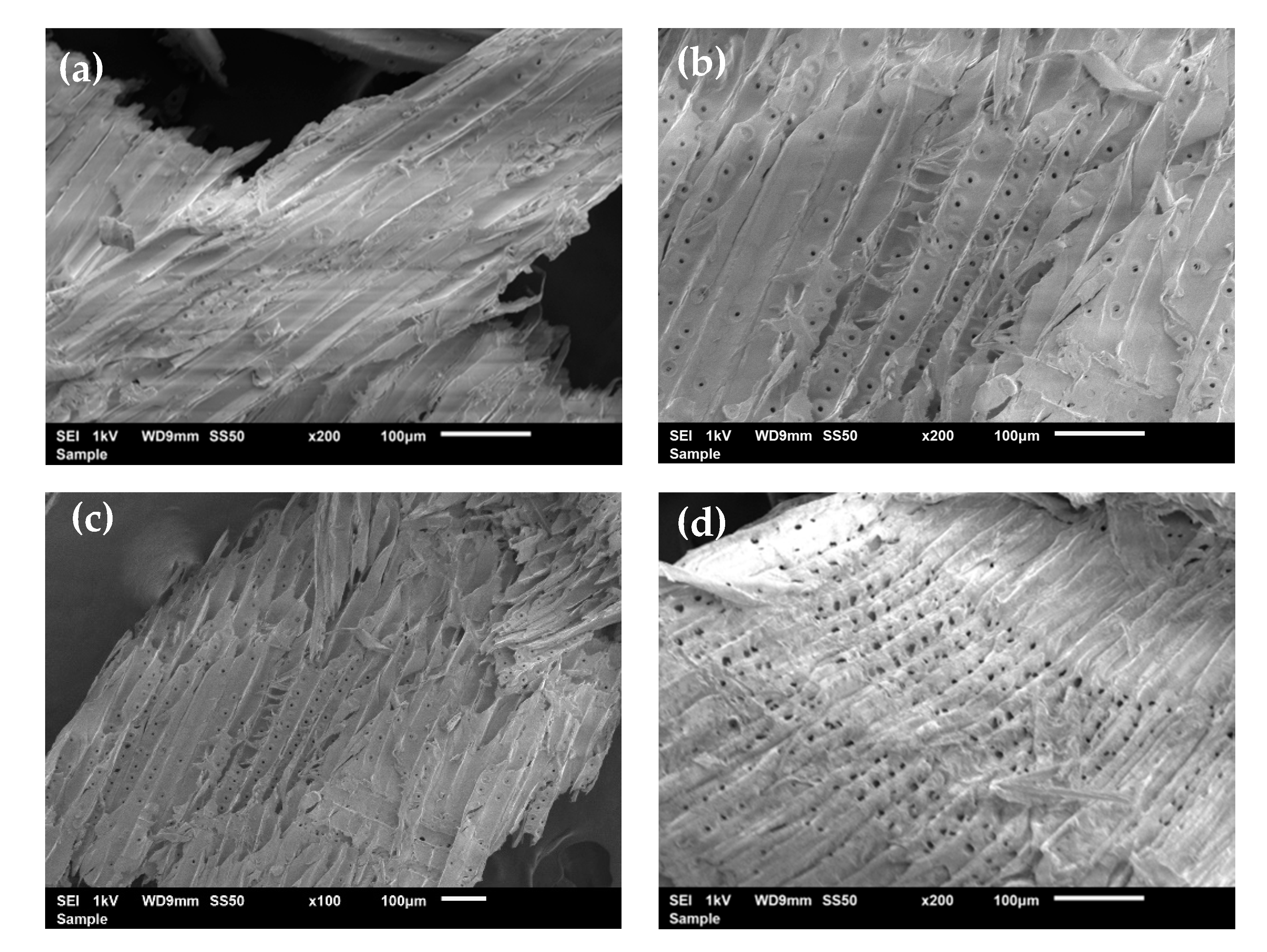
3.3. Scanning Electron Microscopy
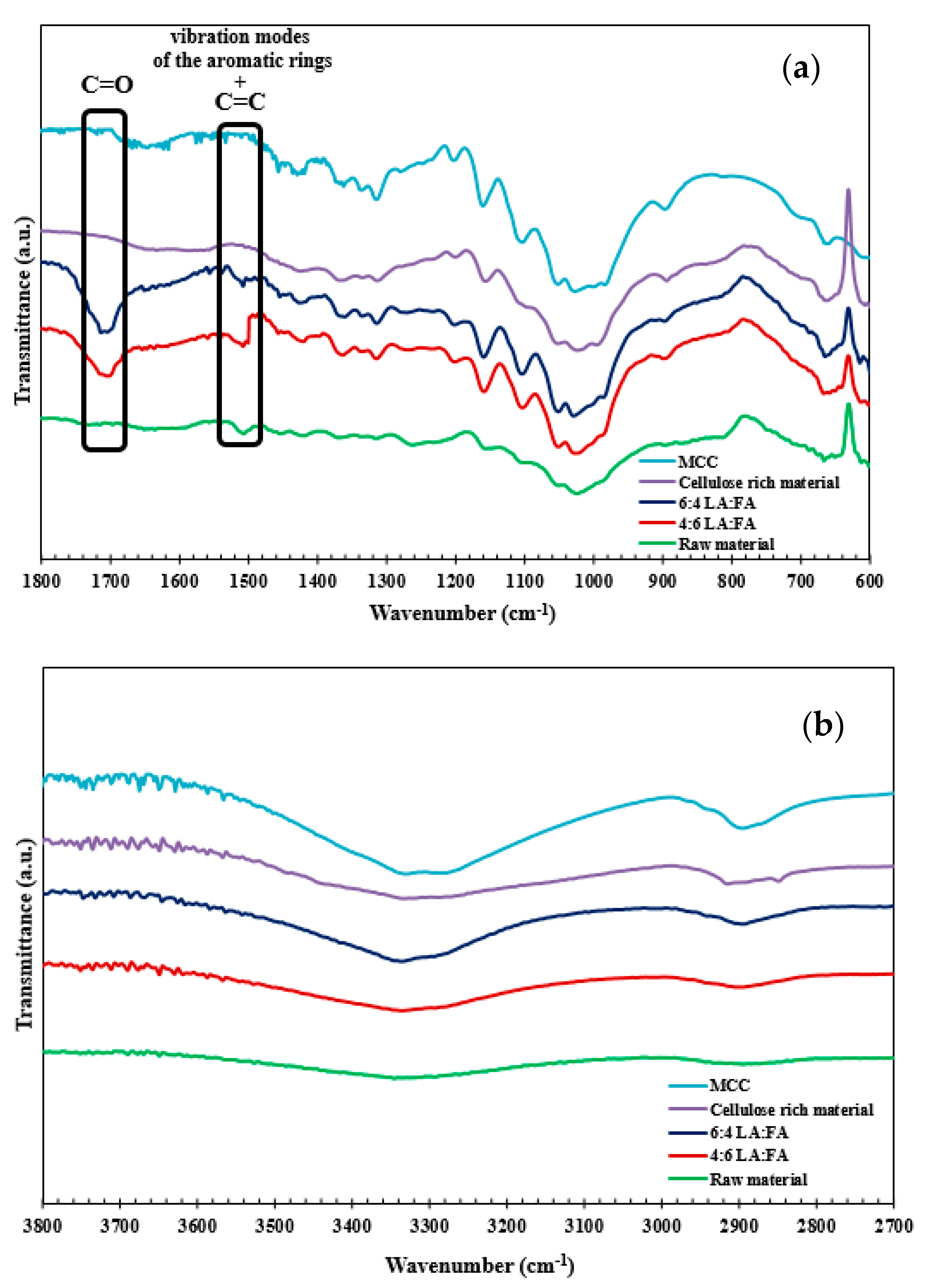
3.4. Fourier Transform Infrared Spectroscopy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Watkins, D.; Nuruddin, M.; Hosur, M.; Tcherbi-Narteh, A.; Jeelani, S. Extraction and characterization of lignin from different biomass resources. J. Mater. Res. Technol. 2015, 4, 26–32. [Google Scholar] [CrossRef]
- Magalhães, S.; Alves, L.; Medronho, B.; Fonseca, A.C.; Romano, A.; Coelho, J.F.J.; Norgren, M. Brief Overview on Bio-Based Adhesives and Sealants. Polymers 2019, 11, 1685. [Google Scholar] [CrossRef]
- Ghaffar, S.H.; Fan, M. Structural analysis for lignin characteristics in biomass straw. Biomass Bioenergy 2013, 57, 264–279. [Google Scholar] [CrossRef]
- Pinkert, A.; Goeke, D.F.; Marsh, K.N.; Pang, S. Extracting wood lignin without dissolving or degrading cellulose: Investigations on the use of food additive-derived ionic liquids. Green Chem. 2011, 13, 3124–3136. [Google Scholar] [CrossRef]
- Duval, A.; Lawoko, M. A review on lignin-based polymeric, micro-and nano-structured materials. React. Funct. Polym. 2014, 85, 78–96. [Google Scholar] [CrossRef]
- Wahlström, R.; Kalliola, A.; Heikkinen, J.; Kyllönen, H.; Tamminen, T. Lignin cationization with glycidyltrimethylammonium chloride aiming at water purification applications. Ind. Crop. Prod. 2017, 104, 188–194. [Google Scholar] [CrossRef]
- Vinardell, M.P.; Ugartondo, V.; Mitjans, M. Potential applications of antioxidant lignins from different sources. Ind. Crop. Prod. 2008, 27, 220–223. [Google Scholar] [CrossRef]
- Melro, E.; Filipe, A.; Sousa, D.; Medronho, B.; Romano, A. Revisiting lignin: A tour through its structural features, characterization methods and applications. N. J. Chem. 2021. [Google Scholar] [CrossRef]
- Zakzeski, J.; Bruijnincx, P.C.A.; Jongerius, A.L.; Weckhuysen, B.M. The Catalytic Valorization of Lignin for the Production of Renewable Chemicals. Chem. Rev. 2010, 110, 3552–3599. [Google Scholar] [CrossRef] [PubMed]
