Fire-Safe Polymer Composites: Flame-Retardant Effect of Nanofillers
Abstract
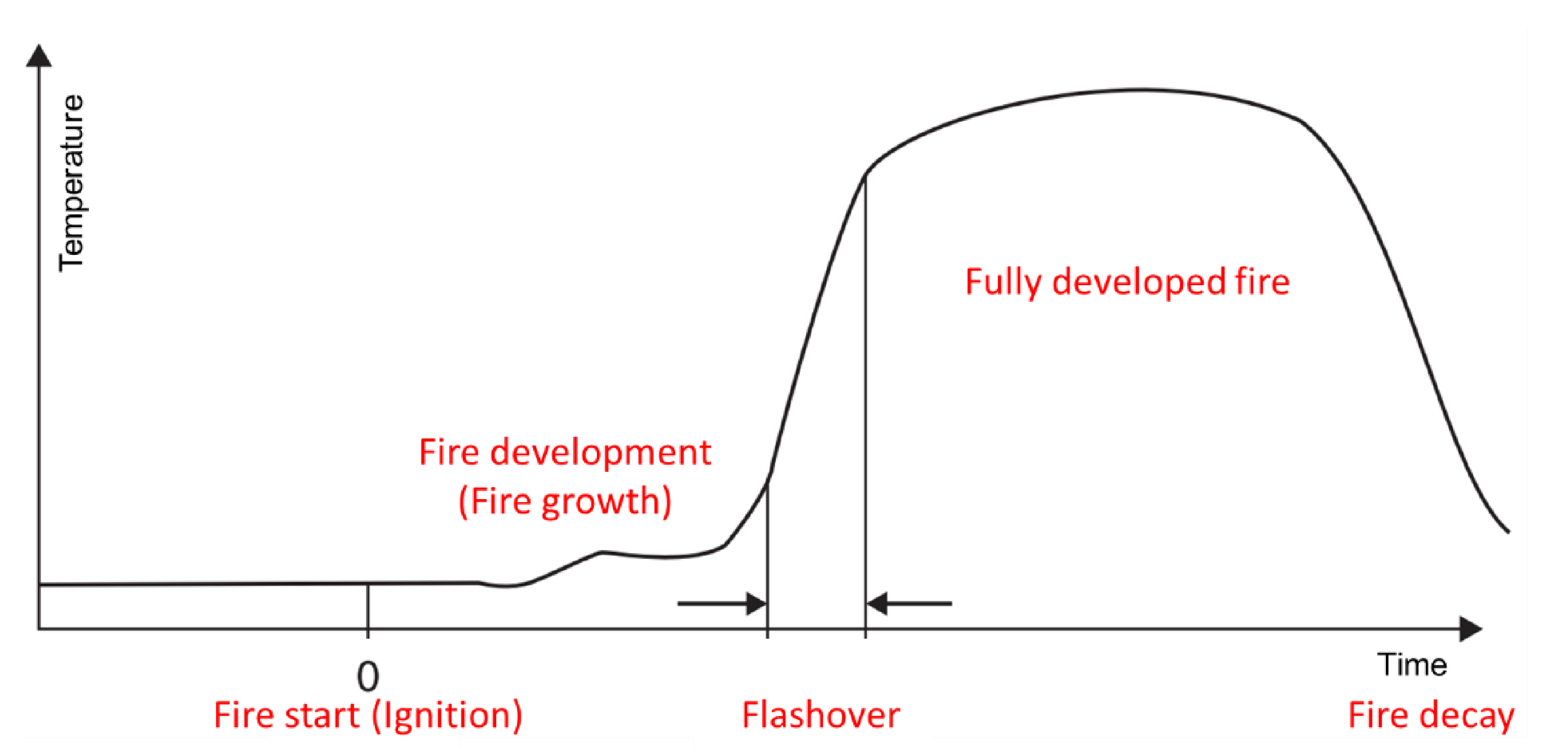
1. Introduction
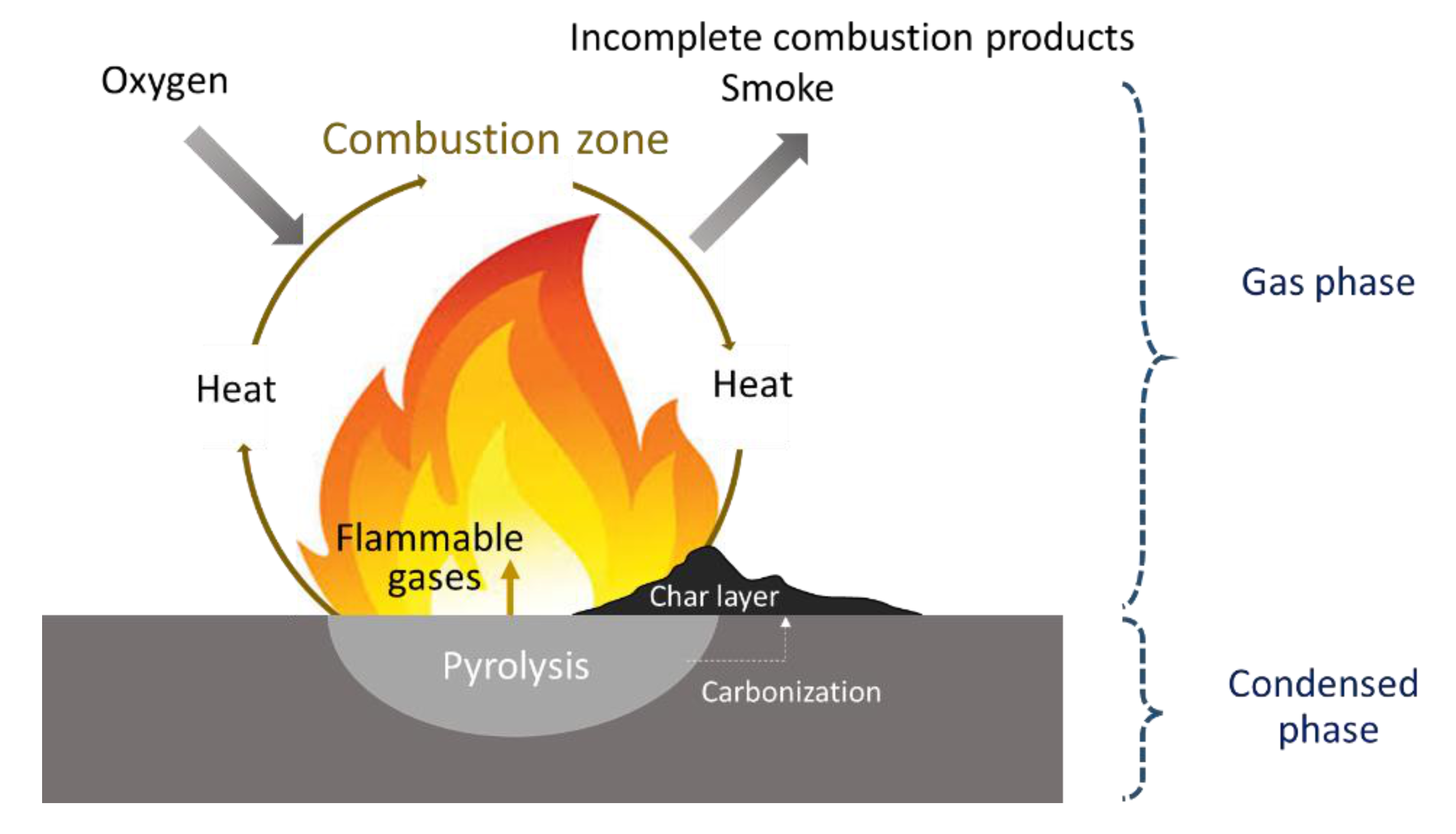
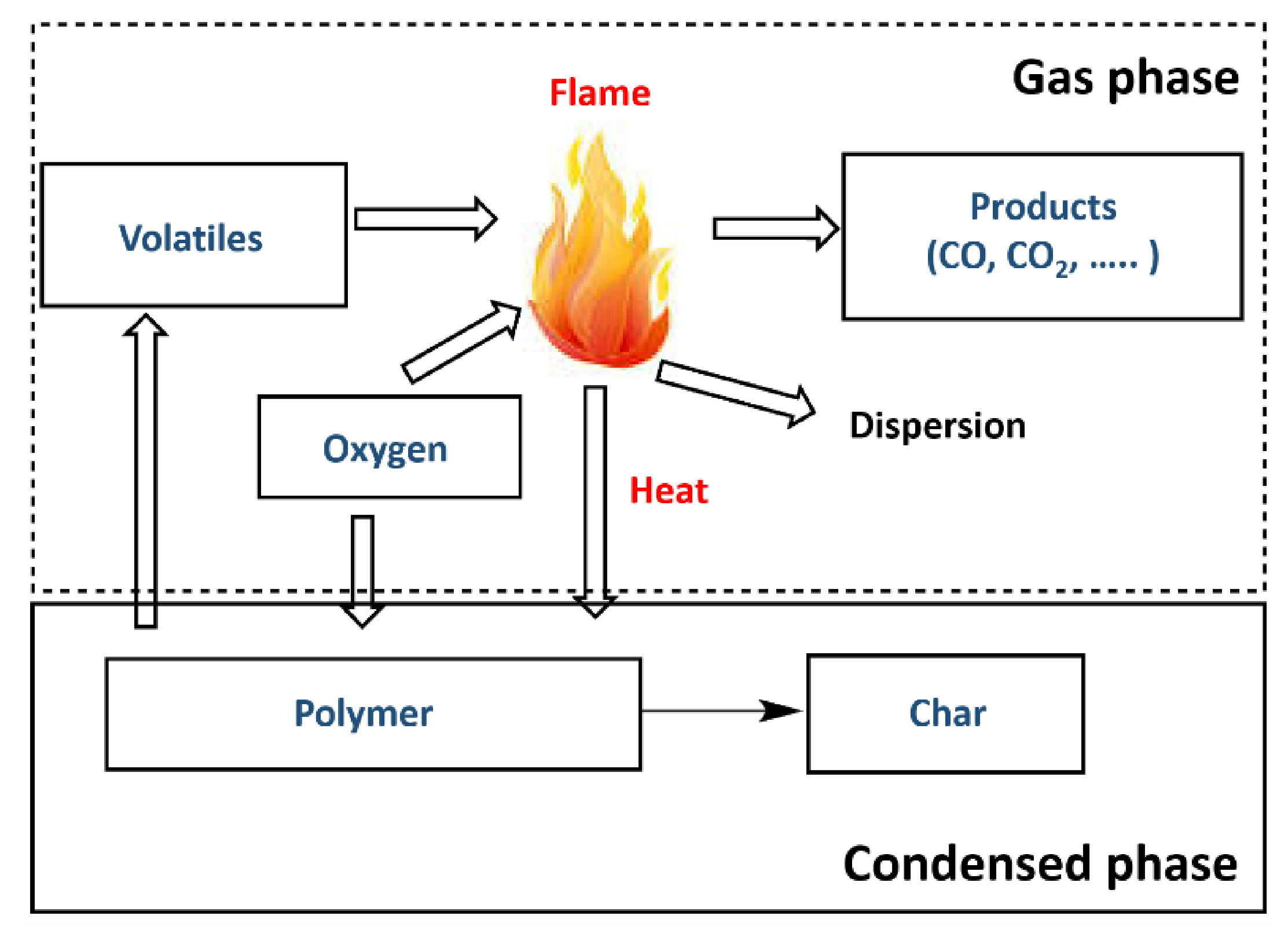
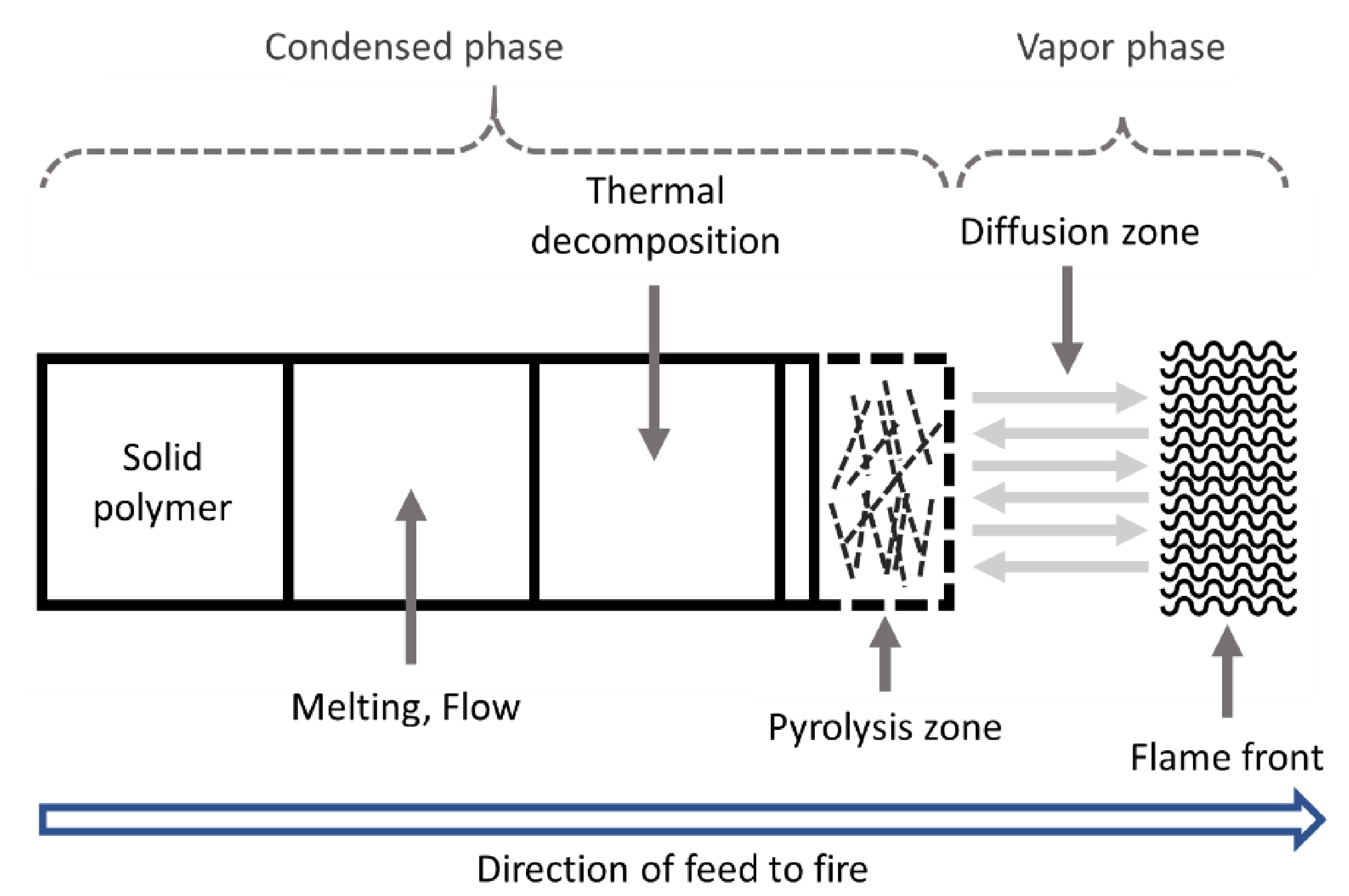
2. Polymer Combustion
3. Types of Flame Retardants
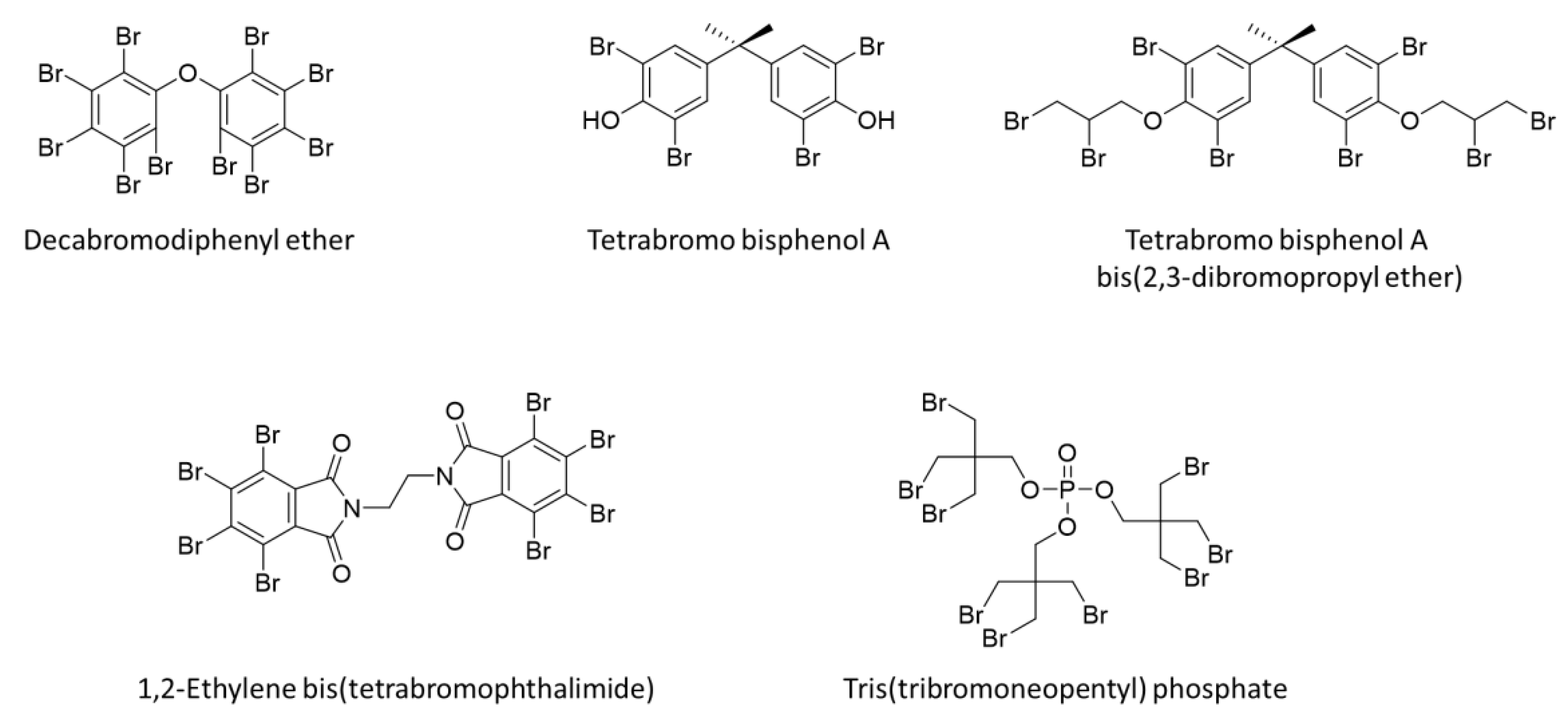
3.1. Additive Flame Retardants
3.2. Reactive Flame Retardants
4. Flammability Testing
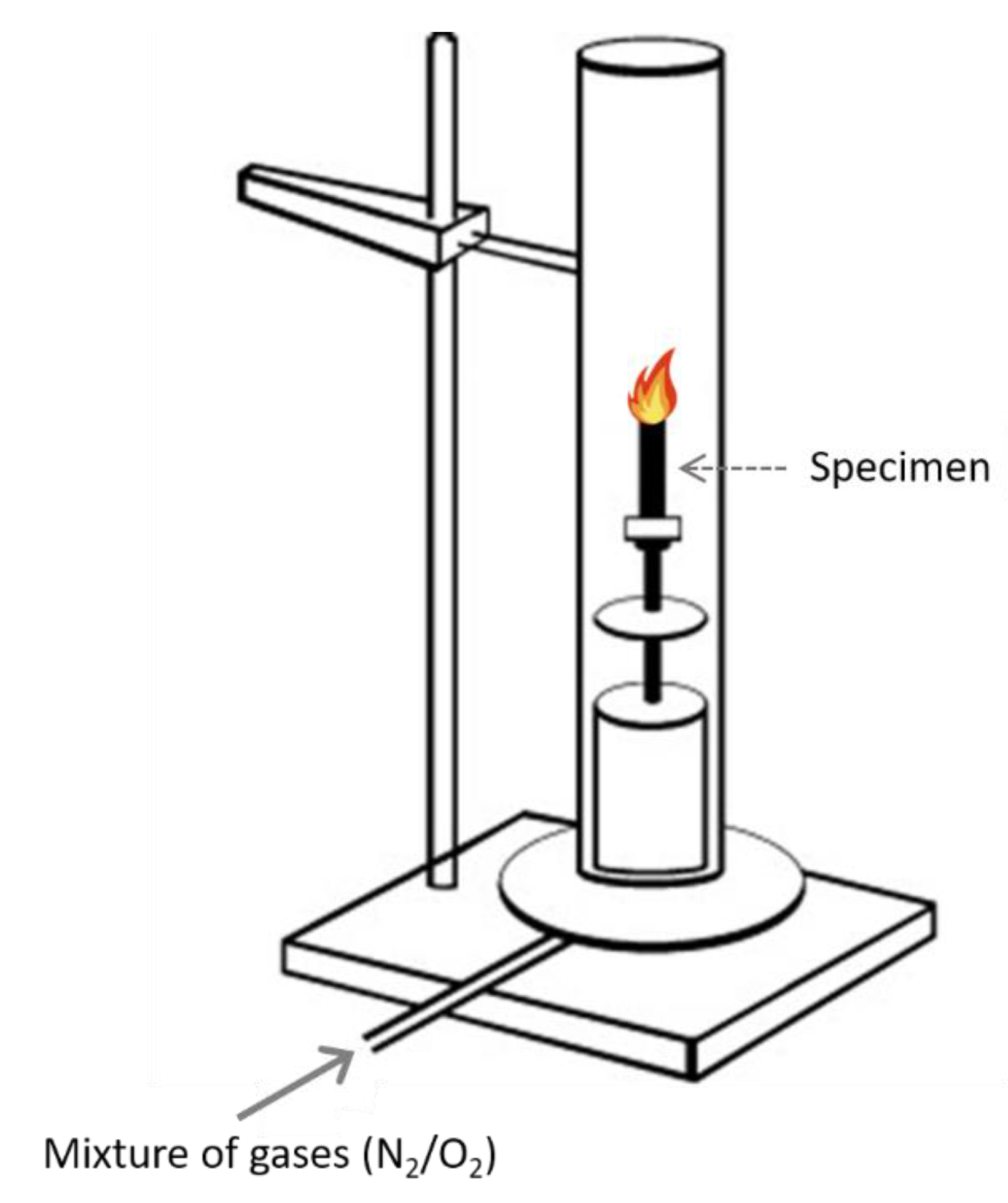
4.1. Limiting Oxygen Index (LOI)
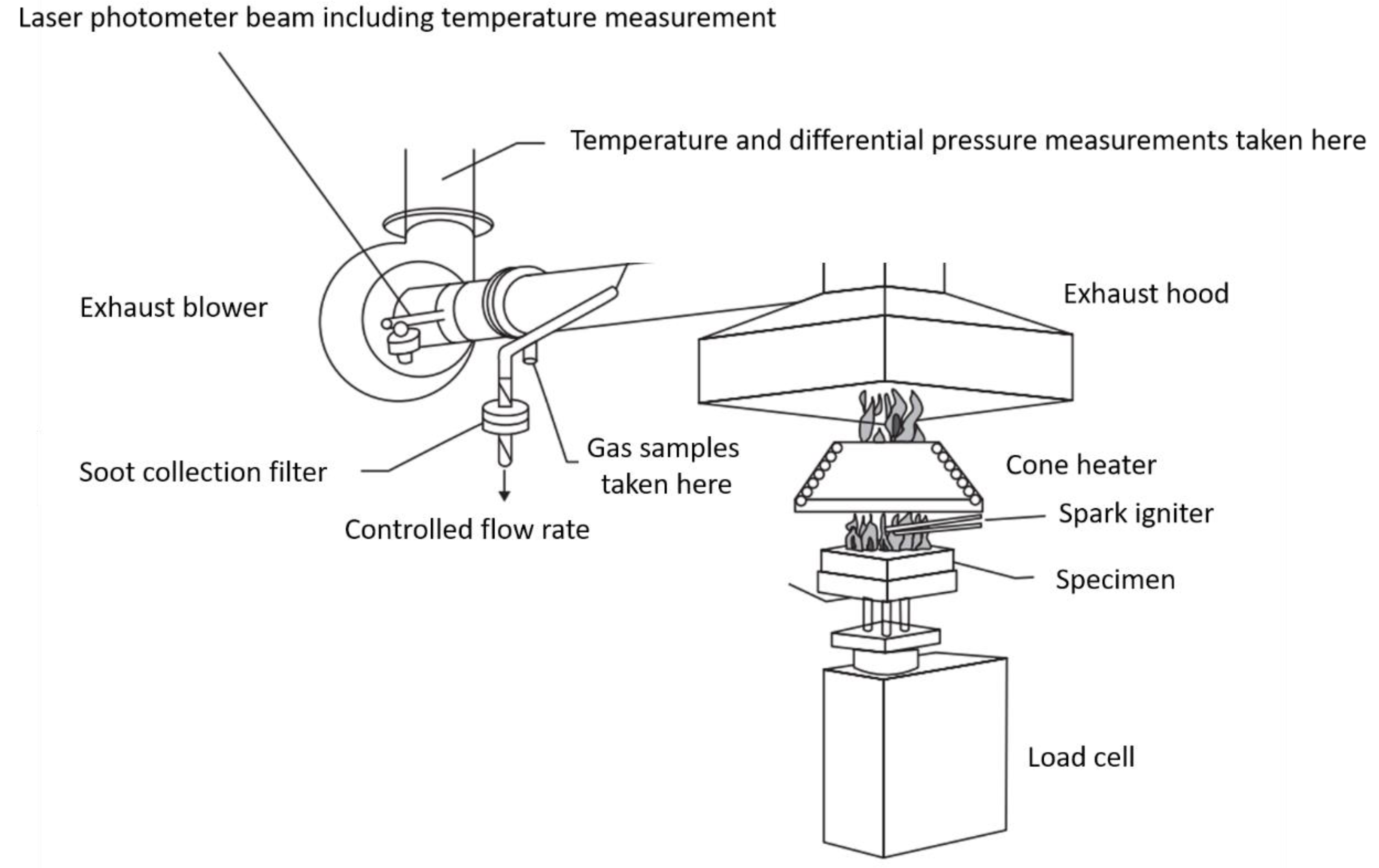
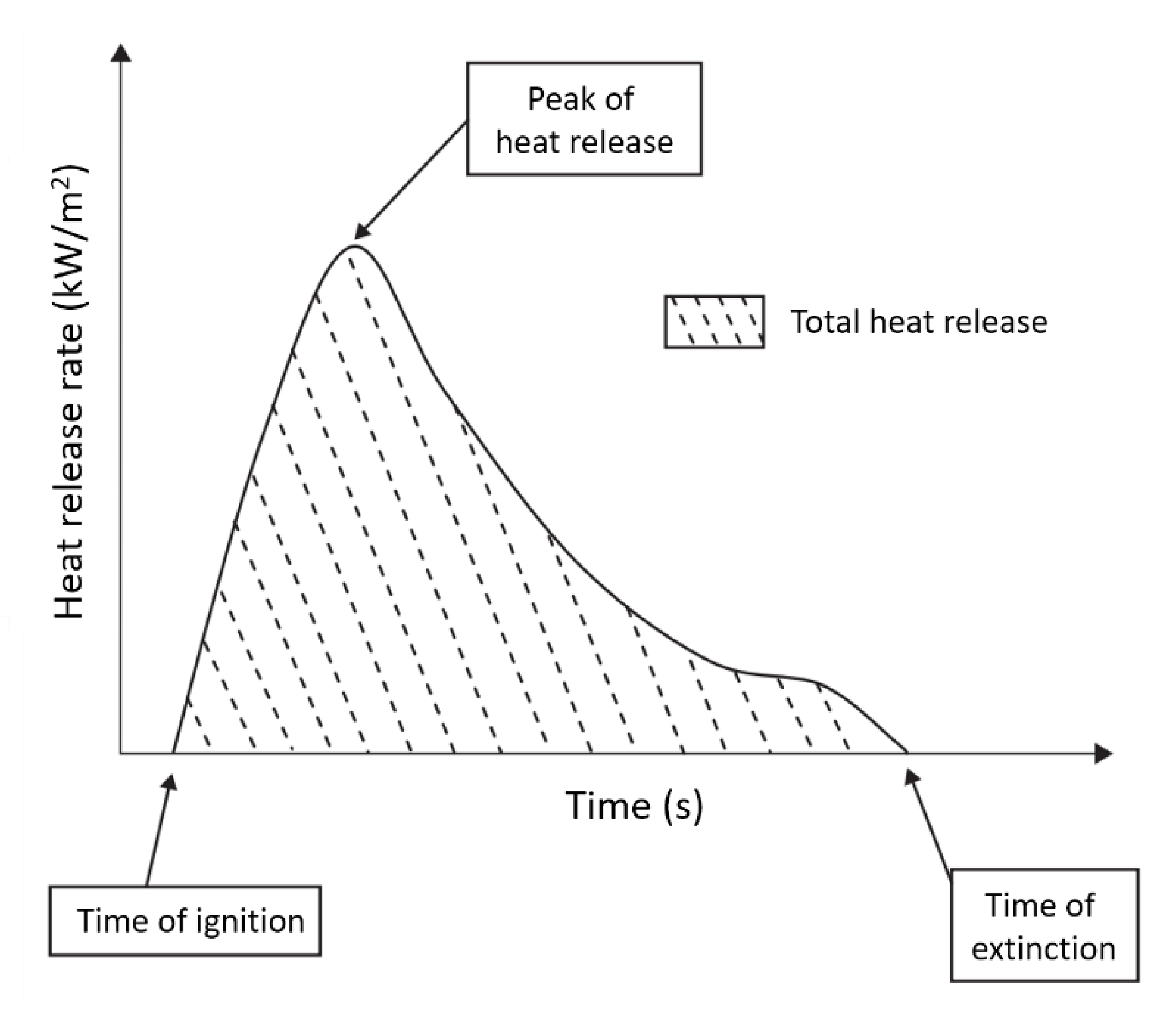
4.2. Cone Calorimetry
4.3. UL 94
4.4. Thermogravimetric Analysis (TGA)
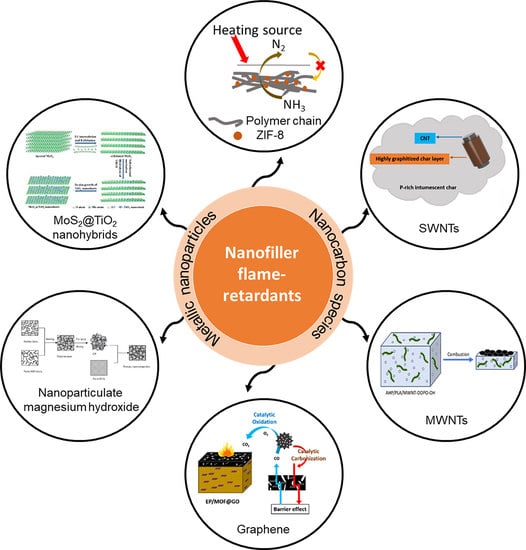
5. Nanoparticles as Flame-Retardant Fillers
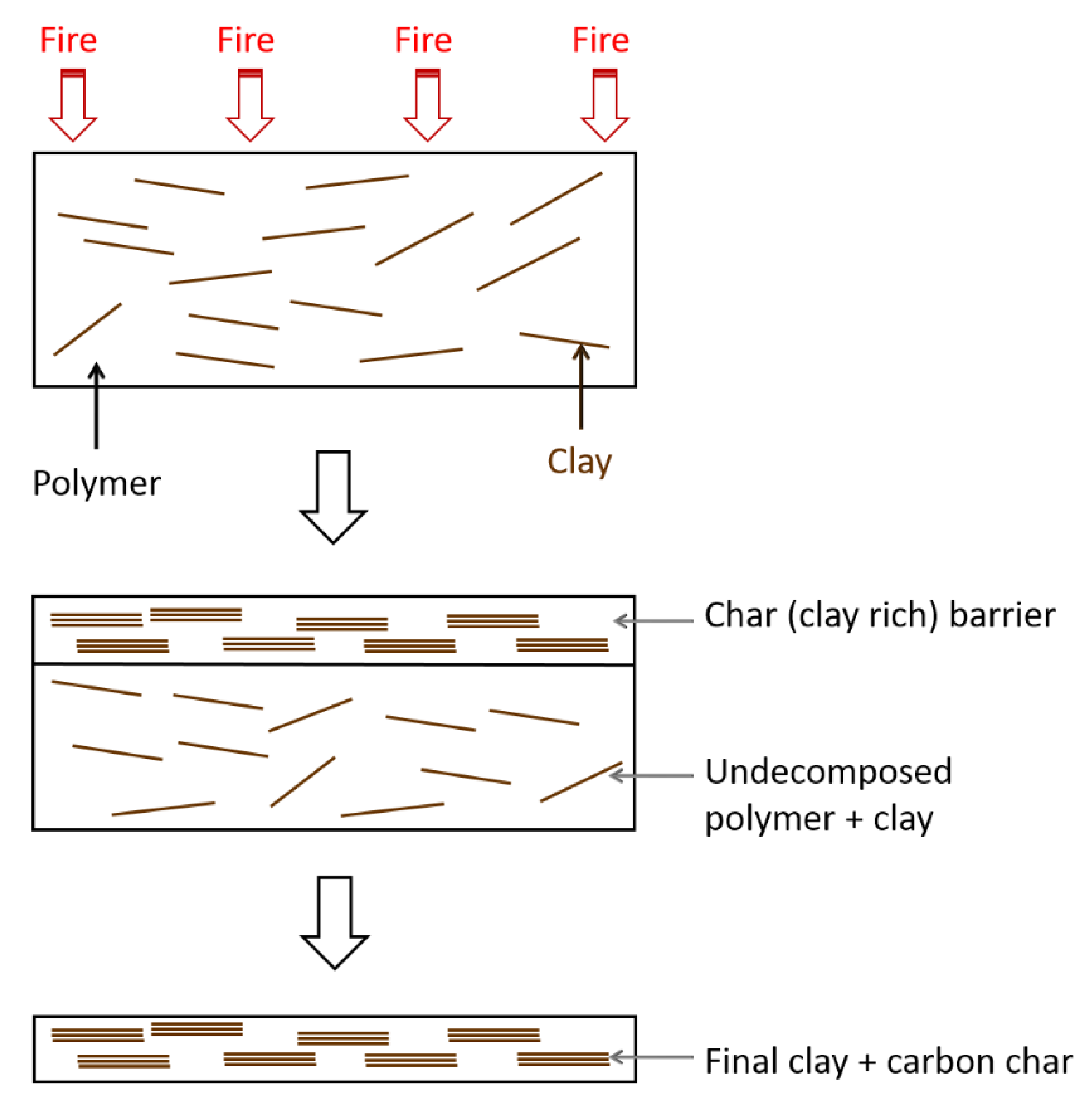
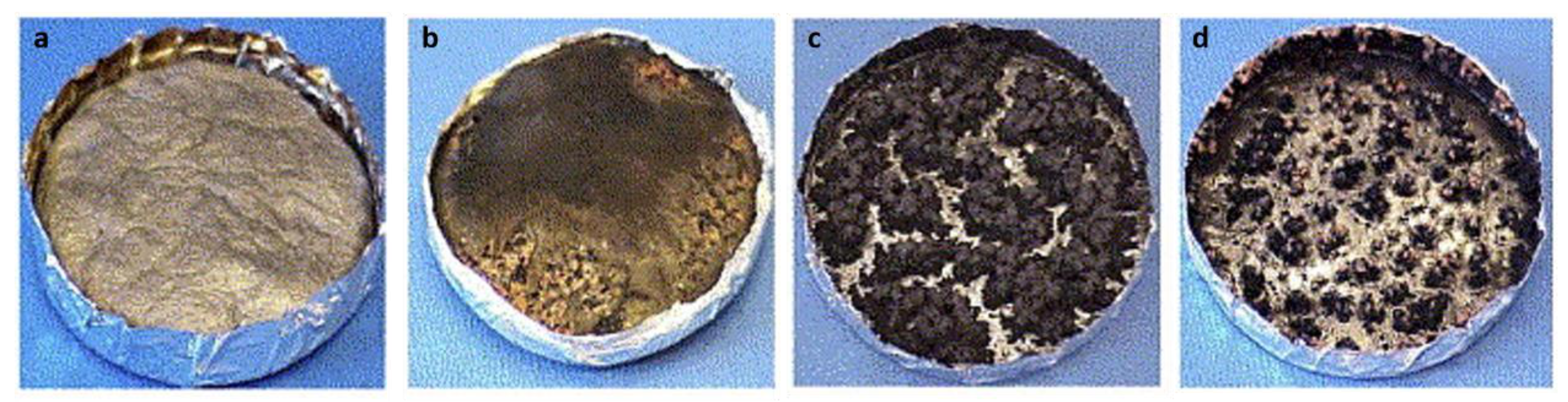
5.1. Nanocarbon Species
5.1.1. Single-Walled Carbon Nanotubes (SWNTs)
5.1.2. Multi-Walled Carbon Nanotubes (MWNTs)
5.1.3. Graphene
5.1.4. Graphitic Carbon Nitrides
5.2. Inorganic Nanoparticles
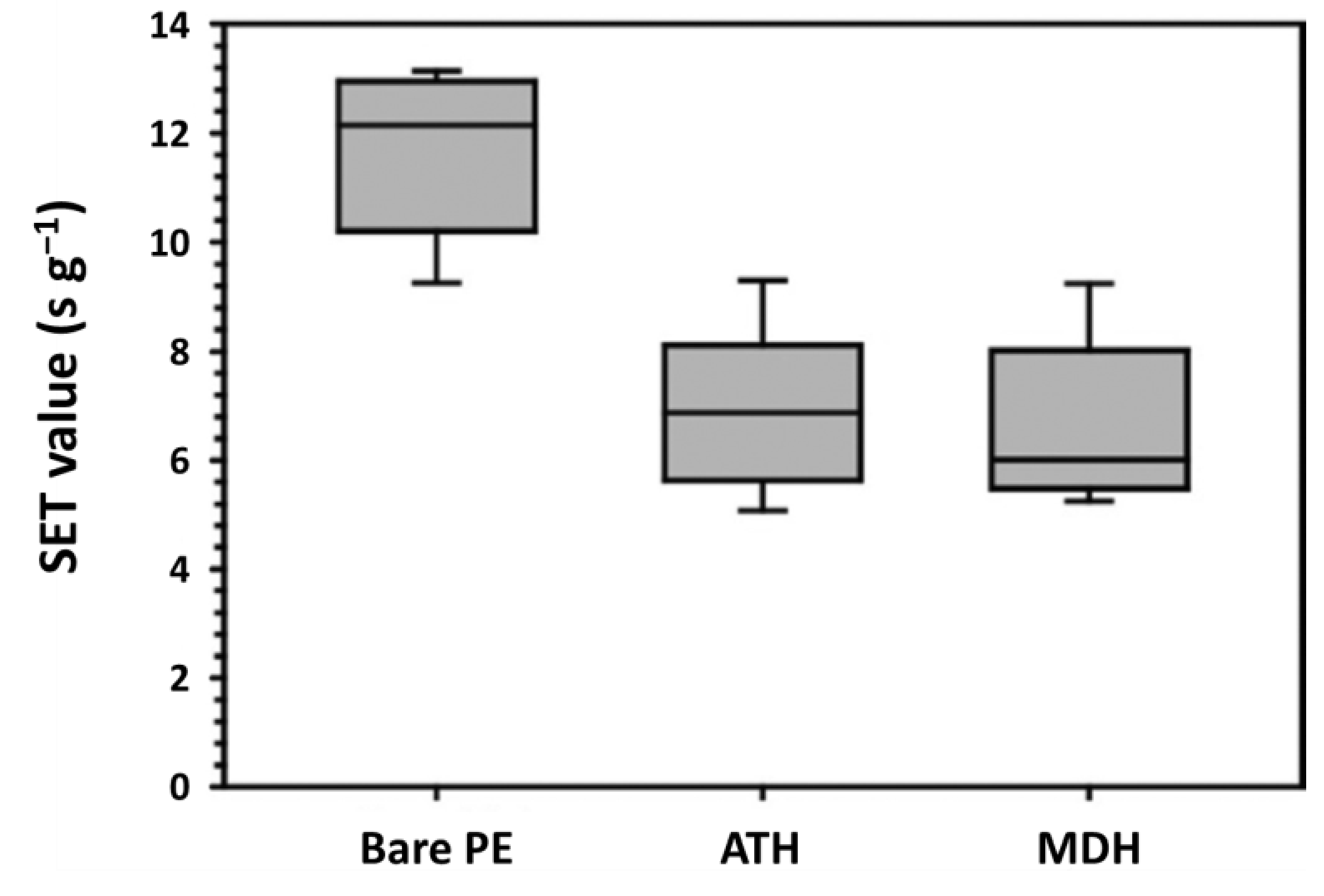
5.2.1. Nanoparticulate Magnesium Hydroxide
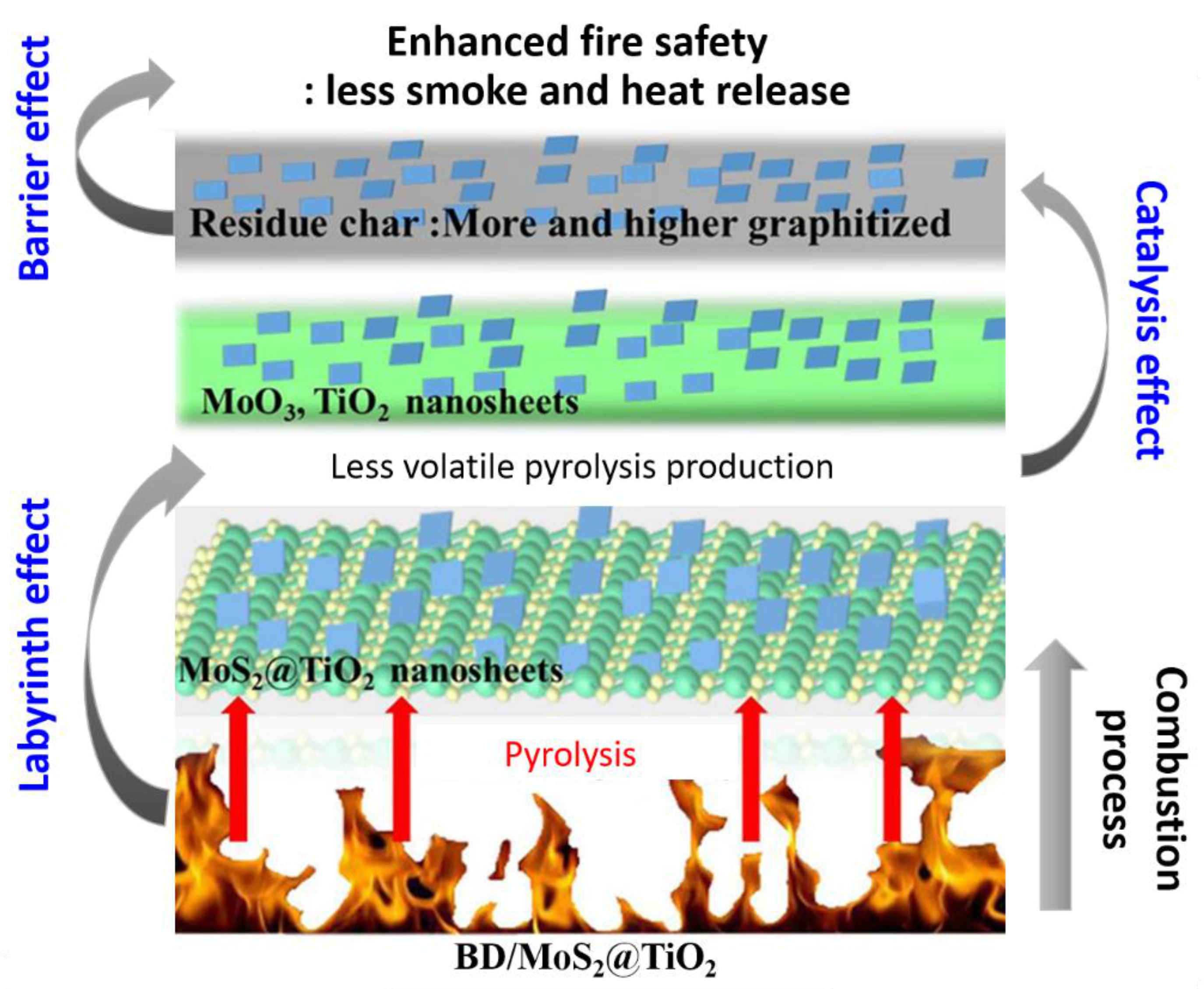
5.2.2. MoS2@TiO2 Nanohybrids
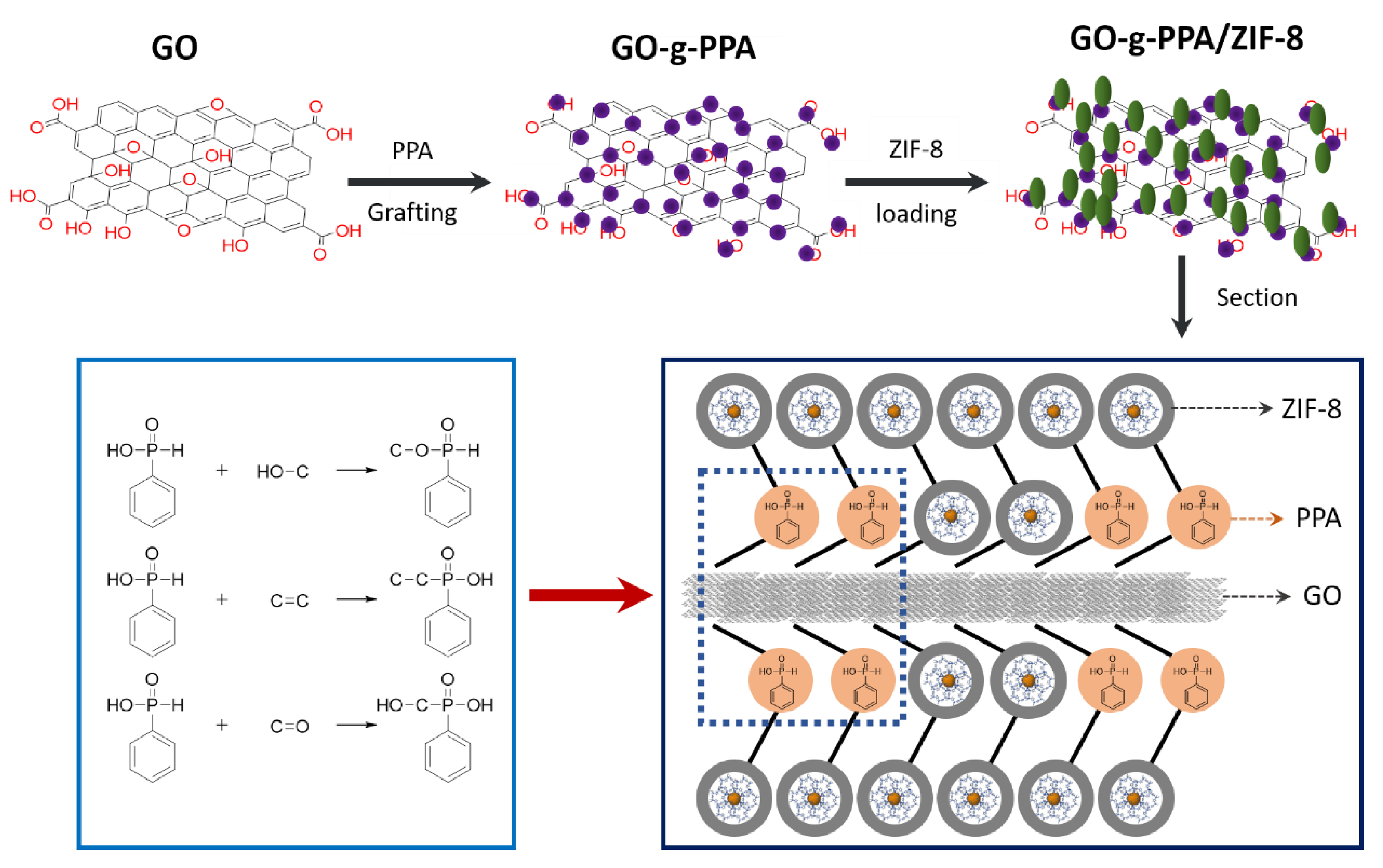
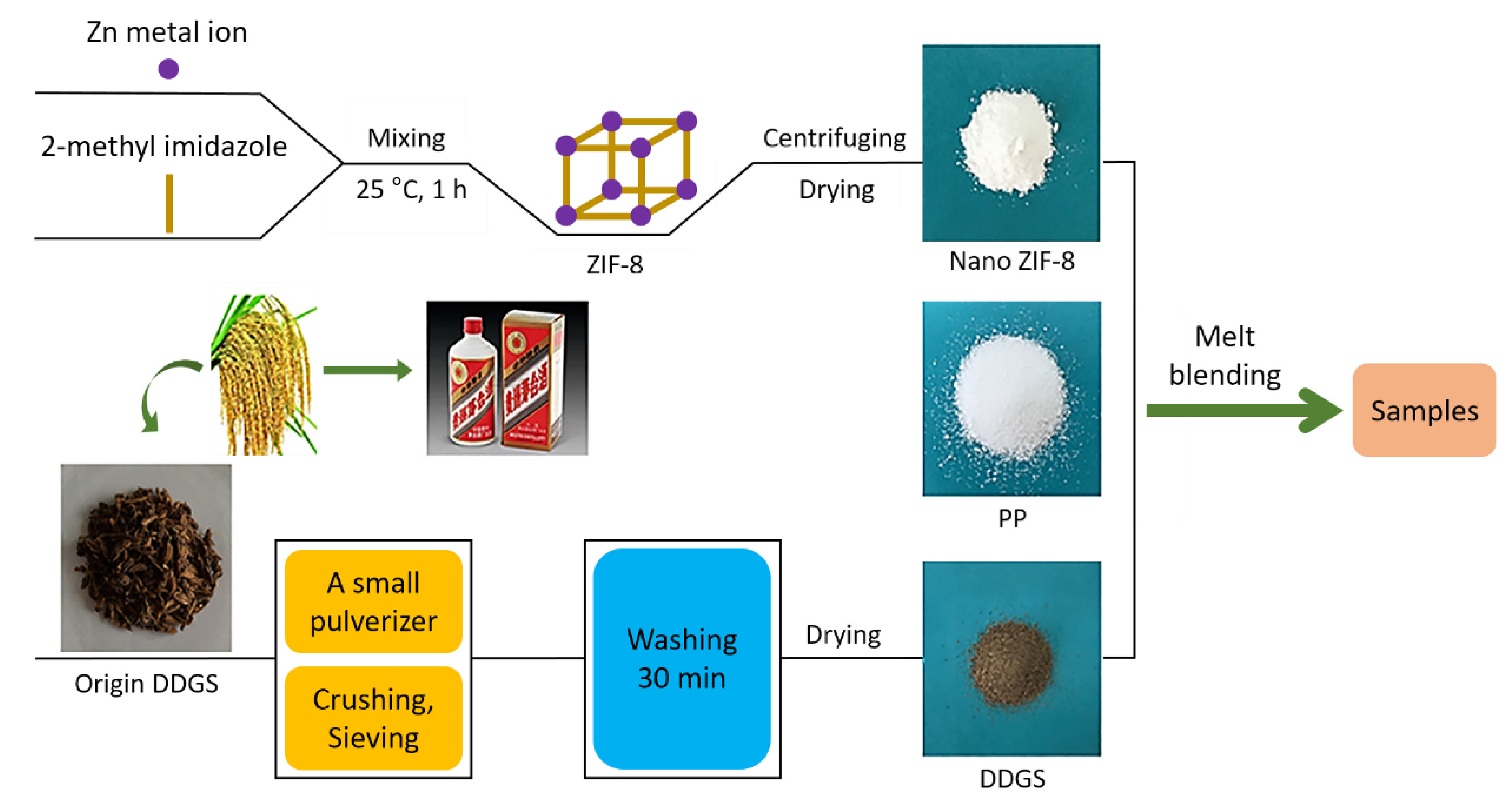
5.2.3. Nanoparticulate Zeolitic Imidazolate Framework-8
5.2.4. Modified Sb2O3 Nanoparticles
5.2.5. MXenes
