The Micellization of Well-Defined Single Graft Copolymers in Block Copolymer/Homopolymer Blends
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paul, D.R.; Bucknall, C.B. (Eds.) Polymer Blends Set: Formulation and Performance; John Wiley & Sons: New York, NY, USA, 2000. [Google Scholar]
- Koning, C.; van Duin, M.; Pagnoulle, C.; Jerome, R. Strategies for compatibilization of polymer blends. Prog. Polym. Sci. 1998, 23, 707–757. [Google Scholar] [CrossRef]
- Adedeji, A.; Jamieson, A.M. Interfacial adhesion in immiscible polymer blends compatibilized with block copolymers. Compos. Interfaces 1995, 3, 51–71. [Google Scholar] [CrossRef]
- Ding, Y.; Feng, W.; Huang, D.; Lu, B.; Wang, P.; Wang, G.; Ji, J. Compatibilization of immiscible PLA-based biodegradable polymer blends using amphiphilic di-block copolymers. Eur. Polym. J. 2019, 118, 45–52. [Google Scholar] [CrossRef]
- Ding, Y.; Feng, W.; Lu, B.; Wang, P.; Wang, G.; Ji, J. PLA-PEG-PLA tri-block copolymers: Effective compatibilizers for promotion of the interfacial structure and mechanical properties of PLA/PBAT blends. Polymer 2018, 146, 179–187. [Google Scholar] [CrossRef]
- Green, P.F.; Russell, T.P. Segregation of low molecular weight symmetric diblock copolymers at the interface of high molecular weight homopolymers. Macromolecules 1991, 24, 2931–2935. [Google Scholar] [CrossRef]
- Dai, K.H.; Kramer, E.J. Molecular weight dependence of diblock copolymer segregation at a polymer/polymer interface. J. Polym. Sci. Part B Polym. Phys. 1994, 32, 1943–1950. [Google Scholar] [CrossRef]
- Reynolds, B.J.; Ruegg, M.L.; Mates, T.E.; Radke, C.J.; Balsara, N.P. Experimental and Theoretical Study of the Adsorption of a Diblock Copolymer to Interfaces between Two Homopolymers. Macromolecules 2005, 38, 3872–3882. [Google Scholar] [CrossRef]
- Eastwood, E.; Viswanathan, S.; O’Brien, C.; Kumar, D.; Dadmun, M. Methods to improve the properties of polymer mixtures: Optimizing intermolecular interactions and compatibilization. Polymer 2005, 46, 3957–3970. [Google Scholar] [CrossRef]
- Shull, K.R.; Kramer, E.J.; Hadziioannou, G.; Tang, W. Segregation of block copolymers to interfaces between immiscible homopolymers. Macromolecules 1990, 23, 4780–4787. [Google Scholar] [CrossRef]
- Russell, T.P.; Anastasiadis, S.H.; Menelle, A.; Felcher, G.P.; Satija, S.K. Segment density distribution of symmetric diblock copolymers at the interface between two homopolymers as revealed by neutron reflectivity. Macromolecules 1991, 24, 1575–1582. [Google Scholar] [CrossRef]
