3.1. TGA Analysis
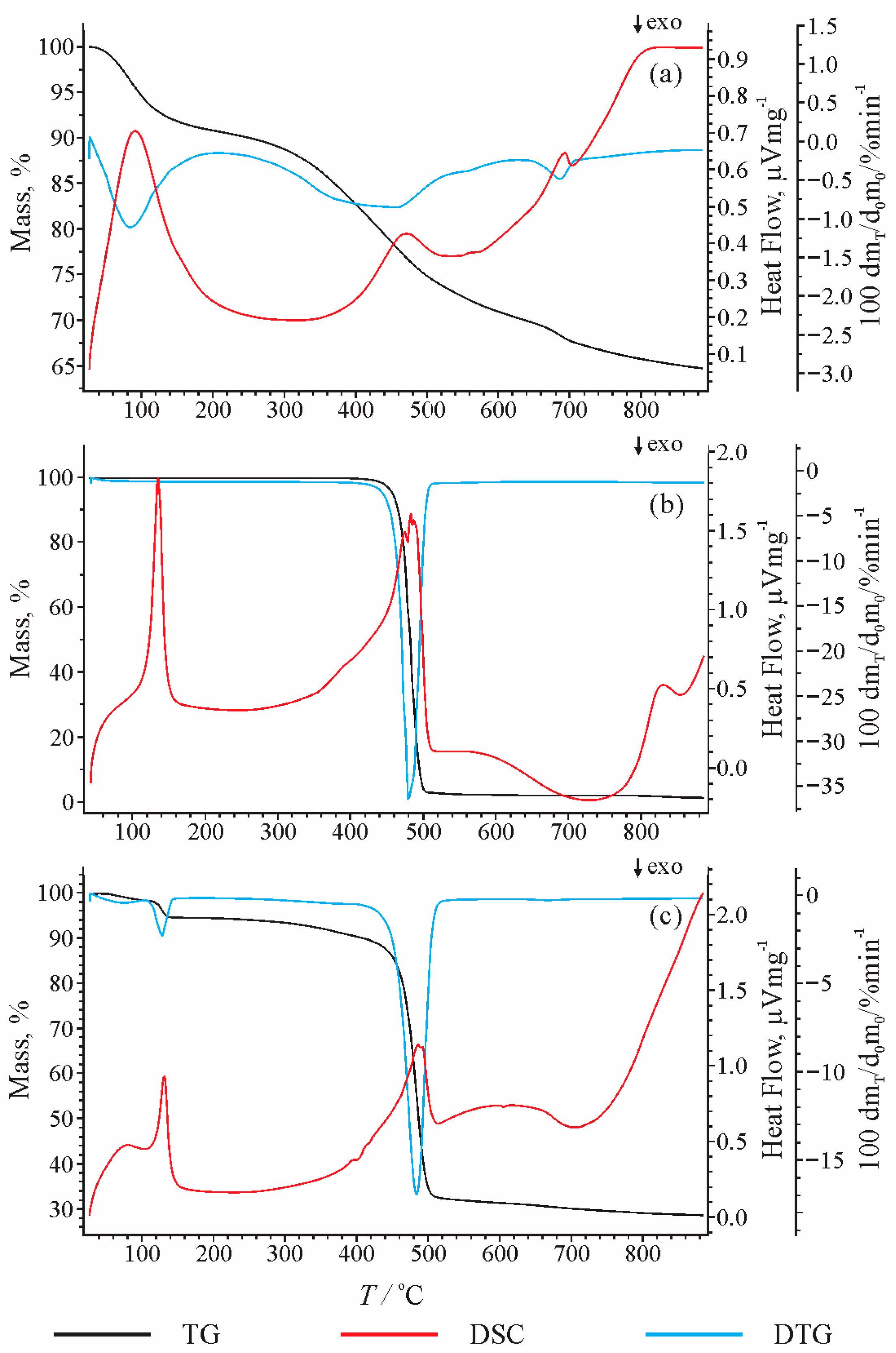
Figure 1a shows the TGA curve and derivative weight loss (DTG) curve of pre-extracted lignite. The first peak at 90.8 °C corresponds to loss of moisture. Degradation of OM starts at about 300 °C and the greatest weight loss is observed in the temperature interval between 400 and 500 °C, with the maximum at 471.2 °C. With further heating from 500 to 800 °C, the weight loss is less intense (<5% with temperature rise of 100 °C). The peak recorded at a temperature of 693 °C represents mostly mass loss caused by thermal alteration of minerals (clay and carbonate) [
50]. The TGA curve shows that only about 25% of the lignite mass decomposes at 500 °C, while at 900 °C, it reaches 35%. The low degree of lignite degradation is in accordance with the low content of OM (total organic carbon of 29.77% and hydrogen of 2.79%;
Section 3.3), as well as a high content of mineral matter (ash content of 47.12%), which is relatively stable and not subjected to significant decomposition even at a temperature >800 °C.
The TGA and DTG results of the plastic bag (
Figure 1b) are consistent with the results of previous studies using other commercial HDPE sources [
20,
51]. The thermal decomposition of HDPE starts at about 430 °C, and almost complete degradation occurs at a temperature of about 500 °C. Differential scanning calorimetry (DSC) indicates the presence of two main peaks. The first peak at about 135 °C corresponds to the melting of HDPE, without loss of mass. The second peak, followed by weight loss, starts at about 390 °C and ends at about 505 °C. The presence of one sharp peak in DTG indicates only one degradation step of HDPE conversion. The temperature corresponding to the maximum release of volatile substances is 483.2 °C, while the largest weight loss, 35.28%, corresponds to a temperature of 479.5 °C. Comparison of the results from
Figure 1a,b indicates that the intense decomposition of selected lignite and HDPE takes place in the same temperature range, from 400 to 500 °C, which was thus chosen for the study of the synergetic effect.
