Synthesis of Di-Block Copolymers Poly (Propylene oxide)-block-Poly (9-(2,3-epoxypropyl) Carbazole) via Sequential Addition of Monomers in One Pot
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of 9-(2,3-epoxypropyl) Carbazole (EPK)
2.3. PPO and PPO-b-PEPK Synthesis
2.4. Methods
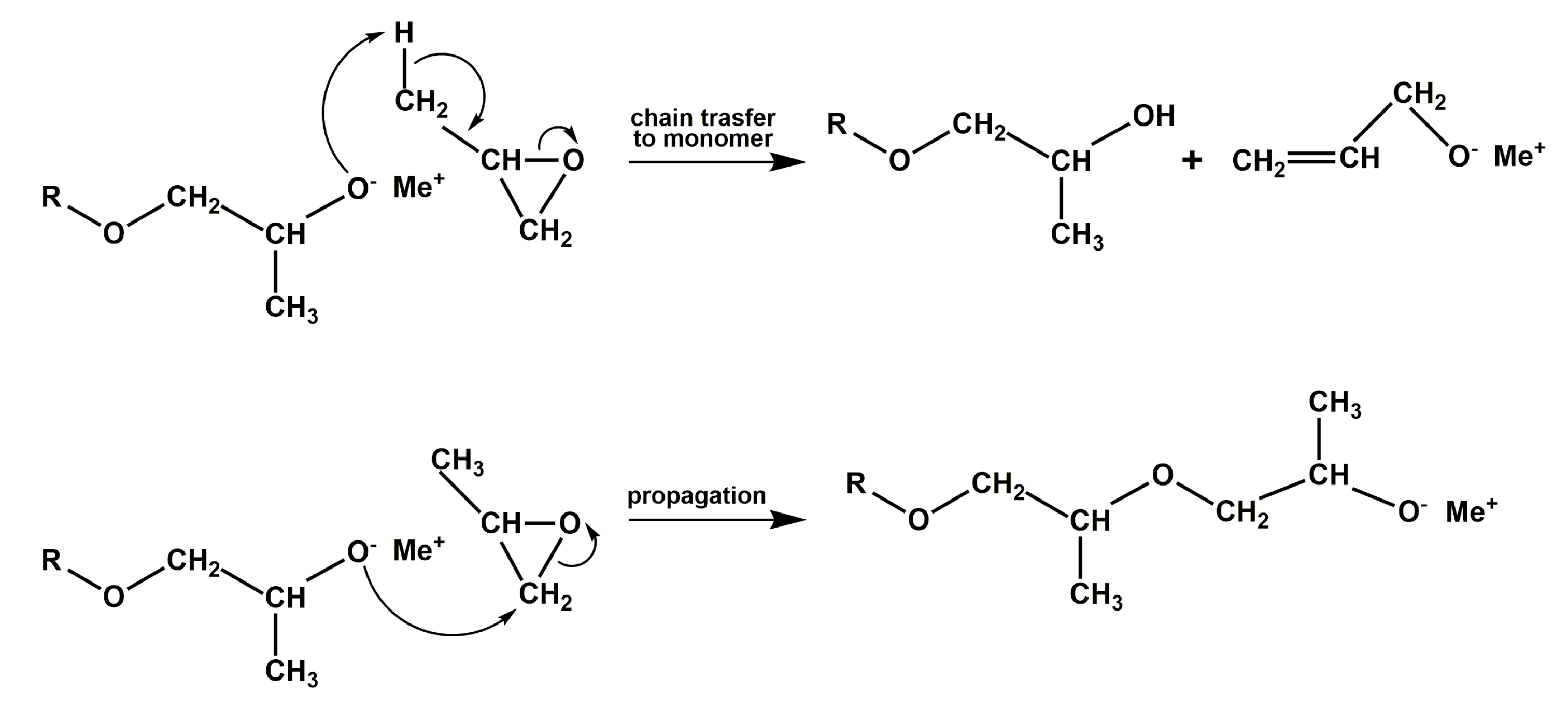
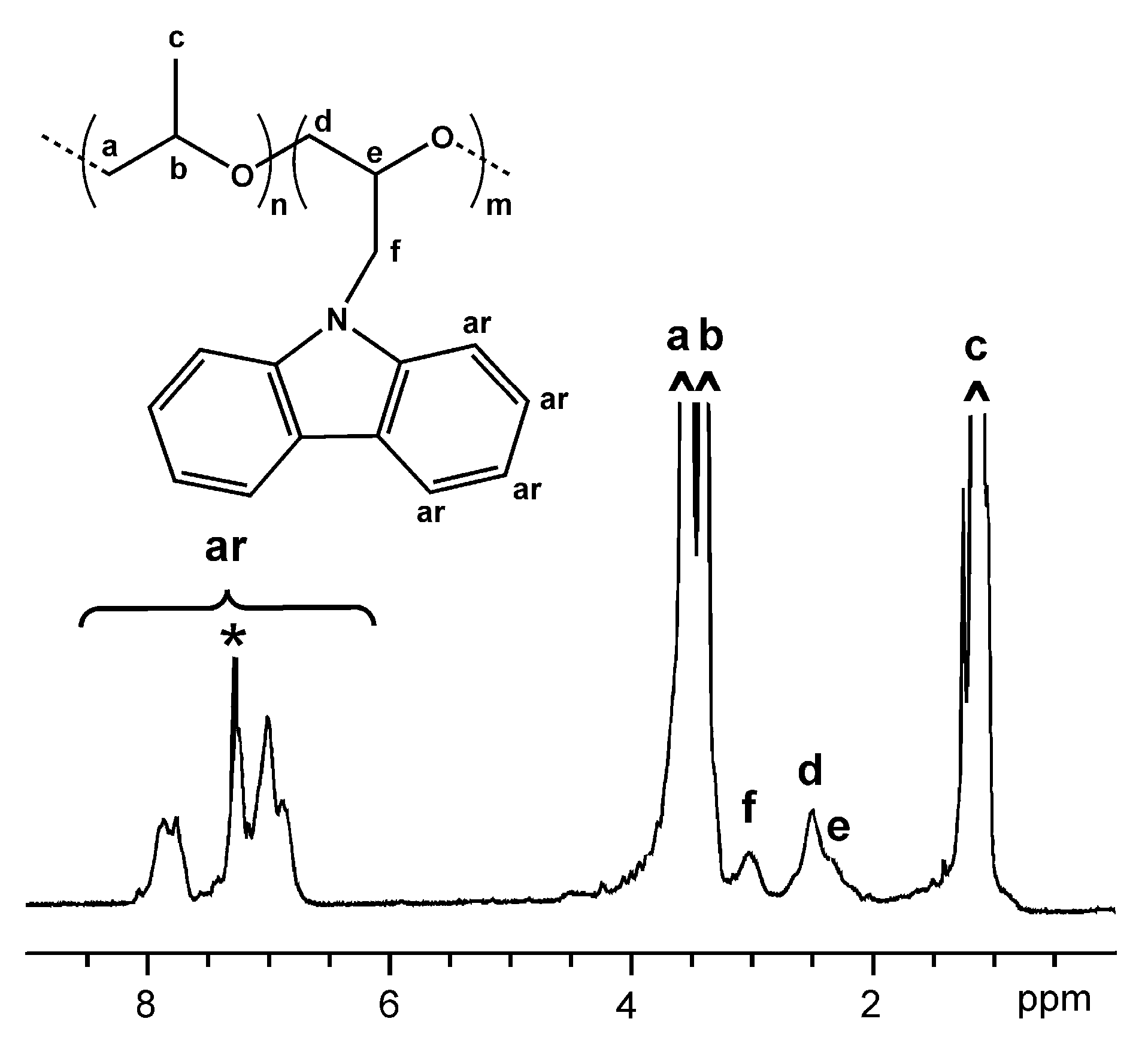
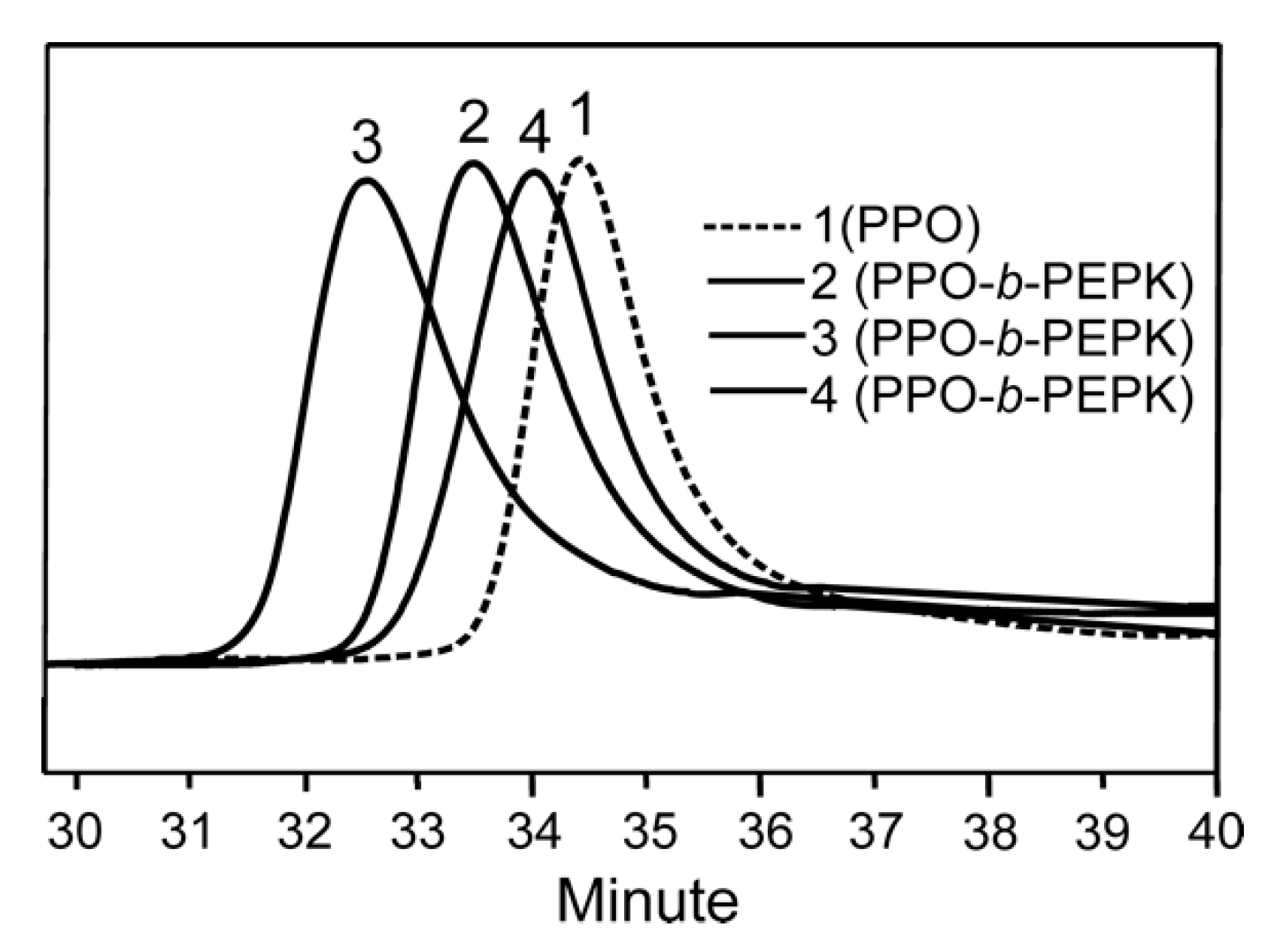
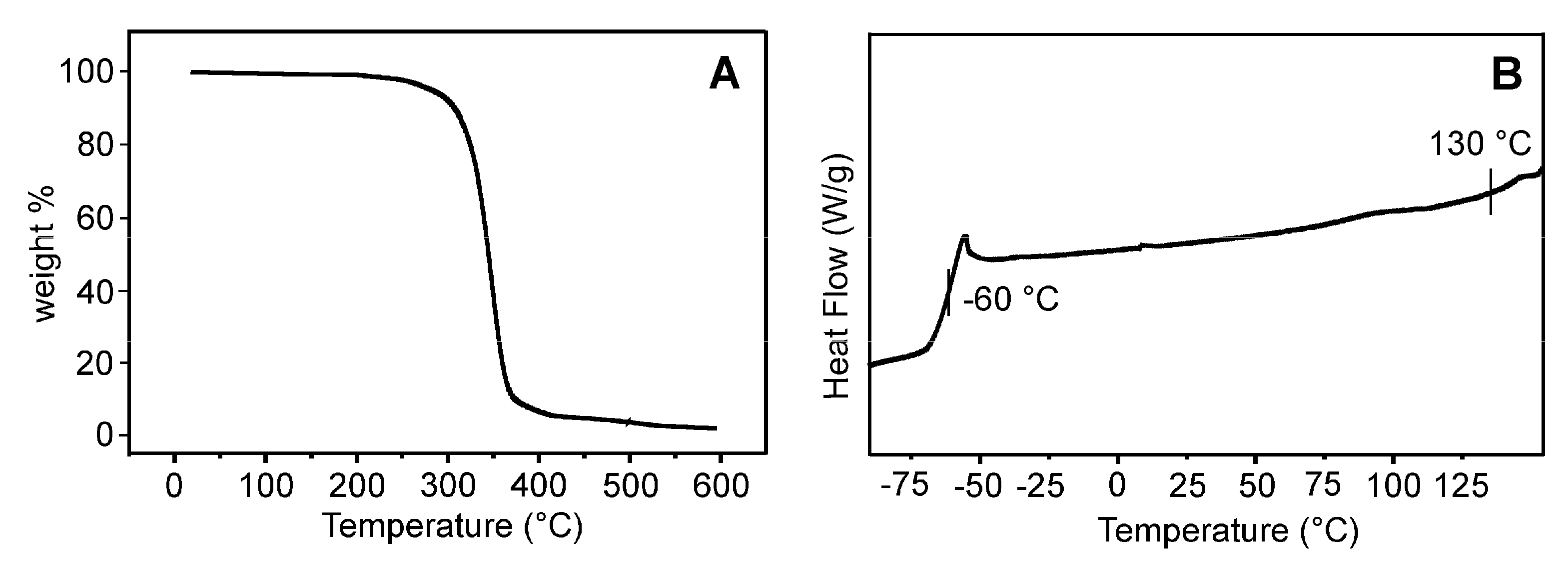
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grazulevicius, J.V.; Strohriegl, P.; Pielichowski, J.; Pielichowski, K. Carbazole-containing polymers: Synthesis, properties and applications. Prog. Polym. Sci. 2003, 28, 1297–1353. [Google Scholar] [CrossRef]
- Liguori, R.; Botta, A.; Rubino, A.; Pragliola, S.; Venditto, V. Stereoregular polymers with pendant carbazolyl groups: Synthesis, properties and optoelectronic applications. Synth. Met. 2018, 246, 185–194. [Google Scholar] [CrossRef]
- Bugatti, V.; Concilio, S.; Iannelli, P.; Piotto, S.P.; Bellone, S.; Ferrara, M.; Neitzert, H.C.; Rubino, A.; Della Sala, D.; Vacca, P. Synthesis and characterization of new electroluminescent molecules containing carbazole and oxadiazole units. Synth. Met. 2006, 156, 13–20. [Google Scholar] [CrossRef]
- Jiang, Z.; Yao, H.; Liu, Z.; Yang, C.; Zhong, C.; Qin, J.; Liu, Y. Bent ladder-type hexaphenylene with carbazole core and spiro linkage as stable and efficient blue emitter. Org. Lett. 2010, 11, 4132–4135. [Google Scholar] [CrossRef]
