Plumbagin-Loaded Glycerosome Gel as Topical Delivery System for Skin Cancer Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Design and Development of Nanosystem
Preparation of PLM-Loaded Nanosystems
2.3. Box–Behnken Design
2.4. Characterization of Nanosystem Dispersion
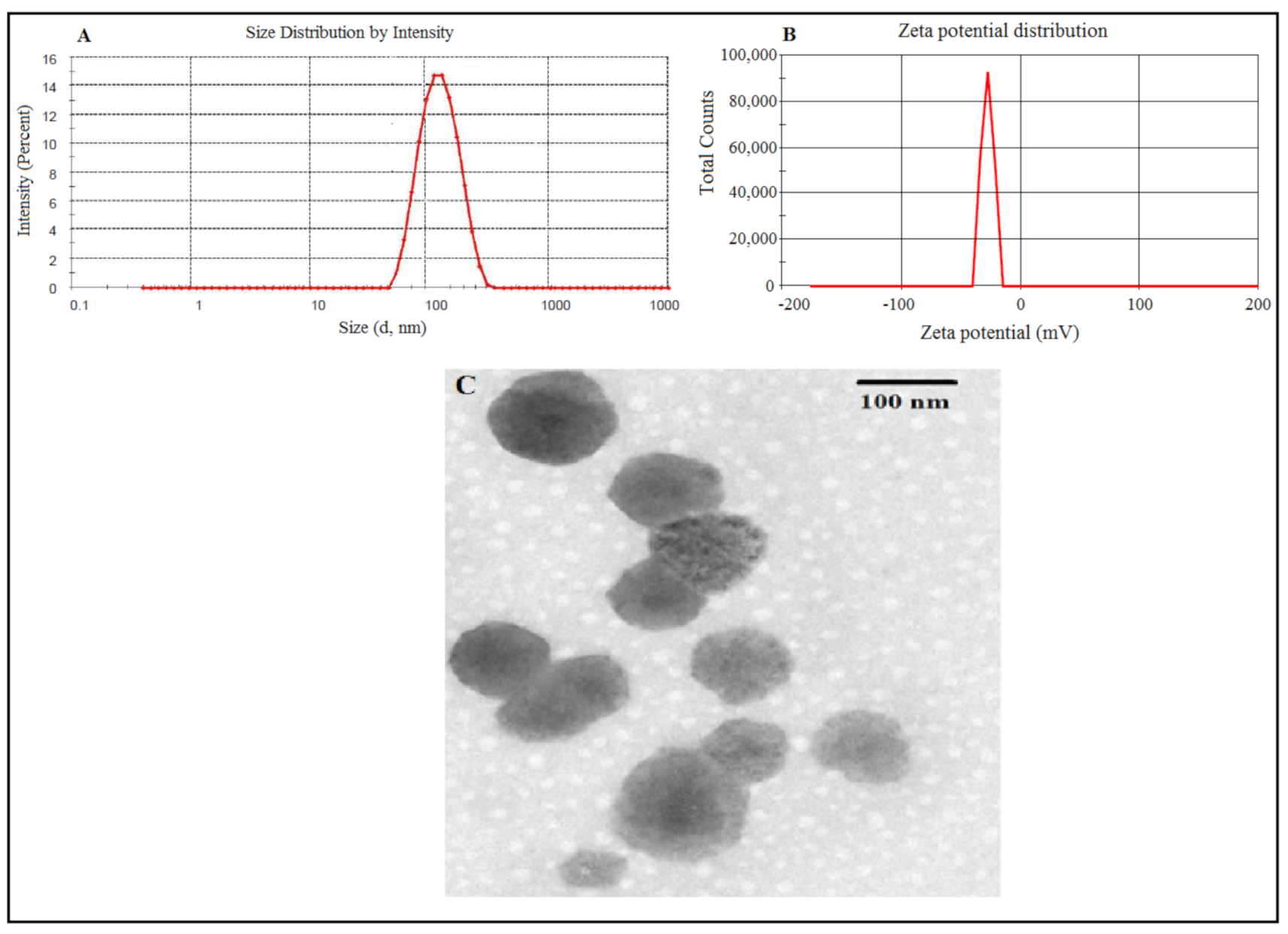
2.4.1. Particle size, Zeta Potential and Morphological Analysis
2.4.2. Entrapment Efficiency and Drug Loading
2.4.3. In Vitro Drug Release and Kinetic Studies
2.5. Encapsulation of the Nanosystem into a Preformed Gels
2.6. Skin Permeation Studies of GM-Loaded Gels
2.7. Drug Retention Study of GM-Loaded Gels
2.8. Confocal Microscopy of Rhodamine B-Loaded GM
2.9. Cytotoxicity Assessment
2.10. In Vitro Radical Scavenging Assay
2.11. Statistical Analysis
3. Results and Discussions
3.1. Optimization of PLM-Loaded GM by Statistical Design
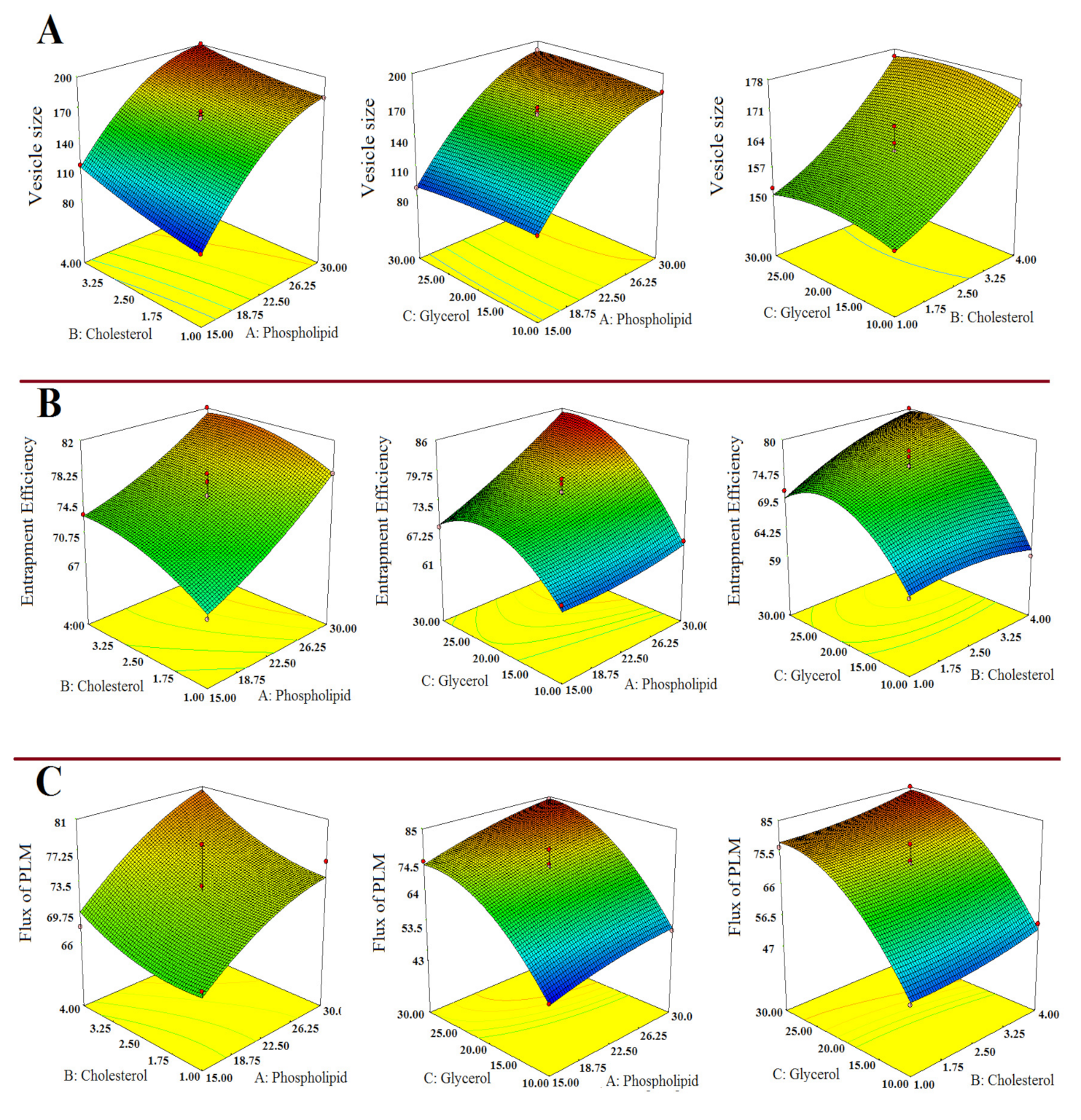
3.1.1. Impact of Independent Variables on Vesicle Size (Y1)
3.1.2. Impact of Independent Variables on % Entrapment Efficiency (Y2)
3.1.3. Impact of Independent Variables on Flux (Y3)
3.2. Characterization of Nanosystems Dispersion
3.2.1. Particle Size, Zeta Potential, Morphology, Entrapment and Drug Loading Efficiency
3.2.2. In Vitro Drug Release and Kinetic Study
3.2.3. Characterization of Gels
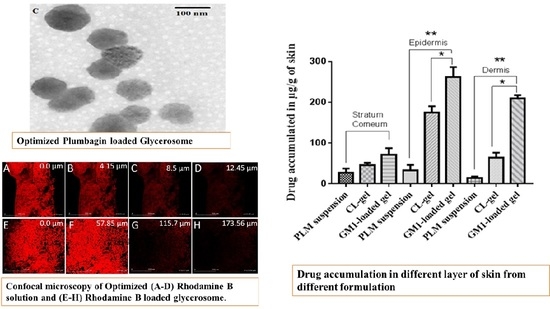
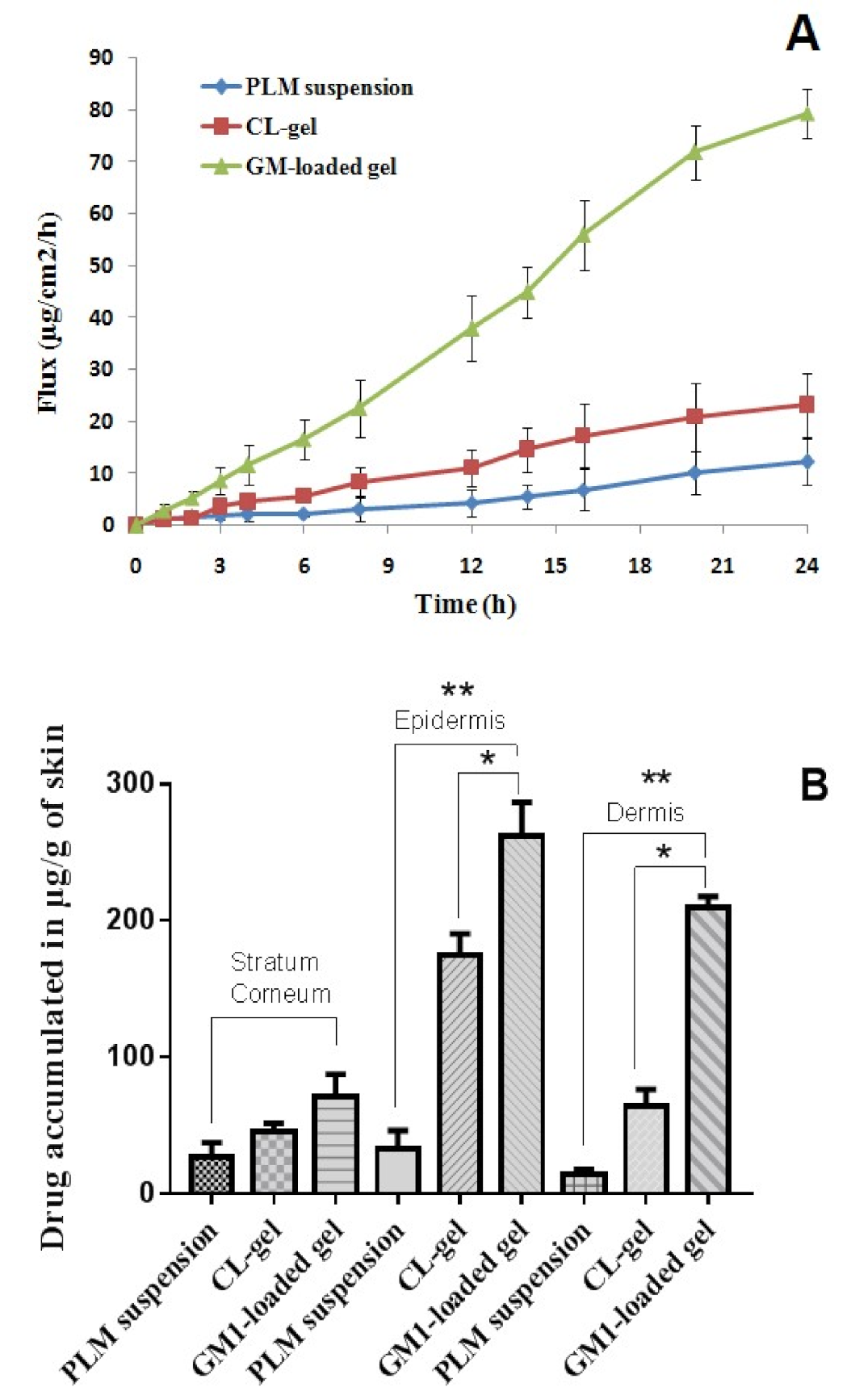
3.2.4. Skin Permeation Study
3.2.5. Drug Retention Study
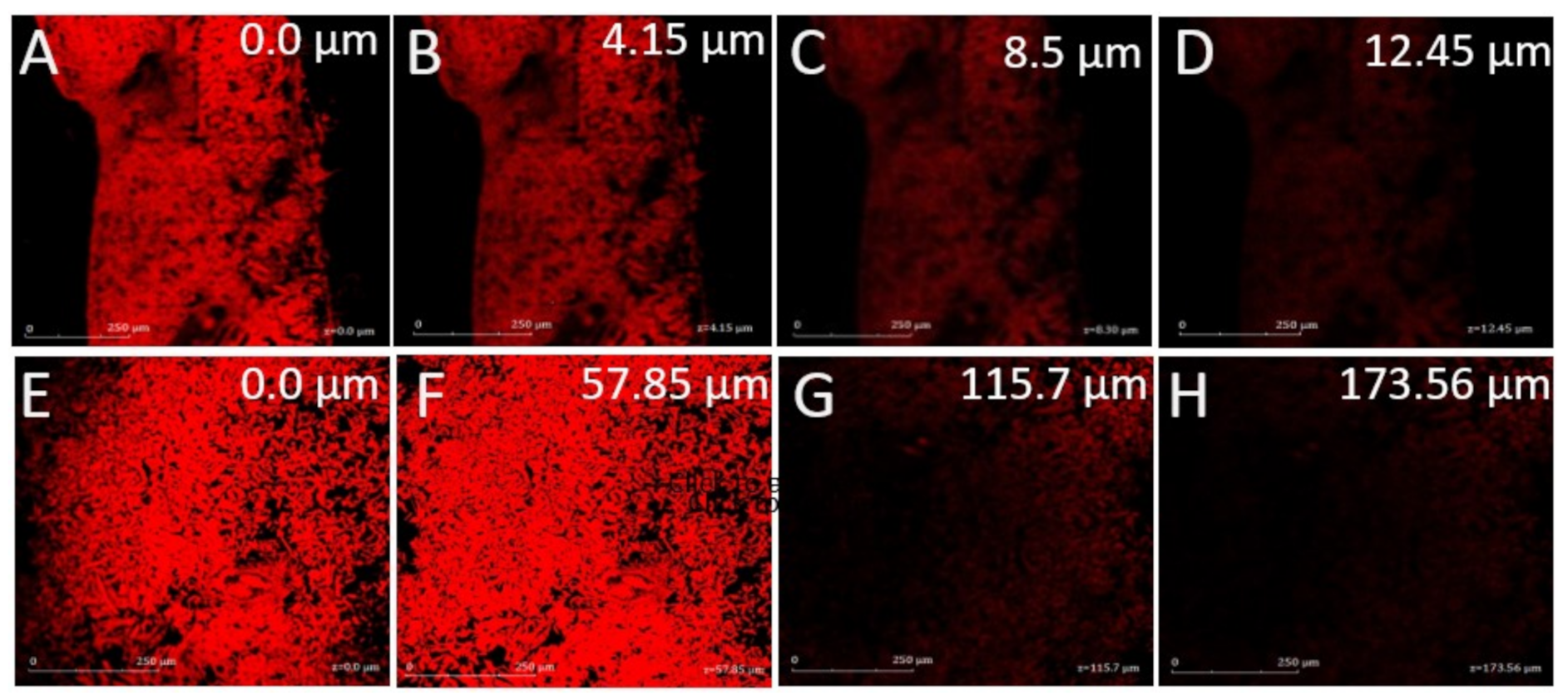
3.2.6. Confocal Laser Microscopy
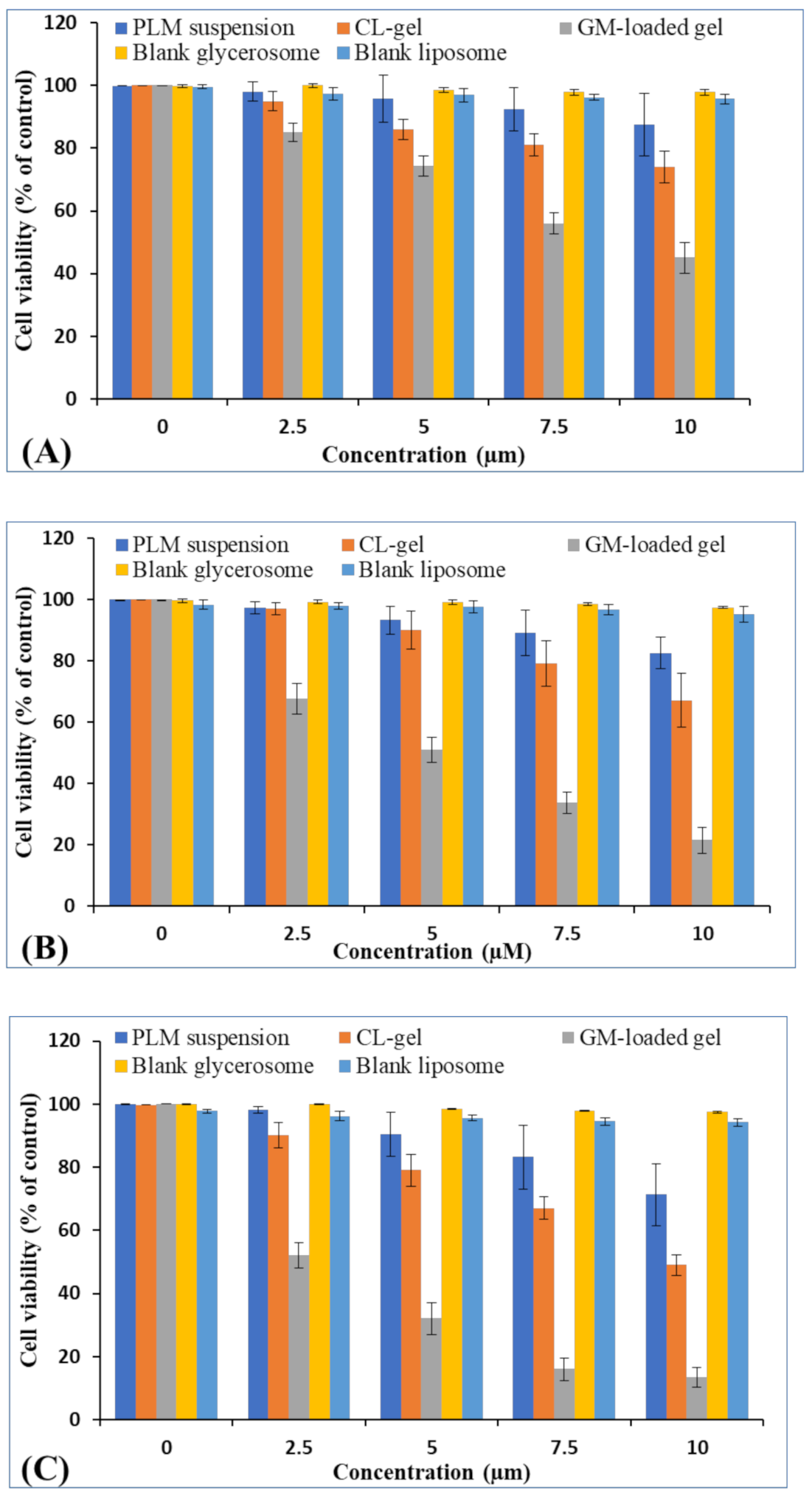
3.2.7. MTT Assay
3.2.8. Antioxidant Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Priya, P.; Raj, R.M.; Vasanthakumar, V.; Raj, V. Curcumin-loaded layer-by-layer folic acid and casein coated carboxymethyl cellulose/casein nanogels for treatment of skin cancer. Arab. J. Chem. 2020, 13, 694–708. [Google Scholar] [CrossRef]
- Akhter, H.; Amin, S. An Investigative Approach to Treatment Modalities for Squamous Cell Carcinoma of Skin. Curr. Drug Deliv. 2017, 14, 597–612. [Google Scholar] [CrossRef]