In the DSC curve of the lignite/HDPE mixture (
Figure 1c), a wide peak at about 90 °C corresponds to the loss of moisture from lignite. In the presence of lignite, HDPE begins to melt at about a 5 °C lower temperature than during thermal decomposition of HDPE alone (
Figure 1b,c). The result implies that release of moisture from the lignite that occurs before the melting of HDPE can accelerate the latter process. The temperature corresponding to onset of the most intense mass loss decreases from 474.7 °C (pure HDPE) to 464.9 °C in the presence of lignite, which is associated with slight widening of the peak. The greatest mass loss per minute is observed at 479.5 °C and 484.0 °C for the HDPE and lignite/HDPE mixture, respectively. The residual mass at 500 °C for lignite alone was 75%, and for HDPE alone was 4.79%, based on which the expected percentage of undecomposed residue for a mixture of lignite and HDPE (1:1) according to the theoretical calculation is (75% + 4.79%)/2 = 39.985%.
However, the experimental data showed that the percentage of undecomposed residue of a mixture of lignite/HDPE at 500 °C amounts to 33.42%, which is ~6% lower than the theoretical calculation (
Figure 1). Previous studies reported a decrease of the experimental percentage of undecomposed residue in comparison with the theoretical value in the range of 2–4% [
37,
38]. The slightly greater reduction of undecomposed residue content observed here can be attributed to characteristics of initial immature lignite enriched in reactive –COOH and –OH groups. At a final temperature of 886 °C, the percent of undecomposed lignite and HDPE is 64.69% and 3.46%, respectively. The theoretically calculated percent of residual mass for the lignite/HDPE mixture at 886 °C is (64.69% + 3.46%)/2 = 34.075%, while the experimental one is 29.15% (
Figure 1). Therefore, the difference between the theoretically calculated and experimental percent of residual mass at 886 °C and 500 °C is almost identical. This implies that, in the temperature range from 500 °C to 900 °C, there is almost no impact of HDPE on the lignite decomposition, as the experimental percentage of undecomposed residue remains about 5–6% lower than theoretical, as at 500 °C. This implies that the volatilisation processes of the lignite/HDPE mixture have been substantially finished at temperatures up to 500 °C.
3.2. The Yields of Pyrolysis Products Obtained by Open System Pyrolysis
The yields of pyrolysis products obtained by open system pyrolysis are given in
Table 1.
As expected, increasing the temperature resulted in an increase of the conversion of all three substrates (lignite, HDPE, and their mixture) into liquid and gaseous products. However, the increase is much more prominent for HDPE and the lignite/HDPE mixture than for lignite alone. The greatest yield of liquid products for all three substrates is recorded at 500 °C, suggesting that, at higher temperatures, their further degradation into gas is favoured. The yield of gas obtained by co-pyrolysis of lignite/HDPE is obviously higher than the yield of gas obtained by pyrolysis of HDPE at 400, 450, and 500 °C, whereas it is opposite at 550 and 600 °C. The yield of gas is lower in the co-pyrolysis experiment lignite/HDPE in comparison with pyrolysis of lignite at 400 °C; at 450 °C, yields are comparable; whereas at temperatures of 500–600 °C, the yield of gas obtained from lignite/HDPE mixture is 1.7–1.9 higher than those from lignite. The yields of liquid products obtained by co-pyrolysis of lignite/HDPE are notably higher than by pyrolysis of lignite (12–15 times at temperatures ≥450 °C), and uniformly lower (for 2.4–8.5%) than by pyrolysis of HDPE. It is noteworthy that the yields of liquid and gaseous products obtained by co-pyrolysis of lignite/HDPE at 450 °C are two times higher than and similar to, respectively, those reported in literature for pyrolysis of lignite of better quality (74.05% of carbon dry, ash-free basis) at 550 °C [
52], whereas our experiment at 500 °C provided two times greater yields of both liquid and gaseous products than in the cited reference. Co-pyrolysis of lignite/HDPE mixture at 450 and 500 °C gave higher yields of liquids and gases than pyrolysis of lignite of better quality (64.35% of carbon, dry basis, and net calorific value of 25.78 MJ/kg) at 600 °C [
16]. The yields of liquid and gaseous products of lignite/HDPE co-pyrolysis at 500 °C are also higher than the yields of these products obtained by mild liquefaction of lignite (temperature of 450 °C and pressure of 11.5 MPa in the presence of solvent, catalyst, and hydrogen) [
17], which contained nearly two times higher amount of carbon than lignite used in the current study. On the other hand, yields of liquid products obtained from pyrolysis of HDPE alone indicate that it cannot be concurrent to well-developed catalytic pyrolytic processes ([
22] and references therein). However, concerning the huge amount of produced HDPE (the third largest produced plastic material in the world, after polyvinylchloride and polypropylene in terms of volume), at least part of it can be used for improved utilization of lignite, particularly in countries where this low-rank coal represents the main energy source.
The evaluation of the interactions between lignite and HDPE wascarried out by comparing the experimental yields of the pyrolysis of the mixture lignite/HDPE and theoretical (calculated) yields = (yield of lignite pyrolysis + yield HDPE pyrolysis)/2 (
Table 1). At a temperature of 400 °C, the experimental and theoretical yields are very similar, indicating almost no synergetic effect between lignite and HDPE. This result can be attributed to the relatively high thermal stability of HDPE up to 400 °C, which is then followed by an abrupt single mass loss step between 400 and 500 °C (
Figure 1b). At temperatures >450 °C, experimental yields of liquid and gas products are obviously higher than theoretical yields (
Table 1), indicating a synergetic effect between lignite and HDPE. At a temperature of 450 °C, the difference between the experimental yields and theoretical yields is greater for liquid products than for gases, whereas at ≥500 °C, the result is inverse, which is consistent with prevalent kerogen type III in lignite, and generally more intense degradation at higher temperatures. The most intense synergetic effect between lignite and HDPE is detected at 500 °C, and reduces with further heating. The elemental analysis and calculated calorific values of solid residues of lignite/HDPE mixtures (
Section 3.3.) also indicate no influence with heating above 500 °C. The mentioned results are consistent with TGA data, which showed that, at temperatures >500 °C, there is almost no impact of HDPE on the lignite decomposition. Because the manuscript is focused on the lignite/HDPE synergetic effect with particular attention devoted to liquid and solid products obtained by co-pyrolysis, specific analyses (GC–MS, Rock-Eval, carbon isotope measurements) were performed on products obtained at 400, 450, and 500 °C.
