Synthesis and Characterization of Novel Anion Exchange Membranes Based on Semi-Interpenetrating Networks of Functionalized Polysulfone: Effect of Ionic Crosslinking
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Synthesis of Anion Exchange Membranes
2.2.1. Synthesis of Chloromethylated Polysulfones
2.2.2. Synthesis of Sulfonated Polysulfones
2.2.3. Functionalization of PSU with 1-Methylimidazole
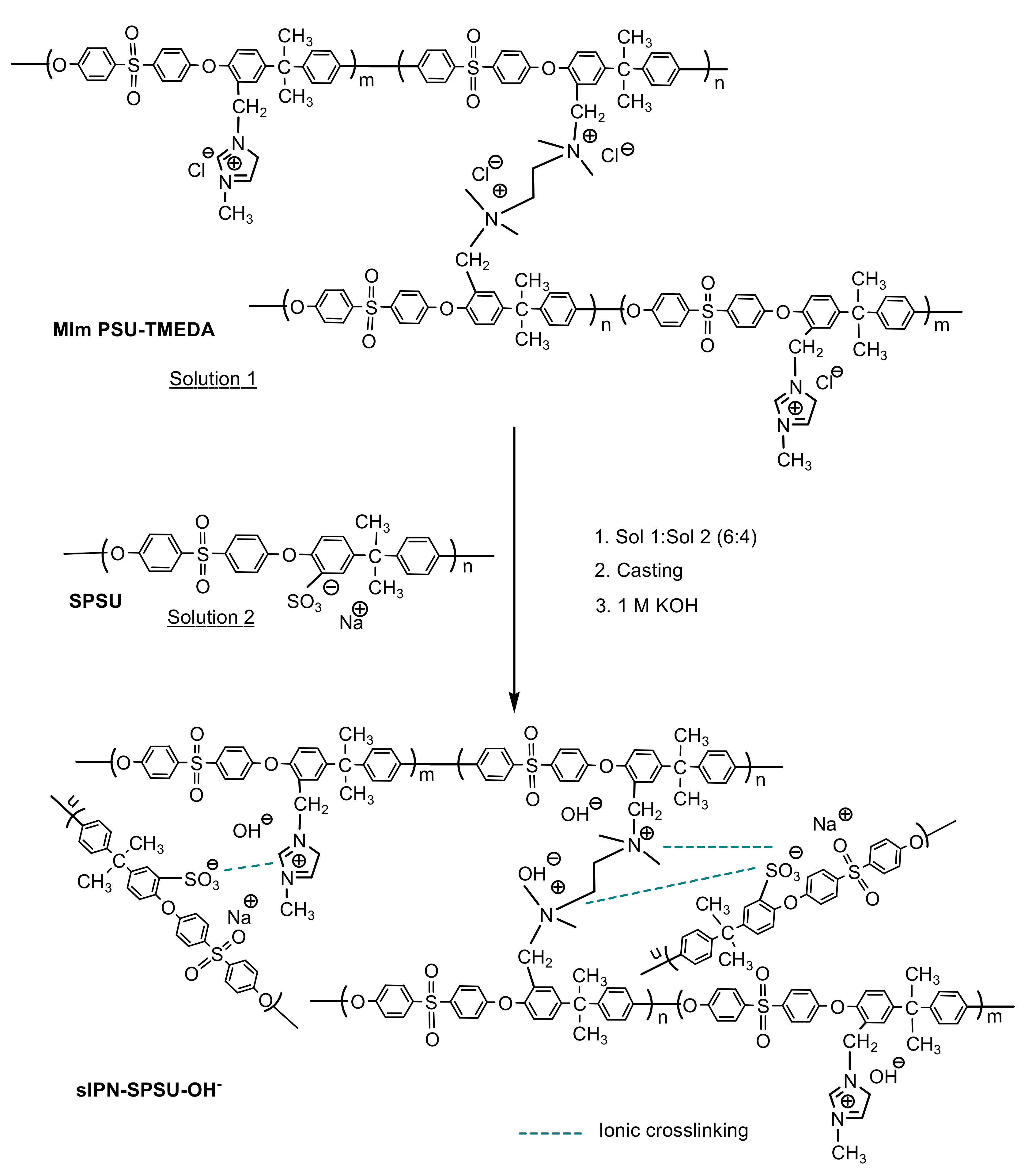
2.2.4. Preparation of Polymer Blends: Ionic Crosslinking
2.2.5. Preparation of Membranes
2.3. Measurements
2.3.1. 1H-NMR
2.3.2. Thermogravimetric Analysis (TGA)
2.3.3. Mechanical Properties
2.3.4. Water Uptake (WU%)
2.3.5. Ion-Exchange Capacity (IEC)
2.3.6. Ionic Conductivity
2.3.7. Alkaline Stability
3. Results and Discussion
3.1. Strategy
3.2. Structural Characterization
3.2.1. Synthesis of Methylimidazolium-Functionalized Polysulfone Crosslinked with TMEDA
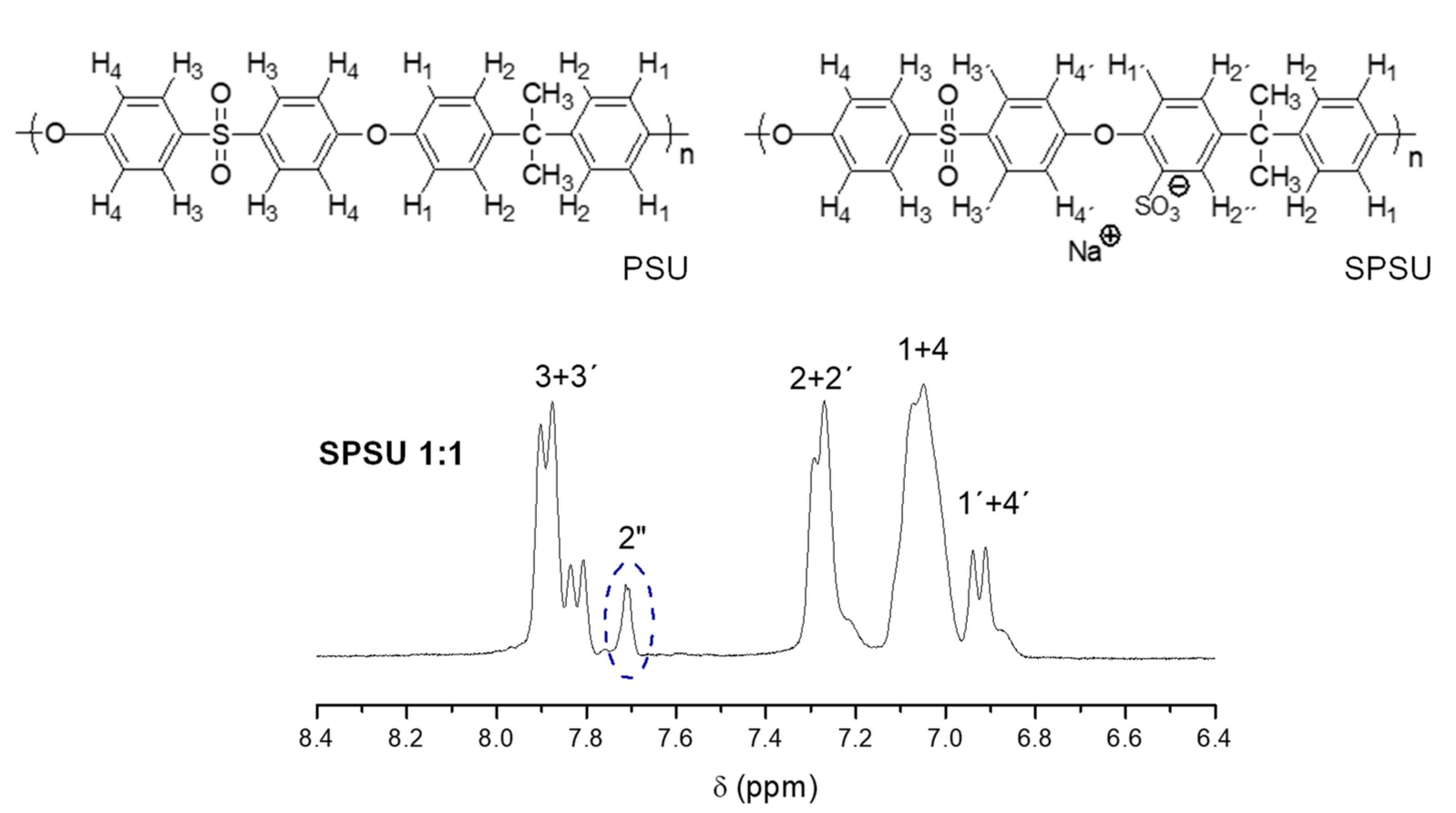
3.2.2. Sulfonation of PSU
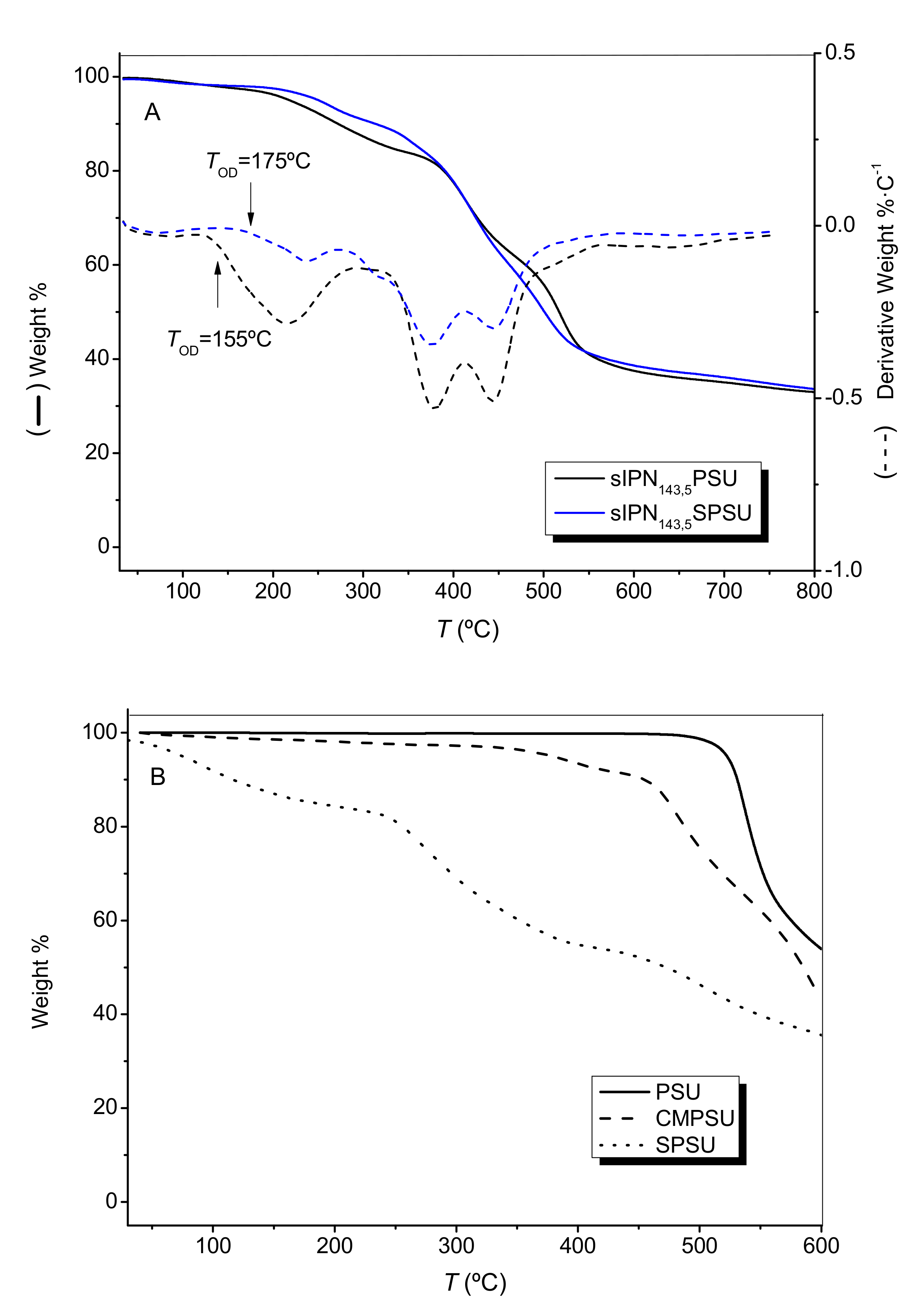
3.3. Thermogravimetric Analysis (TGA)
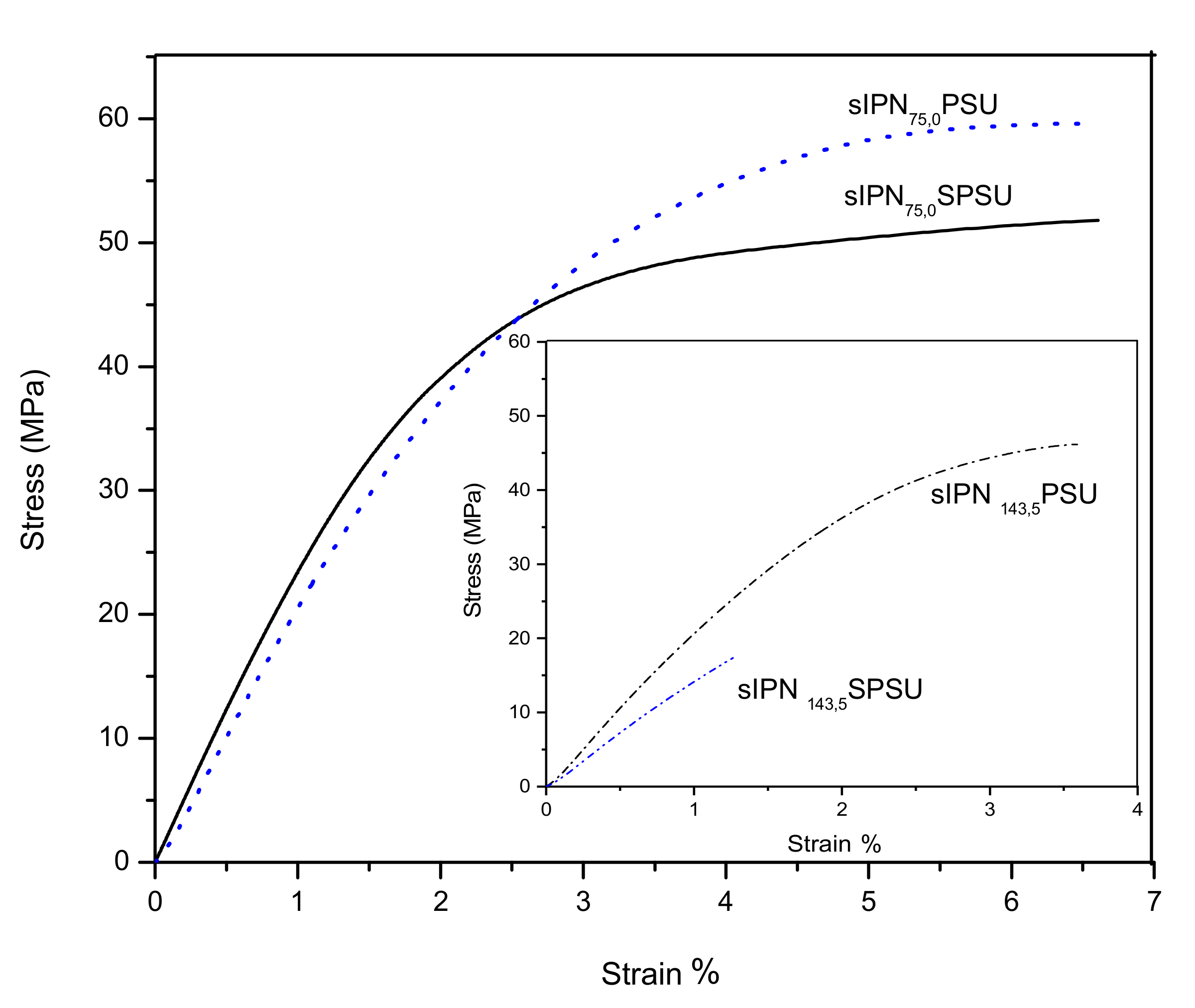
3.4. Mechanical Properties
3.5. Water Uptake
3.6. Ion Exchange Capacity
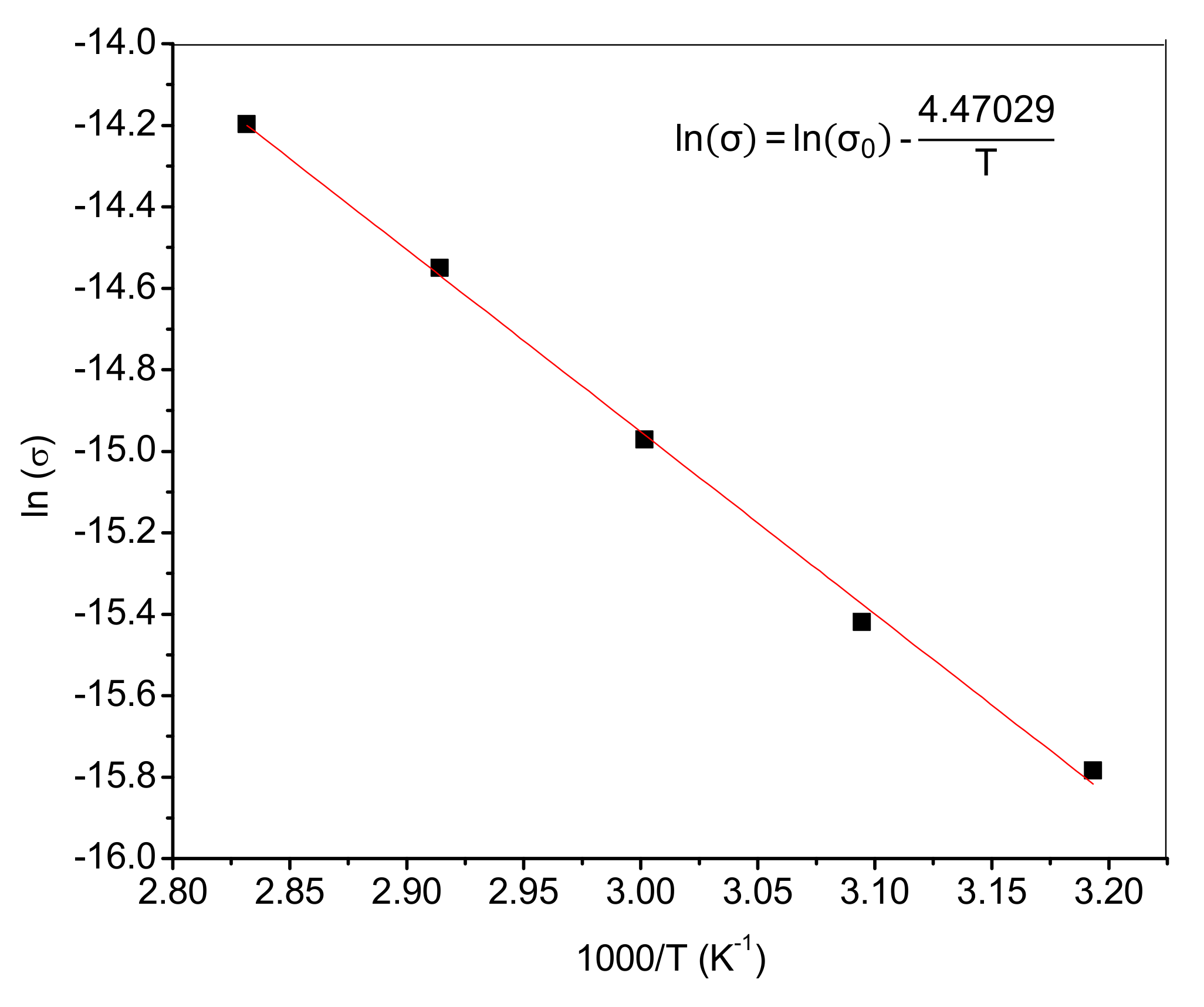
3.7. Ionic Conductivity
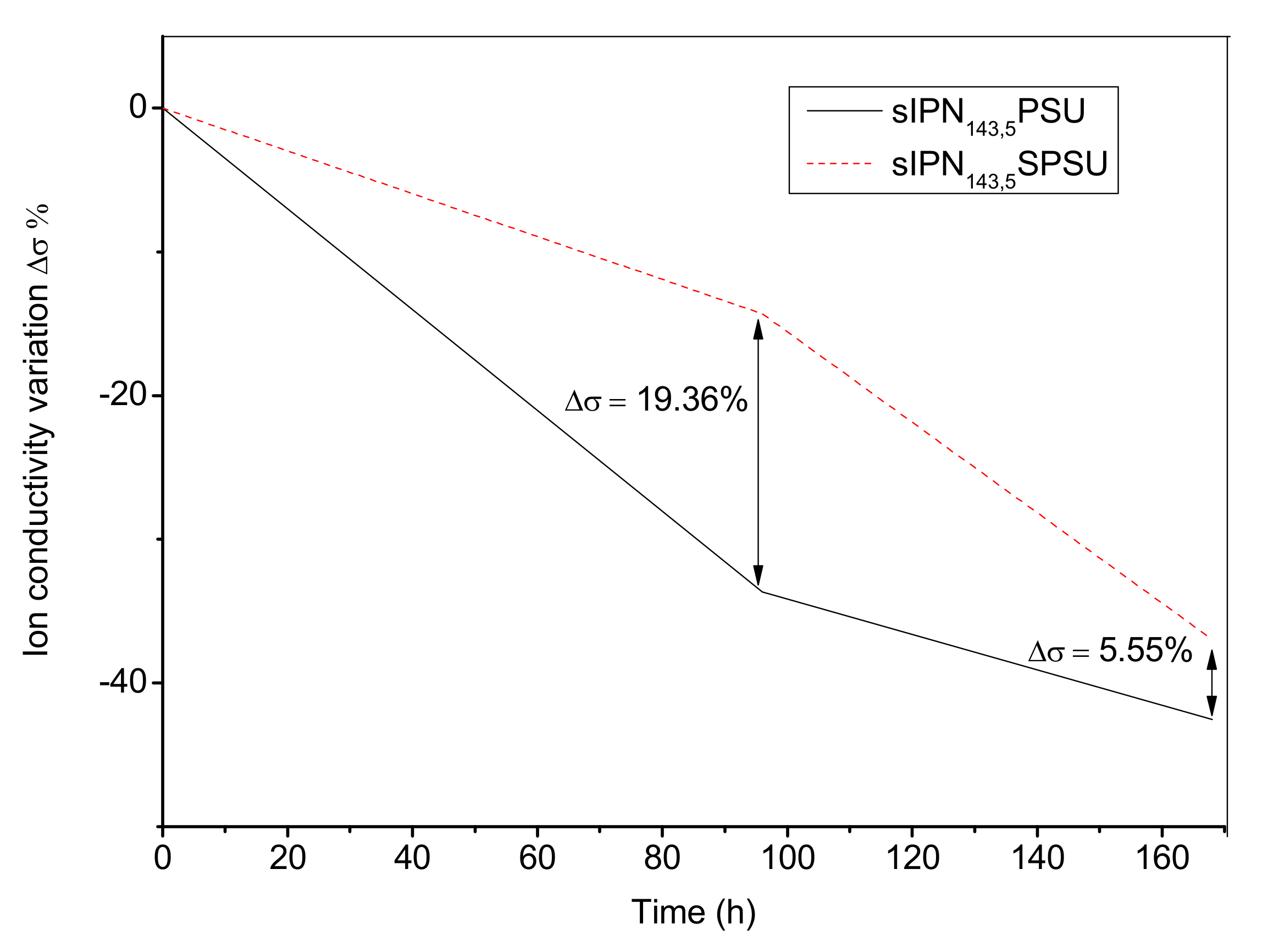
3.8. Alkaline Stability: Effect of Ionic Crosslinking
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PEMFC | Proton exchange membrane fuel cell |
| AEMFC | Anion exchange membrane fuel cell |
| AEM | Anion exchange membrane |
| PEM | Proton exchange membrane |
| DABCO | 1,4-diazabicyclo-[2,2,2]-octane |
| PSU | Polysulfone |
| TMEDA | N,N,N′,N′-tetramethylethylenediamine |
| IPN | Interpenetrating polymer network |
| PVA | Poly (vinyl alcohol) |
| PAADDA | Poly (acrylamide-co-diallyldimethylammonium chloride |
| sIPN | Semi-interpenetrating polymer network |
| BTMA | Benzyltrimethylammonium |
| PS | Poly (styrene) |
| PPO | Poly (2,6-dimethyl-1,4-phenyleneoxide) |
| PEG | Poly (ethylene glycol) |
| SPEEK | Sulfonated poly (ketone ether ether ketone) |
| TS | Tensile strength |