6. Conclusions and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nolan, R.H.; Boer, M.M.; Collins, L.; Resco de Dios, V.; Clarke, H.; Jenkins, M.; Kenny, B.; Bradstock, R.A. Causes and consequences of eastern Australia’s 2019–20 season of mega-fires. Glob. Chang. Biol. 2020, 26, 1039–1041. [Google Scholar] [CrossRef]
- Purnomo, H.; Shantiko, B.; Sitorus, S.; Gunawan, H.; Achdiawan, R.; Kartodihardjo, H.; Dewayani, A.A. Fire economy and actor network of forest and land fires in Indonesia. Policy Econ. 2017, 78, 21–31. [Google Scholar] [CrossRef]
- Moshashaei, P.; Alizadeh, S.S.; Khazini, L.; Asghari-Jafarabadi, M. Investigate the causes of fires and explosions at external floating roof tanks: A comprehensive literature review. J. Fail. Anal. Prev. 2017, 17, 1044–1052. [Google Scholar] [CrossRef]
- Giebułtowicz, J.; Rużycka, M.; Wroczyński, P.; Purser, D.A.; Stec, A.A. Analysis of fire deaths in Poland and influence of smoke toxicity. Forensic Sci. Int. 2017, 277, 77–87. [Google Scholar] [CrossRef]
- Stoll, S.; Roider, G.; Keil, W. Concentrations of cyanide in blood samples of corpses after smoke inhalation of varying origin. Int. J. Leg. Med. 2017, 131, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Janík, M.; Ublová, M.; Kučerová, Š.; Hejna, P. Carbon monoxide-related fatalities: A 60-year single institution experience. J. Forensic Leg. Med. 2017, 48, 23–29. [Google Scholar] [CrossRef] [PubMed]
