Preparation, Characterization and Gelation of a Fungal Nano Chitin Derived from Hericium erinaceus Residue
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of Nano Chitin
2.3. Preparation of Nano Chitin Gels
2.4. Characterization Methods
3. Results and Discussion
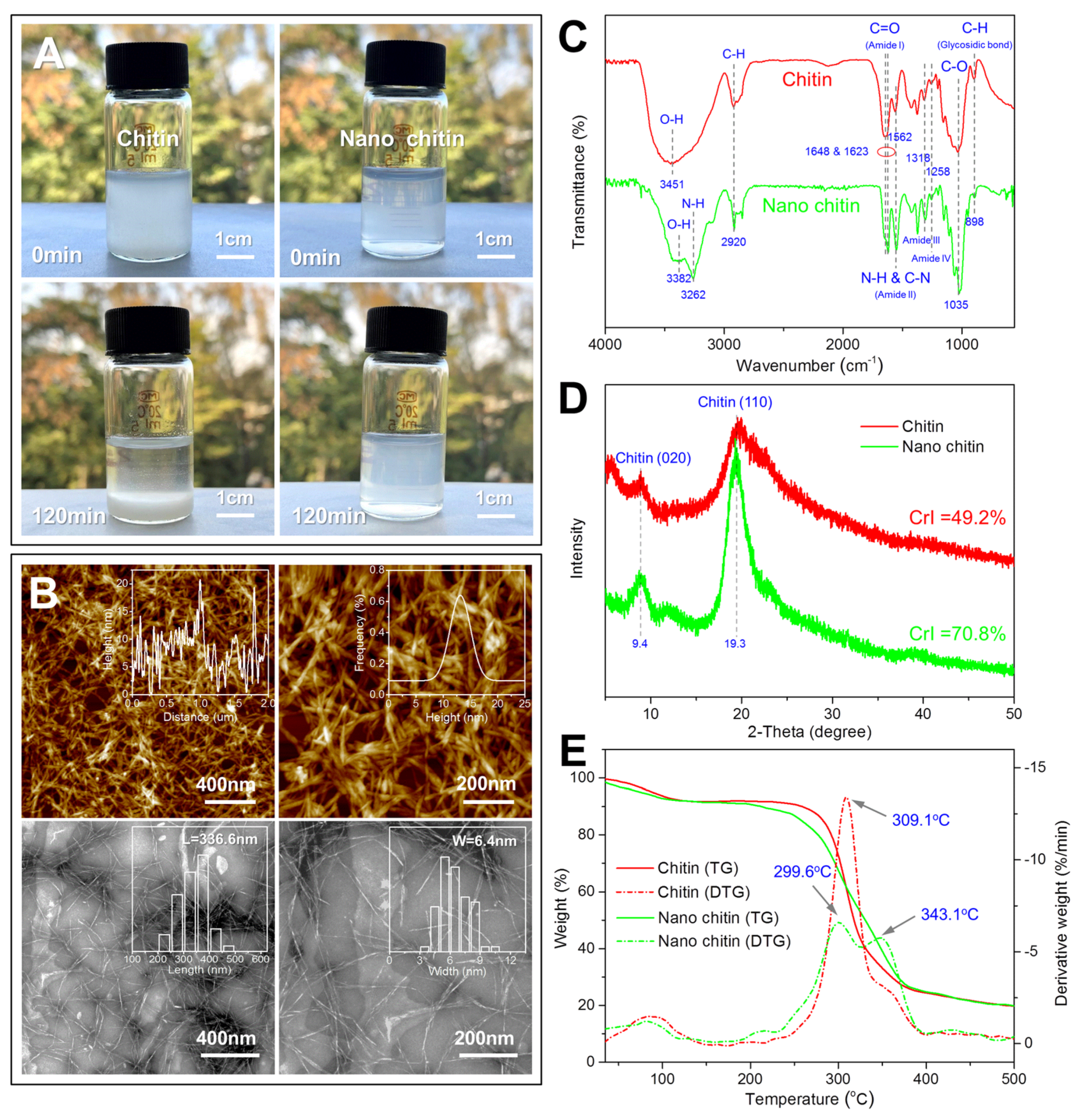
3.1. Characterization of Nano Chitin
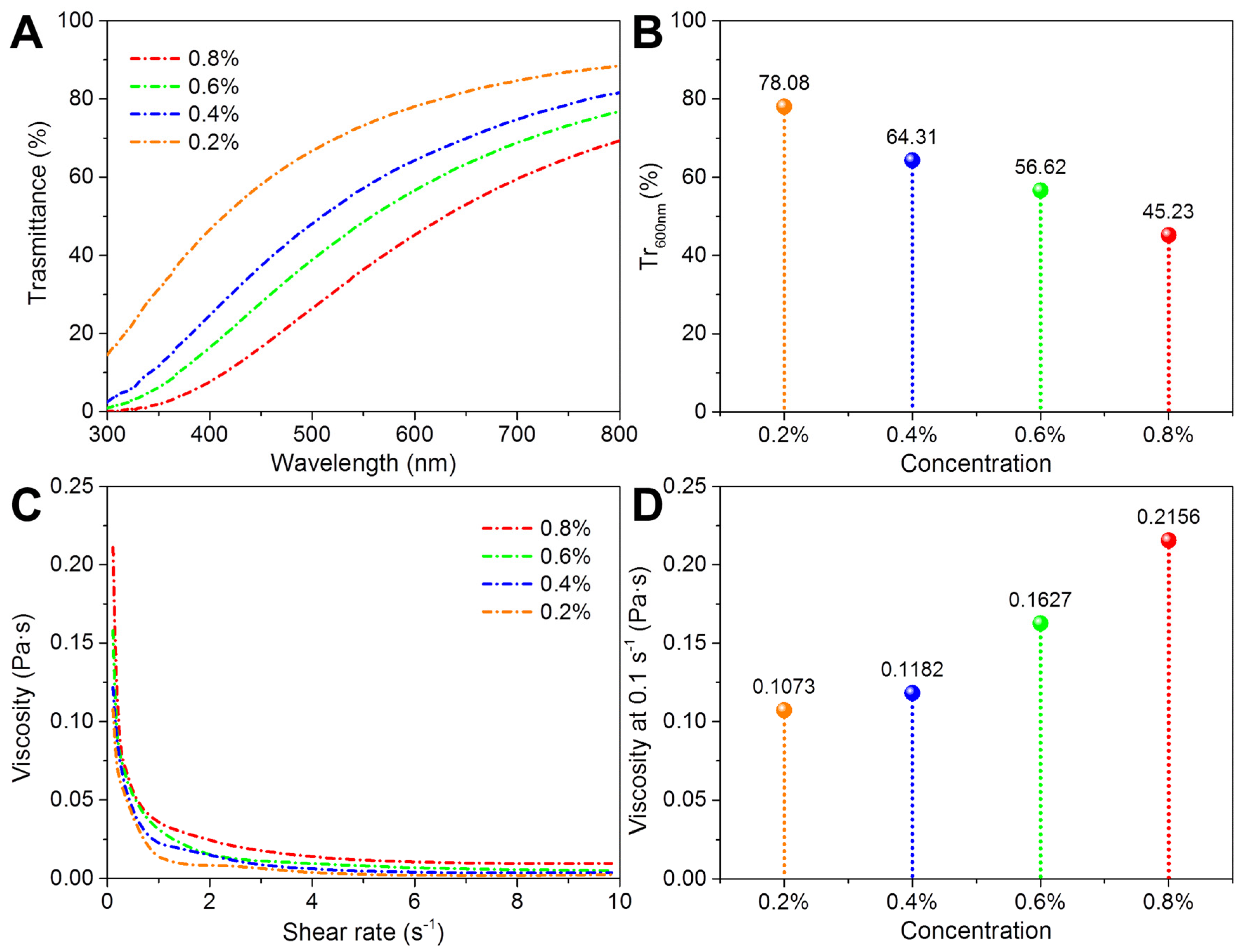
3.2. Dispersion Stability of Nano Chitin
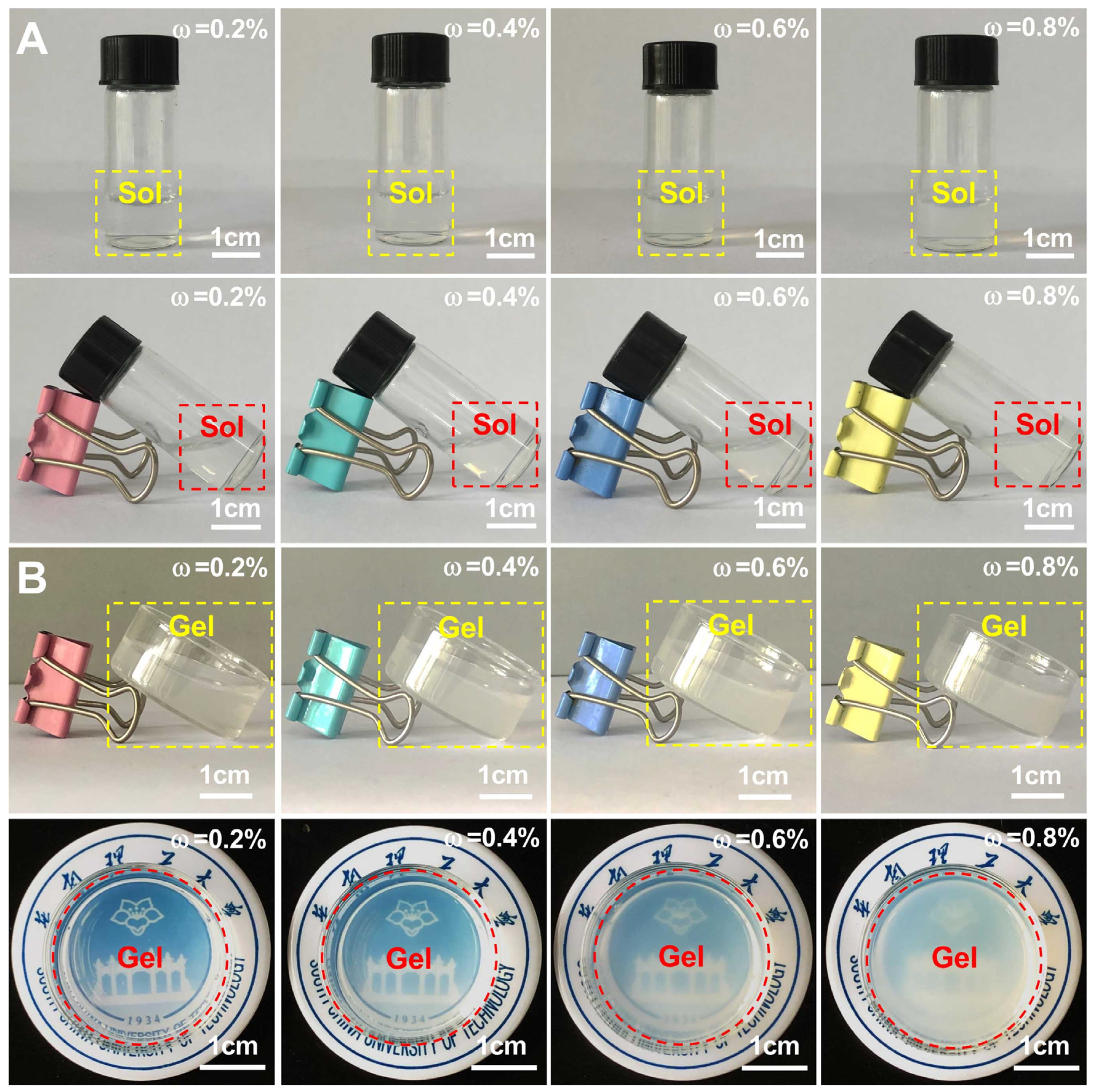
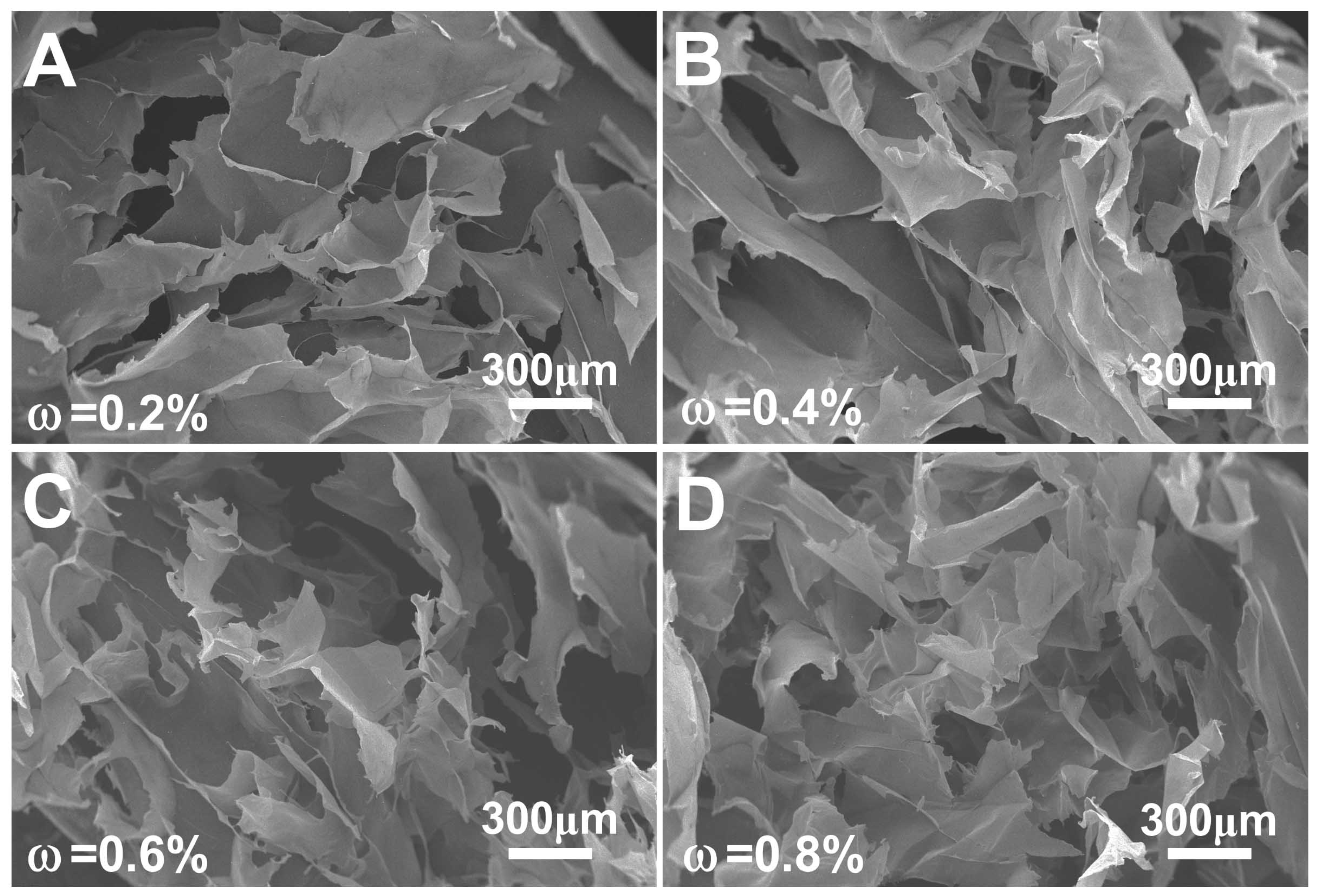
3.3. Gelation of Nano Chitin
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hebeish, A.; Farag, S.; Sharaf, S.; Shaheen, T.I. Thermal responsive hydrogels based on semi interpenetrating network of poly (NIPAm) and cellulose nanowhiskers. Carbohydr. Polym. 2014, 102, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yang, H.; Jiang, X.; Yang, B.; Zhu, K.; Lai, N.C.H.; Huang, C.; Chang, C.; Bian, L.; Zhang, L. Injectable chitin hydrogels with self-healing property and biodegradability as stem cell carriers. Carbohydr. Polym. 2021, 256, 117574. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.M.; Pekdemir, S.; Bilican, I.; Koc-Bilican, B.; Cakmak, B.; Ali, A.; Zang, L.S.; Onses, M.; Kaya, M. Production of natural chitin film from pupal shell of moth: Fabrication of plasmonic surfaces for SERS-based sensing applications. Carbohydr. Polym. 2021, 262, 117909. [Google Scholar] [CrossRef]
- Ybañez, M.G.; Camacho, D.H. Designing hydrophobic bacterial cellulose film composites assisted by sound waves. RSC Adv. 2021, 11, 32873–32883. [Google Scholar] [CrossRef]
- Emam, H.E.; Ahmed, H.B.; Abdelhameed, R.M. Melt intercalation technique for synthesis of hetero-metallic@ chitin bio-composite as recyclable catalyst for prothiofos hydrolysis. Carbohydr. Polym. 2021, 266, 118163. [Google Scholar] [CrossRef] [PubMed]