3.3. Rock Eval Data and Elemental Analysis
The results of Rock-Eval pyrolysis comprising S1, S2, S3, T
max, total organic carbon (TOC), hydrogen index (HI), oxygen index (OI), and production index (PI) are listed in
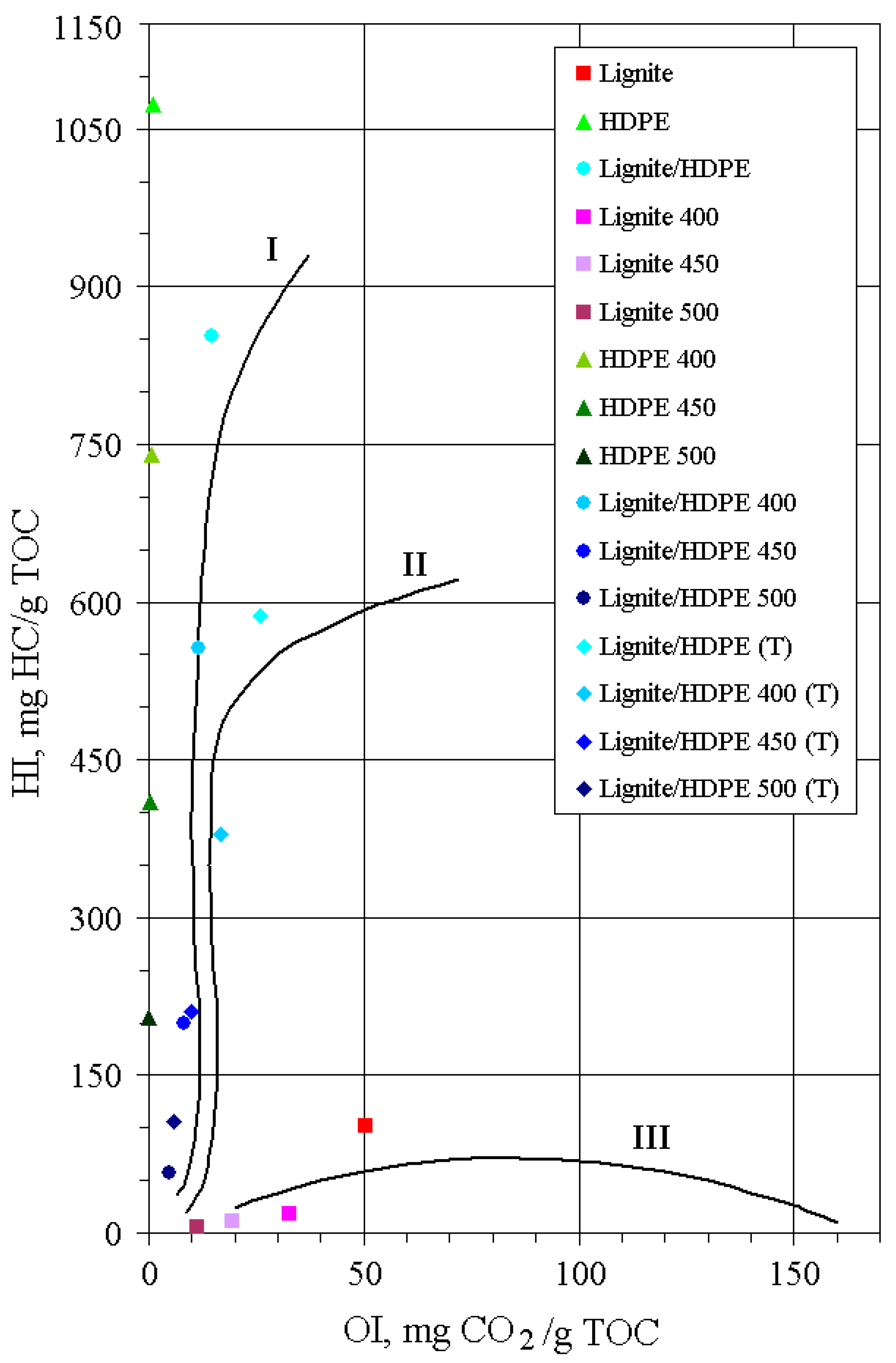
Table 2. As expected, the HI versus OI diagram (
Figure 2) clearly indicates kerogen type III for lignite, and very high liquid generation potential for HDPE exceeding those of immature kerogen type I [
26]. The lignite/HDPE mixture also demonstrated a high liquid generation potential corresponding to kerogen type I. The solid residues of all substrates obtained by the open system pyrolysis show a decreasing trend for HI and OI with the increase of pyrolysis temperature as a result of generation of liquid and gaseous products. An abrupt decrease of HI for HDPE and lignite/HDPE residues between 400 and 500 °C (
Table 2) is consistent with the greatest mass loss in the same temperature range, detected by TGA (
Figure 1).