- Hamada, Y.; Yoshida, K.; Asai, R.-i.; Hayase, S.; Nokami, T.; Izumi, S.; Itoh, T. A possible means of realizing a sacrifice-free three component separation of lignocellulose from wood biomass using an amino acid ionic liquid. Green Chem. 2013, 15, 1863–1868. [Google Scholar] [CrossRef]
- El Seoud, O.A.; Koschella, A.; Fidale, L.C.; Dorn, S.; Heinze, T. Applications of Ionic Liquids in Carbohydrate Chemistry: A Window of Opportunities. Biomacromolecules 2007, 8, 2629–2647. [Google Scholar] [CrossRef] [PubMed]
- Garlapati, V.K.; Chandel, A.K.; Kumar, S.P.J.; Sharma, S.; Sevda, S.; Ingle, A.P.; Pant, D. Circular economy aspects of lignin: Towards a lignocellulose biorefinery. Renew. Sustain. Energy Rev. 2020, 130, 109977. [Google Scholar] [CrossRef]
- Zhang, J.; Cai, D.; Qin, Y.; Liu, D.; Zhao, X. High value-added monomer chemicals and functional bio-based materials derived from polymeric components of lignocellulose by organosolv fractionation. Biofuels Bioprod. Biorefin. 2020, 14, 371–401. [Google Scholar] [CrossRef]
- Dinh Vu, N.; Thi Tran, H.; Bui, N.D.; Duc Vu, C.; Viet Nguyen, H. Lignin and Cellulose Extraction from Vietnam’s Rice Straw Using Ultrasound-Assisted Alkaline Treatment Method. Int. J. Polym. Sci. 2017, 2017, 1063695. [Google Scholar] [CrossRef]
- Hochegger, M.; Trimmel, G.; Cottyn-Boitte, B.; Cézard, L.; Majira, A.; Schober, S.; Mittelbach, M. Influence of Base-Catalyzed Organosolv Fractionation of Larch Wood Sawdust on Fraction Yields and Lignin Properties. Catalysts 2019, 9, 996. [Google Scholar] [CrossRef]
- Zhao, X.; Cheng, K.; Liu, D. Organosolv pretreatment of lignocellulosic biomass for enzymatic hydrolysis. Appl. Microbiol. Biotechnol. 2009, 82, 815–827. [Google Scholar] [CrossRef]
- Tian, D.; Chandra, R.; Lee, J.-S.; Lu, C.; Saddler, J. A comparison of various lignin-extraction methods to enhance the accessibility and ease of enzymatic hydrolysis of the cellulosic component of steam-pretreated poplar. Biotechnol. Biuels 2017, 10. [Google Scholar] [CrossRef]