- Akhter, H.; Ahsan, M.J.; Rahman, M.; Anwar, S. Rizwanullah Advancement in Nanotheranostics for Effective Skin Cancer Therapy: State of the Art. Curr. Nanomed. 2020, 10, 90–104. [Google Scholar] [CrossRef]
- Soni, K.; Mujtaba, A.; Akhter, H.; Zafar, A.; Kohli, K. Optimisation of ethosomal nanogel for topical nano-CUR and sulphoraphane delivery in effective skin cancer therapy. J. Microencapsul. 2019, 37, 91–108. [Google Scholar] [CrossRef] [PubMed]
- Oberli, M.A.; Schoellhammer, C.M.; Langer, R.; Blankschtein, D. Ultrasound-enhanced transdermal delivery: Recent advances and future challenges. Ther. Deliv. 2014, 5, 843–857. [Google Scholar] [CrossRef] [PubMed]
- Rawat, S.; Vengurlekar, S.; Rakesh, B.; Jain, S.; Srikarti, G. Transdermal delivery by iontophoresis. Indian J. Pharm. Sci. 2008, 70, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Daftardar, S.; Neupane, R.; Boddu, S.H.; Renukuntla, J.; Tiwari, A.K. Advances in Ultrasound Mediated Transdermal Drug Delivery. Curr. Pharm. Des. 2019, 25, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Sarheed, O.; Shouqair, D.; Ramesh, K.; Khaleel, T.; Amin, M.; Boateng, J.; Drechsler, M. Formation of stable nanoemulsions by ultrasound-assisted two-step emulsification process for topical drug delivery: Effect of oil phase composition and surfactant concentration and loratadine as ripening inhibitor. Int. J. Pharm. 2020, 576, 118952. [Google Scholar] [CrossRef]
- Song, Y.-Y.; Yuan, Y.; Shi, X.; Che, Y.-Y. Improved drug delivery and anti-tumor efficacy of combinatorial liposomal formulation of genistein and plumbagin by targeting Glut1 and Akt3 proteins in mice bearing prostate tumor. Colloids Surf. B Biointerfaces 2020, 190, 110966. [Google Scholar] [CrossRef] [PubMed]
- Akhter, H.; Rizwanullah, M.; Ahmad, J.; Ahsan, M.J.; Mujtaba, A.; Amin, S. Nanocarriers in advanced drug targeting: Setting novel paradigm in cancer therapeutics. Artif. Cells, Nanomed. Biotechnol. 2018, 46, 873–884. [Google Scholar] [CrossRef]
- Maheshwari, R.G.; Tekade, R.K.; Sharma, P.A.; Darwhekar, G.; Tyagi, A.; Patel, R.P.; Jain, D.K. Ethosomes and ultradeformable liposomes for transdermal delivery of clotrimazole: A comparative assessment. Saudi Pharm. J. 2012, 20, 161–170. [Google Scholar] [CrossRef]
