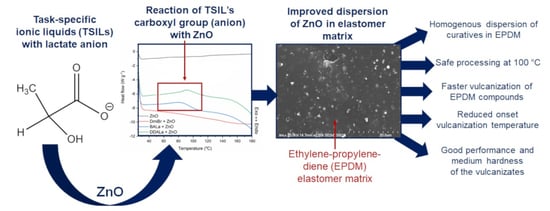
Task-Specific Ionic Liquids with Lactate Anion Applied to Improve ZnO Dispersibility in the Ethylene-Propylene-Diene Elastomer
Abstract
1. Introduction
2. Materials and Methods
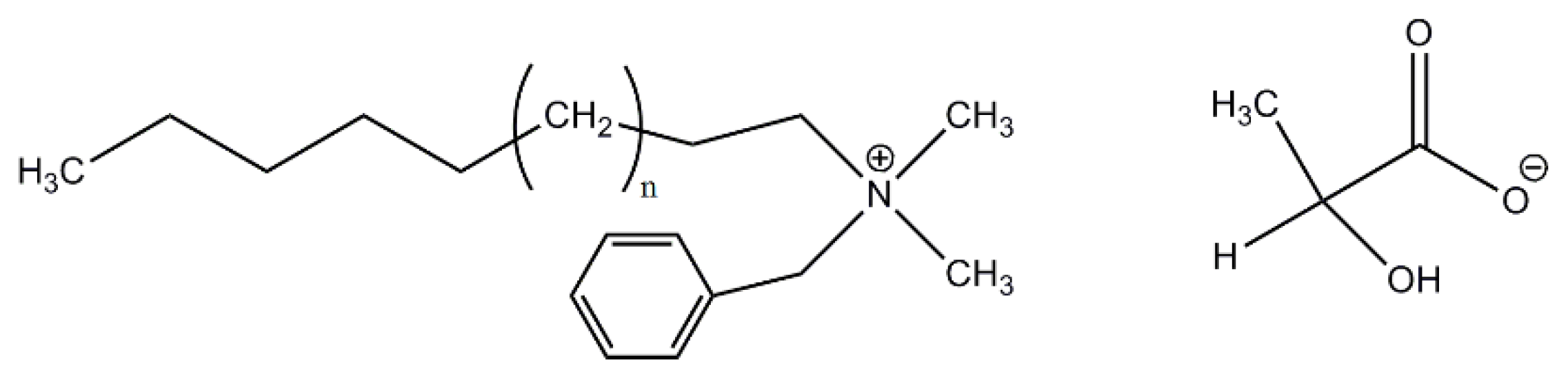
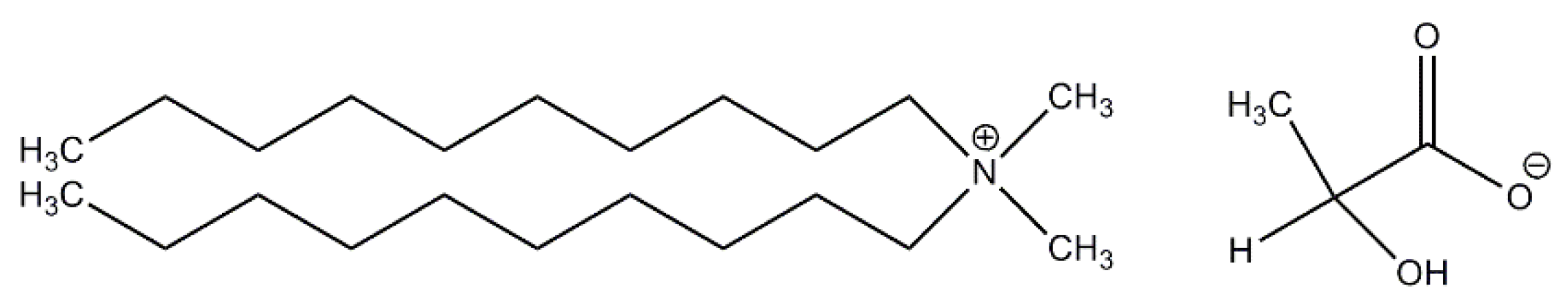
2.1. Materials
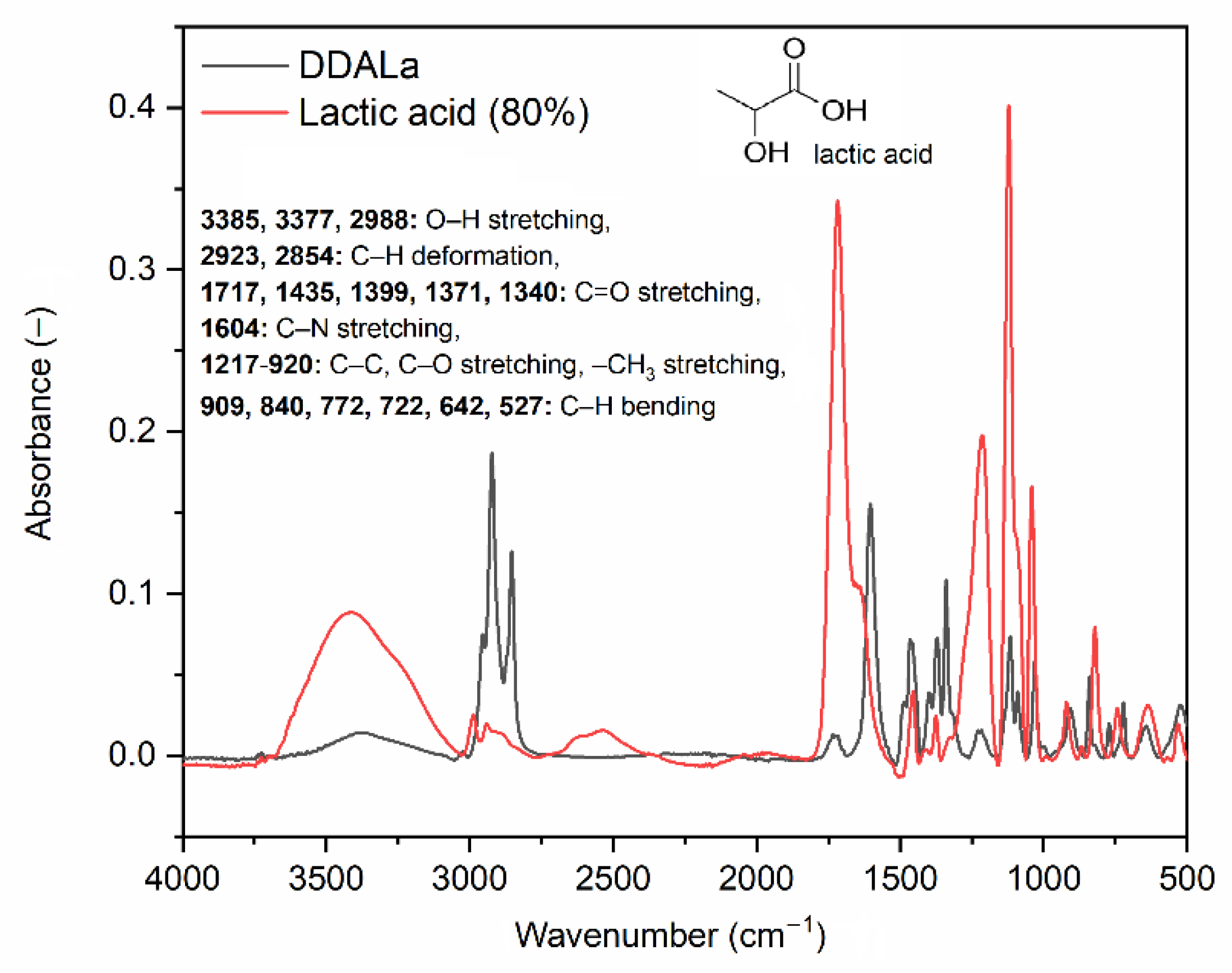
2.2. Characterization of TSILs and Possible Interaction between Their Carboxyl Groups and ZnO
2.3. Preparation and Characterization of EPDM Compounds
3. Results
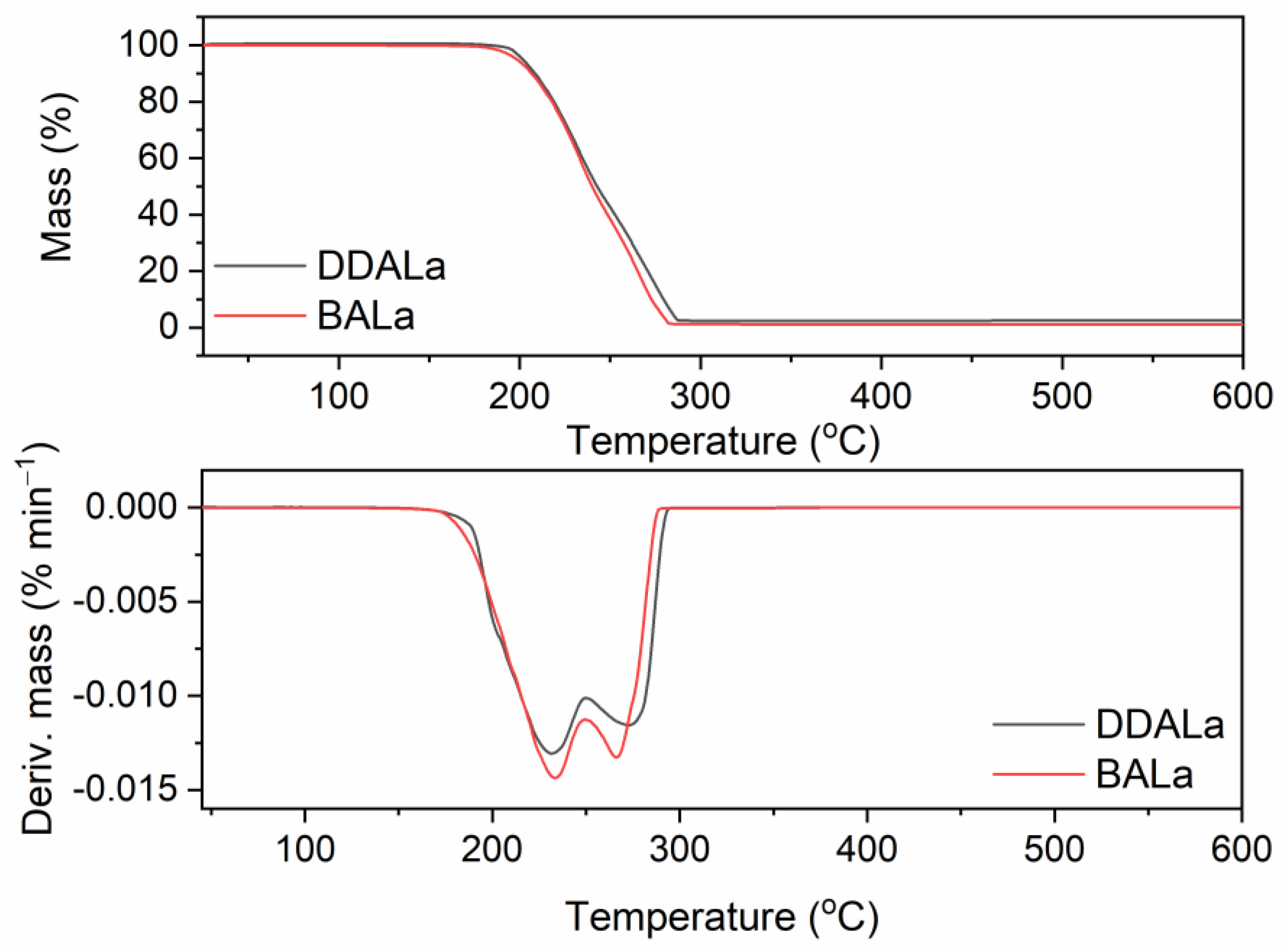
3.1. Thermal Stability of TSILs with Lactate Anione
3.2. Phase Transition of TSILs with Lactate Anion
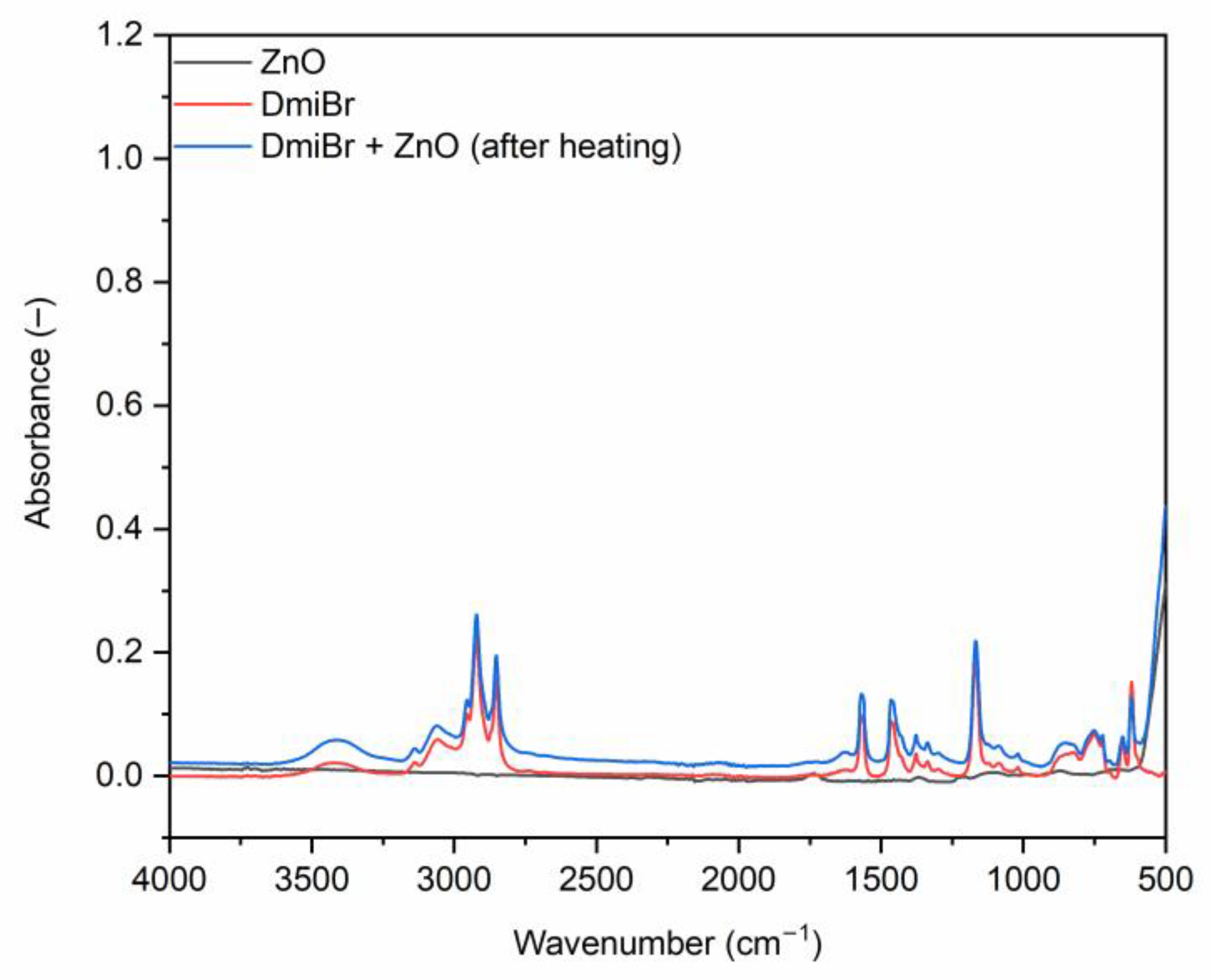
3.3. FTIR Analysis of ZnO and Its Mixtures with ILs
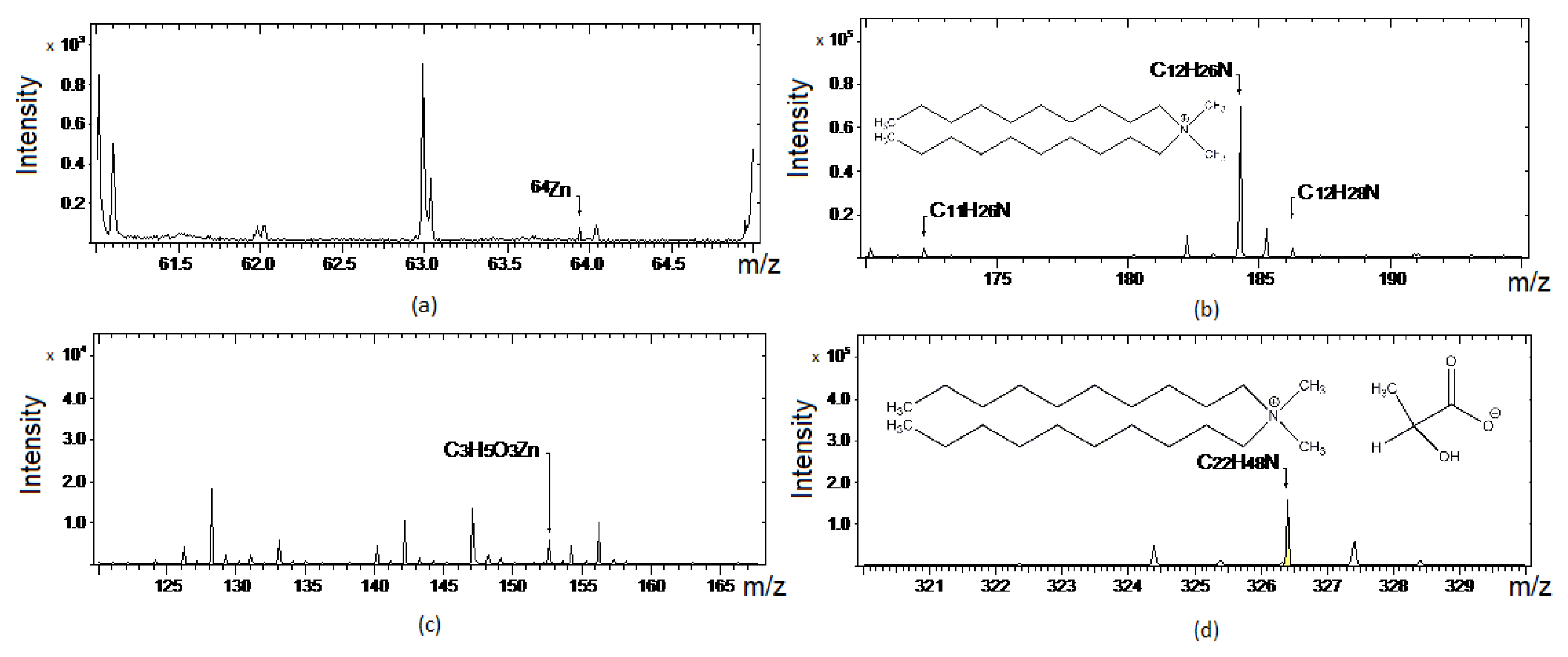
3.4. TOF-SIMS Analysis of DDALa and ZnO Mixture
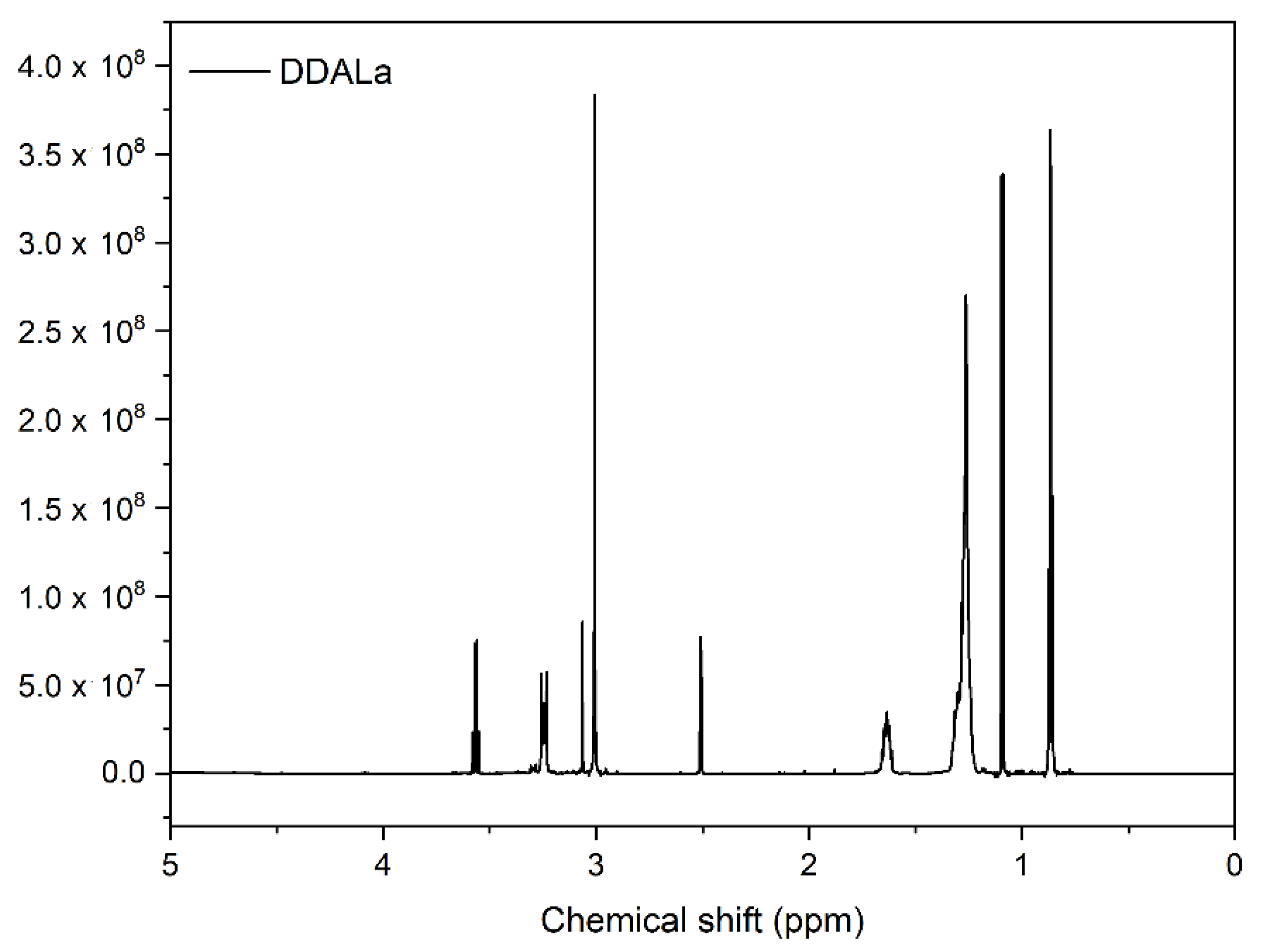
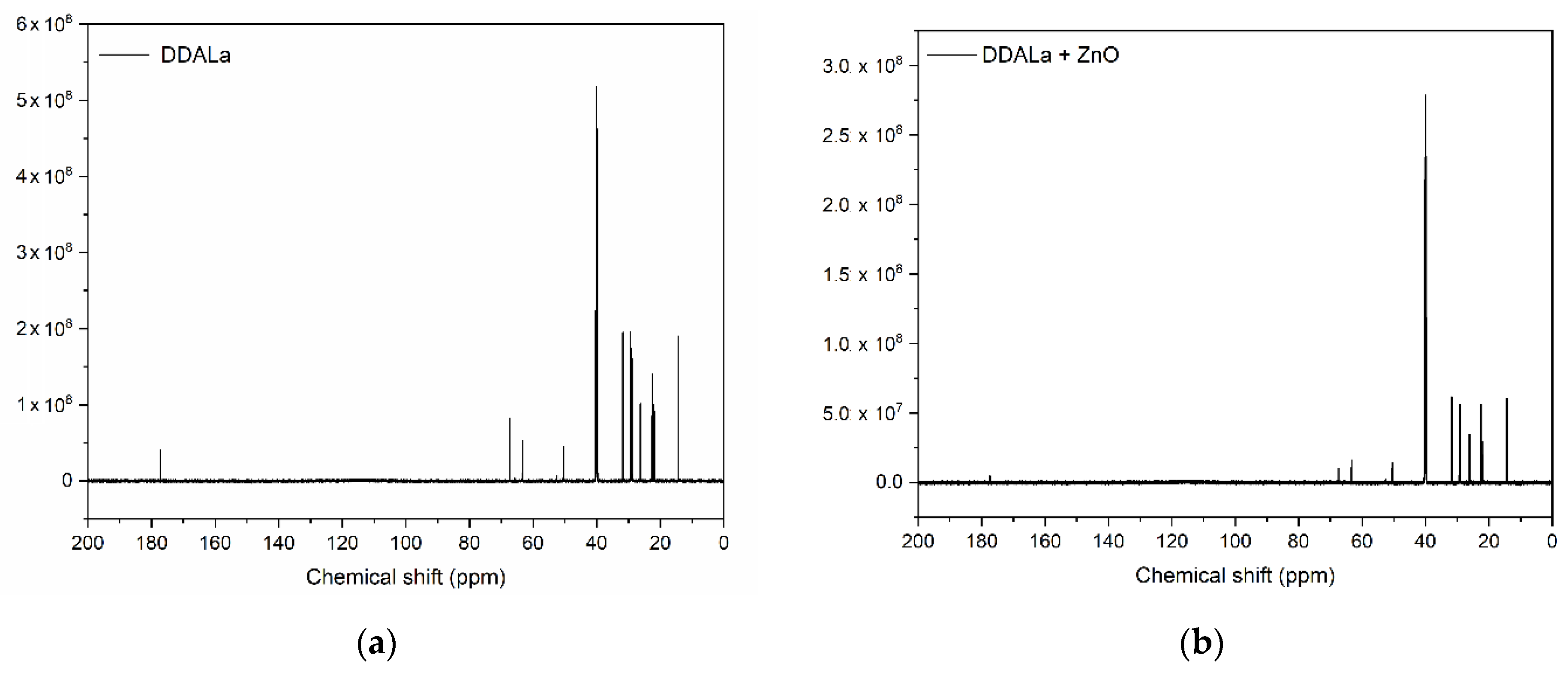
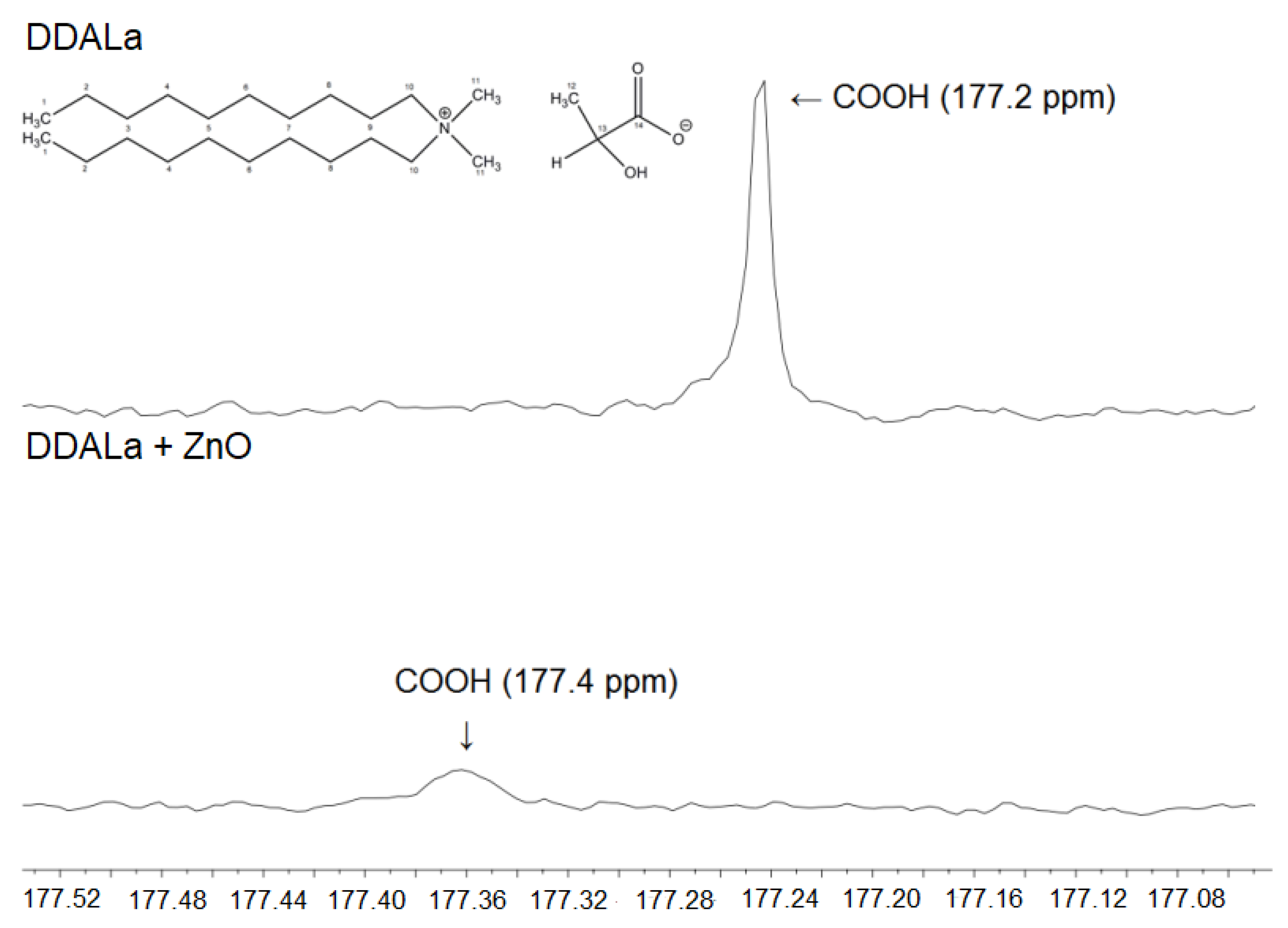
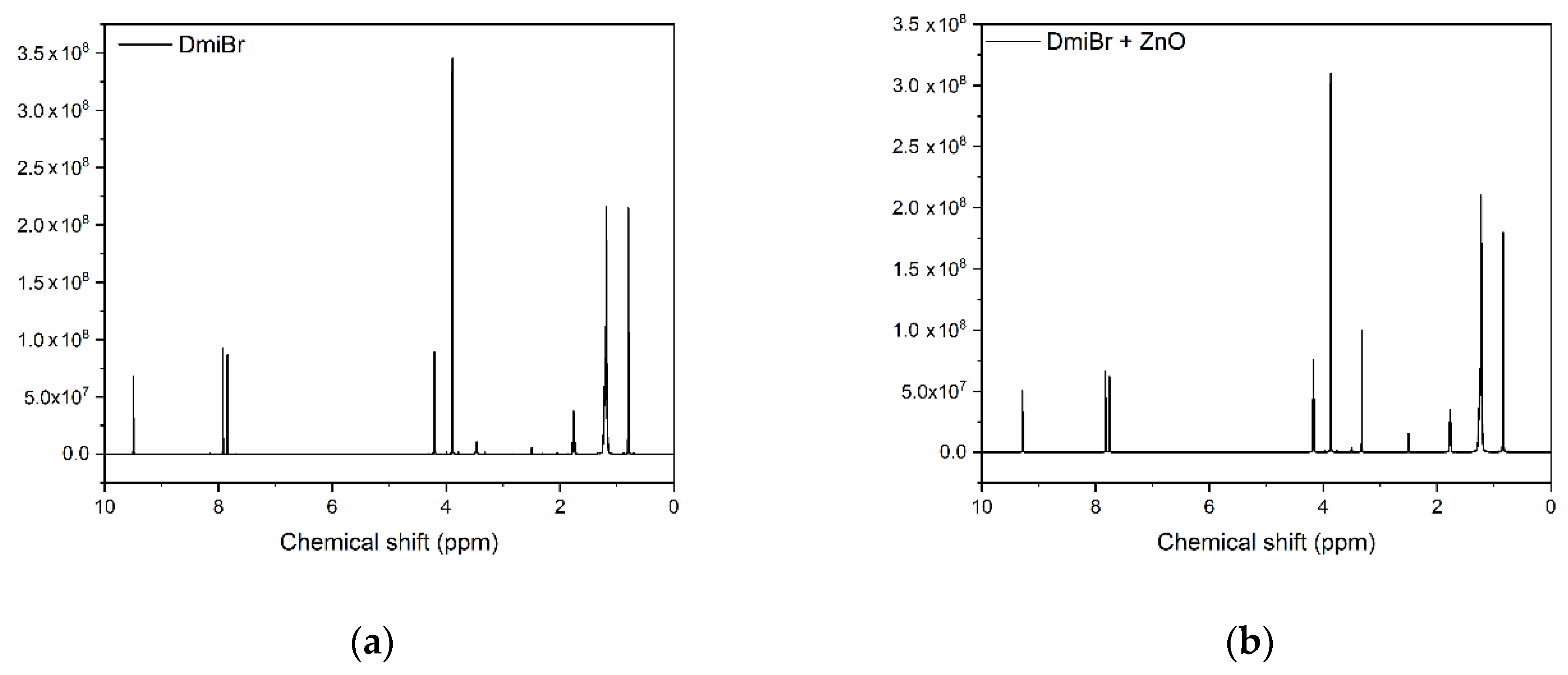
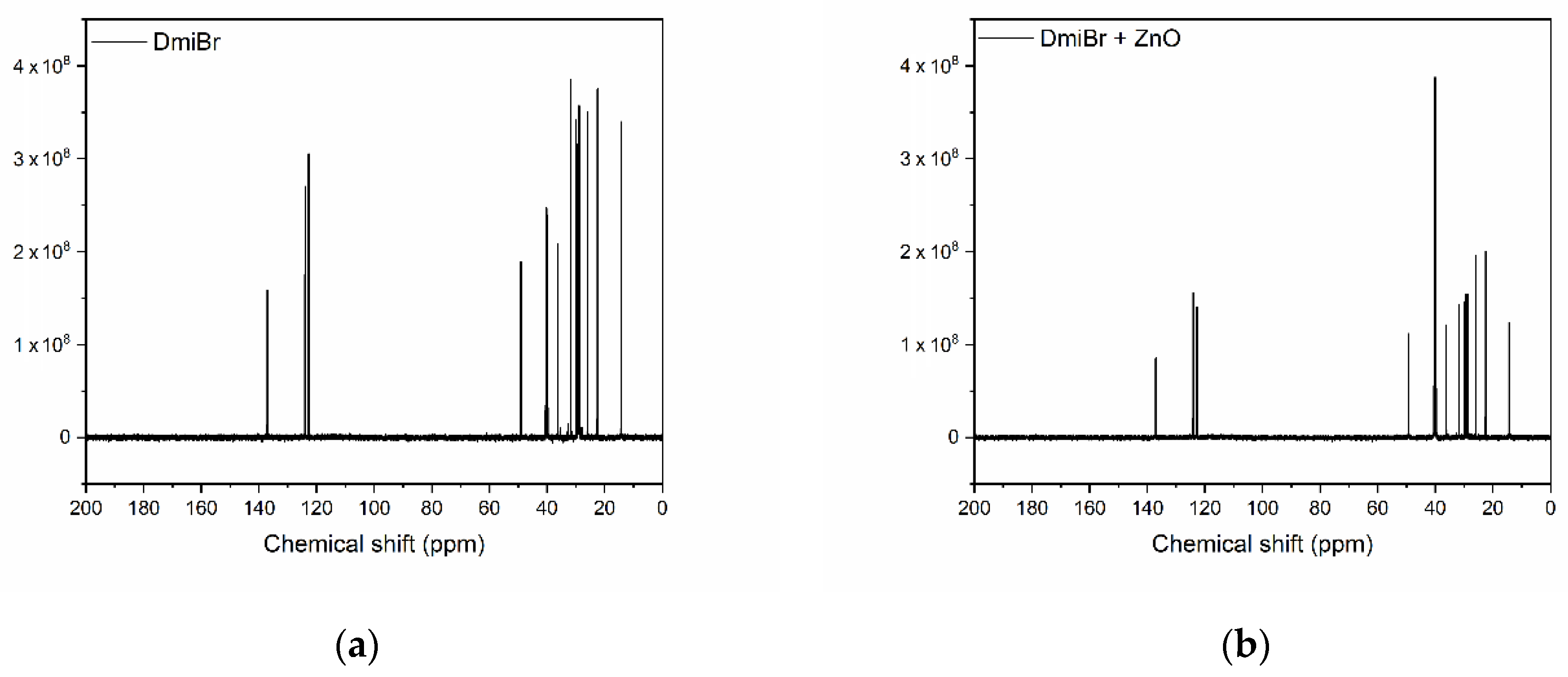
3.5. Nuclear Magnetic Resonance (NMR) Analysis of ZnO and Its Mixtures with ILs
3.6. Effect of TSILs on Dispersion of ZnO and Curatives in the EPDM Matrix
3.7. Effect of TSILs on Cure Characteristics of EPDM Compounds and Crosslink Densities of Vulcanizates
3.8. Effect of TSILs on Temperature and Enthalpy of Vulcanization Studied Using DSC Analysis
3.9. Effect of TSILs on Tensile Properties and Hardness of EPDM Vulcanizates
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wasserscheid, P.; Welton, T. Ionic Liquids in Synthesis, 2nd ed.; Wiley-VCH: New York, NY, USA, 2008. [Google Scholar]
- Rogers, R.D.; Seddon, K.R. Ionic Liquids—Solvents of the Future? Science 2003, 302, 792–793. [Google Scholar] [CrossRef] [PubMed]