- Liu, X.; Li, S.; Liu, Y.; Cao, Y. Formic acid: A versatile renewable reagent for green and sustainable chemical synthesis. Chin. J. Catal. 2015, 36, 1461–1475. [Google Scholar] [CrossRef]
- Dutta, S.; Yu, I.K.M.; Tsang, D.C.W.; Su, Z.; Hu, C.; Wu, K.C.W.; Yip, A.C.K.; Ok, Y.S.; Poon, C.S. Influence of green solvent on levulinic acid production from lignocellulosic paper waste. Bioresour. Technol. 2020, 298, 122544. [Google Scholar] [CrossRef]
- Cocero, M.J.; Cabeza, Á.; Abad, N.; Adamovic, T.; Vaquerizo, L.; Martínez, C.M.; Pazo-Cepeda, M.V. Understanding biomass fractionation in subcritical & supercritical water. J. Supercrit. Fluids 2018, 133, 550–565. [Google Scholar] [CrossRef]
- Tian, D.; Guo, Y.; Hu, J.; Yang, G.; Zhang, J.; Luo, L.; Xiao, Y.; Deng, S.; Deng, O.; Zhou, W.; et al. Acidic deep eutectic solvents pretreatment for selective lignocellulosic biomass fractionation with enhanced cellulose reactivity. Int. J. Biol. Macromol. 2020, 142, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Smink, D.; Kersten, S.R.A.; Schuur, B. Recovery of lignin from deep eutectic solvents by liquid-liquid extraction. Sep. Purif. Technol. 2020, 235, 116127. [Google Scholar] [CrossRef]
- Husanu, E.; Mero, A.; Rivera, J.G.; Mezzetta, A.; Ruiz, J.C.; D’Andrea, F.; Pomelli, C.S.; Guazzelli, L. Exploiting Deep Eutectic Solvents and Ionic Liquids for the Valorization of Chestnut Shell Waste. ACS Sustain. Chem. Eng. 2020, 8, 18386–18399. [Google Scholar] [CrossRef]
- Tan, Y.T.; Chua, A.S.M.; Ngoh, G.C. Deep eutectic solvent for lignocellulosic biomass fractionation and the subsequent conversion to bio-based products—A review. Bioresour. Technol. 2020, 297, 122522. [Google Scholar] [CrossRef]
- Fernandes, C.; Melro, E.; Magalhães, S.; Alves, L.; Craveiro, R.; Filipe, A.; Valente, A.J.; Martins, G.; Antunes, F.E.; Romano, A.; et al. New deep eutectic solvent assisted extraction of highly pure lignin from maritime pine sawdust (Pinus pinaster). Int. J. Biol. Macromol. 2021. [Google Scholar] [CrossRef]