- Sugashini, S.; Gomathi, T.; Devi, R.A.; Sudha, P.N.; Rambabu, K.; Banat, F. Nanochitosan/carboxymethyl cellulose/TiO2 biocomposite for visible-light-induced photocatalytic degradation of crystal violet dye. Environ. Res. 2022, 204, 112047. [Google Scholar] [CrossRef]
- El-Naggar, M.E.; Radwan, E.K.; El-Wakeel, S.T.; Kafafy, H.; Gad-Allah, T.A.; El-Kalliny, A.S.; Shaheen, T.I. Synthesis, characterization and adsorption properties of microcrystalline cellulose based nanogel for dyes and heavy metals removal. Int. J. Biol. Macromol. 2018, 113, 248–258. [Google Scholar] [CrossRef]
- Liao, J.; Huang, H. Magnetic chitin hydrogels prepared from Hericium erinaceus residues with tunable characteristics: A novel biosorbent for Cu2+ removal. Carbohydr. Polym. 2019, 220, 191–201. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, K.; Girouard, N.; Meredith, J.C. Facile route to produce chitin nanofibers as precursors for flexible and transparent gas barrier materials. Biomacromolecules 2014, 15, 4614–4620. [Google Scholar] [CrossRef]
- Sahraee, S.; Milani, J.M.; Ghanbarzadeh, B.; Hamishehkar, H. Effect of corn oil on physical, thermal, and antifungal properties of gelatin-based nanocomposite films containing nano chitin. LWT-Food Sci. Technol. 2017, 76, 33–39. [Google Scholar] [CrossRef]
- Salaberria, A.M.; Diaz, R.H.; Labidi, J.; Fernandes, S.C. Role of chitin nanocrystals and nanofibers on physical, mechanical and functional properties in thermoplastic starch films. Food Hydrocoll. 2015, 46, 93–102. [Google Scholar] [CrossRef]