- Ngo, H.L.; LeCompte, K.; Hargens, L.; McEwen, A.B. Thermal properties of imidazolium ionic liquids. Thermochim. Acta 2000, 357–358, 97–102. [Google Scholar] [CrossRef]
- Hagiwara, R.; Ito, Y. Room temperature ionic liquids of alkylimidazolium cations and fluoroanions. J. Fluorine. Chem. 2000, 105, 221–227. [Google Scholar] [CrossRef]
- Kubisa, P. Ionic liquids in the synthesis and modification of polymers. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 4675–4683. [Google Scholar] [CrossRef]
- Holbrey, J.D.; Seddon, K.R. Ionic liquids. Clean Products Process. 1999, 1, 223–236. [Google Scholar] [CrossRef]
- Singha, N.K.; Pramanik, N.B.; Behera, P.K.; Chakrabarty, A.; Matys, J.W. Tailor-made thermoreversible functional polymer via RAFT polymerization in an ionic liquid: A remarkably fast polymerization process. Green Chem. 2016, 18, 6115–6122. [Google Scholar] [CrossRef]
- Hong, K.; Zhang, H.; Matys, J.W.; Visser, A.E.; Brazel, C.S.; Holbrey, J.D.; Reichert, W.M.; Rogers, R.D. Conventional free radical polymerization in room temperature ionic liquids: A green approach to commodity polymers with practical advantages. Chem. Commun. 2002, 13, 1368–1369. [Google Scholar] [CrossRef]
- Yuan, C.; Guo, J.; Sia, Z.; Yan, F. Polymerization in ionic liquid-based microemulsions. Polym. Chem. 2015, 6, 4059–4066. [Google Scholar] [CrossRef]
- Vijayaraghavan, R.; MacFarlane, D.R. Living cationic polymerisation of styrene in an ionic liquid. Chem. Commun. 2004, 6, 700–701. [Google Scholar] [CrossRef] [PubMed]
- Sarbu, T.; Matyjaszewski, K. ATRP of Methyl Methacrylate in the Presence of Ionic Liquids with Ferrous and Cuprous Anions. Chem. Phys. 2001, 202, 3379–3391. [Google Scholar] [CrossRef]