- McKenna, S.T.; Birtles, R.; Dickens, K.; Walker, R.G.; Spearpoint, M.J.; Stec, A.A.; Hull, T.R. Flame retardants in UK furniture increase smoke toxicity more than they reduce fire growth rate. Chemosphere 2018, 196, 429–439. [Google Scholar] [CrossRef]
- Dewaghe, C.; Lew, C.Y.; Claes, M.; Belgium, S.A.; Dubois, P. 23—Fire-retardant applications of polymer–carbon nanotubes composites: Improved barrier effect and synergism. In Polymer–Carbon Nanotube Composites; McNally, T., Pötschke, P., Eds.; Woodhead Publishing: Cambridge, UK, 2011; pp. 718–745. [Google Scholar]
- Cortés, D.; Gil, D.; Azorín, J.; Vandecasteele, F.; Verstockt, S. A review of modelling and simulation methods for flashover prediction in confined space fires. Appl. Sci. 2020, 10, 5609. [Google Scholar] [CrossRef]
- Cicione, A.; Walls, R.S.; Kahanji, C. Experimental study of fire spread between multiple full scale informal settlement dwellings. Fire Saf. J. 2019, 105, 19–27. [Google Scholar] [CrossRef]
- Till, R.C.; Coon, J.W. Unwanted Fire and Fire Growth. In Fire Protection: Detection, Notification, and Suppression; Till, R.C., Coon, J.W., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 1–10. [Google Scholar]
- Zong, R.; Kang, R.; Zhao, W.; Tao, C. Experimental Study and Model Analysis of Flashover in Confined Compartments. In Fire Science and Technology 2015; Springer: Singapore, 2017. [Google Scholar]