- Tang, H.; Wu, J.; Li, D.; Shi, C.; Chen, G.; He, M.; Tian, J. High-strength paper enhanced by chitin nanowhiskers and its potential bioassay applications. Int. J. Biol. Macromol. 2020, 150, 885–893. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Chen, S.; Chen, D. Preparation and characterization of chitosan based injectable hydrogels enhanced by chitin nano-whiskers. J. Mech. Behav. Biomed. Mater. 2017, 65, 466–477. [Google Scholar] [CrossRef] [PubMed]
- Chantarasataporn, P.; Yoksan, R.; Visessanguan, W.; Chirachanchai, S. Water-based nano-sized chitin and chitosan as seafood additive through a case study of Pacific white shrimp (Litopenaeus vannamei). Food Hydrocoll. 2013, 32, 341–348. [Google Scholar] [CrossRef]
- Lv, S.; Zhou, H.; Bai, L.; Rojas, O.J.; McClements, D.J. Development of food-grade Pickering emulsions stabilized by a mixture of cellulose nanofibrils and nanochitin. Food Hydrocoll. 2021, 113, 106451. [Google Scholar] [CrossRef]
- Zhou, H.; Tan, Y.; Lv, S.; Liu, J.; Mundo, J.L.M.; Bai, L.; Rojas, O.; McClements, D.J. Nanochitin-stabilized pickering emulsions: Influence of nanochitin on lipid digestibility and vitamin bioaccessibility. Food Hydrocoll. 2020, 106, 105878. [Google Scholar] [CrossRef]
- Gopi, S.; Pius, A.; Thomas, S. Enhanced adsorption of crystal violet by synthesized and characterized chitin nano whiskers from shrimp shell. J. Water Process Eng. 2016, 14, 1–8. [Google Scholar] [CrossRef]
- Morganti, P.; Chen, H.D.; Gao, X.H. Chitin Nanofibril: A Natural Eco-Friendly and immunoadjuvant active carrier for medical use. J. Appl. Cosmetol. 2016, 34, 141–154. [Google Scholar]
- Morganti, P.; Morganti, G.; Coltelli, M.B. Chitin Nanomaterials and Nanocomposites for Tissue Repair. In Marine-Derived Biomaterials for Tissue Engineering Applications; Springer: Singapore, 2019; pp. 523–544. [Google Scholar]
