Fabrication and Characterization of Polycaprolactone/Chitosan—Hydroxyapatite Hybrid Implants for Peripheral Nerve Regeneration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
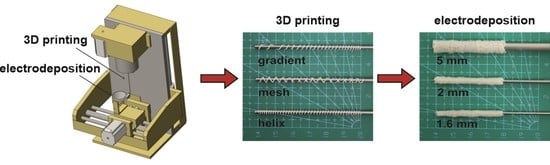
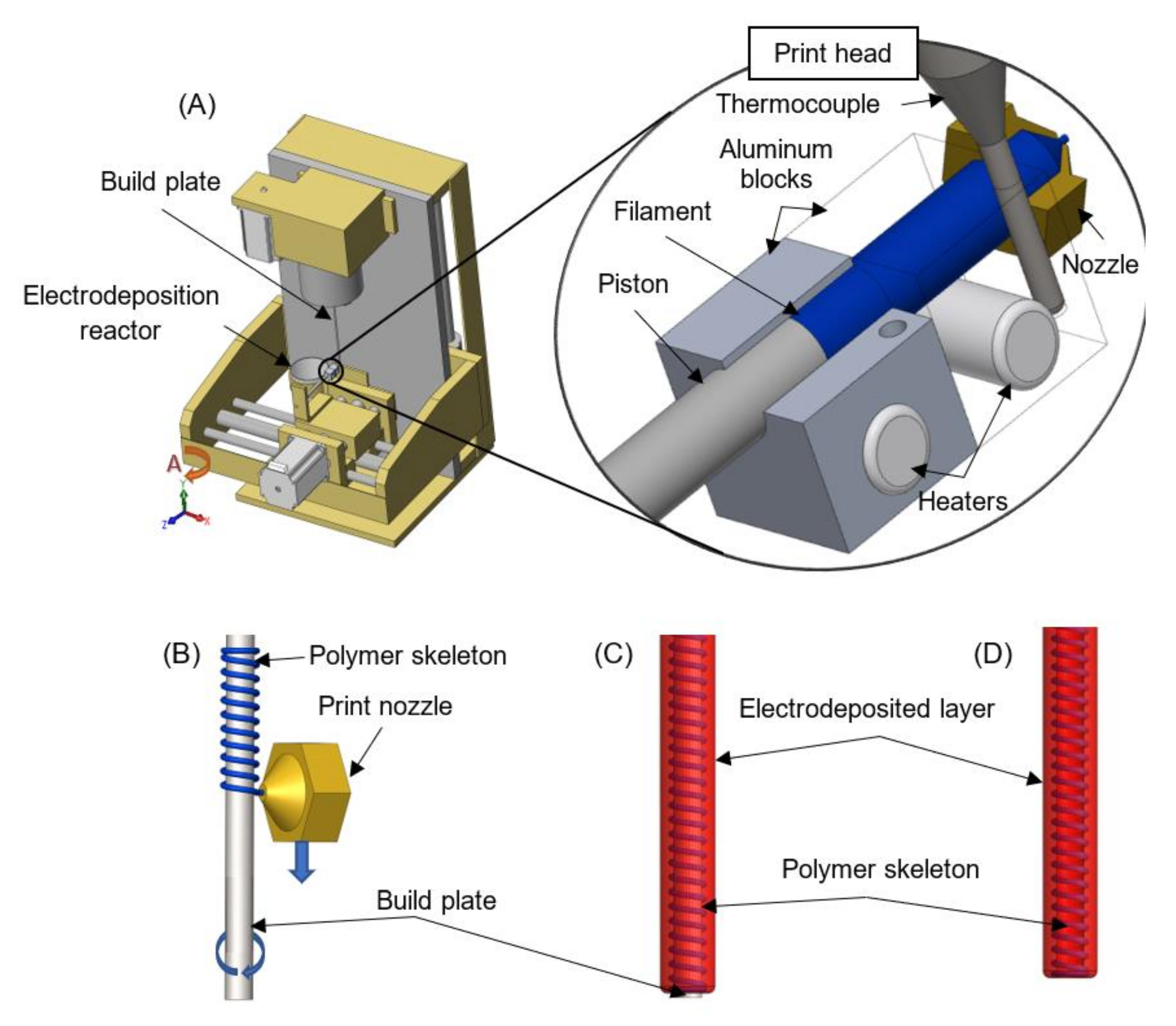
2.2. Apparatus Combining Polymer Extrusion and Electrophoretic Deposition
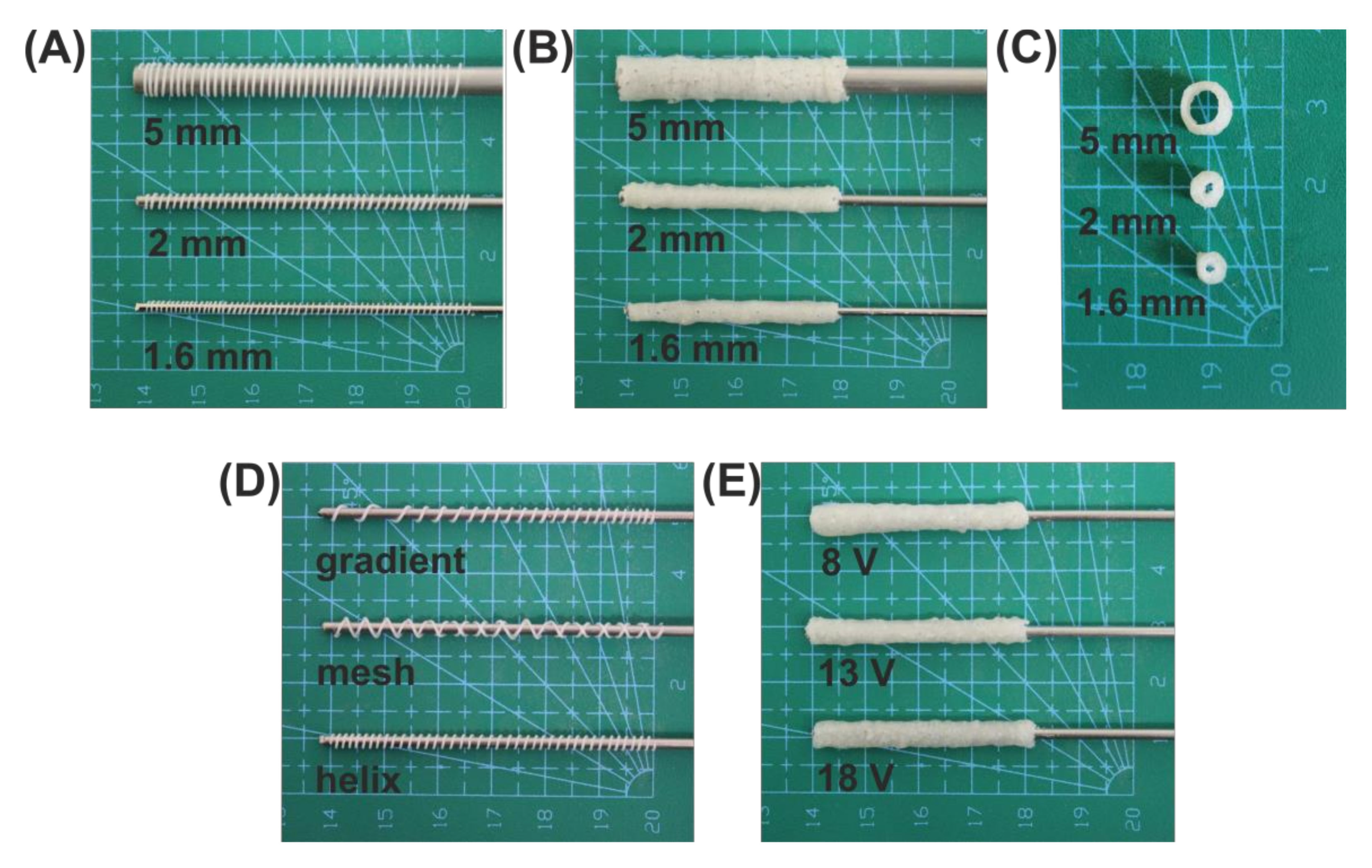
2.3. Implant Fabrication
2.4. Determination of Parameters Influencing the Electrodeposition Process
2.5. Structural Studies
2.6. Degradation Studies and Mechanical Testing
2.7. Statistical Analysis of the Data
3. Results and Discussion
3.1. Electrodeposition Process Characterization
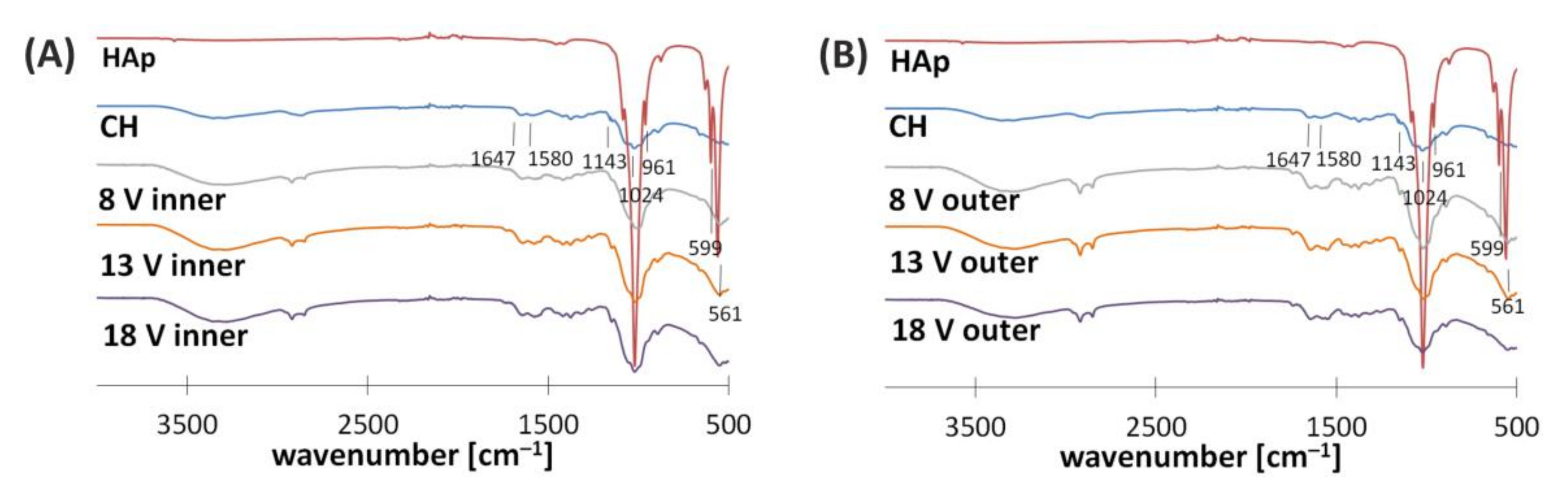
3.2. Structural Characterization
3.3. Degradation and Mechanical Properties
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bellamkonda, R.V. Peripheral nerve regeneration: An opinion on channels, scaffolds and anisotropy. Biomaterials 2006, 27, 3515–3518. [Google Scholar] [CrossRef] [PubMed]
- Saheb-Al-Zamani, M.; Yan, Y.; Farber, S.J.; Hunter, D.A.; Newton, P.; Wood, M.D.; Stewart, S.A.; Johnson, P.J.; Mackinnon, S.E. Limited regeneration in long acellular nerve allografts is associated with increased Schwann cell senescence. Exp. Neurol. 2013, 247, 165–177. [Google Scholar] [CrossRef] [Green Version]
- Dietzmeyer, N.; Förthmann, M.; Grothe, C.; Haastert-Talini, K. Modification of tubular chitosan-based peripheral nerve implants: Applications for simple or more complex approaches. Neural Regen. Res. 2020, 15, 1421. [Google Scholar] [CrossRef] [PubMed]
- Abbasian, M.; Massoumi, B.; Mohammad-Rezaei, R.; Samadian, H.; Jaymand, M. Scaffolding polymeric biomaterials: Are naturally occurring biological macromolecules more appropriate for tissue engineering? Int. J. Biol. Macromol. 2019, 134, 673–694. [Google Scholar] [CrossRef]
- Murdock, M.H.; Badylak, S.F. Biomaterials-based in situ tissue engineering. Curr. Opin. Biomed. Eng. 2017, 1, 4–7. [Google Scholar] [CrossRef] [Green Version]