- Abdelnasser, S.; Park, G.; Han, H.; Toth, R.; Yoon, H. Enhanced photocatalytic performance of poly(3,4-ethylenedioxythiophene)-coated TiO2 nanotube electrodes. Synth. Met. 2019, 251, 120–126. [Google Scholar] [CrossRef]
- Le, T.-H.; Kim, Y.; Yoon, H. Electrical and electrochemical properties of conducting polymers. Polymers 2017, 9, 150. [Google Scholar] [CrossRef]
- Nguyen, D.N.; Yoon, H. Recent advances in nanostructured conducting polymers: From synthesis to practical applications. Polymers 2016, 8, 118. [Google Scholar] [CrossRef]
- Park, S.J.; Park, C.S.; Yoon, H. Chemo-electrical gas sensors based on conducting polymer hybrids. Polymers 2017, 9, 155. [Google Scholar] [CrossRef] [PubMed]
- Elomaa, M.; Sarvaranta, L.; Mikkola, E.; Kallonen, R.; Zitting, A.; Zevenhoven, C.A.P.; Hupa, M. Combustion of polymeric materials. Crit. Rev. Anal. Chem. 1997, 27, 137–197. [Google Scholar] [CrossRef]
- Puliyalil, H.; Filipič, G.; Cvelbar, U. Chapter 9—Selective Plasma Etching of Polymers and Polymer Matrix Composites. In Non-Thermal Plasma Technology for Polymeric Materials; Thomas, S., Mozetič, M., Cvelbar, U., Špatenka, P., K.M, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 241–259. [Google Scholar]