- Hung, Y.W.; Chi, L.C.; Chen, W.J.; Chen, Y.M.; Chou, S.H.; Wong, K.T. A new benzimidazole/carbazole hybrid bipolar material for highly efficient deep-blue electrofluorescence, yellow–green electrophosphorescence, and two-color-based white OLEDs. J. Mater. Chem. 2010, 20, 10113–10119. [Google Scholar] [CrossRef]
- Ye, T.; Chen, J.; Ma, D. Electroluminescence of poly(N-vinylcarbazole) films: Fluorescence, phosphorescence and electromers. Phys. Chem. Chem. Phys. 2010, 12, 15410–15413. [Google Scholar] [CrossRef]
- Fenwick, O.; Fusco, S.; Baig, T.N.; Di Stasio, F.; Steckler, T.T.; Heriksson, P.; Fléchon, C.; Andersson, M.R.; Cacialli, F. Efficient red electroluminescence from diketopyrrolopyrrole copolymerised with a polyfluorene. APL Mater. 2013, 1, 032108. [Google Scholar] [CrossRef]
- Hoegl, H. On Photoelectric Effects in Polymers and Their Sensitization by Dopants. J. Phys. Chem. 1965, 69, 755–766. [Google Scholar] [CrossRef]
- Uryu, T.; Ohkawa, H.; Oshima, R. Synthesis and High Hole Mobility of Isotactic Poly(2-N-carbazolylethyl acrylate). Macromolecules 1987, 20, 712–716. [Google Scholar] [CrossRef]
- Hu, C.J.; Oshima, R.; Sato, S.; Seno, M. Synthesis and photoinduced discharge characteristics of polyacrylates with pendant carbazole group. J. Polym. Sci. C Polym. Lett. 1988, 26, 441–446. [Google Scholar] [CrossRef]
- Keyanpour-Rad, M.; Ledwith, A.; Hallam, A.; North, A.M.; Breton, M.; Hoyle, C.; Guillet, J.E. Some photophysical properties of five new carbazole-containing methacrylate polymers. Macromolecules 1978, 11, 1114–1118. [Google Scholar] [CrossRef]