- Kausar, H.; Mujeeb, M.; Ahad, A.; Moolakkadath, T.; Aqil, M.; Ahmad, A.; Akhter, H. Optimization of ethosomes for topical thymoquinone delivery for the treatment of skin acne. J. Drug Deliv. Sci. Technol. 2019, 49, 177–187. [Google Scholar] [CrossRef]
- Verma, P.; Pathak, K. Therapeutic and cosmeceutical potential of ethosomes: An overview. J. Adv. Pharm. Technol. Res. 2010, 1, 274–282. [Google Scholar] [CrossRef]
- Jain, S.; Tiwary, A.K.; Sapra, B.; Jain, N.K. Formulation and evaluation of ethosomes for transdermal delivery of lamivudine. AAPS PharmSciTech 2007, 8, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Zaru, M.; Manca, M.L.; Fadda, A.M.; Orsini, G. Glycerosomes and Use Thereof in Pharmaceutical and Cosmetic Preparations for Topical Applications. U.S. Patent No. 8,778,367, 5 July 2014. [Google Scholar]
- Kaminski, G.A.; Sierakowski, M.R.; Pontarolo, R.; Dos Santos, L.A.; De Freitas, R.A. Layer-by-layer polysaccharide-coated liposomes for sustained delivery of epidermal growth factor. Carbohydr. Polym. 2016, 140, 129–135. [Google Scholar] [CrossRef]
- Ahmad, J.; Ameeduzzafar; Ahmad, M.Z.; Akhter, H. Surface-Engineered Cancer Nanomedicine: Rational Design and Recent Progress. Curr. Pharm. Des. 2020, 26, 1181–1190. [Google Scholar] [CrossRef]
- Kaewbumrung, S.; Panichayupakaranant, P. Antibacterial activity of plumbagin derivative-rich Plumbago indica root extracts and chemical stability. Nat. Prod. Res. 2014, 28, 835–837. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.K.; Panda, M.; Biswal, B.K. Emerging role of plumbagin: Cytotoxic potential and pharmaceutical relevance towards cancer therapy. Food Chem. Toxicol. 2019, 125, 566–582. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Lv, M.; Chen, X.; Yu, Y.; Zang, G.; Tang, Z. Plumbagin inhibits proliferation and induces apoptosis of hepatocellular carcinoma by downregulating the expression of SIVA. Drug Des. Dev. Ther. 2019, 13, 1289–1300. [Google Scholar] [CrossRef]