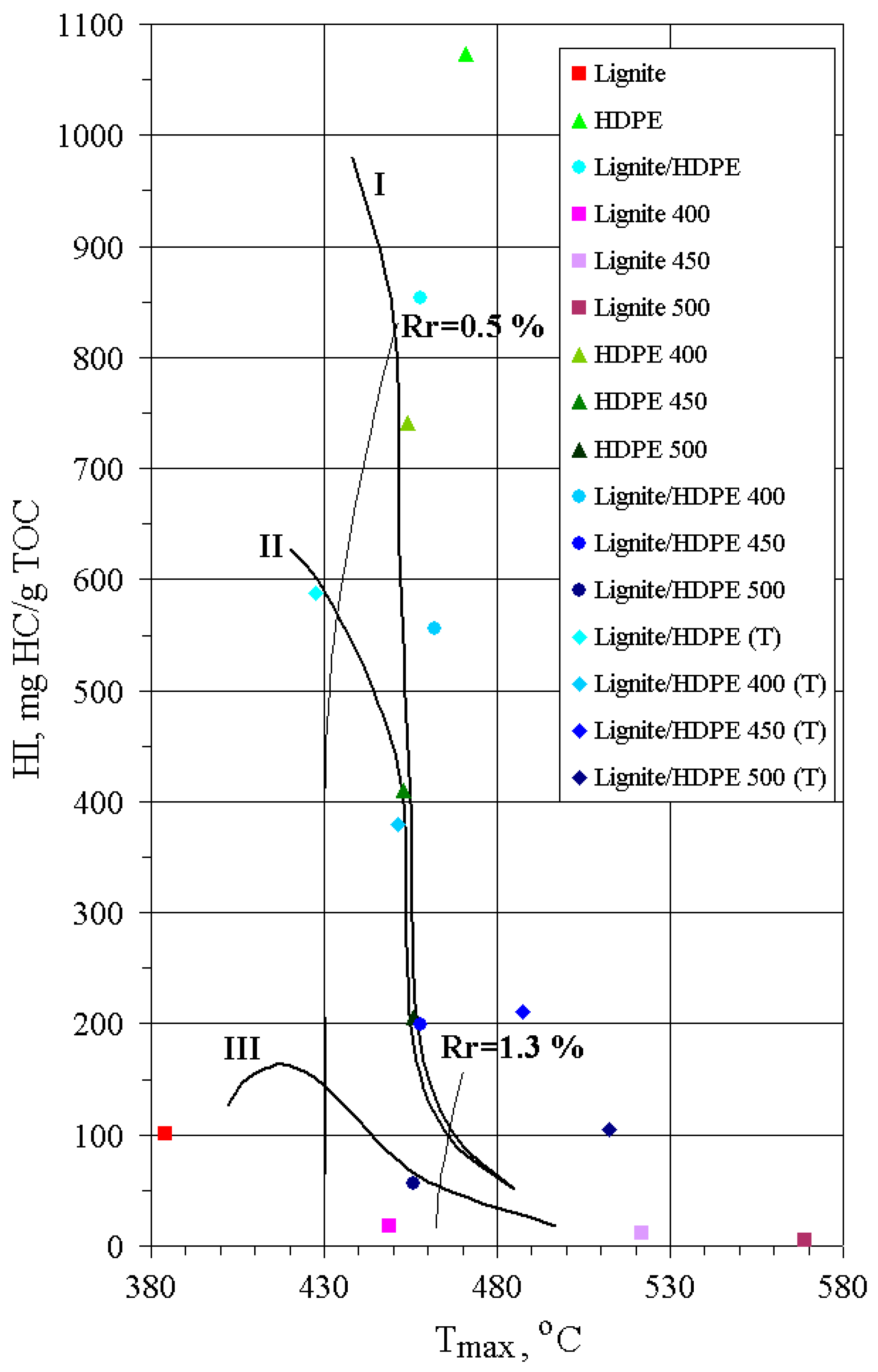
The content of TOC increases in all substrates with the increase of pyrolysis temperature, implying that, during thermal decomposition, aliphatic chains enriched in hydrogen are preferably released, whereas solid residues become enriched in carbon. T
max and PI of solid residues increase with temperature, indicating an increase of OM maturity (
Table 2;
Figure 3). However, this rise is much more pronounced for lignite than for HDPE and lignite/HDPE mixtures owing to the abrupt aromatization of lignite OM. On the other hand, similar T
max temperatures for solid residues of HDPE and lignite/HDPE mixtures indicate that decomposition of HDPE is not accompanied with aromatization; rather, a waxy paraffinic residue is formed.
The experimental values of Rock-Eval parameters (S1, S2, and HI, which indicate hydrocarbon generation potential) of the lignite/HDPE mixture are higher than the theoretical values, (lignite + HDPE)/2, for initial non-heated substrates. On the other hand, the experimental values of the mentioned Rock-Eval parameters of the lignite/HDPE mixture are lower than the theoretical ones for solid residues obtained by pyrolysis at 450 and 500 °C (
Table 2). The results are in concordance with those from open system pyrolysis (
Table 1), indicating the synergetic effect between lignite and HDPE at the aforementioned temperatures, associated with greater production of liquid and gas.
The content of organic carbon (C
org) obtained by elemental analysis is almost identical to the TOC from Rock Eval pyrolysis (
Table 2 and
Table 3). The notable increase of TOC (C
org) in solid residues of lignite/HDPE mixtures in comparison with solid residues of lignite and initial lignite has a positive impact on the calorific value (
Table 4). In accordance with HI (
Table 2), the content of hydrogen shows the following trend: HDPE > lignite/HDPE mixture > lignite, and decreases in all substrates with the rise in temperature (
Table 3). The content of oxygen also shows a decreasing trend with the rise in temperature (
Table 3), resulting from defunctionalization reactions of lignite type III kerogen, enriched in carboxylic, hydroxyl, and carbonyl groups.
Concerning an environmental impact, the substantial feature of solid residues obtained by co-pyrolysis of lignite/HDPE mixture is a lower amount of sulphur than in initial lignite and its solid pyrolysis residues. The content of sulphur continuously decreased with the increase of pyrolysis temperature (
Table 3), most probably due to the higher reactivity of C–S in comparison with C–C bonds. The theoretical (calculated) values and experimental results of elemental analysis are almost identical for untreated mixture lignite/HDPE and very similar for residues obtained at 400 °C. On the other hand, the increase of carbon content associated with a double decrease of hydrogen content is evident for experimental data of lignite/HDPE mixture residues obtained at temperatures ≥450 °C in comparison with theoretical ones, confirming the impact of the synergetic effect.
Net calorific values of lignite and lignite/HDPE solid residues calculated based on 10 formulas [
40,
41,
53,
54,
55,
56,
57,
58,
59] proposed in the literature are listed in
Table 4. Net calorific value of lignite residues continuously increases with the rise in temperature, whereas for lignite/HDPE mixture residues, the influence of pyrolysis temperature was less pronounced, and the greatest values are detected at 500 °C. Net calorific values of lignite/HDPE mixture residues, ranging from 22.87 to 27.48 MJ/kg, are notably higher than those of initial lignite and lignite residues (9.73–15.62 MJ/kg), as well as than the calorific value of char obtained by fast pyrolysis of HDPE waste at 450 °C, reported in the literature (18.83 MJ/kg, [
60]). Furthermore, net calorific values of lignite/HDPE solid residues are greater compared with lignite (7–20 MJ/kg), and similar to sub-bituminous coal (19–26 MJ/kg) and high-volatile bituminous coal (24–35 MJ/kg) [
61,
62]. In comparison with solid residues obtained by HDPE pyrolysis, co-pyrolysis solid products have better combustion properties, as lignite has a stabilizing effect on the plastic residues morphology and prevents its melting [
63]. Containing a relatively high amount of carbon (
Table 3) and clay (from the lignite mineral matter [
2]), the solid residues obtained by lignite/HDPE pyrolysis, in addition to direct combustion, may serve for adsorption of different pollutants from waste water; this would be the objective of our further investigations.