- Du, F.S.; Li, Z.C.; Hong, W.; Cao, Q.Y.; Li, F.M. Vinyl monomers bearing chromophore moieties and their polymers. XI. Synthesis and photochemical behavior of carbazole-containing methacrylic monomers and their polymers. J. Polym. Sci. A Polym. Chem. 2000, 38, 679–688. [Google Scholar] [CrossRef]
- Ledwith, A.; Rowley, N.J.; Walker, S.M. Fluorescence emission from poly[2-(9-ethyl)carbazolyl-methylmethacrylate]. Polymer 1981, 22, 435–436. [Google Scholar] [CrossRef]
- Barik, S.; Valiyaveettil, S. Synthesis and self-assembly of copolymers with pendant electroactive units. Macromolecules 2008, 41, 6376–6386. [Google Scholar] [CrossRef]
- Botta, A.; Pragliola, S.; Capacchione, C.; Rubino, A.; Liguori, R.; De Girolamo Del Mauro, A.; Venditto, V. Synthesis of poly(4-(N-carbazolyl)methyl styrene)s: Tailoring optical properties through stereoregularity. Eur. Polym. J. 2017, 88, 246–256. [Google Scholar] [CrossRef]
- Kanbara, T.; Yokokawa, Y.; Hasegawa, K. Palladium-catalyzed modification of poly(p-bromostyrene) with carbazole and related heteroarenes containing an N-H bond and their properties. J. Polym. Sci. A Polym. Chem. 2000, 38, 28–34. [Google Scholar] [CrossRef]
- Cho, Y.S.; Kim, S.W.; Ihn, C.S.; Lee, J.S. Anionic polymerization of 4-(9-carbazolyl)methylstyrene. Polymer 2001, 42, 7611–7616. [Google Scholar] [CrossRef]
- Botta, A.; Pragliola, S.; Venditto, V.; Rubino, A.; Aprano, S.; De Girolamo Del Mauro, A.; Maglione, M.G.; Minarini, C. Synthesis, characterization, and use as emissive layer of white organic light emitting diodes of the highly isotactic poly(N-pentenyl-carbazole). Polym. Compos. 2015, 36, 1110–1117. [Google Scholar] [CrossRef]