| PEEK | Poly (ketone ether ether ketone) |
| PAEK | Poly (aryl ether ketone) |
| MIm-PSU | 1-Methylimidazolium-functionalized polysulfone |
| DCE | 1,2-dichloroethane |
| TMSCS | Trimethylsilyl chlorosulfonate |
| DMF-d7 | N,N-Dimethylformamide-d7 |
| DMSO-d6 | Dimethyl sulfoxide-d6 |
| NMP | 1-methyl-2-pyrrolidone |
| CMPSU | Chloromethylated polysulfone |
| DC | Degree of chloromethylation |
| SPSU | Sulfonated polysulfone |
| MIm | Methylimidazole |
| DCl | Degree of covalent crosslinking |
| 1H-NMR | Proton nuclear magnetic resonance spectroscopy |
| TMS | Tetramethylsilane |
| TGA | Thermogravimetric analysis |
| TOD | Onset decomposition temperature |
| TFD | Fastest decomposition temperature |
| WU% | Water uptake |
| IEC | Ion-exchange capacity |
| EIS | Electrochemical impedance spectroscopy |
| DS | Degree of sulfonation |
| TMA | Trimethyl ammonium |
| HFA | High frequency arc |
| LFA | Low frequency arc |
Appendix A
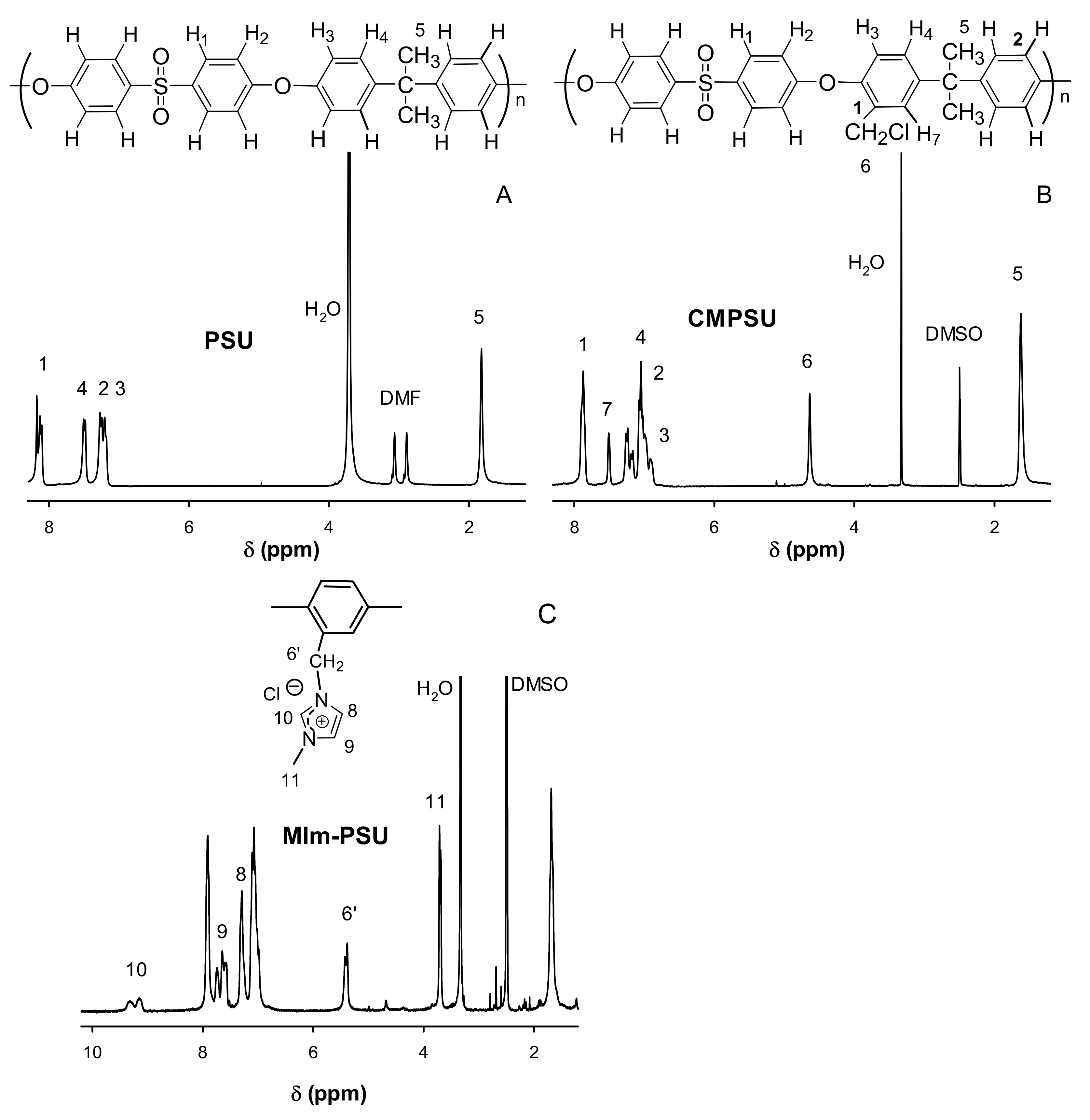
Appendix B
References
- Merle, G.; Wessling, M.; Nijmeijer, K. Anion exchange membranes for alkaline fuel cells: A review. J. Membr. Sci. 2011, 377, 1–35. [Google Scholar] [CrossRef]
- Couture, G.; Alaaeddine, A.; Boschet, F.; Ameduri, B. Polymeric materials as anion-exchange membranes for alkaline fuel cells. Prog. Polym. Sci. 2011, 36, 1521–1557. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, Z.; Tian, H.; Wang, S.; Zhang, B.; Cao, Y.; He, G.; Li, Z.; Wu, H. Preparing alkaline anion exchange membrane with enhanced hydroxide conductivity via blending imidazolium-functionalized and sulfonated poly(ether ether ketone). J. Power Sources 2015, 288, 384–392. [Google Scholar] [CrossRef]
- Díaz, M.; Ortiz, A.; Ortiz, I. Progress in the use of ionic liquids as electrolyte membranes in fuel cells. J. Membr. Sci. 2014, 469, 379–396. [Google Scholar] [CrossRef]
- Chen, H.; Wang, S.; Li, J.; Liu, F.; Tian, X.; Wang, X.; Mao, T.; Xu, J.; Wang, Z. Novel cross-linked membranes based on polybenzimidazole and polymeric ionic liquid with improved proton conductivity for HT-PEMFC applications. J. Taiwan Inst. Chem. Eng. 2019, 95, 185–194. [Google Scholar] [CrossRef]