- Nair, S.; Pitchan, M.K.; Bhowmik, S.; Epaarachchi, J. Development of high temperature electrical conductive polymeric nanocomposite films for aerospace applications. Mater. Res. Express 2018, 6, 026422. [Google Scholar] [CrossRef]
- Su, Y.; Lin, H.; Zhang, S.; Yang, Z.; Yuan, T. One-step synthesis of novel renewable vegetable oil-based acrylate prepolymers and their application in UV-curable coatings. Polymers 2020, 12, 1165. [Google Scholar] [CrossRef]
- Islam, G.M.N.; Ali, A.; Collie, S. Textile sensors for wearable applications: A comprehensive review. Cellulose 2020, 27, 6103–6131. [Google Scholar] [CrossRef]
- Puype, F.; Samsonek, J.; Vilímková, V.; Kopečková, Š.; Ratiborská, A.; Knoop, J.; Egelkraut-Holtus, M.; Ortlieb, M.; Oppermann, U. Towards a generic procedure for the detection of relevant contaminants from waste electric and electronic equipment (WEEE) in plastic food-contact materials: A review and selection of key parameters. Food Addit. Contam. Part A 2017, 34, 1767–1783. [Google Scholar] [CrossRef]
- Chambhare, S.U.; Lokhande, G.P.; Jagtap, R.N. UV-curable behavior of phosphorus- and nitrogen-based reactive diluent for epoxy acrylate oligomer used for flame-retardant wood coating. J. Coat. Technol. Res. 2016, 13, 703–714. [Google Scholar] [CrossRef]
- Mark, H.F.; Atlas, S.M.; Shalaby, S.W.; Pearce, E.M. Combustion of Polymers and its Retardation. In Flame-Retardant Polymeric Materials; Lewin, M., Atlas, S.M., Pearce, E.M., Eds.; Springer: Boston, MA, USA, 1975; pp. 1–17. [Google Scholar]