- Whitmore, M.D.; Noolandi, J. Theory of micelle formation in block copolymer-homopolymer blends. Macromolecules 1985, 18, 657–665. [Google Scholar] [CrossRef]
- Duke, T.A.J. The Dynamics of Polymers at Interfaces and in Electric Fields. Ph.D. Thesis, University of Cambridge, Cambridge, UK, 1989. [Google Scholar]
- Werner, A.; Schmid, F.; Binder, K.; Muller, M. Diblock Copolymers at a Homopolymer–Homopolymer Interface: A Monte Carlo Simulation. Macromolecules 1996, 29, 8241–8248. [Google Scholar] [CrossRef]
- Anastasiadis, S.H.; Gancarz, I.; Koberstein, J.T. Compatibilizing effect of block copolymers added to the polymer/polymer interface. Macromolecules 1989, 22, 1449–1453. [Google Scholar] [CrossRef]
- Wagner, M.; Wolf, B.A. Effect of Block Copolymers on the Interfacial-Tension between 2 Immiscible/Homopolymers. Polymer 1993, 34, 1460–1464. [Google Scholar] [CrossRef]
- Jorzik, U.; Wolf, B.A. Reduction of the Interfacial Tension between Poly(dimethylsiloxane) and Poly(ethylene oxide) by Block Copolymers: Effects of Molecular Architecture and Chemical Composition. Macromolecules 1997, 30, 4713–4718. [Google Scholar] [CrossRef]
- Cho, D.; Hu, W.; Koberstein, J.T.; Lingelser, J.P.; Gallot, Y. Segregation Dynamics of Block Copolymers to Immiscible Polymer Blend Interfaces. Macromolecules 2000, 33, 5245–5251. [Google Scholar] [CrossRef]
- Chang, K.; Macosko, C.W.; Morse, D.C. Ultralow Interfacial Tensions of Polymer/Polymer Interfaces with Diblock Copolymer Surfactants. Macromolecules 2007, 40, 3819–3830. [Google Scholar] [CrossRef]
- Creton, C.; Kramer, E.J.; Hadziioannou, G. Critical molecular weight for block copolymer reinforcement of interfaces in a two-phase polymer blend. Macromolecules 1991, 24, 1846–1853. [Google Scholar] [CrossRef]
- Brown, H.R.; Char, K.; Deline, V.R.; Green, P.F. Effects of a diblock copolymer on adhesion between immiscible polymers. 1. Polystyrene (PS)-PMMA copolymer between PS and PMMA. Macromolecules 1993, 26, 4155–4163. [Google Scholar] [CrossRef]
- Dai, C.-A.; Jandt, K.D.; Iyengar, D.R.; Slack, N.L.; Dai, K.H.; Davidson, A.W.B.; Kramer, E.J.; Hui, C.-Y. Strengthening Polymer Interfaces with Triblock Copolymers. Macromolecules 1997, 30, 549–560. [Google Scholar] [CrossRef]
- Leibler, L. Emulsifying effects of block copolymers in incompatible polymer blends. Makromol. Chemie. Macromol. Symp. 1988, 16, 1–17. [Google Scholar] [CrossRef]
- Leibler, L. Block copolymers at interfaces. Phys. A Stat. Mech. Appl. 1991, 172, 258–268. [Google Scholar] [CrossRef]