- Fan, Y.; Saito, T.; Isogai, A. Individual chitin nano-whiskers prepared from partially deacetylated α-chitin by fibril surface cationization. Carbohydr. Polym. 2010, 79, 1046–1051. [Google Scholar] [CrossRef]
- Ifuku, S.; Nogi, M.; Abe, K.; Yoshioka, M.; Morimoto, M.; Saimoto, H.; Yano, H. Simple preparation method of chitin nanofibers with a uniform width of 10–20 nm from prawn shell under neutral conditions. Carbohydr. Polym. 2011, 84, 762–764. [Google Scholar] [CrossRef]
- Kose, R.; Kondo, T. Favorable 3D-network formation of chitin nanofibers dispersed in water prepared using aqueous counter collision. Sen’i Gakkaishi 2011, 67, 91–95. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.; Ye, W.; Yu, J.; Fan, Y.; Ono, Y.; Saito, T.; Isogai, A. Chitin nanocrystals prepared by oxidation of α-chitin using the O2/laccase/TEMPO system. Carbohydr. Polym. 2018, 189, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Revol, J.F.; Marchessault, R.H. In vitro chiral nematic ordering of chitin crystallites. Int. J. Biol. Macromol. 1993, 15, 329–335. [Google Scholar] [CrossRef]
- Di, M.F.; Rapanà, P.; Tomati, U.; Galli, E. Chitin and chitosan from Basidiomycetes. Int. J. Biol. Macromol. 2008, 43, 8–12. [Google Scholar]
- Chien, R.C.; Yen, M.T.; Mau, J.L. Antimicrobial and antitumor activities of chitosan from shiitake stipes, compared to commercial chitosan from crab shells. Carbohydr. Polym. 2016, 138, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Huang, H. Extraction of a novel fungal chitin from Hericium erinaceus residue using multistep mild procedures. Int. J. Biol. Macromol. 2020, 156, 1279–1286. [Google Scholar] [CrossRef]
- Wu, T.; Zivanovic, S.; Draughon, F.A.; Sams, C.E. Chitin and chitosan value-added products from mushroom waste. J. Agric. Food Chem. 2004, 52, 7905–7910. [Google Scholar] [CrossRef]