- Gomathinayagam, R.; Sowmyalakshmi, S.; Mardhatillah, F.; Kumar, R.; Akbarsha, M.A.; Damodaran, C. Anticancer mechanism of plumbagin, a natural compound, on non-small cell lung cancer cells. Anticancer. Res. 2008, 28, 785–792. [Google Scholar]
- Sand, J.M.; Bin Hafeez, B.; Jamal, M.S.; Witkowsky, O.; Siebers, E.M.; Fischer, J.; Verma, A.K. Plumbagin (5-hydroxy-2-methyl-1,4-naphthoquinone), isolated from Plumbago zeylanica, inhibits ultraviolet radiation-induced development of squamous cell carcinomas. Carcinog. 2011, 33, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Jamal, M.S.; Parveen, S.; Beg, M.A.; Suhail, M.; Chaudhary, A.G.A.; Damanhouri, G.A.; Abuzenadah, A.M.; Rehan, M. Anticancer Compound Plumbagin and Its Molecular Targets: A Structural Insight into the Inhibitory Mechanisms Using Computational Approaches. PLoS ONE 2014, 9, e87309. [Google Scholar] [CrossRef]
- Kortylewski, M.; Jove, R.; Yu, H. Targeting STAT3 affects melanoma on multiple fronts. Cancer Metastasis Rev. 2005, 24, 315–327. [Google Scholar] [CrossRef]
- Oh, T.-I.; Yun, J.-M.; Park, E.-J.; Kim, Y.-S.; Lee, Y.-M.; Lim, J.-H. Plumbagin Suppresses α-MSH-Induced Melanogenesis in B16F10 Mouse Melanoma Cells by Inhibiting Tyrosinase Activity. Int. J. Mol. Sci. 2017, 18, 320. [Google Scholar] [CrossRef] [PubMed]
- Manca, M.L.; Zaru, M.; Manconi, M.; Lai, F.; Valenti, D.; Sinico, C.; Fadda, A.M. Glycerosomes: A new tool for effective dermal and transdermal drug delivery. Int. J. Pharm. 2013, 455, 66–74. [Google Scholar] [CrossRef]
- Gupta, P.; Mazumder, R.; Padhi, S. Glycerosomes: Advanced Liposomal Drug Delivery System. Indian J. Pharm. Sci. 2020, 82, 385–397. [Google Scholar] [CrossRef]
- Lombardo, D.; Calandra, P.; Barreca, D.; Magazù, S.; Kiselev, M.A. Soft Interaction in Liposome Nanocarriers for Therapeutic Drug Delivery. Nanomater. 2016, 6, 125. [Google Scholar] [CrossRef]
- Zhang, H. Thin-Film Hydration Followed by Extrusion Method for Liposome Preparation. NMR-Based Metab. 2017, 1522, 17–22. [Google Scholar] [CrossRef]
- Ahad, A.; Aqil, M.; Kohli, K.; Sultana, Y.; Mujeeb, M. Design, formulation and optimization of valsartan transdermal gel containing iso-eucalyptol as novel permeation enhancer: Preclinical assessment of pharmacokinetics in Wistar albino rats. Expert Opin. Drug Deliv. 2014, 11, 1149–1162. [Google Scholar] [CrossRef]