- Israels, R.; Jasnow, D.; Balazs, A.C.; Guo, L.; Krausch, G.; Sokolov, J.; Rafailovich, M. Compatibilizing A/B blends with AB diblock copolymers: Effect of copolymer molecular weight. J. Chem. Phys. 1995, 102, 8149–8157. [Google Scholar] [CrossRef]
- Gersappe, D.; Harm, P.K.; Irvine, D.; Balazs, A.C. Contrasting the compatibilizing activity of comb and linear copolymers. Macromolecules 1994, 27, 720–724. [Google Scholar] [CrossRef]
- Lyatskaya, Y.; Gersappe, D.; Balazs, A.C. Effect of Copolymer Architecture on the Efficiency of Compatibilizers. Macromolecules 1995, 28, 6278–6283. [Google Scholar] [CrossRef]
- Lyatskaya, Y.; Gersappe, D.; Gross, N.A.; Balazs, A.C. Designing Compatibilizers To Reduce Interfacial Tension in Polymer Blends. J. Phys. Chem. 1996, 100, 1449–1458. [Google Scholar] [CrossRef]
- Russell, T.P.; Mayes, A.M.; Deline, V.R.; Chung, T.C. Hairpin configurations of triblock copolymers at homopolymer interfaces. Macromolecules 1992, 25, 5783–5789. [Google Scholar] [CrossRef]
- Brown, H.R.; Krappe, U.; Stadler, R. Effect of ABC Triblock Copolymers with an Elastomeric Midblock on the Adhesion between Immiscible Polymers. Macromolecules 1996, 29, 6582–6588. [Google Scholar] [CrossRef]
- Cigana, P.; Favis, B.D. The relative efficacy of diblock and triblock copolymers for a polystyrene/ethylene-propylene rubber interface. Polymer 1998, 39, 3373–3378. [Google Scholar] [CrossRef]
- Dadmun, M. Effect of Copolymer Architecture on the Interfacial Structure and Miscibility of a Ternary Polymer Blend Containing a Copolymer and Two Homopolymers. Macromolecules 1996, 29, 3868–3874. [Google Scholar] [CrossRef]
- Dadmun, M.D. Importance of a Broad Composition Distribution in Polymeric Interfacial Modifiers. Macromolecules 2000, 33, 9122–9125. [Google Scholar] [CrossRef]
- Eastwood, E.A.; Dadmun, M.D. Multiblock Copolymers in the Compatibilization of Polystyrene and Poly(methyl methacrylate) Blends: Role of Polymer Architecture. Macromolecules 2002, 35, 5069–5077. [Google Scholar] [CrossRef]
- Dai, C.-A.; Dair, B.J.; Dai, K.H.; Ober, C.K.; Kramer, E.J.; Hui, C.-Y.; Jelinski, L.W. Reinforcement of Polymer Interfaces with Random Copolymers. Phys. Rev. Lett. 1994, 73, 2472–2475. [Google Scholar] [CrossRef] [PubMed]
- Kulasekere, R.; Kaiser, H.; Ankner, J.F.; Russell, T.P.; Brown, H.R.; Hawker, C.J.; Mayes, A.M. Homopolymer Interfaces Reinforced with Random Copolymers. Macromolecules 1996, 29, 5493–5496. [Google Scholar] [CrossRef]