- Botta, A.; Venditto, V.; Rubino, A.; Pragliola, S. Highly isotactic poly(N-butenyl-carbazole): Synthesis, characterization, and optical properties. J. Chem. 2016, 2016, 1656459. [Google Scholar] [CrossRef]
- Liguori, R.; Botta, A.; Pragliola, S.; Venditto, V.; Velardo, A.; Aprano, S.; Maglione, M.G.; Prontera, C.T.; De Girolamo Del Mauro, A. Study of the electroluminescence of highly stereoregular poly(N-pentenylcarbazole) for blue and white OLEDs. Semicond. Sci. Technol. 2017, 32, 065006. [Google Scholar] [CrossRef]
- Botta, A.; Costabile, C.; Venditto, V.; Pragliola, S.; Liguori, R.; Rubino, A.; Alberga, D.; Savarese, M.; Adamo, C. Optoeletronic properties of poly(N-alkenyl-carbazole)s driven by polymer stereoregularity. J. Polym. Sci. A Polym. Chem. 2018, 59, 242–251. [Google Scholar] [CrossRef]
- Mandowska, E.; Mazela, W.; Crub, P.; Mandowski, A.; Pielichowski, J.; Swiatek, J. Luminescence properties of epoxy resins modified with a carbazole derivative. Macromol. Symp. 2004, 212, 269–273. [Google Scholar] [CrossRef]
- Malinauskas, T.; Vilionskiene, I.; Grazulevicius, J.V.; Getautis, V.; Reina, J.A.; Sidaravicius, J. Synthesis and properties of new derivatives of poly[9-(2,3-epoxypropyl)carbazole]. Polym. Int. 2008, 57, 1159–1164. [Google Scholar] [CrossRef]