- Sparks, A.M.; Smith, A.M.S.; Talhelm, A.F.; Kolden, C.A.; Yedinak, K.M.; Johnson, D.M. Impacts of fire radiative flux on mature Pinus ponderosa growth and vulnerability to secondary mortality agents. Int. J. Wildland Fire 2017, 26, 95–106. [Google Scholar] [CrossRef]
- Gričar, J.; Hafner, P.; Lavrič, M.; Ferlan, M.; Ogrinc, N.; Krajnc, B.; Eler, K.; Vodnik, D. Post-fire effects on development of leaves and secondary vascular tissues in quercus pubescens. Tree Physiol. 2020, 40, 796–809. [Google Scholar] [CrossRef] [PubMed]
- Kruse, T.M.; Woo, O.S.; Wong, H.-W.; Khan, S.S.; Broadbelt, L.J. Mechanistic modeling of polymer degradation: A comprehensive study of polystyrene. Macromolecules 2002, 35, 7830–7844. [Google Scholar] [CrossRef]
- Howell, B.A. The Mechanism of Poly(Styrene) Degradation. In Reactions and Mechanisms in Thermal Analysis of Advanced Materials; Scrivener Publishing: Beverly, MA, USA, 2015; pp. 259–267. [Google Scholar]
- Kim, Y.; Le, T.-H.; Kim, S.; Park, G.; Yang, K.S.; Yoon, H. Single-walled carbon nanotube-in-binary-polymer nanofiber structures and their use as carbon precursors for electrochemical applications. J. Phys. Chem. C 2018, 122, 4189–4198. [Google Scholar] [CrossRef]