- Yen, M.T.; Mau, J.L. Selected physical properties of chitin prepared from shiitake stipes. LWT-Food Sci. Technol. 2007, 40, 558–563. [Google Scholar] [CrossRef]
- He, X.; Wang, X.; Fang, J.; Chang, Y.; Ning, N.; Guo, H.; Huang, L.; Huang, X.; Zhao, Z. Structures, biological activities, and industrial applications of the polysaccharides from Hericium erinaceus (Lion’s Mane) mushroom: A review. Int. J. Biol. Macromol. 2017, 97, 228–237. [Google Scholar] [CrossRef]
- Min, B.M.; Lee, S.W.; Lim, J.N.; You, Y.; Lee, T.S.; Kang, P.H.; Park, W.H. Chitin and chitosan nanofibers: Electrospinning of chitin and deacetylation of chitin nanofibers. Polymer 2004, 45, 7137–7142. [Google Scholar] [CrossRef]
- Lu, Y.; Sun, Q.; She, X.; Xia, Y.; Liu, Y.; Li, J.; Yang, D. Fabrication and characterisation of α-chitin nanofibers and highly transparent chitin films by pulsed ultrasonication. Carbohydr. Polym. 2013, 98, 1497–1504. [Google Scholar] [CrossRef] [PubMed]
- Salaberria, A.M.; Fernandes, S.C.; Diaz, R.H.; Labidi, J. Processing of α-chitin nanofibers by dynamic high pressure homogenization: Characterization and antifungal activity against A. niger. Carbohydr. Polym. 2015, 116, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Saito, T.; Isogai, A. Chitin nanocrystals prepared by TEMPO-mediated oxidation of α-chitin. Biomacromolecules 2008, 9, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Saito, T.; Isogai, A. TEMPO-mediated oxidation of β-chitin to prepare individual nanofibrils. Carbohydr. Polym. 2009, 77, 832–838. [Google Scholar] [CrossRef]