- Khetubol, A.; Van Snick, S.; Stanislovaityte, E.; Hassinen, A.; Coutiño-Gonzales, E.; Vanderlinden, W.; Firdaus, Y.; Fron, E.; Vlasselaer, M.; Simokaitiene, J.; et al. Triplet Harvesting in Poly(9-vinylcarbazole) and Poly(9-(2,3-epoxypropyl)carbazole) Doped with CdSe/ZnS Quantum Dots Encapsulated with 16-(N-Carbazolyl) Hexadecanoic Acid Ligands. J. Polym. Sci. B Polym. Phys. 2014, 52, 539–551. [Google Scholar] [CrossRef]
- Akhmedov, K.M.; Karimov, K.S.; Scherbakova, I.M.; Porschnev, Y.N.; Cherkashin, M.I. The synthesis and properties of N-(2,3-epoxypropyl)carbazoles and the oligomers based on them. Russ. Chem. Rev. 1990, 59, 425. [Google Scholar] [CrossRef]
- Grazulevicius, J.V.; Kublickas, R. Polymerization of carbazolyl-containing epoxides by activated monomer mechanism. Eur. Polym. J. 1991, 27, 1411–1416. [Google Scholar] [CrossRef]
- Nakabayashi, K.; Mori, H. Novel Complex Polymers with Carbazole Functionality by Controlled Radical Polymerization. Int. J. Polym. Sci. 2012, 2012, 170912. [Google Scholar] [CrossRef]
- Nakabayashi, K.; Mori, H. Recent progress in controlled radical polymerization of N-vinyl monomers. Eur. Polym. J. 2013, 49, 2808–2838. [Google Scholar] [CrossRef]
- Benoıt, H.L.; Maric, M. Water-Soluble/Dispersible Carbazole-Containing Random and Block Copolymers by Nitroxide-Mediated Radical Polymerisation. Can. J. Chem. Eng. 2013, 91, 618–629. [Google Scholar]
- Kim, M.J.; Yu, Y.G.; Kang, N.G.; Kang, B.G.; Lee, J.S. Precise Synthesis of Functional Block Copolymers by Living Anionic Polymerization of Vinyl Monomers Bearing Nitrogen Atoms in the Side Chain. Macromol. Chem. Phys. 2017, 218, 1600445. [Google Scholar] [CrossRef]