- Akhter, H.; Kumar, S.; Nomani, S. Sonication tailored enhance cytotoxicity of naringenin nanoparticle in pancreatic cancer: Design, optimization, and in vitro studies. Drug Dev. Ind. Pharm. 2020, 46, 659–672. [Google Scholar] [CrossRef] [PubMed]
- Ahad, A.; Al-Saleh, A.A.; Al-Mohizea, A.M.; Al-Jenoobi, F.I.; Raish, M.; Yassin, A.E.B.; Alam, M.A. Pharmacodynamic study of eprosartan mesylate-loaded transfersomes Carbopol ® gel under Dermaroller ® on rats with methyl prednisolone acetate-induced hypertension. Biomed. Pharmacother. 2017, 89, 177–184. [Google Scholar] [CrossRef]
- Ahad, A.; Raish, M.; Al-Jenoobi, F.I.; Al-Mohizea, A.M. Sorbitane Monostearate and Cholesterol based Niosomes for Oral Delivery of Telmisartan. Curr. Drug Deliv. 2018, 15, 260–266. [Google Scholar] [CrossRef]
- Unnikrishnan, K.P.; Raja, S.S.; Balachandran, I. A reverse phase HPLC-UV and HPTLC methods for determination of plumbagin inPlumbago indicaandPlumbago zeylanica. Indian J. Pharm. Sci. 2008, 70, 844–847. [Google Scholar] [CrossRef] [PubMed]
- Sabitha, M.; Rejinold, N.S.; Nair, A.; Lakshmanan, V.-K.; Nair, S.V.; Jayakumar, R. Development and evaluation of 5-fluorouracil loaded chitin nanogels for treatment of skin cancer. Carbohydr. Polym. 2013, 91, 48–57. [Google Scholar] [CrossRef]
- Pierre, M.B.R.; Tedesco, A.C.; Marchetti, J.M.; Bentley, M.V.L. Stratum corneum lipids liposomes for the topical delivery of 5-aminolevulinic acid in photodynamic therapy of skin cancer: Preparation and in vitro permeation study. BMC Dermatol. 2001, 1, 5. [Google Scholar] [CrossRef]
- Bhattamisra, S.K.; Shak, A.T.; Xi, L.W.; Safian, N.H.; Choudhury, H.; Lim, W.M.; Shahzad, N.; Alhakamy, N.A.; Anwer, K.; Radhakrishnan, A.K.; et al. Nose to brain delivery of rotigotine loaded chitosan nanoparticles in human SH-SY5Y neuroblastoma cells and animal model of Parkinson’s disease. Int. J. Pharm. 2020, 579, 119148. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.P.; Prabha, R.; Verma, S.; Meena, K.K.; Yandigeri, M. Antioxidant properties and polyphenolic content in terrestrial cyanobacteria. 3 Biotech 2017, 7, 134. [Google Scholar] [CrossRef] [PubMed]