- Liang, M.; Liu, Y.; Xiao, B.; Yang, S.; Wang, Z.; Han, H. An analytical model for the transverse permeability of gas diffusion layer with electrical double layer effects in proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2018, 43, 17880–17888. [Google Scholar] [CrossRef]
- Xiao, B.; Wang, W.; Zhang, X.; Long, G.; Chen, H.; Cai, H.; Deng, L. A novel fractal model for relative permeability of gas diffusion layer in proton exchange membrane fuel cell with capillary pressure effect. Fractals 2019, 27, 27. [Google Scholar] [CrossRef]
- Wang, K.; Wu, Q.; Yan, X.; Liu, J.; Gao, L.; Hu, L.; Zhang, N.; Pan, Y.; Zheng, W.; He, G. Branched poly(ether ether ketone) based anion exchange membrane for H2/O2 fuel cell. Int. J. Hydrogen Energy 2019, 44, 23750–23761. [Google Scholar] [CrossRef]
- Dai, P.; Mo, Z.-H.; Xu, R.-W.; Zhang, S.; Wu, Y.-X. Cross-Linked Quaternized Poly(styrene-b-(ethylene-co-butylene)-b-styrene) for Anion Exchange Membrane: Synthesis, Characterization and Properties. ACS Appl. Mater. Interfaces 2016, 8, 20329–20341. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; An, L.; Zhao, T.; Tang, Z. Advances and challenges in alkaline anion exchange membrane fuel cells. Prog. Energy Combust. Sci. 2018, 66, 141–175. [Google Scholar] [CrossRef]
- Vijayakumar, V.; Nam, S.Y. Recent advancements in applications of alkaline anion exchange membranes for polymer electrolyte fuel cells. J. Ind. Eng. Chem. 2019, 70, 70–86. [Google Scholar] [CrossRef]
- Lu, S.; Pan, J.; Huang, A.; Zhuang, L.; Lu, J. Alkaline polymer electrolyte fuel cells completely free from noble metal catalysts. Proc. Natl. Acad. Sci. USA 2008, 105, 20611–20614. [Google Scholar] [CrossRef]
- Zhou, J.; Zuo, P.; Liu, Y.; Yang, Z.; Xu, T. Ion exchange membranes from poly(2,6-dimethyl-1,4-phenylene oxide) and related applications. Sci. China Ser. B Chem. 2018, 61, 1062–1087. [Google Scholar] [CrossRef]