- Kwon, O.S.; Park, C.S.; Park, S.J.; Noh, S.; Kim, S.; Kong, H.J.; Bae, J.; Lee, C.-S.; Yoon, H. Carboxylic acid-functionalized conducting-polymer nanotubes as highly sensitive nerve-agent chemiresistors. Sci. Rep. 2016, 6, 33724. [Google Scholar] [CrossRef]
- Lee, J.E.; Lee, Y.; Ahn, K.-J.; Huh, J.; Shim, H.W.; Sampath, G.; Im, W.B.; Huh, Y.I.; Yoon, H. Role of Co-vapors in vapor deposition polymerization. Sci. Rep. 2015, 5, 8420. [Google Scholar] [CrossRef]
- Noh, S.; Nguyen, D.N.; Park, C.S.; Kim, Y.; Kong, H.J.; Kim, S.; Kim, S.; Hur, S.-M.; Yoon, H. Development of effective porosity in carbon nanofibers based on phase behavior of ternary polymer blend precursors: Toward high-performance electrode materials. J. Phys. Chem. C 2017, 121, 18480–18489. [Google Scholar] [CrossRef]
- Shim, H.W.; Ahn, K.-J.; Im, K.; Noh, S.; Kim, M.-S.; Lee, Y.; Choi, H.; Yoon, H. Effect of hydrophobic moieties in water-soluble polymers on physical exfoliation of graphene. Macromolecules 2015, 48, 6628–6637. [Google Scholar] [CrossRef]
- Yoon, H. Current trends in sensors based on conducting polymer nanomaterials. Nanomaterials 2013, 3, 524–549. [Google Scholar] [CrossRef]
- Malucelli, G. Surface-engineered fire protective coatings for fabrics through sol-gel and layer-by-layer methods: An overview. Coatings 2016, 6, 33. [Google Scholar] [CrossRef]
