Marine Algae Incorporated Polylactide Acid Patch: Novel Candidate for Targeting Osteosarcoma Cells without Impairing the Osteoblastic Proliferation
Abstract
:1. Introduction
2. Materials and Methods
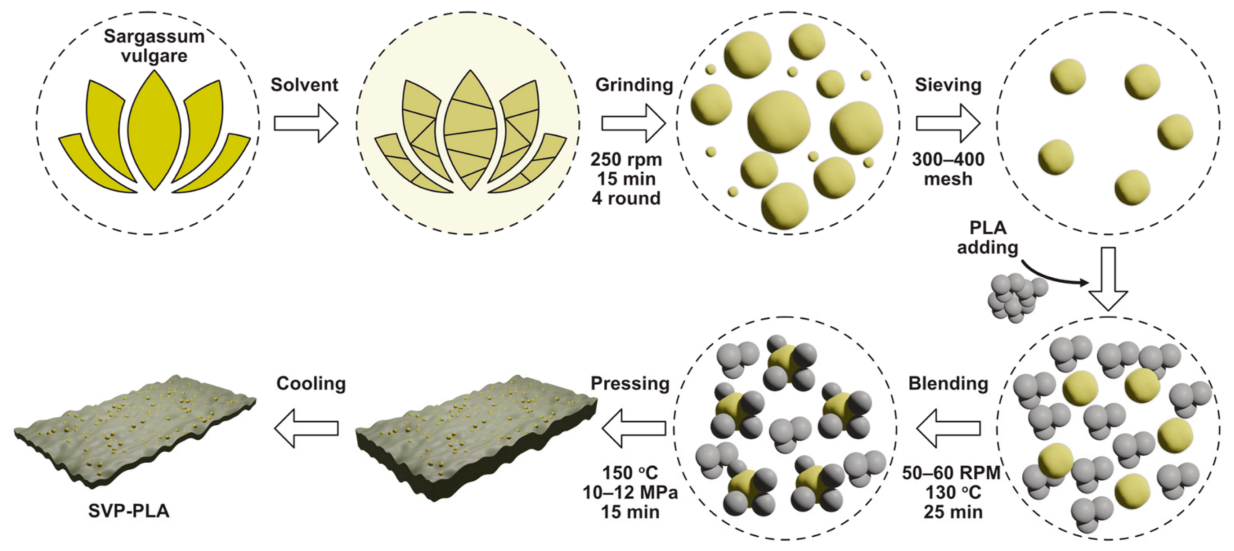
2.1. Preparation of SVP
2.2. Preparation of SVP-PLA Composite
2.3. Preparation of PLA Patches
2.4. Material Characterization
2.5. Cytocompatibility Analysis
2.6. Analysis of Cell Viability—MTT Assay
2.7. Analysis of Cell Proliferation—5-Bromo-2′-deoxyuridine (BrdU) Assay
2.8. Immunostaining and Florescence Imaging
2.9. Cell Morphology Analysis
2.10. Statistical Analysis
3. Results and Discussions
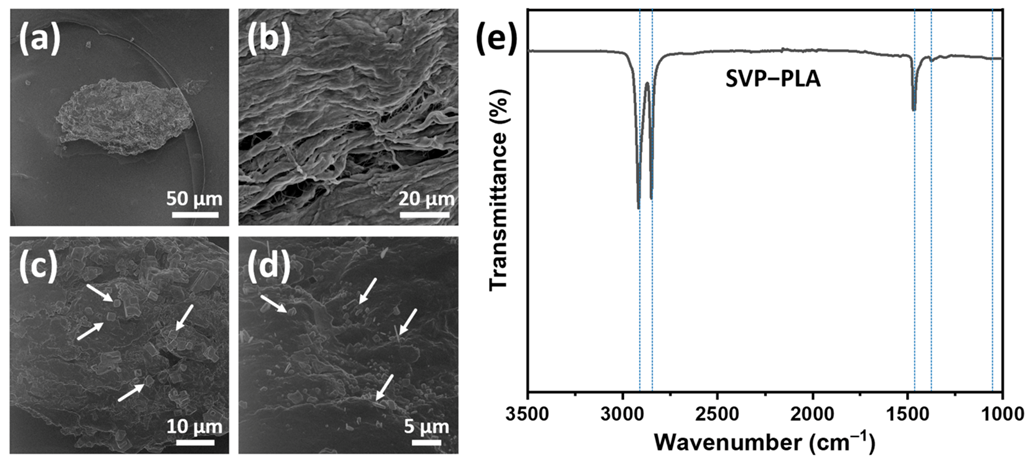
3.1. Material Characteristics
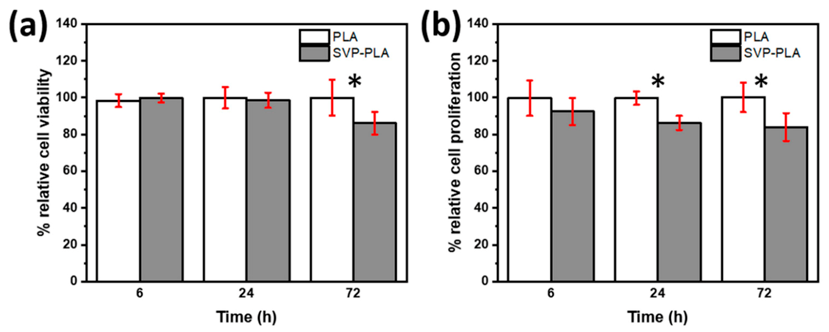
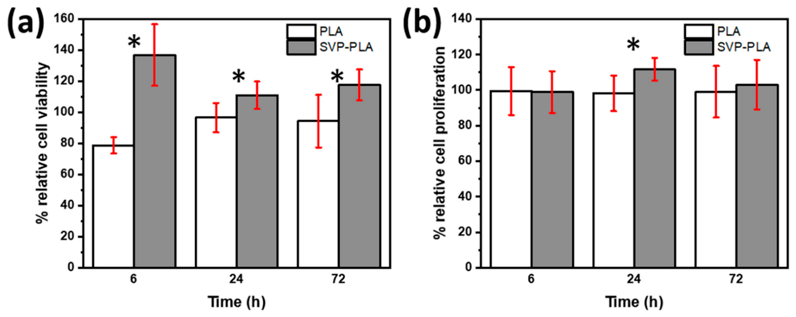
3.2. Cell Viability and Proliferation
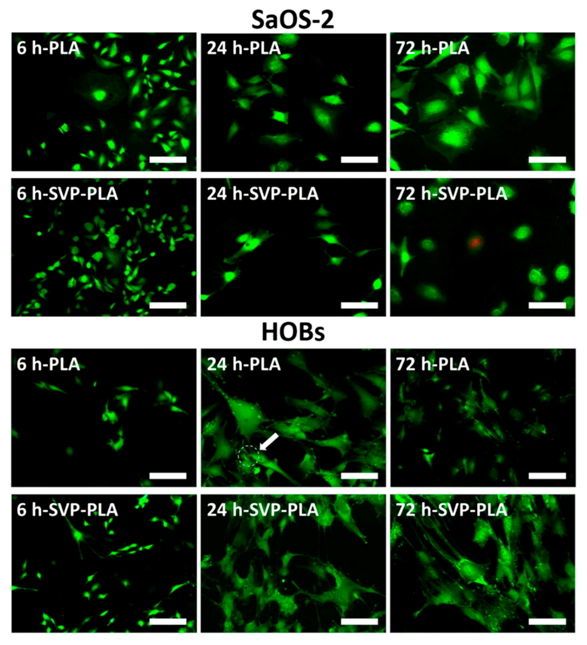
3.3. Immunostaining and Fluorescence Imaging
3.4. Morphological Analysis of Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Terriza, A.; Vilches-Pérez, J.I.; González-Caballero, J.L.; de la Orden, E.; Yubero, F.; Barranco, A.; Gonzalez-Elipe, A.R.; Vilches, J.; Salido, M. Osteoblasts interaction with PLGA membranes functionalized with titanium film nanolayer by PECVD. In vitro assessment of surface influence on cell adhesion during initial cell to material interaction. Materials (Basel) 2014, 7, 1687–1708. [Google Scholar] [CrossRef] [Green Version]
- Shi, C.; Yuan, Z.; Han, F.; Zhu, C.; Li, B. Polymeric biomaterials for bone regeneration. Ann. Jt. 2016, 1, 27. [Google Scholar] [CrossRef]
- Filippi, M.; Born, G.; Chaaban, M.; Scherberich, A. Natural Polymeric Scaffolds in Bone Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 474. [Google Scholar] [CrossRef] [PubMed]
- Souza, P.M.S.; Morales, A.R.; Marin-Morales, M.A.; Mei, L.H.I. PLA and Montmorilonite Nanocomposites: Properties, Biodegradation and Potential Toxicity. J. Polym. Environ. 2013, 21, 738–759. [Google Scholar] [CrossRef]
- Goonoo, N.; Bhaw-Luximon, A.; Bowlin, G.L.; Jhurry, D. An assessment of biopolymer- and synthetic polymer-based scaffolds for bone and vascular tissue engineering. Polym. Int. 2013, 62, 523–533. [Google Scholar] [CrossRef]
- Xiao, L.; Wang, B.; Yang, G.; Gauthier, M. Poly(Lactic Acid)-Based Biomaterials: Synthesis, Modification and Applications. In Biomedical Science, Engineering and Technology; Ghista, D.N., Ed.; InTech: Rijeka, Croatia, 2012. [Google Scholar]