- Cheng, J.; He, G.; Zhang, F. A mini-review on anion exchange membranes for fuel cell applications: Stability issue and addressing strategies. Int. J. Hydrogen Energy 2015, 40, 7348–7360. [Google Scholar] [CrossRef]
- Yokota, N.; Ono, H.; Miyake, J.; Nishino, E.; Asazawa, K.; Watanabe, M.; Miyatake, K. Anion Conductive Aromatic Block Copolymers Containing Diphenyl Ether or Sulfide Groups for Application to Alkaline Fuel Cells. ACS Appl. Mater. Interfaces 2014, 6, 17044–17052. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, H.; Qu, C. Influence of Solvent on Polymer Prequaternization toward Anion-Conductive Membrane Fabrication for All-Vanadium Flow Battery. J. Phys. Chem. B 2012, 116, 9016–9022. [Google Scholar] [CrossRef]
- Xue, J.; Liu, L.; Liao, J.; Shen, Y.; Li, N. Semi-interpenetrating polymer networks by azide–alkyne cycloaddition as novel anion exchange membranes. J. Mater. Chem. A 2018, 6, 11317–11326. [Google Scholar] [CrossRef]
- Pérez-Prior, M.T.; Ureña, N.; Tannenberg, M.; Del Río, C.; Levenfeld, B. DABCO-functionalized polysulfones as anion-exchange membranes for fuel cell applications: Effect of crosslinking. J. Polym. Sci. Part B Polym. Phys. 2017, 55, 1326–1336. [Google Scholar] [CrossRef]
- Park, J.-S.; Park, S.-H.; Yim, S.-D.; Yoon, Y.-G.; Lee, W.-Y.; Kim, C.-S. Performance of solid alkaline fuel cells employing anion-exchange membranes. J. Power Sources 2008, 178, 620–626. [Google Scholar] [CrossRef]
- Zhao, D.; Kim, J.F.; Ignacz, G.; Pogany, P.; Lee, Y.M.; Szekely, G. Bio-Inspired Robust Membranes Nanoengineered from Interpenetrating Polymer Networks of Polybenzimidazole/Polydopamine. ACS Nano 2019, 13, 125–133. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Z.; Yang, F.; Wang, J. Improving the permselectivity and antifouling performance of reverse osmosis membrane based on a semi-interpenetrating polymer network. Desalination 2021, 502, 114910. [Google Scholar] [CrossRef]
- Li, H.Q.; Liu, X.J.; Wang, H.; Yang, H.; Wang, Z.; He, J. Proton exchange membranes with cross-linked interpenetrating network of sulfonated polyvinyl alcohol and poly(2-acrylamido-2-methyl-1-propanesulfonic acid): Excellent relative selectivity. J. Membr. Sci. 2020, 595, 117511. [Google Scholar] [CrossRef]