- Westh, P. Unilamellar DMPC Vesicles in Aqueous Glycerol: Preferential Interactions and Thermochemistry. Biophys. J. 2003, 84, 341–349. [Google Scholar] [CrossRef]
- Gupta, B.; Poudel, B.K.; Pathak, S.; Tak, J.W.; Lee, H.H.; Jeong, J.-H.; Choi, H.-G.; Yong, C.S.; Kim, J.O. Effects of Formulation Variables on the Particle Size and Drug Encapsulation of Imatinib-Loaded Solid Lipid Nanoparticles. AAPS PharmSciTech 2015, 17, 652–662. [Google Scholar] [CrossRef] [PubMed]
- Akhter, H.; Ahmad, A.; Ali, J.; Mohan, G. Formulation and Development of CoQ10-Loaded s-SNEDDS for Enhancement of Oral Bioavailability. J. Pharm. Innov. 2014, 9, 121–131. [Google Scholar] [CrossRef]
- Ramadon, D.; Wirarti, G.A.; Anwar, E. Novel Transdermal Ethosomal Gel Containing Green Tea (Camellia sinensis L. Kuntze) Leaves Extract: Formulation and In vitro Penetration Study. J. Young- Pharm. 2017, 9, 336–340. [Google Scholar] [CrossRef]
- Sakpakdeejaroen, I.; Somani, S.; Laskar, P.; Mullin, M.; Dufès, C. Transferrin-bearing liposomes entrapping plumbagin for targeted cancer therapy. J. Interdiscip. Nanomed. 2019, 4, 54–71. [Google Scholar] [CrossRef] [PubMed]
- Tzeyung, A.S.; Md, S.; Bhattamisra, S.K.; Madheswaran, T.; Alhakamy, N.A.; Aldawsari, H.M.; Radhakrishnan, A.K. Fabrication, Optimization, and Evaluation of Rotigotine-Loaded Chitosan Nanoparticles for Nose-To-Brain Delivery. Pharmaceutics 2019, 11, 26. [Google Scholar] [CrossRef] [PubMed]
- Subongkot, T.; Wonglertnirant, N.; Songprakhon, P.; Rojanarata, T.; Opanasopit, P.; Ngawhirunpat, T. Visualization of ultradeformable liposomes penetration pathways and their skin interaction by confocal laser scanning microscopy. Int. J. Pharm. 2013, 441, 151–161. [Google Scholar] [CrossRef]
- Moolakkadath, T.; Aqil, M.; Ahad, A.; Imam, S.S.; Iqbal, B.; Sultana, Y.; Mujeeb, M.; Iqbal, Z. Development of transethosomes formulation for dermal fisetin delivery: Box–Behnken design, optimization, in vitro skin penetration, vesicles–skin interaction and dermatokinetic studies. Artif. Cells, Nanomedicine, Biotechnol. 2018, 46, 755–765. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]