- Stammer, A.; Wolf, B.A. Effect of random copolymer additives on the interfacial tension between incompatible polymers. Macromol. Rapid Commun. 1998, 19, 123–126. [Google Scholar] [CrossRef]
- Benkoski, J.J.; Fredrickson, G.H.; Kramer, E.J. Effects of composition drift on the effectiveness of random copolymer reinforcement at polymer-polymer interfaces. J. Polym. Sci. Part B Polym. Phys. 2001, 39, 2363–2377. [Google Scholar] [CrossRef]
- Vilgis, T.A.; Noolandi, J. On the compatibilization of polymer blends. Makromol. Chemie. Macromol. Symp. 1988, 16, 225–234. [Google Scholar] [CrossRef]
- Vilgis, T.A.; Noolandi, J. Theory of homopolymer-block copolymer blends. The search for a universal compatibilizer. Macromolecules 1990, 23, 2941–2947. [Google Scholar] [CrossRef]
- Semenov, A.N. Theory of diblock-copolymer segregation to the interface and free surface of a homopolymer layer. Macromolecules 1992, 25, 4967–4977. [Google Scholar] [CrossRef]
- Retsos, H.; Margiolaki, I.; Messaritaki, A.; Anastasiadis, S.H. Interfacial Tension in Binary Polymer Blends in the Presence of Block Copolymers: Effects of Additive MW. Macromolecules 2001, 34, 5295–5305. [Google Scholar] [CrossRef]
- Adedeji, A.; Lyu, S.; Macosko, C.W. Block Copolymers in Homopolymer Blends: Interface vs. Micelles. Macromolecules 2001, 34, 8663–8668. [Google Scholar] [CrossRef]
- Chang, K.; Morse, D.C. Diblock Copolymer Surfactants in Immiscible Homopolymer Blends: Swollen Micelles and Interfacial Tension. Macromolecules 2006, 39, 7746–7756. [Google Scholar] [CrossRef]
- Retsos, H.; Anastasiadis, S.H.; Pispas, S.; Mays, J.W.; Hadjichristidis, N. Interfacial Tension in Binary Polymer Blends in the Presence of Block Copolymers. 2. Effects of Additive Architecture and Composition. Macromolecules 2003, 37, 524–537. [Google Scholar] [CrossRef]
- Leibler, L.; Orland, H.; Wheeler, J.C. Theory of Critical Micelle Concentration for Solutions of Block Co-Polymers. J. Chem. Phys. 1983, 79, 3550–3557. [Google Scholar] [CrossRef]
- Mayes, A.M.; De La Cruz, M.O. Cylindrical versus spherical micelle formation in block copolymer/homopolymer blends. Macromolecules 1988, 21, 2543–2547. [Google Scholar] [CrossRef]
- Greenall, M.J.; Buzza, D.M.A.; McLeish, T.C.B. Micelle Formation in Block Copolymer/Homopolymer Blends: Comparison of Self-Consistent Field Theory with Experiment and Scaling Theory. Macromolecules 2009, 42, 5873–5880. [Google Scholar] [CrossRef][Green Version]
- Selb, J.; Marie, P.; Rameau, A.; Duplessix, R.; Gallot, Y. Study of the structure of block copolymer-homopolymer blends using small angle neutron scattering. Polym. Bull. 1983, 10, 444–451. [Google Scholar] [CrossRef]