3.4. Characteristics of Liquid Pyrolysates
The bulk composition of EOM of initial lignite and liquid pyrolysis products is shown in
Table 5. The EOM of lignite is notably dominated by polar, NSO compounds, and asphaltenes, typical for immature terrestrial OM. The liquid products obtained by pyrolysis of lignite also have a relatively low percent of hydrocarbons that does not exceed 31%. The liquid pyrolysates of HDPE at all temperatures consist of aliphatic hydrocarbons only. The liquid products obtained by lignite/HDPE co-pyrolysis have ~2.3 times higher content of hydrocarbons than corresponding liquid pyrolysates of lignite, indicating an improvement in composition. The contents of hydrocarbons in lignite/HDPE pyrolysates are higher than the theoretical ones at temperatures ≥450 °C, where the synergetic effect was observed (
Table 5).
Furthermore, the content of hydrocarbons in lignite/HDPE pyrolysates obtained at 450 and 500 °C is similar or even higher than in crude oils. HDPE serves as a hydrogen donor to lignite OM, which is a clue for the generation of liquid hydrocarbons. This is also evident from eight times higher liquid hydrocarbon generation potential, expressed by HI from Rock-Eval, of the lignite/HDPE mixture in comparison with lignite (
Table 2). HDPE also serves as a solvent, because it melts at about 135 °C and, in the temperature range between 450 and 500 °C, it is in a liquid state. Decomposition of both lignite and HDPE under applied pyrolysis conditions was carried out via free radical mechanism. Radicals formed from lignite kerogen can be easily stabilized in the presence of HDPE, capturing the H radicals from HDPE. This prevents the secondary processes (further cracking, coking on carbon residue) and contributes to increasing the content of liquid hydrocarbons. Simultaneously, capture of hydrogen radicals by lignite OM results in the formation of new reactive radical places in HDPE that promotes further cracking of polymer. Because different zeolite catalysts (e.g., clinoptilolite, HZSM-5) showed a positive impact on HDPE pyrolysis [
64], the certain influence of clays that prevail in lignite mineral matter [
2] on thermal decomposition of HDPE cannot be excluded. Termination reactions between radicals formed from kerogen and HDPE also occur. Furthermore, longer radicals can be disproportionated into alkene (
Table 6 and
Table 7) and new reactive radical.
Diterpenoids prevail in the aliphatic and aromatic fraction of lignite EOM. The main constituents of aliphatic fraction of all liquid pyrolysates at each temperature are
n-alkanes and terminal
n-alkenes, having similar distributions (
Figures S1–S3;
Table 6). Aliphatic fractions of lignite pyrolysates contain diterpenoids (pimarane, 16α(H)-phyllocladane), isoprenoids (pristene, norpristane, pristane, and phytane), and hopanes, whereas in liquid pyrolysates of lignite/HDPE, these biomarkers cannot be identified owing to the extremely low concentrations, even using their typical fragmentation ions
m/z 123, 183, and 191, respectively. In addition to
n-alkanes and terminal
n-alkenes, liquid pyrolysates of HDPE and lignite/HDPE mixtures contain terminal
n-dienes.
In the liquid pyrolysates of lignite,
n-alkanes prevail over terminal
n-alkenes; in pyrolysates of HDPE, the opposite trend is observed; whereas in pyrolysates of lignite/HDPE, they are present in very similar amounts. With the increase of temperature pyrolysis, the ratio of
n-alkenes to
n-alkanes slightly increases, consistent with data from the literature [
65], implying more intense cracking and further disproportionation of the formed radicals (
Table 7;
Figures S1–S3).
In contrast to initial lignite EOM, which is characterized by notable predominance of long-chain odd
n-alkane homologues (carbon preference index [
66], CPI > 4), aliphatic fractions of all liquid pyrolysates display uniform distributions of odd and even
n-alkane and
n-alkene homologues (CPI ~1;
Table 7;
Figures S1–S3). Pyrolysates of lignite obtained at all temperatures are characterized by dominance of mid-chain C
21-C
25 normal hydrocarbon homologues, indicating almost no impact of temperature on
n-alkane and
n-alkene distributions (
Figure S1;
Table 6). Pyrolysates of HDPE and lignite/HDPE mixtures obtained at 400 °C have higher abundance of long chain-homologues with a broad maximum in the C
24 to C
32 range. This prevalence became slightly less prominent in pyrolysates of HDPE and lignite/HDPE mixtures at 450 °C, whereas in pyrolysate of HDPE and co-pyrolysate of lignite/HDPE at 500 °C, the increase of short-chain homologues is evident (
Figures S2 and S3;
Table 6).
Among the present hydrocarbons with a normal skeleton (alkanes, alkenes, and dienes),
n-alkane series can be the most accurately separated from alkenes and dienes, using the typical ion fragmentogram
m/z 71 (
Figure S4) that enabled precise peak integration up to C
33. Therefore, for more detailed interpretation of lignite/HDPE interactions, typical geochemical parameters based on distributions of
n-alkanes were used (
Table 7). Liquid pyrolysates of lignite at all three temperatures have almost equal distributions of
n-alkanes with prevalence of mid-chain homologues. In all three HDPE liquid pyrolysates, domination of long-chain homologues is observed; however, with the increase in temperature, the decrease in abundance of long-chain homologues associated with the increase of short-chain
n-alkanes and without a change in the content of mid-chain homologues is noticed (
Table 7).
Table 7.
Values of Σn-alk-1-enes/Σn-alkanes ratio and values of common organic geochemical parameters based on distributions of n-alkanes in lignite EOM and the liquid products obtained in open system pyrolysis.
Table 7.