| Independent Variables | Level Used | ||
|---|---|---|---|
| Low (−1) | Medium (0) | High (+1) | |
| X1: Phospholipid conc. (mg) | 15 | 22.5 | 30 |
| X2: Cholesterol (mg) | 1 | 2.5 | 4 |
| X3: Glycerol | 10 | 20 | 30 |
| Dependent variables | |||
| Y1: Vesicles size (nm) | Minimize | ||
| Y2: Entrapment efficiency (%) | Maximize | ||
| Y3: Drug permeation/Flux (μg/cm2/h) | Maximize | ||
| Formulation No | Independent Variables | Observe Responses | ||||
|---|---|---|---|---|---|---|
| X1 (mg) | X2 (% w/v) | X3 (% w/v) | Y1 (nm) | Y2 (%) | Y3 | |
| FL1 | 22.50 | 2.50 | 20.00 | 160.00 ± 16.30 | 74.00 ± 3.20 | 69.00 ± 6.80 |
| FL2 | 22.50 | 2.50 | 20.00 | 167.00 ± 17.10 | 73.00 ± 5.30 | 73.00 ± 6.10 |
| FL3 | 15.00 | 2.50 | 10.00 | 96.00 ± 9.40 | 63.00 ± 7.50 | 44.00 ± 4.30 |
| FL4 | 22.50 | 2.50 | 20.00 | 163.00 ± 15.20 | 77.00 ± 8.60 | 72.00 ± 6.50 |
| FL5 | 30.00 | 2.50 | 30.00 | 190.00 ± 18.30 | 84.00 ± 9.10 | 84.00 ± 9.90 |
| FL6 | 30.00 | 4.00 | 20.00 | 200.00 ± 18.40 | 82.00 ± 8.60 | 80.00 ± 8.60 |
| FL7 | 15.00 | 2.50 | 30.00 | 88.00 ± 6.60 | 68.00 ± 7.40 | 74.00 ± 5.80 |
| FL8 | 30.00 | 2.50 | 10.00 | 182.00 ± 14.40 | 65.00 ± 6.60 | 50.00 ± 4.60 |
| FL9 | 22.50 | 1.00 | 10.00 | 150.00 ± 12.70 | 61.00 ± 6.90 | 47.00 ± 4.60 |
| FL10 | 22.50 | 1.00 | 30.00 | 152.00 ± 10.50 | 71.00 ± 7.40 | 77.00 ± 8.60 |
| FL11 | 22.50 | 4.00 | 10.00 | 172.00 ± 16.80 | 59.00 ± 4.20 | 54.00 ± 5.90 |
| FL12 | 30.00 | 1.00 | 20.00 | 180.00 ± 15.00 | 78.00 ± 9.50 | 76.00 ± 9.50 |
| FL13 | 22.50 | 4.00 | 30.00 | 176.00 ± 16.80 | 80.00 ± 7.80 | 85.00 ± 9.90 |
| FL14 | 15.00 | 1.00 | 20.00 | 82.23 ± 4.70 | 67.00 ± 7.60 | 67.00 ± 6.70 |
| FL15 | 15.00 | 4.00 | 20.00 | 115.00 ± 8.50 | 73.00 ± 6.80 | 68.00 ± 7.90 |
| FL16 | 22.50 | 2.50 | 20.00 | 157.00 ± 10.80 | 78.00 ± 7.30 | 78.00 ± 8.80 |
| FL17 | 22.50 | 2.50 | 20.00 | 161.00 ± 13.50 | 75.30 ± 9.20 | 72.00 ± 8.30 |
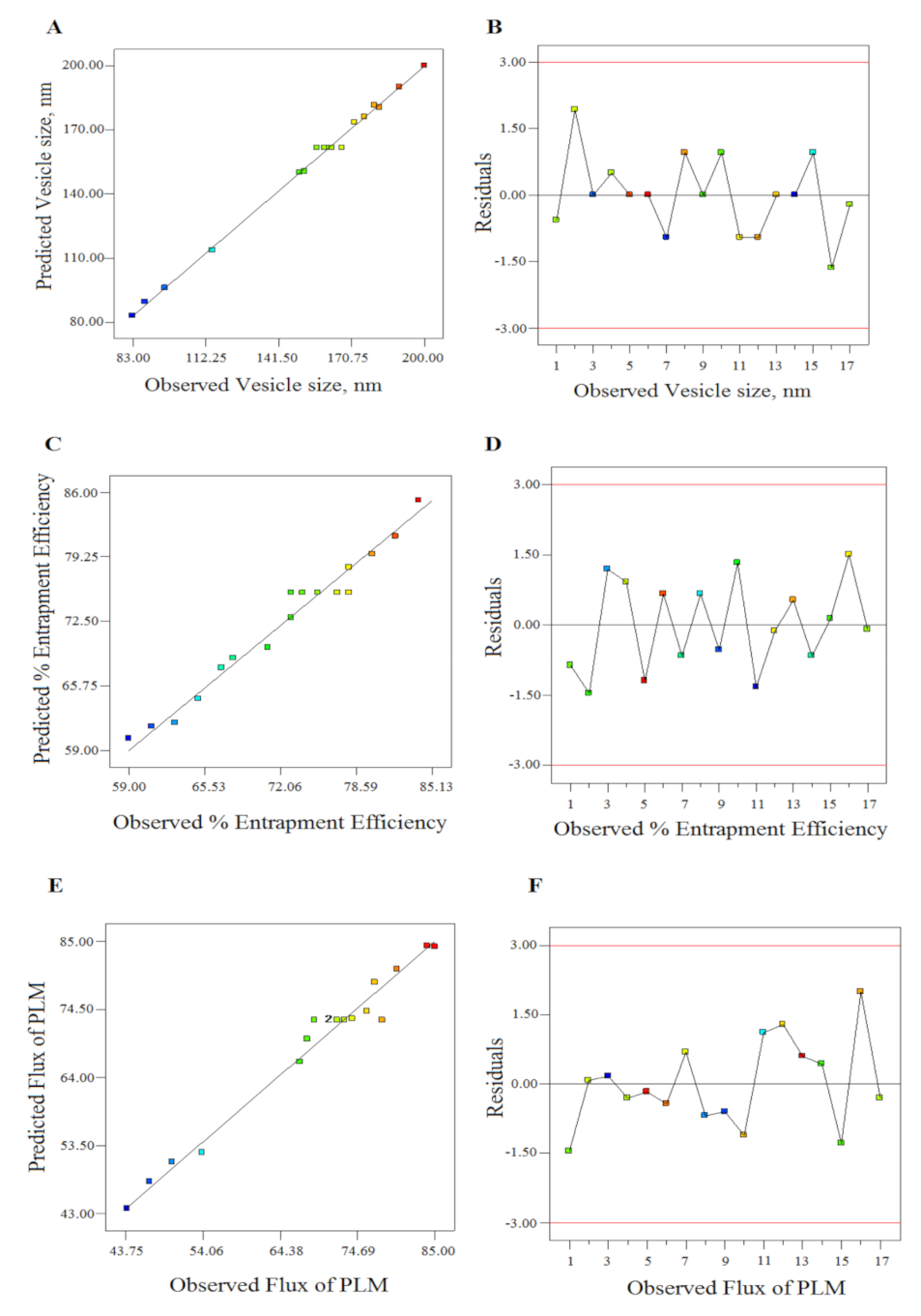
| Response Surface Quadratic Model | R-Squared | Adj R-Squared | Pred R-Squared | Adeq Precision | PRESS | % CV | Mean | SD |