- Mi, H.Y.; Salick, M.R.; Jing, X.; Jacques, B.R.; Crone, W.C.; Peng, X.F.; Turng, L.S. Characterization of thermoplastic polyurethane/polylactic acid (TPU/PLA) tissue engineering scaffolds fabricated by microcellular injection molding. Mater. Sci. Eng. C 2013, 33, 4767–4776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardea, S.; Baldino, L.; Scognamiglio, M.; Reverchon, E. 3D PLLA/Ibuprofen composite scaffolds obtained by a supercritical fluids assisted process. J. Mater. Sci. Mater. Med. 2014, 25, 989–998. [Google Scholar] [CrossRef]
- Pinese, C.; Gagnieu, C.; Nottelet, B.; Rondot-Couzin, C.; Hunger, S.; Coudane, J.; Garric, X. In vivo evaluation of hybrid patches composed of PLA based copolymers and collagen/chondroitin sulfate for ligament tissue regeneration. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 1778–1788. [Google Scholar] [CrossRef]
- Kale, G.; Auras, R.; Singh, S.P. Degradation of commercial biodegradable packages under real composting and ambient exposure conditions. J. Polym. Environ. 2006, 14, 317–334. [Google Scholar] [CrossRef]
- Bulota, M.; Budtova, T. PLA/algae composites: Morphology and mechanical properties. Compos. Part A Appl. Sci. Manuf. 2015, 73, 109–115. [Google Scholar] [CrossRef]
- Wu, C.S. Preparation, Characterisation, and Controlled-Release of Biodegradable Polyester and Marine-Algae Composite. J. Polym. Environ. 2015, 23, 356–366. [Google Scholar] [CrossRef]
- Reichstein, W.; Sommer, L.; Veziroglu, S.; Sayin, S.; Schröder, S.; Mishra, Y.K.; Saygili, E.İ.; Karayürek, F.; Açil, Y.; Wiltfang, J.; et al. Initiated Chemical Vapor Deposition (iCVD) Functionalized Polylactic Acid–Marine Algae Composite Patch for Bone Tissue Engineering. Polymers (Basel) 2021, 13, 186. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.Y.; Yang, M.C.; Hsu, Y.C. Improvement of cytocompatibility of polylactide by filling with marine algae powder. Mater. Sci. Eng. C 2015, 50, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.J.; Kwon, M.J.; Kim, I.H.; Nam, T.J. Chemoprotective effects of a protein from the red algae Porphyra yezoensis on acetaminophen-induced liver injury in rats. Phyther. Res. 2008, 22, 1149–1153. [Google Scholar] [CrossRef]