- Ye, W.; Hu, Y.; Ma, H.; Liu, L.; Yu, J.; Fan, Y. Comparison of cast films and hydrogels based on chitin nanofibers prepared using TEMPO/NaBr/NaClO and TEMPO/NaClO/NaClO2 systems. Carbohydr. Polym. 2020, 237, 116125. [Google Scholar] [CrossRef]
- Abe, K.; Ifuku, S.; Kawata, M.; Yano, H. Preparation of tough hydrogels based on β-chitin nanofibers via NaOH treatment. Cellulose 2014, 21, 535–540. [Google Scholar] [CrossRef]
- Liu, L.; Wang, R.; Yu, J.; Jiang, J.; Zheng, K.; Hu, L.; Wang, Z.; Fan, Y. Robust self-standing chitin nanofiber/nanowhisker hydrogels with designed surface charges and ultralow mass content via gas phase coagulation. Biomacromolecules 2016, 17, 3773–3781. [Google Scholar] [CrossRef]
- Paulino, A.T.; Simionato, J.I.; Garcia, J.C.; Nozaki, J. Characterization of chitosan and chitin produced from silkworm crysalides. Carbohydr. Polym. 2006, 64, 98–103. [Google Scholar] [CrossRef]
- Sun, C.; Fu, D.; Jin, L.; Chen, M.; Zheng, X.; Yu, T. Chitin isolated from yeast cell wall induces the resistance of tomato fruit to Botrytis cinerea. Carbohydr. Polym. 2018, 199, 341–352. [Google Scholar] [CrossRef]
- Lavall, R.L.; Assis, O.B.; Campana-Filho, S.P. β-Chitin from the pens of Loligo sp.: Extraction and characterization. Bioresour. Technol. 2007, 98, 2465–2472. [Google Scholar] [CrossRef]
- Sugimoto, M.; Morimoto, M.; Sashiwa, H.; Saimoto, H.; Shigemasa, Y. Preparation and characterization of water-soluble chitin and chitosan derivatives. Carbohydr. Polym. 1998, 36, 49–59. [Google Scholar] [CrossRef]
- Cárdenas, G.; Cabrera, G.; Taboada, E.; Miranda, S.P. Chitin characterization by SEM, FTIR, XRD, and 13C cross polarization/mass angle spinning NMR. J. Appl. Polym. Sci. 2004, 93, 1876–1885. [Google Scholar] [CrossRef]