- Ullah, S.; Chen, X. Fabrication, applications and challenges of natural biomaterials in tissue engineering. Appl. Mater. Today 2020, 20, 100656. [Google Scholar] [CrossRef]
- He, D.; Li, H. Biomaterials affect cell-cell interactions in vitro in tissue engineering. J. Mater. Sci. Technol. 2021, 63, 62–72. [Google Scholar] [CrossRef]
- Ates, B.; Koytepe, S.; Ulu, A.; Gurses, C.; Thakur, V.K. Chemistry, structures, and advanced applications of nanocomposites from biorenewable resources. Chem. Rev. 2020, 120, 9304–9362. [Google Scholar] [CrossRef] [PubMed]
- Reverchon, E.; Baldino, L.; Cardea, S.; De Marco, I. Biodegradable synthetic scaffolds for tendon regeneration. Muscles Ligaments Tendons J. 2012, 2, 181–186. [Google Scholar] [PubMed]
- Liu, X.; Ma, L.; Mao, Z.; Gao, C. Chitosan-based biomaterials for tissue repair and regeneration. Adv. Polym. Sci. 2011, 244, 81–128. [Google Scholar]
- Sultankulov, B.; Berillo, D.; Sultankulova, K.; Tokay, T.; Saparov, A. Progress in the Development of Chitosan-Based Biomaterials for Tissue Engineering and Regenerative Medicine. Biomolecules 2019, 9, 470. [Google Scholar] [CrossRef] [Green Version]
- Muxika, A.; Etxabide, A.; Uranga, J.; Guerrero, P.; de la Caba, K. Chitosan as a bioactive polymer: Processing, properties and applications. Int. J. Biol. Macromol. 2017, 105, 1358–1368. [Google Scholar] [CrossRef]
- Jiang, M.; Zhuge, X.; Yang, Y.; Gu, X.; Ding, F. The promotion of peripheral nerve regeneration by chitooligosaccharides in the rat nerve crush injury model. Neurosci. Lett. 2009, 454, 239–243. [Google Scholar] [CrossRef]
- Haastert-Talini, K.; Geuna, S.; Dahlin, L.B.; Meyer, C.; Stenberg, L.; Freier, T.; Heimann, C.; Barwig, C.; Pinto, L.F.V.; Raimondo, S.; et al. Chitosan tubes of varying degrees of acetylation for bridging peripheral nerve defects. Biomaterials 2013, 34, 9886–9904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Yin, S.; Ren, L.; Zhao, L. Surface characterization of the chitosan membrane after oxygen plasma treatment and its aging effect. Biomed. Mater. 2009, 4, 035003. [Google Scholar] [CrossRef]