- Walden, P. Molecular weights and electrical conductivity of several fused salts. Bull. Acad. Imper. Sci. 1914, 8, 405–422. [Google Scholar]
- Wilkes, J.S. A short history of ionic liquids—from molten salts to neoteric solvents. Green Chem. 2002, 4, 73–80. [Google Scholar] [CrossRef]
- Wilkes, J.S. Properties of ionic liquid solvents for catalysis. J. Mol. Catal. A 2004, 214, 11–17. [Google Scholar] [CrossRef]
- Chippe, C.; Pierracini, D. Ionic liquids: Solvent properties and organic reactivity. J. Phys. Org. Chem. 2005, 18, 275–297. [Google Scholar] [CrossRef]
- Fei, Z.; Geldbach, T.J.; Zhao, D.; Dyson, P. From dysfunction to bis-function: On the design and applications of functionalised ionic liquids. J. Chem. Eur. J. 2006, 12, 2122–2130. [Google Scholar] [CrossRef]
- Bodo, E.; Migliorati, V. Theoretical Description of Ionic Liquids. In Ionic Liquids—Classes and Properties; Handy, S., Ed.; InTechOpen: Rijeka, Croatia, 2011. [Google Scholar] [CrossRef]
- Yacob, Z.; Liebscher, J. 1,2,3-Triazolium Salts as a Versatile New Class of Ionic Liquids. In Ionic Liquids—Classes and Properties; Handy, S., Ed.; InTechOpen: Rijeka, Croatia, 2011. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, J. Facile EG/ionic liquid interfacial synthesis of uniform RE3+ doped NaYF4 nanocubes. Chem. Commun. 2010, 46, 592–594. [Google Scholar] [CrossRef] [PubMed]
- Fei, Z.; Zhao, D.; Geldbach, T.J.; Scopelliti, R.; Dyson, P.J. Brønsted Acidic Ionic Liquids and Their Zwitterions: Synthesis, Characterization and pKa Determination. Chem. Eur. J. 2004, 10, 4886–4893. [Google Scholar] [CrossRef]
- Xiao, L.; Lv, D.; Wu, W. Brønsted Acidic Ionic Liquids Mediated Metallic Salts Catalytic System for the Chemical Fixation of Carbon Dioxide to Form Cyclic Carbonates. Catal. Lett. 2011, 141, 1838–1844. [Google Scholar] [CrossRef]