Values of Σn-alk-1-enes/Σn-alkanes ratio and values of common organic geochemical parameters based on distributions of n-alkanes in lignite EOM and the liquid products obtained in open system pyrolysis.
| Sample | Σn-alk-1-enes/Σn-alkanes | CPI | n-C15 − n-C20 (%) | n-C21 − n-C25 (%) | n-C26 − n-C33 (%) | n-C17/n-C27 |
|---|
| Lignite EOM | N.D. | 4.61 | 1 | 17 | 82 | 0.003 |
| Lignite 400 | 0.38 | 1.12 | 23 | 49 | 28 | 0.14 |
| Lignite 450 | 0.41 | 1.08 | 24 | 45 | 31 | 0.26 |
| Lignite 500 | 0.64 | 1.05 | 24 | 48 | 28 | 0.29 |
| HDPE 400 | 1.25 | 1.03 | 27 | 24 | 49 | 0.93 |
| HDPE 450 | 1.37 | 0.96 | 32 | 23 | 45 | 1.17 |
| HDPE 500 | 1.47 | 0.95 | 37 | 24 | 39 | 1.53 |
| Lignite/HDPE 400 | 0.93 | 1.00 | 24 | 27 | 49 | 0.83 |
| Lignite/HDPE 450 | 0.96 | 1.03 | 25 | 26 | 49 | 0.82 |
| Lignite/HDPE 500 | 0.97 | 1.02 | 39 | 23 | 38 | 1.39 |
Liquid pyrolysate of lignite HDPE/mixture at 400 °C has an almost identical composition of
n-alkanes to pyrolysate of HDPE at this temperature, consistent with a much higher impact of HDPE than lignite in liquid pyrolysate (
Table 1), confirming no significant interaction between lignite OM and HDPE at 400 °C. In lignite/HDPE liquid pyrolysate at 450 °C, the distribution of
n-alkanes is still similar to those in HDPE pyrolysate at the same temperature, although the former has a slightly elevated content of long-chain homologues and lower amount of short-chain homologues. In comparison with lignite pyrolysate at 450 °C, lignite/HDPE pyrolysate has a higher content of long-chain homologues and proportionally lower amount of mid
n-alkanes. The mentioned data indicate that, at 450 °C, the interaction between lignite and HDPE resulted in more intense release of long-chain homologues. This is consistent with the observation that lower molecular weight units of the normal chain are more stable than the initial polymer [
67] and immature kerogen [
26]. At a temperature of 500 °C, the greater percent of short-chain
n-alkanes in lignite/HDPE pyrolysate is detected than in both lignite and HDPE pyrolysate (
Table 7;
Figures S1d, S2c, and S3c).
The interactions between lignite OM and HDPE were further evaluated by measurement of the stable carbon isotope composition of individual
n-alkane/
n-alkene pairs (sufficient precise separation of their peaks was not possible in total ion chromatograms (TICs), even manually, for that analysis), which represented the most abundant compounds in all liquid pyrolysis products (
Figures S1–S3). The results are presented in
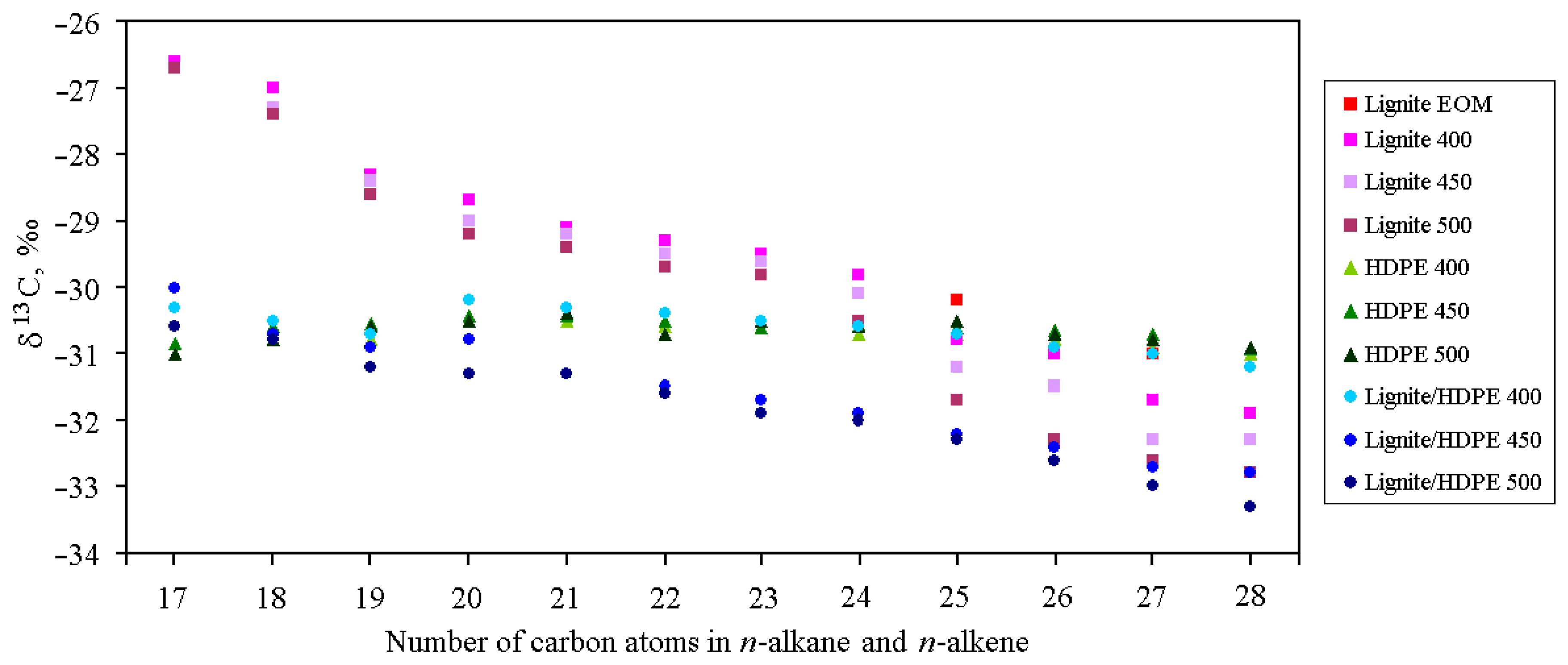
Figure 4.