|---|---|---|---|---|---|---|---|---|
| Response 1 (y1) | 0.9966 | 0.9923 | 0.9851 | 48.695 | 302.25 | 2.05 | 152.47 | 3.13 |
| Response 2 (y2) | 0.9719 | 0.9358 | 0.8294 | 17.237 | 150.61 | 2.60 | 72.25 | 1.88 |
| Response 3 (y3) | 0.9767 | 0.9467 | 0.8689 | 18.105 | 334.87 | 4.24 | 68.82 | 2.92 |
| Variables | Optimum Composition | Response | Observed Value of Response | Predicted Value of Response | Percentage Error |
|---|---|---|---|---|---|
| X1 | 15 mg | Y1 | 119.20 ± 15.67 | 110.53 | 8.18 |
| X2 | 4 mg | Y2 | 76.42 ± 9.98 | 74.09 | 3.14 |
| X3 | 26.8% w/v | Y3 | 79.43 ± 12.43 | 76.12 | 4.34 |
| Incubation Time (h) | PLM Suspension (µm) | CL Gel (µm) | GM-Loaded Gel (µm) |
|---|---|---|---|
| 24 | 41.7 ± 2.3 | 19.0 ± 1.2 | 8.9 ± 0.6 |
| 48 | 29.8 ± 1.4 | 16.0 ± 1.3 | 5.6 ± 0.4 |
| 72 | 19.1 ± 1.7 | 10.4 ± 0.8 | 4.1 ± 0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Md, S.; Alhakamy, N.A.; Aldawsari, H.M.; Husain, M.; Khan, N.; Alfaleh, M.A.; Asfour, H.Z.; Riadi, Y.; Bilgrami, A.L.; Akhter, M.H. Plumbagin-Loaded Glycerosome Gel as Topical Delivery System for Skin Cancer Therapy. Polymers 2021, 13, 923. https://doi.org/10.3390/polym13060923
Md S, Alhakamy NA, Aldawsari HM, Husain M, Khan N, Alfaleh MA, Asfour HZ, Riadi Y, Bilgrami AL, Akhter MH. Plumbagin-Loaded Glycerosome Gel as Topical Delivery System for Skin Cancer Therapy. Polymers. 2021; 13(6):923. https://doi.org/10.3390/polym13060923
Chicago/Turabian StyleMd, Shadab, Nabil A. Alhakamy, Hibah M. Aldawsari, Mohammad Husain, Nazia Khan, Mohamed A. Alfaleh, Hani Z. Asfour, Yassine Riadi, Anwar L. Bilgrami, and Md Habban Akhter. 2021. "Plumbagin-Loaded Glycerosome Gel as Topical Delivery System for Skin Cancer Therapy" Polymers 13, no. 6: 923. https://doi.org/10.3390/polym13060923
APA StyleMd, S., Alhakamy, N. A., Aldawsari, H. M., Husain, M., Khan, N., Alfaleh, M. A., Asfour, H. Z., Riadi, Y., Bilgrami, A. L., & Akhter, M. H. (2021). Plumbagin-Loaded Glycerosome Gel as Topical Delivery System for Skin Cancer Therapy. Polymers, 13(6), 923. https://doi.org/10.3390/polym13060923