- Liu, X.; Wang, S.; Cao, S.; He, X.; Qin, L.; He, M.; Yang, Y.; Hao, J.; Mao, W. Structural characteristics and anticoagulant property in vitro and in vivo of a seaweed Sulfated Rhamnan. Mar. Drugs 2018, 16, 243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, N.; Lee, J.H.; Lee, W.W.; Ko, J.Y.; Kim, E.A.; Kim, J.S.; Heu, M.S.; Kim, G.H.; Jeon, Y.J. Gallic acid isolated from Spirogyra sp. improves cardiovascular disease through a vasorelaxant and antihypertensive effect. Environ. Toxicol. Pharmacol. 2015, 39, 764–772. [Google Scholar] [CrossRef]
- Dore, C.M.P.G.; Faustino Alves, M.G.D.C.; Pofírio Will, L.S.E.; Costa, T.G.; Sabry, D.A.; De Souza Rêgo, L.A.R.; Accardo, C.M.; Rocha, H.A.O.; Filgueira, L.G.A.; Leite, E.L. A sulfated polysaccharide, fucans, isolated from brown algae Sargassum vulgare with anticoagulant, antithrombotic, antioxidant and anti-inflammatory effects. Carbohydr. Polym. 2013, 91, 467–475. [Google Scholar] [CrossRef]
- Fujihara, M.; Iizima, N.; Yamamoto, I.; Nagumo, T. Purification and chemical and physical characterisation of an antitumour polysaccharide from the brown seaweed Sargassum fulvellum. Carbohydr. Res. 1984, 125, 97–106. [Google Scholar] [CrossRef]
- Fujihara, M.; Nagumo, T. The effect of the content of d-mannuronic acid and l-guluronic acid blocks in alginates on antitumor activity. Carbohydr. Res. 1992, 224, 343–347. [Google Scholar] [CrossRef]
- Fujihara, M.; Nagumo, T. An influence of the structure of alginate on the chemotactic activity of macrophages and the antitumor activity. Carbohydr. Res. 1993, 243, 211–216. [Google Scholar] [CrossRef]
- Namvar, F.; Mohamad, R.; Baharara, J.; Zafar-Balanejad, S.; Fargahi, F.; Rahman, H.S. Antioxidant, antiproliferative, and antiangiogenesis effects of polyphenol-rich seaweed (sargassum muticum). BioMed Res. Int. 2013, 2013, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Domblides, C.; Lartigue, L.; Faustin, B. Control of the Antitumor Immune Response by Cancer Metabolism. Cells 2019, 8, 104. [Google Scholar] [CrossRef] [Green Version]
- Ghania, A.; Nabila, B.-B.; Larbi, B.; Elisabeth, M.; Philippe, G.; Mariem, B.; Khadidja, K.-K.; Wacila, B.-R.; Fawzia, A.-B. Antimicrobial and antiparasitic activities of three algae from the northwest coast of Algeria. Nat. Prod. Res. 2019, 33, 742–745. [Google Scholar] [CrossRef] [PubMed]
- Nojima, K.; Mizukami, T.; Sobata, R.; Matsumoto, C.; Kuramitsu, M.; Okuma, K.; Satake, M.; Tadokoro, K.; Yamaguchi, K.; Hamaguchi, I. Development of an effective prevention method for t-cell lymphotropic virus type I (HTLV-1) infection using HTLV-1 sero-positive serum in vitro. Transfusion 2013, 53, 215A. [Google Scholar]