Homologues up to C
28 have been involved in interpretation, owing to the possible impact of hopanes on δ
13C of higher homologues in lignite pyrolysates (
Figure S1). δ
13C values of normal hydrocarbons in lignite pyrolysates ranging from −26.6 to −32.8‰ are typical for precursor lipids of C3-terrestrial plants [
68], showing z decreasing trend with chain length in all samples, as usual for coals [
2,
69,
70,
71]. Long-chain homologues exhibit slight enrichment in
12C with temperature increase, whereas this change was less pronounced for short-chain counterparts.
δ
13C values of individual normal hydrocarbons in HDPE pyrolysates are quite uniform, ranging from −30.5‰ to −31.0‰, exhibiting almost no change with temperature (
Figure 4). This is in accordance with their origin from polymer material, not from natural sedimentary OM, where short-, mid-, and long-chain homologues have different precursors [
26]. Consistent with very similar distributions of normal hydrocarbons in liquid pyrolysates of HDPE and lignite/HDPE mixture at 400 °C (
Figures S2a and S3a), their δ
13C signatures also reveal notable similarity (
Figure 4). In pyrolysates of lignite/HDPE mixture at 450 °C, δ
13C values of short-chain normal hydrocarbons are still much closer to those of HDPE pyrolysate than lignite pyrolysate, signifying their prevalent origin from HDPE, whereas δ
13C values of mid- and long-chain homologues are more negative than in both lignite pyrolysate and HDPE pyrolysate. In lignite/HDPE pyrolysate at 500 °C, this effect of enrichment of normal hydrocarbons in
12C isotope (in comparison with lignite and HDPE pyrolysate) is evident in the whole range C
17–C
28 (
Figure 4). Data confirm the interaction between lignite and HDPE at 450 °C and particularly at 500 °C, which promotes degradation of both HDPE and kerogen, associated with preferred cleavage of more labile
12C–
12C bonds.
Liquid pyrolysates of HDPE do not contain aromatic hydrocarbons;
n-alkylbenzenes were identified in traces via characteristic
m/z 91 mass fragmentogram, however, in an insufficient quantity for integration and interpretation. Solely compounds present in lignite extracts and lignite pyrolysis products are cadalene and retene (
Figure S5,
Table 8).
Aromatic fractions of lignite and lignite/HDPE pyrolysates consist of naphthalene, phenanthrene, fluorene, pyrene, chrysene, dibenzofuran, and their methylated derivatives (
Figures S5 and S6,
Table 8). As expected, an increase of pyrolysis temperature resulted in a rise of common geochemical maturity parameters calculated from distributions of naphthalene and phenanthrene derivatives in lignite pyrolysates (
Table 9) [
72,
73,
74,
75,
76]. The exception is MPI 1 (containing unsubstituted phenanthrene in the denominator), which showed an opposite trend. This can be attributed to preferable releasing of unsubstituted aromatics from lignite, rather than their methylated counterparts, with the increase of pyrolysis temperature, which is documented by an apparent decrease of the PAI 1 ratio from 400 °C to 500 °C (
Table 9). Although HDPE pyrolysates do not contain aromatic compounds detected in lignite and its pyrolysates, differences in TICs of aromatic fractions of lignite and lignite/HDPE pyrolysates are evident (
Figures S5 and S6;
Table 8). This is reflected through the variations in contents of phenanthrene relative to its methylated derivatives, as well as the phenanthrene to cadalene ratio, assuring the influence of HDPE on cracking of lignite kerogen. The impact of HDPE on distributions of aromatic hydrocarbons derived from lignite kerogen cracking is also indicative based on the differences in values of geochemical parameters (
Table 9;
Figures S5–S7). In accordance with the weak impact of HDPE on lignite OM at 400 °C, differences in geochemical ratios are negligible, whereas they become more evident with the temperature increase, confirming the synergetic effect during lignite/HDPE co-pyrolysis at 450 °C and 500 °C.
The compositions of individual hydrocarbons in the aliphatic and the aromatic fractions of liquid lignite/HDPE co-pyrolysis product at 500 °C are similar to those of higher rank coal and crude oils of terrestrial origin. The main difference between the compositions of liquid lignite/HDPE pyrolysate and crude oil is reflected through the presence of
n-alkenes and
n-dienes in pyrolysate (
Figure S3;
Table 6), which are absent in crude oil. However, the presence of these unsaturated hydrocarbons in liquid co-pyrolysate does not represent a significant problem, as these hydrocarbons can be more easily converted into branched and cyclic hydrocarbons than
n-alkanes, via reforming processes, giving an opportunity for producing high octane rating gasoline. On the other hand, terminal
n-alkenes can be separated and used as chemical feedstock for plastic and detergent manufacture [
77].
3.5. The Composition of Gaseous Products
Gaseous products produced in the open system pyrolysis could not be collected and analyzed. The qualitative composition of gases was, therefore, estimated based on TGA coupled to Fourier transform infrared spectroscopy (FTIR) [
78,
79] (
Figure 5 and
Figure S8). However, it should be mentioned that certain differences in the composition of gases obtained by TGA and the open system pyrolysis are expected as it depends on pyrolysis conditions and the heating rate.
The TGA-FTIR thermogram of lignite (
Figure S8a) indicates that the produced gas mainly consists of water vapour (O–H stretching peaks around 3700 cm
−1 and O–H bending peaks from 1400 to 1700 cm
−1), CO
2 (peaks between 2300 cm
−1 and 2400 cm
−1 corresponding to C=O stretching), and CO (peaks between 2100 cm
−1 and 2200 cm
−1 representing C–O stretching). Peaks corresponding to water vapour are visible at 100 °C, indicating the removal of moisture from lignite, whereas peaks in the temperature range from 420 °C to 620 °C correspond to removal of H
2O by dehydration of lignite OM (e.g., alcohol groups) and clay minerals, which represent the main inorganic constituents of lignite [
2]. Release of CO
2 starts above 220 °C and can be attributed to decarboxylation reactions of lignite OM, which is rich in carboxylic groups, whereas CO
2 formed at temperatures >650 °C corresponds to degradation of carbonates. Release of CO starts at about 500 °C, being the most pronounced at around 700 °C. This reflects decarbonylation reactions, but CO also can be formed by reaction between CO
2 and a small amount of generated hydrocarbons at high temperatures. Hydrocarbons in lignite gas are observed in traces only, and they are represented mainly by gaseous alkenes, as documented by very small peaks at around 3100 cm
−1, corresponding to C–H stretching; around 1650 cm
−1 corresponding to C=C stretching; and around 950 cm
−1, corresponding to C–H bending (
Figure S8a).