- Nockeman, P.; Thijs, B.; Pittois, S.; Thoen, J.; Glorieux, C.; Van Hecke, K.; Van Meervelt, L.; Kirchner, B.; Binnemans, K. Task-specific ionic liquid for solubilizing metal oxides. J. Phys. Chem. B. 2006, 110, 20978–20992. [Google Scholar] [CrossRef]
- Nockemann, P.; Thijs, B.; Lunstroot, L.; Parac-Vogt, T.N.; Gorller-Warland, C.; Binnemans, K.; Van Hecke, K.; Van Meervelt, L.; Nikitenko, S.; Daniels, S.; et al. Speciation of rare-earth metal complexes in ionic liquids: A multiple-technique approach. Chem. Eur. J. 2009, 15, 1449–1461. [Google Scholar] [CrossRef]
- Heideman, G.; Datta, R.N.; Noordermeer, J.W.M.; van Baarle, B. Activators in Accelerated Sulfur Vulcanization. Rubber Chem. Technol. 2004, 77, 514–541. [Google Scholar] [CrossRef]
- Wolfe, J.R.; Pugh, T.L.; Killian, A.S. The Chemistry of Sulfur Curing. III. Effects of Zinc Oxide on the Mechanism of the Reaction of Cyclohexene with Sulfur. Rubber Chem. Technol. 1968, 41, 1329–1338. [Google Scholar] [CrossRef]
- Kresja, M.R.; Koenig, J.L. A Review of Sulfur Crosslinking Fundamentals for Accelerated and Unaccelerated Vulcanization. Rubber Chem. Technol. 1993, 66, 376–410. [Google Scholar] [CrossRef]
- Coran, A.Y. Vulcanization of Rubber. In Science & Technology of Rubber, 2nd ed.; Mark, J., Erman, B., Eirich, F., Eds.; Academic Press: New York, NY, USA, 1978; pp. 291–338. [Google Scholar]
- Saville, B.; Watson, A.A. Structural Characterization of Sulfur-Vulcanized Rubber Networks. Rubber Chem. Technol. 1967, 40, 100–148. [Google Scholar] [CrossRef]
- Manik, S.P.; Banerjee, S. Sulfenamide Accelerated Sulfur Vulcanization of Natural Rubber in Presence and Absence of Dicumyl Peroxide. Rubber Chem. Technol. 1970, 43, 1311–1326. [Google Scholar] [CrossRef]