- Boileau, S. Comprehensive Polymer Science, Chain Polymerization; Pergamon: Anatolia, Turkey, 1989; Volume 3, pp. 467–487, part 1. [Google Scholar]
- Gagnon, S.D. Encyclopedia of Polymer Science and Engineering, 2nd ed.; Kruschwitz, J.I., Ed.; Wiley-Interscience: New York, NY, USA, 1985; Volume 6, pp. 273–307. [Google Scholar]
- Quirk, R.P.; Lizarraga, G.M. Anionic synthesis of well-defined, poly[(styrene)-block-(propylene oxide)] block copolymers. Macromol. Chem. Phys. 2000, 201, 1395–1404. [Google Scholar] [CrossRef]
- Billouard, C.; Carlotti, S.; Desbois, P.; Deffieux, A. Controlled High-Speed Anionic Polymerization of Propylene Oxide Initiated by Alkali Metal Alkoxide/Trialkylaluminum Systems. Macromolecules 2004, 37, 4038–4043. [Google Scholar] [CrossRef]
- Zang, Y.; Tangadanchu, V.K.R.; Cheng, Y.; Yang, R.G.; Lin, J.M.; Zhu, C.H. Potential Antimicrobial Isopropanol-Conjugated Carbazole Azoles as Dual Targeting Inhibitors of Enterococcus faecalis. ACS Med. Chem. Lett. 2018, 9, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Berlman, I.B. Handbook of Fluorescence Spectra of Aromatic Molecules Academic; Elsevier: Amsterdam, The Netherlands, 1971. [Google Scholar]
- Johnson, P.C.; Offen, H.W. Excimer Fluorescence of Poly(N-vinylcarbazole). Chem. Phys. 1971, 55, 2945–2949. [Google Scholar] [CrossRef]
- Gaidelis, V.; Krisciunas, V.; Montrimas, E. Optical and Photoelectric properties of Thin Layers of Poly-N-epoxypropylcarbazole. Thin Solid Films 1976, 38, 9–14. [Google Scholar] [CrossRef]