- Qiao, J.; Fu, J.; Liu, L.; Liu, Y.; Sheng, J. Highly stable hydroxyl anion conducting membranes poly(vinyl alcohol)/poly(acrylamide-co-diallyldimethylammonium chloride) (PVA/PAADDA) for alkaline fuel cells: Effect of cross-linking. Int. J. Hydrogen Energy 2012, 37, 4580–4589. [Google Scholar] [CrossRef]
- Alemán, J.V.; Chadwick, A.V.; He, J.; Hess, M.; Horie, K.; Jones, R.G.; Kratochvíl, P.; Meisel, I.; Mita, I.; Moad, G.; et al. Definitions of terms relating to the structure and processing of sols, gels, networks, and inorganic-organic hybrid materials (IUPAC Recommendations 2007). Pure Appl. Chem. 2007, 79, 1801–1829. [Google Scholar] [CrossRef]
- He, S.S.; Strickler, A.L.; Frank, C.W. A Semi-Interpenetrating Network Approach for Dimensionally Stabilizing Highly-Charged Anion Exchange Membranes for Alkaline Fuel Cells. ChemSusChem 2015, 8, 1472–1483. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Zhu, L.; Han, J.; Hickner, M.A. Mechanically Tough and Chemically Stable Anion Exchange Membranes from Rigid-Flexible Semi-Interpenetrating Networks. Chem. Mater. 2015, 27, 6689–6698. [Google Scholar] [CrossRef]
- Tashvigh, A.A.; Luo, L.; Chung, T.-S.; Weber, M.; Maletzko, C. A novel ionically cross-linked sulfonated polyphenylsulfone (sPPSU) membrane for organic solvent nanofiltration (OSN). J. Membr. Sci. 2018, 545, 221–228. [Google Scholar] [CrossRef]
- Xu, Y.; Ye, N.; Zhang, D.; Yang, J.; He, R. Ionic crosslinking of imidazolium functionalized poly(aryl ether ketone) by sulfonated poly(ether ether ketone) for anion exchange membranes. J. Coll. Interface Sci. 2017, 497, 333–342. [Google Scholar] [CrossRef]
- Hande, V.R.; Rath, S.K.; Rao, S.; Praveen, S.; Sasane, S.; Patri, M. Effect of constrained amorphous region on properties of acid–base polyelectrolyte membranes based on sulphonated poly(ether ether ketone) and a nonconjugated diamine. J. Membr. Sci. 2016, 499, 1–11. [Google Scholar] [CrossRef]