- Shelton, J.R.; McDonel, E.T. Investigation of Radical and Polar Mechanisms in Vulcanization Reactions. Rubber Chem. Technol. 1960, 33, 342–356. [Google Scholar] [CrossRef]
- Morita, E.; Young, E.J. A Study of Sulfenamide Acceleration. Rubber Chem. Technol. 1963, 36, 844–862. [Google Scholar] [CrossRef]
- Heideman, G. Reduced Zinc Oxide Levels in Sulphur Vulcanization of Rubber Compounds. Ph.D. Thesis, University of Twente, Enschede, The Netherlands, 2004. [Google Scholar]
- Sowinska, A.; Maciejewska, M.; Guo, L.; Delebecq, E. Thermal Analysis and SEM Microscopy Applied to Studying the Efficiency of Ionic Liquid Immobilization on Solid Supports. Materials 2019, 12, 1579. [Google Scholar] [CrossRef] [PubMed]
- Sowinska, A.; Maciejewska, M.; Guo, L.; Delebecq, E. Effect of SILPs on the Vulcanization and Properties of Ethylene–Propylene–Diene Elastomer. Polymers 2020, 12, 1220. [Google Scholar] [CrossRef]
- International Organization for Standardization. ISO 1817:2015, Rubber, Vulcanized or Thermoplastic—Determination of Effect of Liquids; International Organization for Standardization: Geneva, Switzerland, 2017. [Google Scholar]
- Flory, P.J.; Rehner, J. Statistical mechanics of cross-linked polymer networks. II. Swelling. J. Chem. Phys. 1943, 11, 521–526. [Google Scholar] [CrossRef]
- Zamboni, V.; Flisi, U.; Giunchi, G. Crosslink density evaluation of EPDM vulcanizates. Rubber Chem. Technol. 1971, 44, 1109–1129. [Google Scholar] [CrossRef]
- International Organization for Standardization. ISO 37:2017, Rubber, Vulcanized or Thermoplastic—Determination of Tensile Stress-Strain Properties; International Organization for Standardization: Geneva, Switzerland, 2017. [Google Scholar]