- Luo, W.; Deka, U.; Beale, A.M.; van Eck, E.R.H.; Bruijnincx, P.C.A.; Weckhuysen, B.M. Ruthenium-catalyzed hydrogenation of levulinic acid: Influence of the support and solvent on catalyst selectivity and stability. J. Catal. 2013, 301, 175–186. [Google Scholar] [CrossRef]
- Li, X.; Lu, X.; Nie, S.; Liang, M.; Yu, Z.; Duan, B.; Yang, J.; Xu, R.; Lu, L.; Si, C. Efficient catalytic production of biomass-derived levulinic acid over phosphotungstic acid in deep eutectic solvent. Ind. Crops Prod. 2020, 145, 112154. [Google Scholar] [CrossRef]
- Li, M.-F.; Sun, S.N.; Xu, F.; Sun, R.-C. Formic acid based organosolv pulping of bamboo ( Phyllostachys acuta): Comparative characterization of the dissolved lignins with milled wood lignin. Chem. Eng. J. Chem. Eng. J 2012, 179. [Google Scholar] [CrossRef]
- Moreira, R.; Mendes, C.V.T.; Banaco, M.B.F.; Carvalho, M.G.V.S.; Portugal, A. New insights in the fractionation of Pinus pinaster wood: Sequential autohydrolysis, soda ethanol organosolv and acidic precipitation. Ind. Crops Prod. 2020, 152, 112499. [Google Scholar] [CrossRef]
- Dong, D.; Fricke, A.L. Intrinsic viscosity and the molecular weight of kraft lignin. Polymer 1995, 36, 2075–2078. [Google Scholar] [CrossRef]
- Kwaambwa, H.M.; Goodwin, J.W.; Hughes, R.W.; Reynolds, P.A. Viscosity, molecular weight and concentration relationships at 298 K of low molecular weight cis-polyisoprene in a good solvent. Colloids Surf. A Physicochem. Eng. Asp. 2007, 294, 14–19. [Google Scholar] [CrossRef]
- Kamlet, M.J.; Abboud, J.L.; Taft, R.W. The solvatochromic comparison method. 6. The.pi.* scale of solvent polarities. J. Am. Chem. Soc. 1977, 99, 6027–6038. [Google Scholar] [CrossRef]
- González-Arjona, D.; López-Pérez, G.; Domínguez, M.; González, A. Solvatochromism: A Comprehensive Project for the Final Year Undergraduate Chemistry Laboratory. J. Lab. Chem. Educ. 2016, 2016, 45–52. [Google Scholar] [CrossRef]