- Rigby, D.; Roe, R.J. Small-Angle X-Ray-Scattering Study of Micelle Formation in Mixtures of Butadiene Homopolymer and Styrene Butadiene Block Copolymer. Macromolecules 1984, 17, 1778–1785. [Google Scholar] [CrossRef]
- Rigby, D.; Roe, R.J. Small-Angle X-Ray-Scattering Study of Micelle Formation in Mixtures of Butadiene Homopolymer and Styrene Butadiene Block Copolymer.2. Effects of Block Lengths. Macromolecules 1986, 19, 721–728. [Google Scholar] [CrossRef]
- Kinning, D.J.; Winey, K.I.; Thomas, E.L. Structural transitions from spherical to nonspherical micelles in blends of poly(styrene-butadiene) diblock copolymer and polystyrene homopolymers. Macromolecules 1988, 21, 3502–3506. [Google Scholar] [CrossRef]
- Kinning, D.J.; Thomas, E.L.; Fetters, L.J. Morphological studies of micelle formation in block copolymer/homopolymer blends. J. Chem. Phys. 1989, 90, 5806–5825. [Google Scholar] [CrossRef]
- Kinning, D.J.; Thomas, E.L.; Fetters, L.J. Morphological studies of micelle formation in block copolymer/homopolymer blends: Comparison with theory. Macromolecules 1991, 24, 3893–3900. [Google Scholar] [CrossRef]
- Gohr, K.; Pakula, T.; Tsutsumi, K.; Schärtl, W. Dynamics of Copolymer Micelles in an Entangled Homopolymer Matrix. Macromolecules 1999, 32, 7156–7165. [Google Scholar] [CrossRef]
- Schartl, W. Structure and diffusion of copolymer micelles in entangled homopolymer chains. Macromol. Chem. Phys. 1999, 200, 481–500. [Google Scholar] [CrossRef]
- Gohr, K.; Schärtl, W.; Willner, L.; Pyckhout-Hintzen, W. SANS Investigation of PS–PB Block Copolymer Micelles in a Short Chain PB Homopolymer Matrix. Macromolecules 2002, 35, 9110–9116. [Google Scholar] [CrossRef][Green Version]
- Major, M.D.; Torkelson, J.M.; Brearley, A.M. Fluorescence energy-transfer studies of bulk styrene-isoprene diblock copolymers and their blends with polyisoprene: Applications to microphase separation. Macromolecules 1990, 23, 1711–1717. [Google Scholar] [CrossRef]
- Wong, C.L.H.; Kim, J.; Roth, C.B.; Torkelson, J.M. Comparison of Critical Micelle Concentrations of Gradient Copolymer and Block Copolymer in Homopolymer: Novel Characterization by Intrinsic Fluorescence. Macromolecules 2007, 40, 5631–5633. [Google Scholar] [CrossRef]
- Sandoval, R.W.; Williams, D.E.; Kim, J.; Roth, C.B.; Torkelson, J.M. Critical micelle concentrations of block and gradient copolymers in homopolymer: Effects of sequence distribution, composition, and molecular weight. J. Polym. Sci. Part B Polym. Phys. 2008, 46, 2672–2682. [Google Scholar] [CrossRef]