- Pérez-Prior, M.T.; Varez, A.; Levenfeld, B. Synthesis and characterization of benzimidazolium-functionalized polysulfones as anion-exchange membranes. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 2363–2373. [Google Scholar] [CrossRef]
- Iojoiu, C.; Genova-Dimitrova, P.; Maréchal, M.; Sanchez, J.-Y. Chemical and physicochemical characterizations of ionomers. Electrochim. Acta 2006, 51, 4789–4801. [Google Scholar] [CrossRef]
- Ureña, N.; Pérez-Prior, M.T.; del Río, C.; Várez, A.; Levenfeld, B. New Amphiphilic Semi-Interpenetrating Networks Based on Polysulfone for Anion-Exchange Membrane Fuel Cells with Improved Alkaline and Mechanical Stabilities. Polymer 2021. Accepted to publish. [Google Scholar]
- Benavente, J.; García, J.M.; Riley, R.; Lozano, A.E.; de Abajo, J. Sulfonated Poly(Ether Ether Sulfones). J. Membr. Sci. 2000, 175. [Google Scholar] [CrossRef]
- Jenkins, A.D.; Kratochvíl, P.; Stepto, R.F.T.; Suter, U.W. Glossary of Basic Terms in Polymers Science. Pure Appl. Chem. 1996, 68, 2287–2311. [Google Scholar] [CrossRef]
- Atkins, P.; Jones, L. Principios de Química: Los Caminos Del Descubrimiento, 5th ed.; Bookman: Porto Alegre, Brazil, 2012. [Google Scholar]
- Pantamas, N.; Khonkeng, C.; Krachodnok, S.; Chaisena, A. Ecofriendly and Simplified Synthetic Route for Polysulfone-Based Solid-State Alkaline Electrolyte Membrane. Am. J. Appl. Sci. 2012, 9, 1577–1582. [Google Scholar]
- Martos, A.M.; Sanchez, J.-Y.; Várez, A.; Levenfeld, B. Electrochemical and Structural Characterization of Sulfonated Polysulfone. Polym. Test. 2015, 45, 185–193. [Google Scholar] [CrossRef]
- Li, T.; Yan, X.; Liu, J.; Wu, X.; Gong, X.; Zhen, D.; Sun, S.; Chen, W.; He, G. Friedel-Crafts Alkylation Route for Preparation of Pendent Side Chain Imidazolium-Functionalized Polysulfone Anion Exchange Membranes for Fuel Cells. J. Membr. Sci. 2019, 573, 157–166. [Google Scholar] [CrossRef]
- Lu, W.; Shao, Z.-G.; Zhang, G.; Zhao, Y.; Yi, B. Crosslinked Poly(Vinylbenzyl Chloride) with a Macromolecular Crosslinker for Anion Exchange Membrane Fuel Cells. J. Power Sources 2014, 248, 905–914. [Google Scholar] [CrossRef]