- Perepichka, D.F.; Perepichka, I.F.; Bryce, M.R.; Sokolovc, N.I.; Moore, A.J. π-Extended nitrofluorene-1,3-dithiole chromophore: Enhancing the photoresponse of holographic materials through the balance of intramolecular charge transfer and electron affinity. J. Mater. Chem. 2001, 11, 1772–1774. [Google Scholar] [CrossRef]
- Itaya, A.; Okamoto, K.; Kusabayashi, S. Singlet Excitation Energy Transfer in the Vinyl Polymers with Pendant Carbazolyl Groups. Bull. Chem. Soc. Jpn. 1977, 50, 22–26. [Google Scholar] [CrossRef]
- Johnson, G.E. Emission properties of vinylcarbazole polymers. J. Chem. Phys. 1975, 62, 4697–4709. [Google Scholar] [CrossRef]
- Evers, F.; Kobs, K.; Memming, R.; Terrell, D.R. Intramolecular excimer emission of poly(N-vinylcarbazole) and rac- and meso-2,4-di-N-carbazolylpentane. Model substances for its syndiotactic and isotactic dyads. J. Am. Chem. Soc. 1983, 105, 5988–5995. [Google Scholar] [CrossRef]
- Rivaton, A.; Mailhot, B.; Derderian, G.; Bussiere, P.O.; Gardette, J.L. Investigation of the Photophysical Processes and Photochemical Reactions Involved in PVK Films Irradiated at λ > 300 nm. Macromolecules 2003, 36, 5815–5824. [Google Scholar] [CrossRef]





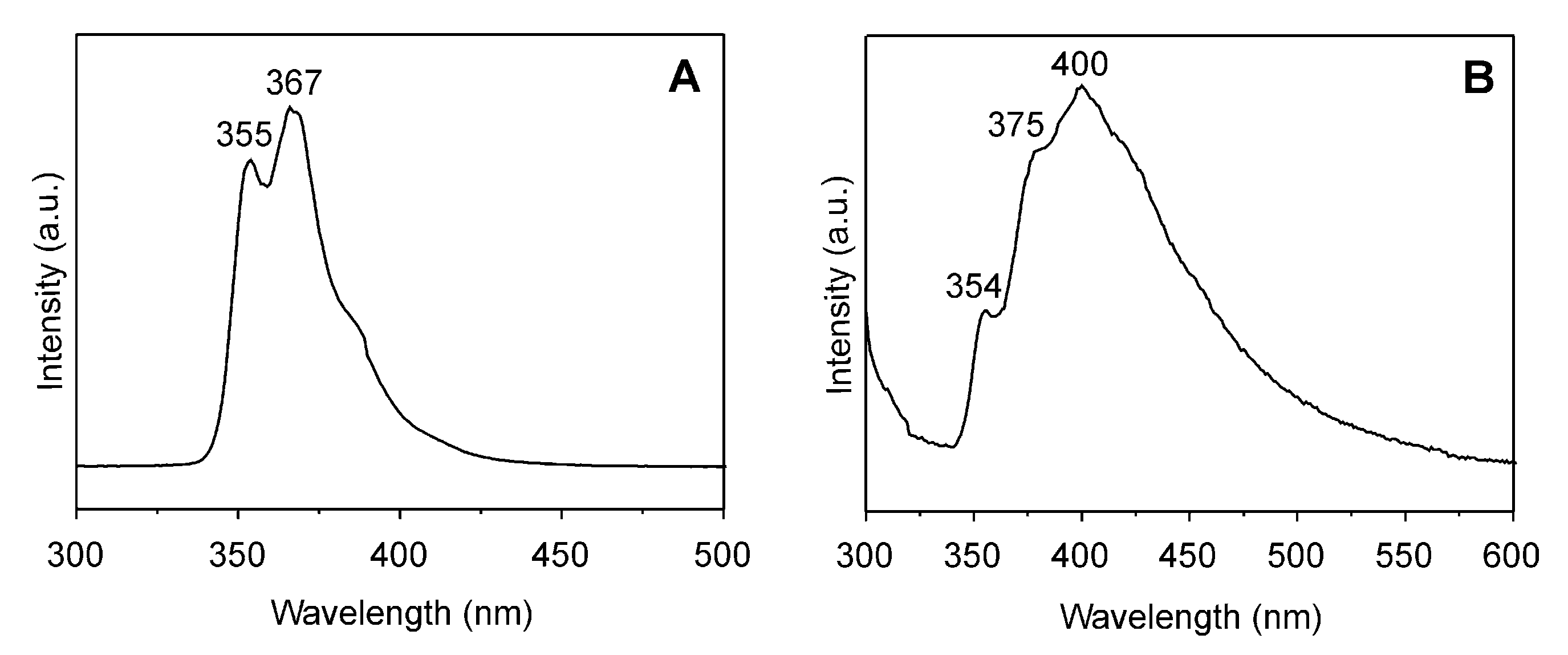
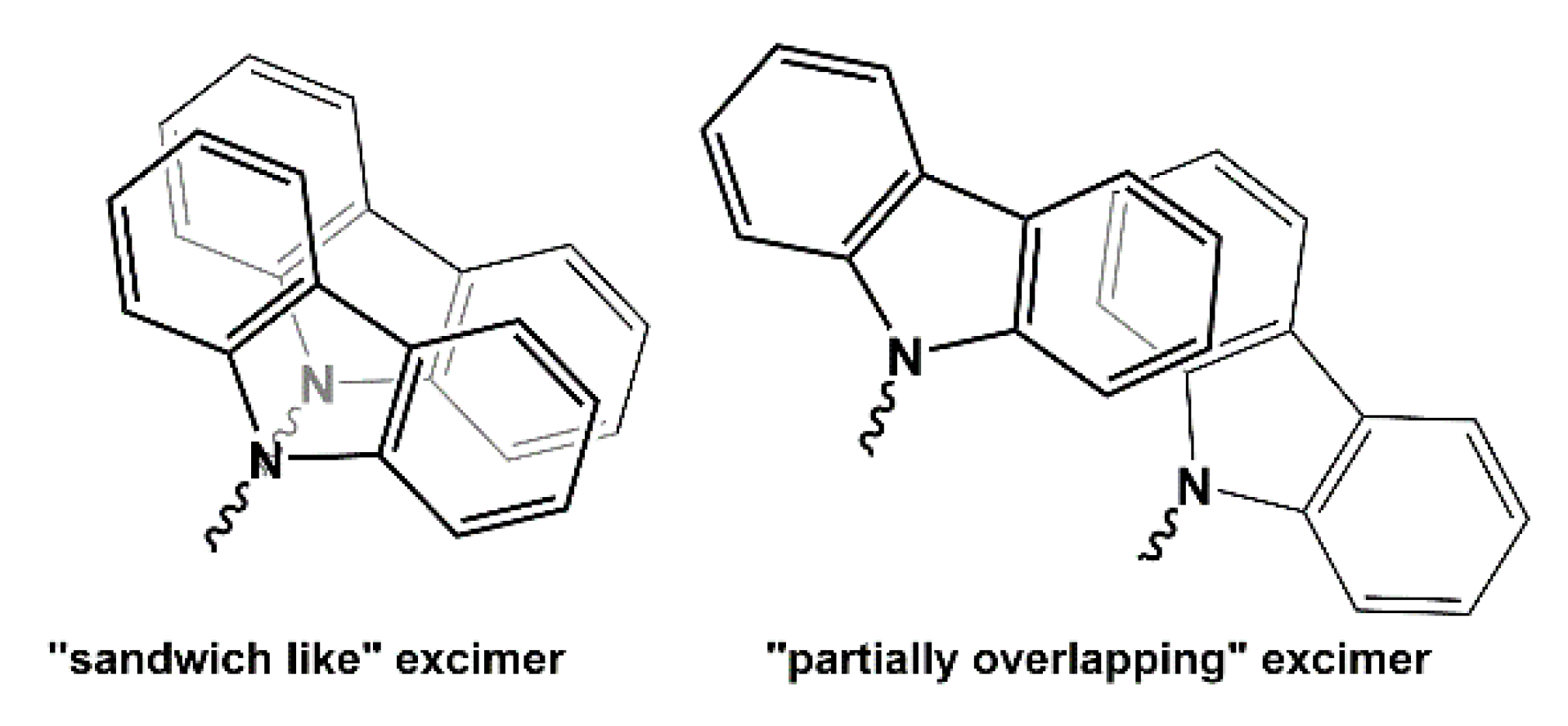
| Run | PO/ i-PrONa | i-Bu3Al/ i-PrONa | EPK/ i-PrONa | Conv. (%) | Mn (teor) (g/mol) | Mn (exp) b (g/mol) | Ð b | Tg (°C) Tg(PPO)/Tg(PEPK)c |
|---|---|---|---|---|---|---|---|---|
| 1 a | 390 | 7.8 | - | 100 | 22,651 | 24,100 | 1.05 | −60/- |