- International Organization for Standardization. ISO 868:2003, Plastics and Ebonite—Determination of Indentation Hardness by Means of a durometer (Shore Hardness); International Organization for Standardization: Geneva, Switzerland, 2003. [Google Scholar]
- Nockemann, P.; Thijs, B.; Parac-Vogt, T.N.; Van Hecke, L.; VanMeervelt, L.; Tinant, B.; Hartenbach, I.; Schleid, T.; Ngan, V.T.; Nguyen, M.T.; et al. Carboxyl-Functionalized Task-Specific Ionic Liquids for Solubilizing Metal Oxides. Inorg. Chem. 2008, 47, 9987–9999. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, H.; Kotlyar, V.; Nudelman, A. NMR Chemical Shifts of Common Laboratory Solvents as Trace Impurities. J. Org. Chem. 1997, 62, 7512–7515. [Google Scholar] [CrossRef] [PubMed]
- Heideman, G.; Datta, R.N.; Noordermeer, J.W.M.; van Baarle, B. Influence of Zinc Oxide during Different Stages of Sulfur Vulcanization. Elucidated by Model Compound Vulcanization. J. Appl. Polym. Sci. 2005, 95, 1388–1404. [Google Scholar] [CrossRef]
- Heideman, G.; Noordermeer, J.W.M.; Datta, R.N.; van Baarle, B. Various Ways to Reduce Zinc Oxide Levels in S-SBR Rubber Compounds. Macromol. Symp. 2006, 245–246, 657–667. [Google Scholar] [CrossRef]
- Prochon, M.; Janowska, G.; Przepiorkowska, A.; Kucharska-Jastrząbek, A. Thermal properties and combustibility of elastomer-protein composites Part, I. Composites SBR-keratin. J. Therm. Anal. Calorim. 2012, 109, 1563–1570. [Google Scholar] [CrossRef]










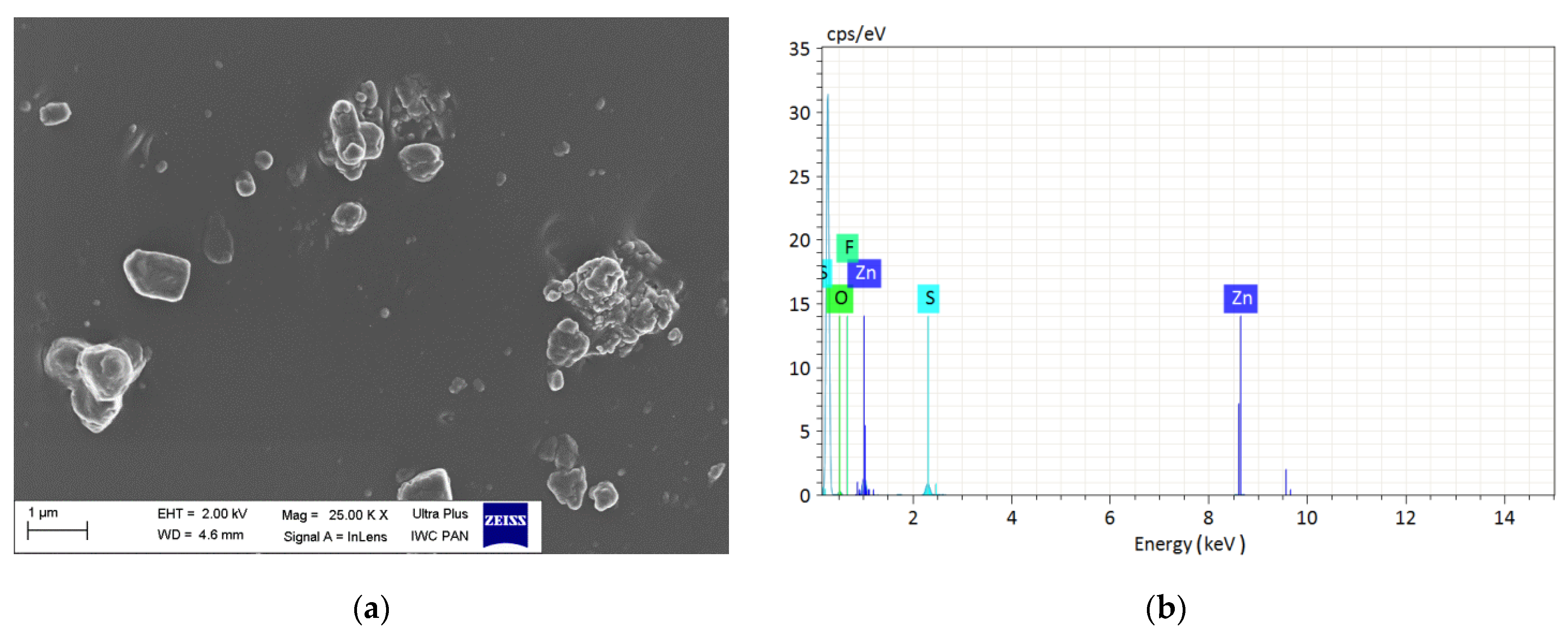

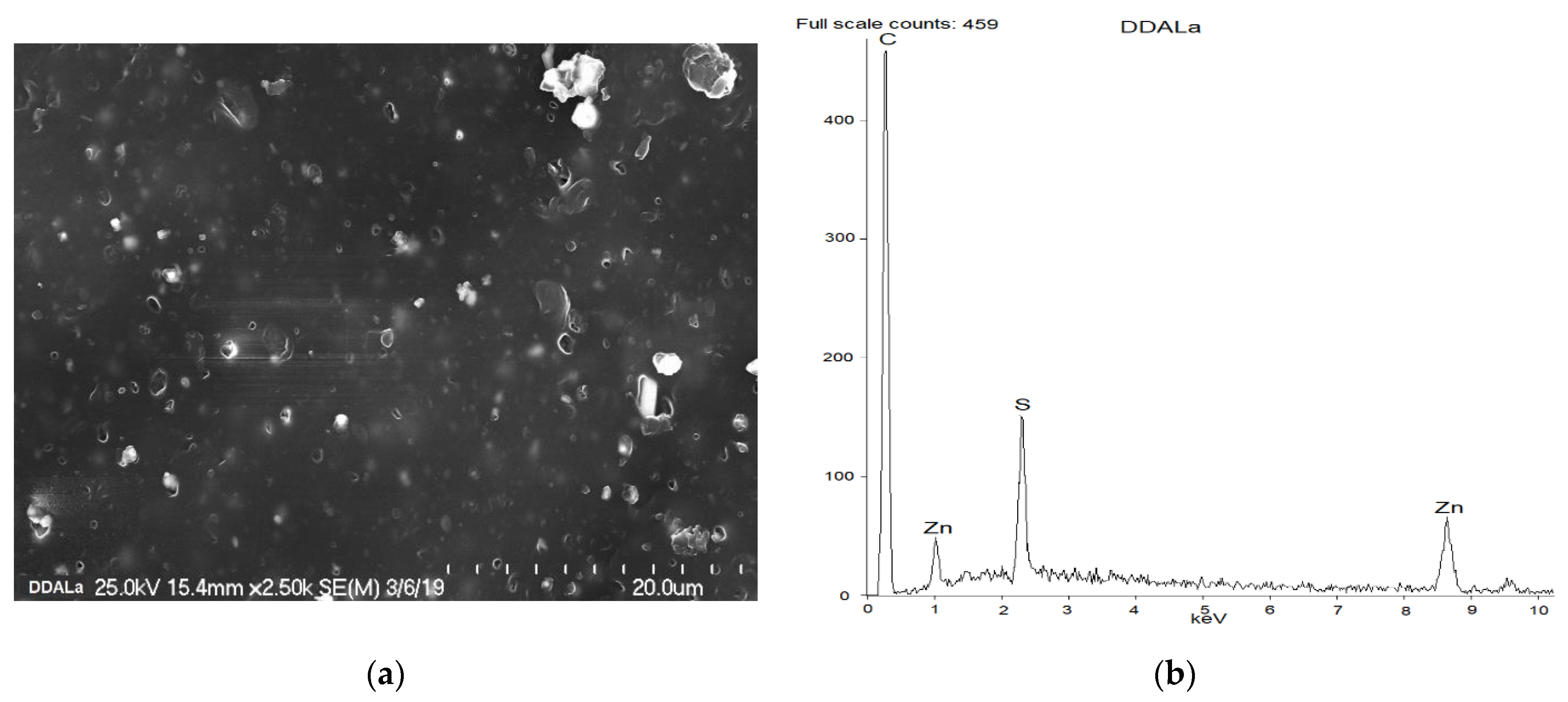

| Components, phr | Masterbatch |
|---|---|
| EPDM | 100.0 |
| Carbon black | 125.0 |
| Mineral oil | 65.0 |
| Chalk | 40.0 |
| CaO | 7.5 |
| ZnO | 7.5 |
| Stearic acid | 1.5 |