- Pispas, S.; Hadjichristidis, N.; Potemkin, I.; Khokhlov, A. Effect of Architecture on the Micellization Properties of Block Copolymers: A2B Miktoarm Stars vs. AB Diblocks. Macromolecules 2000, 33, 1741–1746. [Google Scholar] [CrossRef]
- Sotiriou, K.; Nannou, A.; Velis, G.; Pispas, S. Micellization Behavior of PS(PI)3Miktoarm Star Copolymers. Macromolecules 2002, 35, 4106–4112. [Google Scholar] [CrossRef]
- Voulgaris, D.; Tsitsilianis, C.; Grayer, V.; Esselink, F.; Hadziioannou, G. Amphiphile micelles formed by polystyrene/poly(2-vinyl pyridine) heteroarm star copolymers in toluene. Polymer 1999, 40, 5879–5889. [Google Scholar] [CrossRef]
- Pispas, S.; Poulos, A.Y.; Hadjichristidis, N. Micellization Behavior of (PS)8(PI)8Miktoarm (Vergina) Star Copolymers. Macromolecules 1998, 31, 4177–4181. [Google Scholar] [CrossRef]
- Mountrichas, G.; Mpiri, M.; Pispas, S. Micelles of Star Block (PSPI)8and PSPI Diblock Copolymers (PS = Polystyrene, PI = Polyisoprene): Structure and Kinetics of Micellization. Macromolecules 2005, 38, 940–947. [Google Scholar] [CrossRef]
- Iatrou, H.; Willner, L.; Hadjichristidis, N.; Halperin, A.; Richter, D. Aggregation Phenomena of Model PS/PI Super-H-Shaped Block Copolymers. Influence of the Architecture. Macromolecules 1996, 29, 581–591. [Google Scholar] [CrossRef]
- Pochan, D.J.; Gido, S.P.; Pispas, S.; Mays, J.W.; Ryan, A.J.; Fairclough, J.P.A.; Hamley, A.I.W.; Terrill, N.J. Morphologies of Microphase-Separated A2B Simple Graft Copolymers. Macromolecules 1996, 29, 5091–5098. [Google Scholar] [CrossRef]
- Pitsikalis, M.; Woodward, J.; Mays, J.W.; Hadjichristidis, N. Micellization of Model Graft Copolymers in Dilute Solution. Macromolecules 1997, 30, 5384–5389. [Google Scholar] [CrossRef]
- Zamurovic, M.; Christodoulou, S.; Vazaios, A.; Iatrou, E.; Pitsikalis, A.M.; Hadjichristidis, N. Micellization Behavior of Complex Comblike Block Copolymer Architectures. Macromolecules 2007, 40, 5835–5849. [Google Scholar] [CrossRef]
- Liu, H.; Xu, J.; Jiang, J.; Yin, J.; Narain, R.; Cai, Y.; Liu, S. Syntheses and micellar properties of well-defined amphiphilic AB2 and A2B Y-shaped miktoarm star copolymers of ε-caprolactone and 2-(dimethylamino)ethyl methacrylate. J. Polym. Sci. 2007, 45, 1446–1462. [Google Scholar] [CrossRef]
- Yin, Y.; Jiang, R.; Li, B.; Jin, Q.; Ding, D.; Shi, A.-C. Self-assembly of grafted Y-shaped ABC triblock copolymers in solutions. J. Chem. Phys. 2008, 129, 154903. [Google Scholar] [CrossRef]