- Ragauskas, A.; Beckham, G.; Biddy, M.J.; Chandra, R.; Chen, F.; Davis, M.F.; Davison, B.H.; Dixon, R.; Gilna, P.; Keller, M.; et al. Lignin valorization: Improving lignin processing in the biorefinery. Science 2014, 344, 709–719. [Google Scholar] [CrossRef]
- da Silva, S.H.F.; dos Santos, P.S.B.; Gatto, D.A.; Andres, M.A.; Egüés, I. Liquefaction of Kraft Lignin at Atmospheric Pressure. J. Renew. Mater. 2019, 7, 527–534. [Google Scholar] [CrossRef]
- Wang, P.; Fu, Y.; Shao, Z.; Zhang, F.; Qin, M. Structural Changes to Aspen Wood Lignin during Autohydrolysis Pretreatment. BioResources 2016, 11, 18. [Google Scholar] [CrossRef]
- Rusanen, A.; Lappalainen, K.; Kärkkäinen, J.; Tuuttila, T.; Mikola, M.; Lassi, U. Selective hemicellulose hydrolysis of Scots pine sawdust. Biomass Convers. Biorefinery 2018, 9. [Google Scholar] [CrossRef]
- Zhang, H.; Fu, S.; Chen, Y. Basic understanding of the color distinction of lignin and the proper selection of lignin in color-depended utilizations. Int. J. Biol. Macromol. 2020, 147, 607–615. [Google Scholar] [CrossRef]
- Wang, J.; Deng, Y.; Qian, Y.; Qiu, X.; Ren, Y.; Yang, D. Reduction of lignin color via one-step UV irradiation. Green Chem. 2016, 18, 695–699. [Google Scholar] [CrossRef]
- Wang, H.; Tucker, M.; Ji, Y. Recent Development in Chemical Depolymerization of Lignin: A Review. J. Appl. Chem. 2013, 2013, 838645. [Google Scholar] [CrossRef]
- Melro, E.; Filipe, A.; Valente, A.J.M.; Antunes, F.E.; Romano, A.; Norgren, M.; Medronho, B. Levulinic acid: A novel sustainable solvent for lignin dissolution. Int. J. Biol. Macromol. 2020, 164, 3454–3461. [Google Scholar] [CrossRef]
- Cheong, W.J.; Carr, P.W. Kamlet-Taft.pi.* polarizability/dipolarity of mixtures of water with various organic solvents. Anal. Chem. 1988, 60, 820–826. [Google Scholar] [CrossRef]