- Sayin, S.; Kohlhaas, T.; Veziroglu, S.; Okudan, E.Ş.; Naz, M.; Schröder, S.; Saygili, E.I.; Açil, Y.; Faupel, F.; Wiltfang, J.; et al. Marine Algae-PLA composites as de novo alternative to porcine derived collagen membranes. Mater. Today Chem. 2020, 17, 100276. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A Rapid Method Of Total Lipid Extraction And Purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vollenweider, R.A.; Talling, J.F.; Westlake, D.F. Manual on Methods for Measuring Primary Production in Aquatic Environments (Handbooks/International Biological Programme); Blackwell Scientific Publications: Oxford, UK, 1974. [Google Scholar]
- Corobea, M.C.; Vuluga, Z.; Florea, D.; Miculescu, F.; Voicu, S.I. Composites and Nanocomposites Based on Polylactic Acid. In Handbook of Composites from Renewable Materials; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017; Volume 1–8, pp. 327–360. [Google Scholar]
- Liu, L.; Yuan, W. A hierarchical functionalized biodegradable PLA electrospun nanofibrous membrane with superhydrophobicity and antibacterial properties for oil/water separation. New J. Chem. 2018, 42, 17615–17624. [Google Scholar] [CrossRef]
- Murakami, D.; Jinnai, H.; Takahara, A. Wetting transition from the cassie-baxter state to the wenzel state on textured polymer surfaces. Langmuir 2014, 30, 2061–2067. [Google Scholar] [CrossRef]
- Kolsi, R.B.A.; Salah, H.B.; Jardak, N.; Chaaben, R.; Jribi, I.; Feki, A.E.; Rebai, T.; Jamoussi, K.; Allouche, N.; Blecker, C.; et al. Sulphated polysaccharide isolated from Sargassum vulgare: Characterization and hypolipidemic effects. Carbohydr. Polym. 2017, 170, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Lv, J.; Feng, J. Spectral Characterization of Four Kinds of Biodegradable Plastics: Poly (Lactic Acid), Poly (Butylenes Adipate-Co-Terephthalate), Poly (Hydroxybutyrate-Co-Hydroxyvalerate) and Poly (Butylenes Succinate) with FTIR and Raman Spectroscopy. J. Polym. Environ. 2013, 21, 108–114. [Google Scholar] [CrossRef]
- Kacuráková, M. FT-IR study of plant cell wall model compounds: Pectic polysaccharides and hemicelluloses. Carbohydr. Polym. 2000, 43, 195–203. [Google Scholar] [CrossRef]
- Phan, L.M.; Yeung, S.C.J.; Lee, M.H. Cancer metabolic reprogramming: Importance, main features, and potentials for precise targeted anti-cancer therapies. Cancer Biol. Med. 2014, 11, 1–19. [Google Scholar] [CrossRef]