- Tsiamantas, C.; Psarros, C.; Mays, J.W.; Pitsikalis, M. Micellization behavior of model asymmetric miktoarm star copolymers of the AA′B type, where A is polyisoprene and B is polystyrene. Polym. J. 2013, 45, 1216–1223. [Google Scholar] [CrossRef]
- Lebedeva, I.O.; Zhulina, E.B.; Borisov, O.V. Theory of Linear–Dendritic Block Copolymer Micelles. ACS Macro Lett. 2017, 7, 42–46. [Google Scholar] [CrossRef]
- Vazaios, A.; Touris, A.; Echeverria, M.; Zorba, G.; Pitsikalis, M. Micellization Behaviour of Linear and Nonlinear Block Copolymers Based on Poly(n-hexyl isocyanate) in Selective Solvents. Polymers 2020, 12, 1678. [Google Scholar] [CrossRef]
- Pispas, S.; Hadjichristidis, N.; Mays, J.W. Micellization of model graft copolymers of the H and pi type in dilute solution. Macromolecules 1996, 29, 7378–7385. [Google Scholar] [CrossRef]
- Pavlopoulou, E.; Anastasiadis, S.H.; Iatrou, H.; Moshakou, M.; Hadjichristidis, N.; Portale, G.; Bras, W. The Micellization of Miktoarm Star SnIn Copolymers in Block Copolymer / Homopolymer Blends. Macromolecules 2009, 42, 5285–5295. [Google Scholar] [CrossRef]
- Iatrou, H.; Hadjichristidis, N. Synthesis of a model 3-miktoarm star terpolymer. Macromolecules 1992, 25, 4649–4651. [Google Scholar] [CrossRef]
- Iatrou, H.; Siakali-Kioulafa, E.; Hadjichristidis, N.; Roovers, J.; Mays, J. Hydrodynamic properties of model 3-miktoarm star copolymers. J. Polym. Sci. Part B Polym. Phys. 1995, 33, 1925–1932. [Google Scholar] [CrossRef]
- Pochan, D.J.; Gido, S.P.; Pispas, S.; Mays, J.W. Morphological Transitions in an I2S Simple Graft Block Copolymer: From Folded Sheets to Folded Lace to Randomly Oriented Worms at Equilibrium. Macromolecules 1996, 29, 5099–5105. [Google Scholar] [CrossRef]
- Yang, L.; Gido, S.P.; Mays, J.W.; Pispas, S.; Hadjichristidis, N. Phase Behavior of I2S Single Graft Block Copolymer/Homopolymer Blends. Macromolecules 2001, 34, 4235–4243. [Google Scholar] [CrossRef]
- Anastasiadis, S.H.; Chrissopoulou, A.K.; Fytas, G.; Fleischer, G.; Pispas, S.; Pitsikalis, A.M.; Mays, J.W.; Hadjichristidis‡, N. Self-Diffusivity in Block Copolymer Solutions. 2. A2B Simple Grafts. Macromolecules 1997, 30, 2445–2453. [Google Scholar] [CrossRef]
- Borsboom, M.; Bras, W.; Cerjak, I.; Detollenaere, D.; Van Loon, D.G.; Goedtkindt, P.; Konijnenburg, M.; Lassing, P.; Levine, Y.K.; Munneke, B.; et al. The Dutch–Belgian beamline at the ESRF. J. Synchrotron Radiat. 1998, 5, 518–520. [Google Scholar] [CrossRef]