| Components, phr | Reference Sample | Rubber Compounds with TSILs |
|---|---|---|
| Masterbatch | 346.5 | 346.5 |
| Sulfur | 1.9 | 1.9 |
| MBTS | 1.0 | 1.0 |
| Rhenogran TP 50 | 1.5 | 1.5 |
| CBS | 0.6 | 0.6 |
| Stearic acid | 1.5 | 1.5 |
| TSIL | - | 3.0 |
| Components, phr | Reference Sample | Rubber Compounds with Ionic Liquid |
|---|---|---|
| EPDM | 100.0 | 100.0 |
| Sulfur | 1.5 | 1.5 |
| MBTS | 1.0 | 1.0 |
| Rhenogran TP 50 | 1.5 | 1.5 |
| CBS | 0.6 | 0.6 |
| Stearic acid | 1.5 | 1.5 |
| ZnO | 7.5 | 7.5 |
| TSIL | - | 3.0 |
| Sample | Temperature Range of Exothermic Peak (°C) | Tpeak (°C) | ∆H (J/g) |
|---|---|---|---|
| ZnO | - | - | - |
| DmiBr + ZnO | - | - | - |
| BALa + ZnO | 81–112 | 90 | 3.6 |
| DDALa + ZnO | 63–109 | 92 | 4.2 |
| Carbon | Chemical Shift (ppm) | |
|---|---|---|
| DDALa | DDALa + ZnO | |
| C1 | 14.4 | 14.4 |
| C2 | 22.1 | 22.1 |
| C3 | 31.8 | 31.8 |
| C4 | 28.9 | 28.9 |
| C5 | 29.4 | 29.4 |
| C6 | 29.3 | 29.3 |
| C7 | 29.1 | 29.1 |
| C8 | 26.2 | 26.2 |
| C9 | 22.6 | 22.6 |
| C10 | 63.3 | 63.3 |
| C11 | 50.4 | 50.4 |
| C12 | 21.9 | 21.9 |
| C13 | 67.3 | 67.4 |
| C14 | 177.2 | 177.4 |
| DMSO | 40.0 | 40.0 |
| Carbon | Chemical Shift (ppm) | |
|---|---|---|
| DmiBr | DmiBr + ZnO | |
| C1 | 13.7 | 13.7 |
| C2 | 21.8 | 21.8 |
| C3 | 29.2 | 29.2 |
| C4 | 28.6 | 28.6 |
| C5 | 28.7 | 28.7 |
| C6 | 28.4 | 28.4 |
| C7 | 28.2 | 28.2 |
| C8 | 25.3 | 25.3 |
| C9 | 31.0 | 31.0 |
| C10 | 48.5 | 48.5 |
| C11 | 122.1 | 122.1 |
| C12 | 123.3 | 123.3 |
| C13 | 136.3 | 136.3 |
| C14 | 35.6 | 35.6 |
| DMSO | 40.0 | 40.0 |
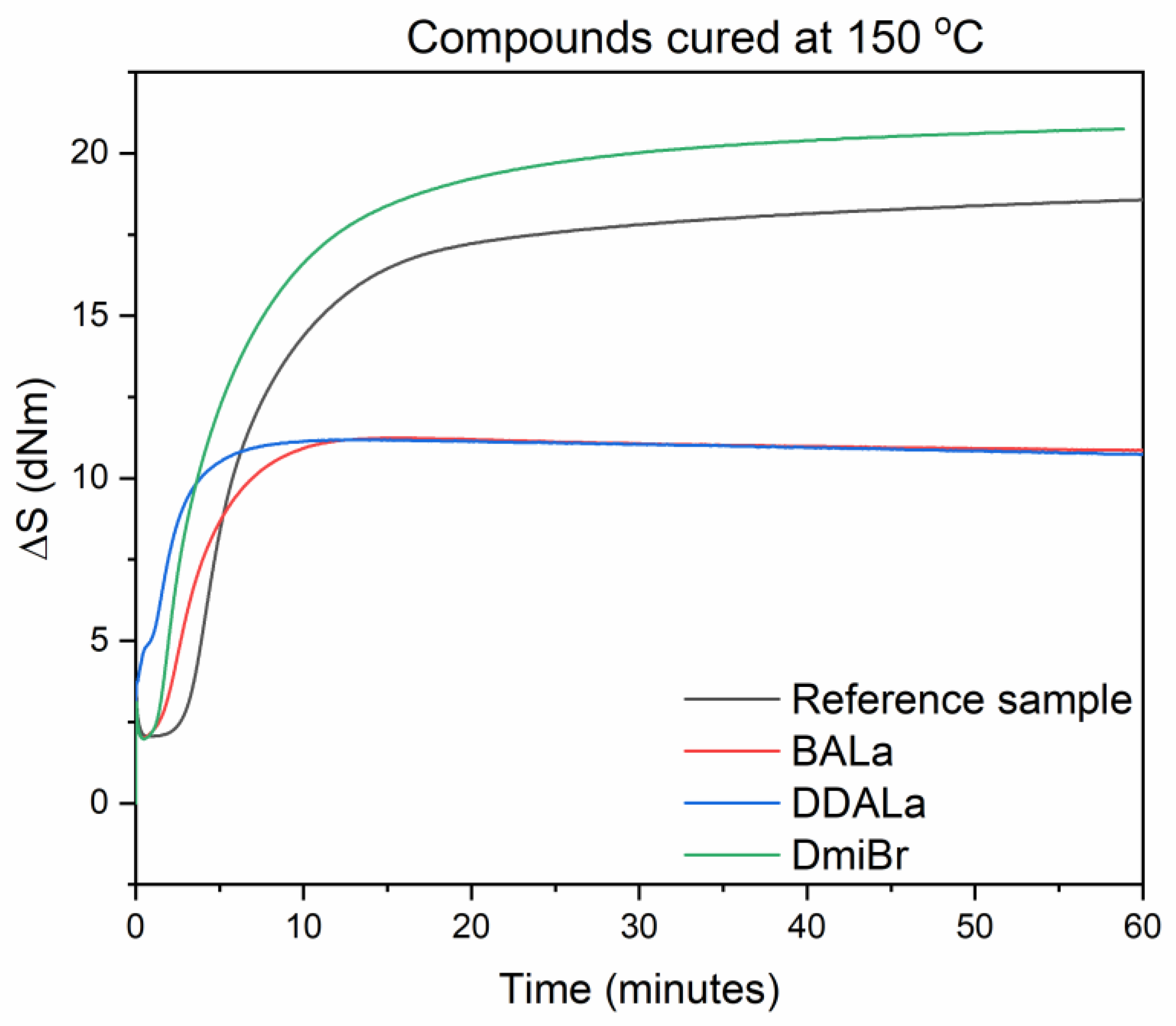
| EPDM Compounds | Smin (dNm) | Smax (dNm) | ∆S (dNm) | t05 at 100 °C (min) | t95 (min) | νe (× 10−5 mole/cm3) |
|---|---|---|---|---|---|---|