- Sameni, J.; Krigstin, S.; Sain, M. Solubility of Lignin and Acetylated Lignin in Organic Solvents. Bioresources 2017, 12, 1548–1565. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, X.; Fu, S.; Chen, Y. High-value utilization of kraft lignin: Color reduction and evaluation as sunscreen ingredient. Int. J. Biol. Macromol. 2019, 133, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Jin, D.; Ouyang, X.; Zhao, L.; Qiu, X.; Wang, F. Effect of structural characteristics on the depolymerization of lignin into phenolic monomers. Fuel 2018, 223, 366–372. [Google Scholar] [CrossRef]
- Ufodike, C.O.; Eze, V.O.; Ahmed, M.F.; Oluwalowo, A.; Park, J.G.; Liang, Z.; Wang, H. Investigation of molecular and supramolecular assemblies of cellulose and lignin of lignocellulosic materials by spectroscopy and thermal analysis. Int. J. Biol. Macromol. 2020, 146, 916–921. [Google Scholar] [CrossRef] [PubMed]
- Derkacheva, O.; Sukhov, D. Investigation of Lignins by FTIR Spectroscopy. Macromol. Symp. 2008, 265, 61–68. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magalhães, S.; Filipe, A.; Melro, E.; Fernandes, C.; Vitorino, C.; Alves, L.; Romano, A.; Rasteiro, M.G.; Medronho, B. Lignin Extraction from Waste Pine Sawdust Using a Biomass Derived Binary Solvent System. Polymers 2021, 13, 1090. https://doi.org/10.3390/polym13071090
Magalhães S, Filipe A, Melro E, Fernandes C, Vitorino C, Alves L, Romano A, Rasteiro MG, Medronho B. Lignin Extraction from Waste Pine Sawdust Using a Biomass Derived Binary Solvent System. Polymers. 2021; 13(7):1090. https://doi.org/10.3390/polym13071090
Chicago/Turabian StyleMagalhães, Solange, Alexandra Filipe, Elodie Melro, Catarina Fernandes, Carla Vitorino, Luís Alves, Anabela Romano, Maria G. Rasteiro, and Bruno Medronho. 2021. "Lignin Extraction from Waste Pine Sawdust Using a Biomass Derived Binary Solvent System" Polymers 13, no. 7: 1090. https://doi.org/10.3390/polym13071090
APA StyleMagalhães, S., Filipe, A., Melro, E., Fernandes, C., Vitorino, C., Alves, L., Romano, A., Rasteiro, M. G., & Medronho, B. (2021). Lignin Extraction from Waste Pine Sawdust Using a Biomass Derived Binary Solvent System. Polymers, 13(7), 1090. https://doi.org/10.3390/polym13071090