- Richardson, M.; Savill, N. Volumetric properties of polystyrene: Influence of temperature, molecular weight and thermal treatment. Polymer 1977, 18, 3–9. [Google Scholar] [CrossRef]
- Förster, S.; Burger, C. Scattering Functions of Polymeric Core−Shell Structures and Excluded Volume Chains. Macromolecules 1998, 31, 879–891. [Google Scholar] [CrossRef]

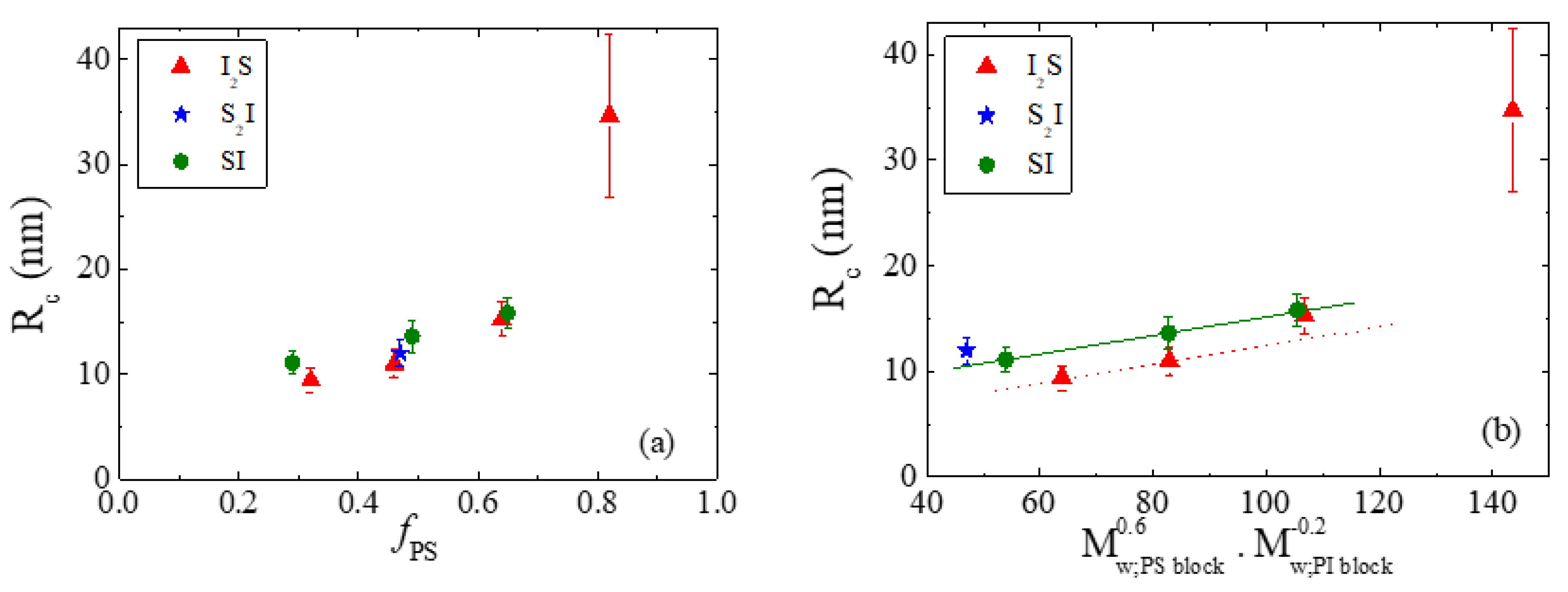
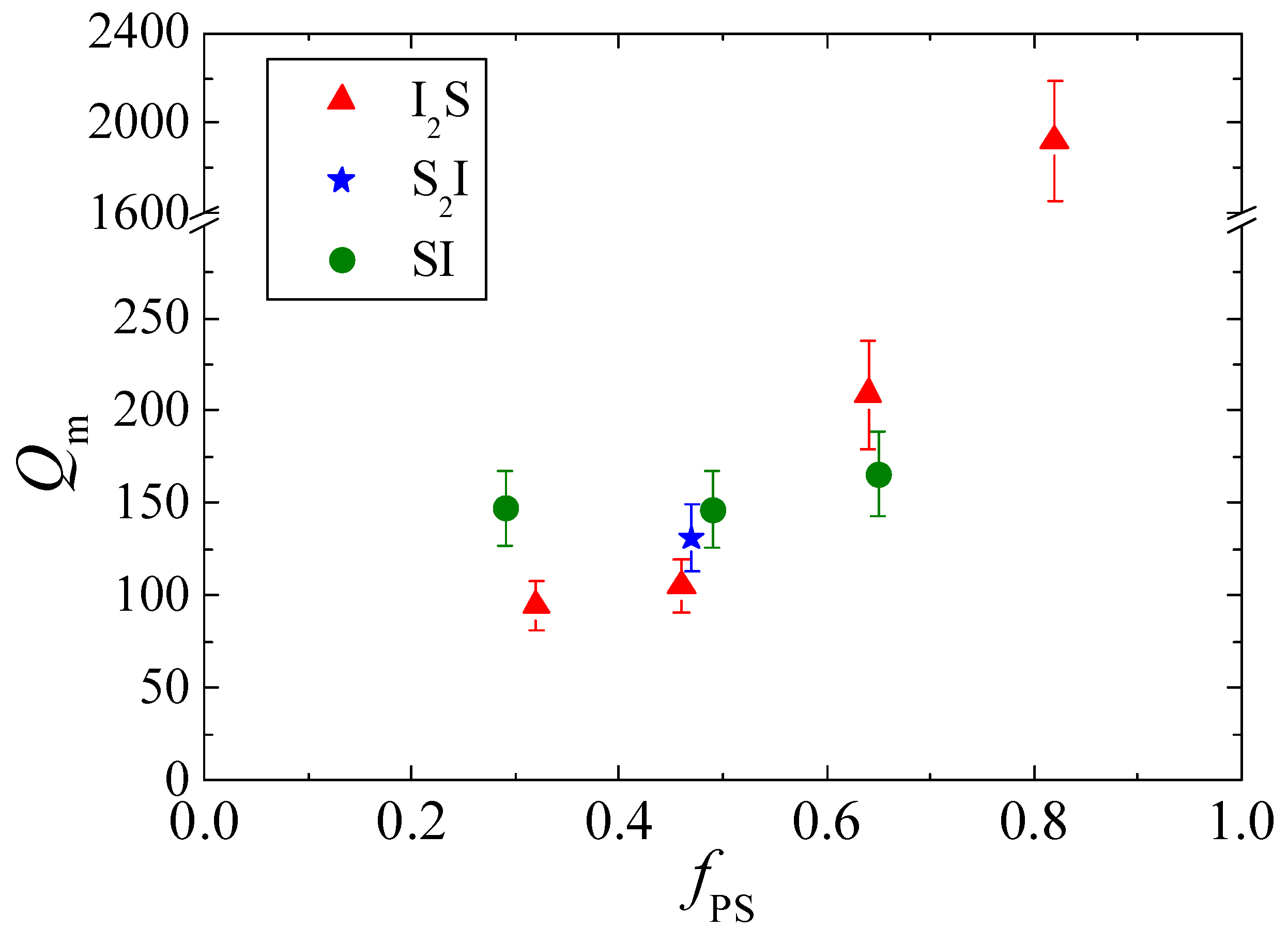
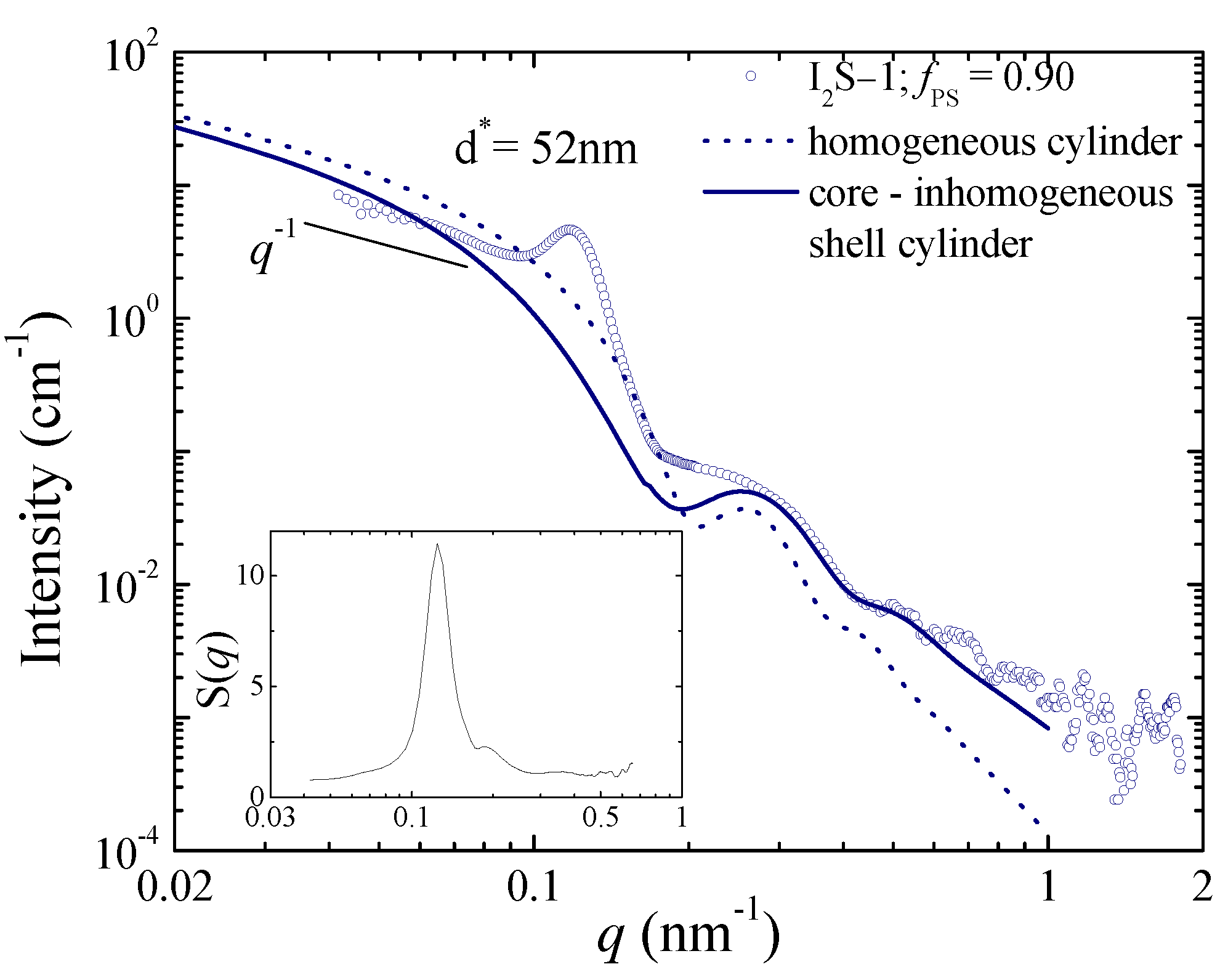
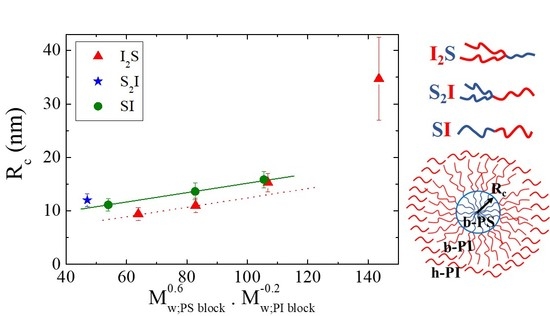
| Species | Mwa | Mwb | Ɖ = Mw/Mn b | wPSc | Nd | fPSe |
|---|---|---|---|---|---|---|
| I2S−1 | 106,000 | 94,100 | 1.04 | 0.91 | 1191 | 0.90 |
| I2S−2 | 90,800 | 83,700 | 1.04 | 0.84 | 1031 | 0.82 |
| I2S−3 | 87,400 | 88,100 | 1.04 | 0.67 | 1017 | 0.64 |
| I2S−4 | 92,000 | 101,200 | 1.04 | 0.49 | 1098 | 0.46 |
| I2S−5 | 89,800 | 104,300 | 1.04 | 0.35 | 1093 | 0.32 |
| I2S−7 | 91,300 | 118,400 | 1.06 | 0.10 | 1149 | 0.09 |
| S2I−4 | 93,000 | 102,500 | 1.06 | 0.48 | 1112 | 0.47 |
| SI−3 | 116,300 | 138,600 | 1.02 | 0.68 | 1354 | 0.65 |
| SI−4 | 113,700 | 146,400 | 1.05 | 0.52 | 1351 | 0.49 |
| SI−5 | 99,500 | 140,100 | 1.03 | 0.32 | 1213 | 0.29 |
| PI | - | 4000 | 1.06 | 0.00 | 81 | 0.00 |
| Species | Mw,PS block | Mw,PI block | Rc (nm) | Qm | Qm×(PS arms) |
|---|---|---|---|---|---|
| I2S−4 | 45,080 | 23,460 | 11.0 | 105 | 105 |
| S2I−4 | 22,320 | 48,360 | 12.0 | 131 | 262 |
| SI−4 | 59,120 | 54,580 | 13.6 | 147 | 147 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavlopoulou, E.; Chrissopoulou, K.; Pispas, S.; Hadjichristidis, N.; Anastasiadis, S.H. The Micellization of Well-Defined Single Graft Copolymers in Block Copolymer/Homopolymer Blends. Polymers 2021, 13, 833. https://doi.org/10.3390/polym13050833
Pavlopoulou E, Chrissopoulou K, Pispas S, Hadjichristidis N, Anastasiadis SH. The Micellization of Well-Defined Single Graft Copolymers in Block Copolymer/Homopolymer Blends. Polymers. 2021; 13(5):833. https://doi.org/10.3390/polym13050833
Chicago/Turabian StylePavlopoulou, Eleni, Kiriaki Chrissopoulou, Stergios Pispas, Nikos Hadjichristidis, and Spiros H. Anastasiadis. 2021. "The Micellization of Well-Defined Single Graft Copolymers in Block Copolymer/Homopolymer Blends" Polymers 13, no. 5: 833. https://doi.org/10.3390/polym13050833
APA StylePavlopoulou, E., Chrissopoulou, K., Pispas, S., Hadjichristidis, N., & Anastasiadis, S. H. (2021). The Micellization of Well-Defined Single Graft Copolymers in Block Copolymer/Homopolymer Blends. Polymers, 13(5), 833. https://doi.org/10.3390/polym13050833