- Diaz-Pulido, G.; Barrón, C. CO2 Enrichment Stimulates Dissolved Organic Carbon Release in Coral Reef Macroalgae. J. Phycol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Haghshenas, V.; Kafaei, R.; Tahmasebi, R.; Dobaradaran, S.; Hashemi, S.; Sahebi, S.; Sorial, G.A.; Ramavandi, B. Potential of green/brown algae for monitoring of metal(loid)s pollution in the coastal seawater and sediments of the Persian Gulf: Ecological and health risk assessment. Environ. Sci. Pollut. Res. 2020. [Google Scholar] [CrossRef]
- Su, L.; Shi, W.; Chen, X.; Meng, L.; Yuan, L.; Chen, X.; Huang, G. Simultaneously and quantitatively analyze the heavy metals in Sargassum fusiforme by laser-induced breakdown spectroscopy. Food Chem. 2021. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veziroglu, S.; Ayna, M.; Kohlhaas, T.; Sayin, S.; Fiutowski, J.; Mishra, Y.K.; Karayürek, F.; Naujokat, H.; Saygili, E.I.; Açil, Y.; et al. Marine Algae Incorporated Polylactide Acid Patch: Novel Candidate for Targeting Osteosarcoma Cells without Impairing the Osteoblastic Proliferation. Polymers 2021, 13, 847. https://doi.org/10.3390/polym13060847
Veziroglu S, Ayna M, Kohlhaas T, Sayin S, Fiutowski J, Mishra YK, Karayürek F, Naujokat H, Saygili EI, Açil Y, et al. Marine Algae Incorporated Polylactide Acid Patch: Novel Candidate for Targeting Osteosarcoma Cells without Impairing the Osteoblastic Proliferation. Polymers. 2021; 13(6):847. https://doi.org/10.3390/polym13060847
Chicago/Turabian StyleVeziroglu, Salih, Mustafa Ayna, Theresa Kohlhaas, Selin Sayin, Jacek Fiutowski, Yogendra Kumar Mishra, Fatih Karayürek, Hendrik Naujokat, Eyüp Ilker Saygili, Yahya Açil, and et al. 2021. "Marine Algae Incorporated Polylactide Acid Patch: Novel Candidate for Targeting Osteosarcoma Cells without Impairing the Osteoblastic Proliferation" Polymers 13, no. 6: 847. https://doi.org/10.3390/polym13060847
APA StyleVeziroglu, S., Ayna, M., Kohlhaas, T., Sayin, S., Fiutowski, J., Mishra, Y. K., Karayürek, F., Naujokat, H., Saygili, E. I., Açil, Y., Wiltfang, J., Faupel, F., Aktas, O. C., & Gülses, A. (2021). Marine Algae Incorporated Polylactide Acid Patch: Novel Candidate for Targeting Osteosarcoma Cells without Impairing the Osteoblastic Proliferation. Polymers, 13(6), 847. https://doi.org/10.3390/polym13060847