| Reference sample | 2.1 | 18.6 | 16.5 | no curing | 29 | 7.7 |
| BALa | 2.0 | 11.2 | 9.2 | no curing | 9 | 4.2 |
| DDALa | 3.0 | 11.2 | 8.2 | no curing | 6 | 4.7 |
| DmiBr | 2.0 | 20.8 | 18.8 | 28 | 26 | 7.7 |
| EPDM Compounds | Temperature of Vulcanization (°C) | ∆H (J/g) |
|---|---|---|
| Reference sample | 134–220 | 4.8 |
| BALa | 93–196 | 6.0 |
| DDALa | 87–195 | 6.4 |
| DmiBr | 111–195 | 3.3 |
| EPDM Vulcanizates | SE100 (MPa) | TS (MPa) | EB (%) | H (ShA) |
|---|---|---|---|---|
| Reference sample | 4.3 ± 0.1 | 10.9 ± 1.4 | 321 ± 57 | 70 ± 1 |
| BALa | 2.1 ± 0.1 | 8.1 ± 0.2 | 581 ± 34 | 59 ± 1 |
| DDALa | 2.9 ± 0.1 | 7.0 ± 0.2 | 387 ± 24 | 60 ± 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sowińska, A.; Maciejewska, M.; Guo, L.; Delebecq, E. Task-Specific Ionic Liquids with Lactate Anion Applied to Improve ZnO Dispersibility in the Ethylene-Propylene-Diene Elastomer. Polymers 2021, 13, 774. https://doi.org/10.3390/polym13050774
Sowińska A, Maciejewska M, Guo L, Delebecq E. Task-Specific Ionic Liquids with Lactate Anion Applied to Improve ZnO Dispersibility in the Ethylene-Propylene-Diene Elastomer. Polymers. 2021; 13(5):774. https://doi.org/10.3390/polym13050774
Chicago/Turabian StyleSowińska, Anna, Magdalena Maciejewska, Laina Guo, and Etienne Delebecq. 2021. "Task-Specific Ionic Liquids with Lactate Anion Applied to Improve ZnO Dispersibility in the Ethylene-Propylene-Diene Elastomer" Polymers 13, no. 5: 774. https://doi.org/10.3390/polym13050774
APA StyleSowińska, A., Maciejewska, M., Guo, L., & Delebecq, E. (2021). Task-Specific Ionic Liquids with Lactate Anion Applied to Improve ZnO Dispersibility in the Ethylene-Propylene-Diene Elastomer. Polymers, 13(5), 774. https://doi.org/10.3390/polym13050774