- Yang, J.; Li, Q.; Jensen, J.O.; Pan, C.; Cleemann, L.N.; Bjerrum, N.J.; He, R. Phosphoric Acid Doped Imidazolium Polysulfone Membranes for High Temperature Proton Exchange Membrane Fuel Cells. J. Power Sources 2012, 205, 114–121. [Google Scholar] [CrossRef]
- Gong, Y.; Liao, X.; Xu, J.; Chen, D.; Zhang, H. Novel Anion-Conducting Interpenetrating Polymer Network of Quaternized Polysulfone and Poly(Vinyl Alcohol) for Alkaline Fuel Cells. Int. J. Hydrogen Energy 2016, 41, 5816–5823. [Google Scholar] [CrossRef]
- Narducci, R.; Chailan, J.-F.; Fahs, A.; Pasquini, L.; di Vona, M.L.; Knauth, P. Mechanical Properties of Anion Exchange Membranes by Combination of Tensile Stress-Strain Tests and Dynamic Mechanical Analysis. J. Polym. Sci. Part B Polym. Phys. 2016, 54, 1180–1187. [Google Scholar] [CrossRef]
- Tuan, C.M.; Cong Tinh, V.D.; Kim, D. Anion Exchange Membranes Prepared from Quaternized Polyepichlorohydrin Cross-Linked with 1-(3-Aminopropyl)Imidazole Grafted Poly(Arylene Ether Ketone) for Enhancement of Toughness and Conductivity. Membranes 2020, 10, 138. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, K.H.; Chu, J.Y.; Kim, A.R.; Yoo, D.J. Enhanced Hydroxide Conductivity and Dimensional Stability with Blended Membranes Containing Hyperbranched PAES/Linear PPO as Anion Exchange Membranes. Polymers 2020, 12, 3011. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Lu, S.; Li, Y.; Huang, A.; Zhuang, L.; Lu, J. High-Performance Alkaline Polymer Electrolyte for Fuel Cell Applications. Adv. Funct. Mater. 2010, 20, 312–319. [Google Scholar] [CrossRef]
- Yan, X.; He, G.; Gu, S.; Wu, X.; Du, L.; Wang, Y. Imidazolium-Functionalized Polysulfone Hydroxide Exchange Membranes for Potential Applications in Alkaline Membrane Direct Alcohol Fuel Cells. Int. J. Hydrogen Energy 2012, 37, 5216–5224. [Google Scholar] [CrossRef]
- Serbanescu, O.S.; Voicu, S.I.; Thakur, V.K. Polysulfone Functionalized Membranes: Properties and Challenges. Mater. Today Chem. 2020, 17, 100302. [Google Scholar] [CrossRef]