The main components in the gaseous product of HDPE decomposition are light hydrocarbons (alkanes and alkenes). Gaseous alkanes are characterized by peaks around 2950 cm
−1, representing C–H stretching, and peaks around 1370 cm
−1, representing C–H scissoring and bending. Gaseous alkenes are represented by peaks at around 3100 cm
−1, corresponding to C–H stretching; peaks around 1450 cm
−1, corresponding to C=C stretching; as well as by peaks observed around 950 cm
−1, corresponding to C–H bending (
Figure S8b). More pronounced peaks of C–H than of C–C or C=C vibrations indicate that main gaseous products are methane and ethene. The intense releasing of hydrocarbons begins at a temperature higher than 450 °C. A small amount of produced CO
2 (peaks between 2300 cm
−1 and 2400 cm
−1 corresponding to C=O stretching) can be explained by the presence of minor amount of oxygen in initial HDPE [
42], most probably originating from additives that are intercalated to improve the properties of HDPE [
80]. Further reaction of released CO
2 with hydrocarbons at high temperatures (above 850 °C) resulted in the formation of CO, whose presence is documented by C–O stretching peaks between 2100 cm
−1 and 2200 cm
−1 (
Figure S8b). The obtained result suggests that pyrolysis of HDPE produces a valuable gaseous product rich in hydrocarbons.
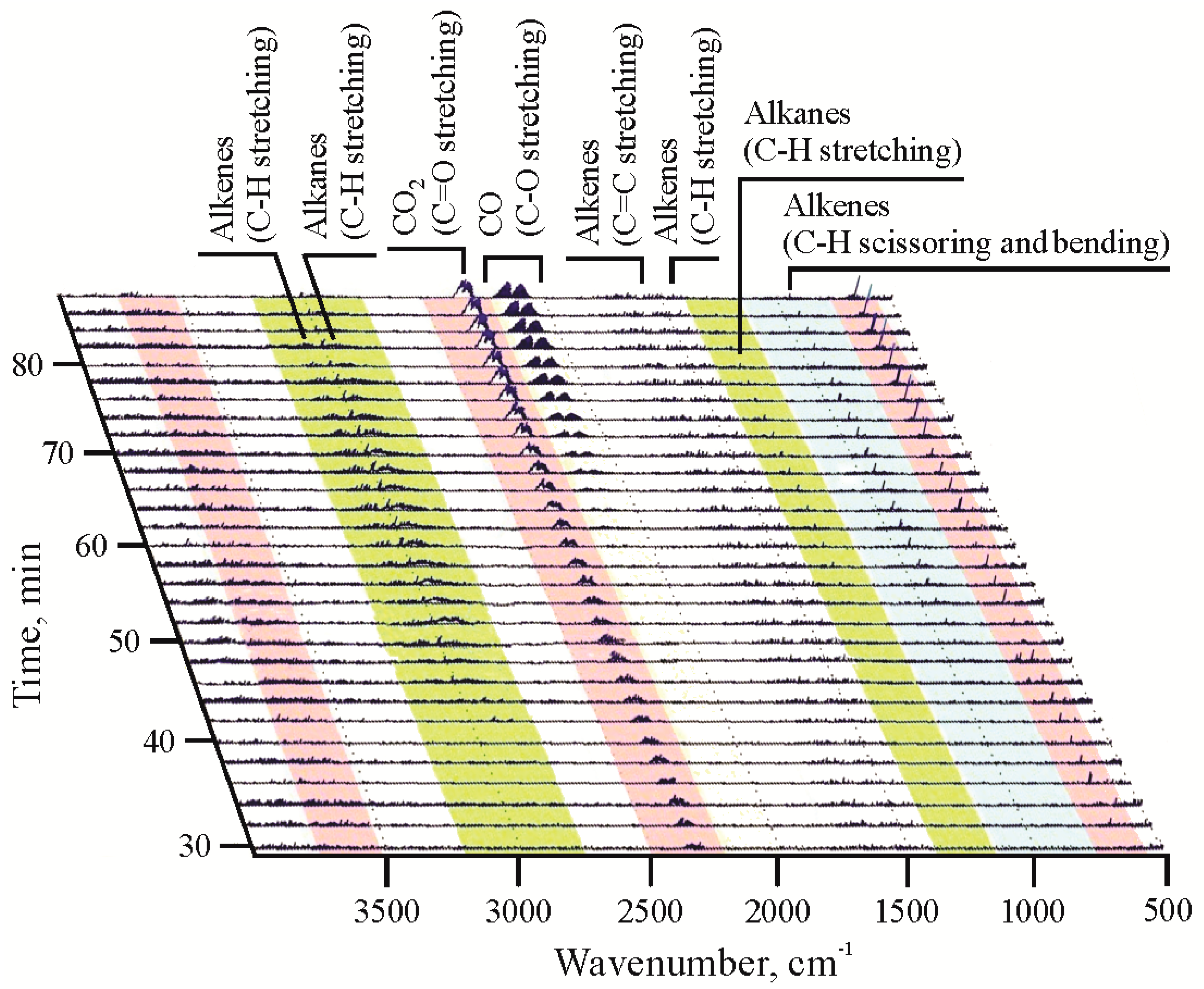
TGA-FTIR thermogram of lignite/HDPE mixture reveals that gas contains all of the above-mentioned components (light hydrocarbons, CO
2, CO, and water vapour) detected in TGA-FTIR thermograms of pure lignite and pure HDPE, with prevalence of CO
2 and CO (
Figure 5 and
Figure S8c). The obtained results suggest that the presence of HDPE can significantly improve the quality of the gas composition in comparison with gas produced from lignite alone, which is primarily reflected through the elevated content of hydrocarbons. Although TGA-FTIR thermograms enable the qualitative gas composition assessment only, the obtained results are in concordance with more precise published results of GC analysis of gaseous products obtained by pyrolysis of coal, HDPE, and their mixture [
35,
52].