- Farinha, I.; Duarte, P.; Pimentel, A.; Plotnikova, E.; Chagas, B.; Mafra, L.; Grandfils, C.; Freitas, F.; Fortunato, E.; Reis, M.A. Chitin–glucan complex production by Komagataella pastoris: Downstream optimization and product characterization. Carbohydr. Polym. 2015, 130, 455–464. [Google Scholar] [CrossRef]
- Sajomsang, W.; Gonil, P. Preparation and characterization of α-chitin from cicada sloughs. Mater. Sci. Eng. C 2010, 30, 357–363. [Google Scholar] [CrossRef]
- Liu, S.; Sun, J.; Yu, L.; Zhang, C.; Bi, J.; Zhu, F.; Qu, M.; Jiang, C.; Yang, Q. Extraction and characterization of chitin from the beetle Holotrichia parallela Motschulsky. Molecules 2012, 17, 4604–4611. [Google Scholar] [CrossRef] [PubMed]
- Wijesena, R.N.; Tissera, N.D.; Rathnayaka, V.W.S.G.; de Silva, R.M.; de Silva, K.N. Colloidal stability of chitin nanofibers in aqueous systems: Effect of pH, ionic strength, temperature & concentration. Carbohydr. Polym. 2020, 235, 116024. [Google Scholar] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, J.; Huang, H. Preparation, Characterization and Gelation of a Fungal Nano Chitin Derived from Hericium erinaceus Residue. Polymers 2022, 14, 474. https://doi.org/10.3390/polym14030474
Liao J, Huang H. Preparation, Characterization and Gelation of a Fungal Nano Chitin Derived from Hericium erinaceus Residue. Polymers. 2022; 14(3):474. https://doi.org/10.3390/polym14030474
Chicago/Turabian StyleLiao, Jing, and Huihua Huang. 2022. "Preparation, Characterization and Gelation of a Fungal Nano Chitin Derived from Hericium erinaceus Residue" Polymers 14, no. 3: 474. https://doi.org/10.3390/polym14030474
APA StyleLiao, J., & Huang, H. (2022). Preparation, Characterization and Gelation of a Fungal Nano Chitin Derived from Hericium erinaceus Residue. Polymers, 14(3), 474. https://doi.org/10.3390/polym14030474