| 2 | 390 | 7.8 | 39 | 100 | 31,352 | 33,500 | 1.05 | −60/130 |
| 3 | 390 | 7.8 | 78 | 100 | 40,053 | 43,000 | 1.10 | −60/136 |
| 4 | 390 | 7.8 | 20 | 100 | 27,113 | 28,000 | 1.05 | −60/97 |
| Carbon | Chem. Shift |
|---|---|
| C1 | 73.79 |
| C2 | 75.38 |
| C3 | 17.75 |
| C4 | 69.20 |
| C5 | 67.40 |
| C6 | 44.47 |
| C7 | 140.59 |
| C8 | 109.48 |
| C9 | 125.43 |
| C10 | 119.05 |
| C11 | 120.25 |
| C12 | 122.84 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Izzo, L.; Lisa, P.; Sacco, O.; Pragliola, S. Synthesis of Di-Block Copolymers Poly (Propylene oxide)-block-Poly (9-(2,3-epoxypropyl) Carbazole) via Sequential Addition of Monomers in One Pot. Polymers 2021, 13, 763. https://doi.org/10.3390/polym13050763
Izzo L, Lisa P, Sacco O, Pragliola S. Synthesis of Di-Block Copolymers Poly (Propylene oxide)-block-Poly (9-(2,3-epoxypropyl) Carbazole) via Sequential Addition of Monomers in One Pot. Polymers. 2021; 13(5):763. https://doi.org/10.3390/polym13050763
Chicago/Turabian StyleIzzo, Lorella, Paola Lisa, Olga Sacco, and Stefania Pragliola. 2021. "Synthesis of Di-Block Copolymers Poly (Propylene oxide)-block-Poly (9-(2,3-epoxypropyl) Carbazole) via Sequential Addition of Monomers in One Pot" Polymers 13, no. 5: 763. https://doi.org/10.3390/polym13050763
APA StyleIzzo, L., Lisa, P., Sacco, O., & Pragliola, S. (2021). Synthesis of Di-Block Copolymers Poly (Propylene oxide)-block-Poly (9-(2,3-epoxypropyl) Carbazole) via Sequential Addition of Monomers in One Pot. Polymers, 13(5), 763. https://doi.org/10.3390/polym13050763