- Nawrotek, K.; Tylman, M.; Rudnicka, K.; Gatkowska, J.; Balcerzak, J. Tubular electrodeposition of chitosan-carbon nanotube implants enriched with calcium ions. J. Mech. Behav. Biomed. Mater. 2016, 60, 256–266. [Google Scholar] [CrossRef]
- Tao, J.; Zhang, J.; Du, T.; Xu, X.; Deng, X.; Chen, S.; Liu, J.; Chen, Y.; Liu, X.; Xiong, M.; et al. Rapid 3D printing of functional nanoparticle-enhanced conduits for effective nerve repair. Acta Biomater. 2019, 90, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Liu, S.; Zhang, C.; Wang, X.; Pu, J.; Ba, F.; Xue, S.; Ye, H.; Zhao, T.; Li, K.; et al. Programmable and printable Bacillus subtilis biofilms as engineered living materials. Nat. Chem. Biol. 2019, 15, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Restrepo, D.; Naleway, S.E.; Thomas, V.; Schniepp, H.C. Advanced Manufacturing for Biomaterials and Biological Materials, Part I. JOM 2020, 72, 1151–1153. [Google Scholar] [CrossRef] [Green Version]
- Johnson, B.N.; Lancaster, K.Z.; Zhen, G.; He, J.; Gupta, M.K.; Kong, Y.L.; Engel, E.A.; Krick, K.D.; Ju, A.; Meng, F.; et al. 3D Printed Anatomical Nerve Regeneration Pathways. Adv. Funct. Mater. 2015, 25, 6205–6217. [Google Scholar] [CrossRef]
- Song, S.; Wang, X.; Wang, T.; Yu, Q.; Hou, Z.; Zhu, Z.; Li, R. Additive Manufacturing of Nerve Guidance Conduits for Regeneration of Injured Peripheral Nerves. Front. Bioeng. Biotechnol. 2020, 8, 1–12. [Google Scholar] [CrossRef]
- Coolbear, T.; Daniel, R.M.; Morgan, H.W. The enzymes from extreme thermophiles: Bacterial sources, thermostabilities and industrial relevance. Adv. Biochem. Eng. Biotechnol. 1992, 45, 57–98. [Google Scholar] [CrossRef]