| Membrane | TS (MPa) | ε % |
|---|---|---|
| sIPN143,5-PSU | 15 ± 4 | 1.8 ± 0.9 |
| sIPN143,5-SPSU | 12 ± 3 | 2.6 ± 0.4 |
| sIPN75,5-PSU | 49 ± 3 | 5.3 ± 0.6 |
| sIPN75,5-SPSU | 44 ± 2 | 3.5 ± 0.4 |
| sIPN75,0-PSU | 58 ± 2 | 7 ± 3 |
| sIPN75,0-SPSU | 52 ± 2 | 10 ± 2 |
| Membrane | WU% | σm (mS·cm−1) a |
|---|---|---|
| sIPN143,5-PSU | 22.0 | 1.29 × 10−2 |
| sIPN143,5-SPSU | 25.0 | 1.10 × 10−1 |
| sIPN143,0 SPSU | - | - |
| sIPN75,5-PSU | 8.8 | 1.24 × 10−3 |
| sIPN75,5-SPSU | 5.0 | 2.21 × 10−4 |
| sIPN75,0-PSU | 8.4 | 7.32 10−2 |
| sIPN75,0-SPSU | 11.0 | 1.15 × 10−4 |
| Membrane | Δσ % (96 h) | Δσ % (168 h) |
|---|---|---|
| sIPN143,5-PSU | −33.67 | −42.52 |
| sIPN143,5-SPSU | −14.31 | −36.97 |
| sIPN75,5-PSU | 0 | −11.97 |
| sIPN75,5-SPSU | 0 | −11.88 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Swaby, S.; Ureña, N.; Pérez-Prior, M.T.; Várez, A.; Levenfeld, B. Synthesis and Characterization of Novel Anion Exchange Membranes Based on Semi-Interpenetrating Networks of Functionalized Polysulfone: Effect of Ionic Crosslinking. Polymers 2021, 13, 958. https://doi.org/10.3390/polym13060958
Swaby S, Ureña N, Pérez-Prior MT, Várez A, Levenfeld B. Synthesis and Characterization of Novel Anion Exchange Membranes Based on Semi-Interpenetrating Networks of Functionalized Polysulfone: Effect of Ionic Crosslinking. Polymers. 2021; 13(6):958. https://doi.org/10.3390/polym13060958
Chicago/Turabian StyleSwaby, Sydonne, Nieves Ureña, María Teresa Pérez-Prior, Alejandro Várez, and Belén Levenfeld. 2021. "Synthesis and Characterization of Novel Anion Exchange Membranes Based on Semi-Interpenetrating Networks of Functionalized Polysulfone: Effect of Ionic Crosslinking" Polymers 13, no. 6: 958. https://doi.org/10.3390/polym13060958
APA StyleSwaby, S., Ureña, N., Pérez-Prior, M. T., Várez, A., & Levenfeld, B. (2021). Synthesis and Characterization of Novel Anion Exchange Membranes Based on Semi-Interpenetrating Networks of Functionalized Polysulfone: Effect of Ionic Crosslinking. Polymers, 13(6), 958. https://doi.org/10.3390/polym13060958