- Daly, W.; Yao, L.; Zeugolis, D.; Windebank, A.; Pandit, A. A biomaterials approach to peripheral nerve regeneration: Bridging the peripheral nerve gap and enhancing functional recovery. J. R. Soc. Interface 2012, 9, 202–221. [Google Scholar] [CrossRef] [Green Version]
- Malikmammadov, E.; Tanir, T.E.; Kiziltay, A.; Hasirci, V.; Hasirci, N. PCL and PCL-Based Materials in Biomedical Applications; Taylor & Francis: Abingdon, UK, 2018; Volume 29, ISBN 9031221031. [Google Scholar]
- Cheng, Y.; Luo, X.; Betz, J.; Buckhout-White, S.; Bekdash, O.; Payne, G.F.; Bentley, W.E.; Rubloff, G.W. In situ quantitative visualization and characterization of chitosan electrodeposition with paired sidewall electrodes. Soft Matter 2010, 6, 3177–3183. [Google Scholar] [CrossRef]
- Mąkiewicz, M.; Wach, R.A.; Nawrotek, K. Investigation of Parameters Influencing Tubular-Shaped Chitosan-Hydroxyapatite Layer Electrodeposition. Molecules 2020, 26, 104. [Google Scholar] [CrossRef]
- Wilcox, M.; Gregory, H.; Powell, R.; Quick, T.J.; Phillips, J.B. Strategies for Peripheral Nerve Repair. Curr. Tissue Microenviron. Rep. 2020, 1, 49–59. [Google Scholar] [CrossRef]
- Nawrotek, K.; Tylman, M.; Rudnicka, K.; Balcerzak, J.; Kamiński, K. Chitosan-based hydrogel implants enriched with calcium ions intended for peripheral nervous tissue regeneration. Carbohydr. Polym. 2016, 136, 764–771. [Google Scholar] [CrossRef] [PubMed]
- Danilchenko, S.N. Chitosan–hydroxyapatite composite biomaterials made by a one step co-precipitation method: Preparation, characterization and in vivo tests. J. Biol. Phys. Chem. 2009, 9, 119–126. [Google Scholar] [CrossRef] [Green Version]
- Manoj, M.; Mangalaraj, D.; Ponpandian, N.; Viswanathan, C. Core-shell hydroxyapatite/Mg nanostructures: Surfactant free facile synthesis, characterization and their in vitro cell viability studies against leukaemia cancer cells (K562). RSC Adv. 2015, 5, 48705–48711. [Google Scholar] [CrossRef]
- Khachani, M.; El Hamidi, A.; Halim, M.; Arsalane, S. Non-isothermal kinetic and thermodynamic studies of the dehydroxylation process of synthetic calcium hydroxide Ca(OH)2. J. Mater. Environ. Sci. 2014, 5, 615–624. [Google Scholar]
- Jennings, J.A.; Bumgardner, J.D. Chitosan Based Biomaterials Volume 1: Fundamentals, 1st ed.; Jennings, J.A., Bumgardner, J.D., Eds.; Woodhead Publishing: Sawtson, UK, 2016; Volume 1. [Google Scholar]
- Borschel, G.H.; Kia, K.F.; Kuzon, W.M.; Dennis, R.G. Mechanical properties of acellular peripheral nerve. J. Surg. Res. 2003, 114, 133–139. [Google Scholar] [CrossRef] [Green Version]




| Electrodeposition at the Build Plate | |||
|---|---|---|---|
| U [V] | 8 | 13 | 18 |
| Qt [C] | 94.4 | 102 | 81.9 |
| [μmol] | 294 | 316 | 340 |
| X [%] | 75.9 | 76.4 | 73.7 |
| Electrodeposition at the Build Plate with the PCL Skeleton | |||
| Qt [C] | 80.9 | 84.1 | 99.0 |
| [μmol] | 361 | 360 | 377 |
| X [%] | 77.7 | 76.2 | 74.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nawrotek, K.; Mąkiewicz, M.; Zawadzki, D. Fabrication and Characterization of Polycaprolactone/Chitosan—Hydroxyapatite Hybrid Implants for Peripheral Nerve Regeneration. Polymers 2021, 13, 775. https://doi.org/10.3390/polym13050775
Nawrotek K, Mąkiewicz M, Zawadzki D. Fabrication and Characterization of Polycaprolactone/Chitosan—Hydroxyapatite Hybrid Implants for Peripheral Nerve Regeneration. Polymers. 2021; 13(5):775. https://doi.org/10.3390/polym13050775
Chicago/Turabian StyleNawrotek, Katarzyna, Mariusz Mąkiewicz, and Dawid Zawadzki. 2021. "Fabrication and Characterization of Polycaprolactone/Chitosan—Hydroxyapatite Hybrid Implants for Peripheral Nerve Regeneration" Polymers 13, no. 5: 775. https://doi.org/10.3390/polym13050775
APA StyleNawrotek, K., Mąkiewicz, M., & Zawadzki, D. (2021). Fabrication and Characterization of Polycaprolactone/Chitosan—Hydroxyapatite Hybrid Implants for Peripheral Nerve Regeneration. Polymers, 13(5), 775. https://doi.org/10.3390/polym13050775