- Im, K.; Nguyen, D.N.; Kim, S.; Kong, H.J.; Kim, Y.; Park, C.S.; Kwon, O.S.; Yoon, H. Graphene-embedded hydrogel nanofibers for detection and removal of aqueous-phase dyes. Acs Appl. Mater. Interfaces 2017, 9, 10768–10776. [Google Scholar] [CrossRef]
- Kang, M.; Lee, J.E.; Shim, H.W.; Jeong, M.S.; Im, W.B.; Yoon, H. Intrinsically conductive polymer binders for electrochemical capacitor application. Rsc Adv. 2014, 4, 27939–27945. [Google Scholar] [CrossRef]
- Lee, J.E.; Park, S.J.; Kwon, O.S.; Shim, H.W.; Jang, J.; Yoon, H. Systematic investigation on charge storage behaviour of multidimensional poly(3,4-ethylenedioxythiophene) nanostructures. Rsc Adv. 2014, 4, 37529–37535. [Google Scholar] [CrossRef]
- Lahoti, M.; Tan, K.H.; Yang, E.-H. A critical review of geopolymer properties for structural fire-resistance applications. Constr. Build. Mater. 2019, 221, 514–526. [Google Scholar] [CrossRef]
- Zhang, M.; Shi, X.; Dai, X.; Huo, C.; Xie, J.; Li, X.; Wang, X. Improving the crystallization and fire resistance of poly(lactic acid) with nano-ZIF-8@GO. J. Mater. Sci. 2018, 53, 7083–7093. [Google Scholar] [CrossRef]
- Miao, J.; Fang, Y.; Guo, Y.; Zhu, Y.; Hu, A.; Wang, G. Interpenetrating polymer networks of porous organic polymers and polyurethanes for flame resistance and high mechanical properties. Acs Appl. Polym. Mater. 2019, 1, 2692–2702. [Google Scholar] [CrossRef]
- Baldissera, A.F.; Silveira, M.R.d.S.; Beraldo, C.H.; Tocchetto, N.S.; Ferreira, C.A. Polymeric organic coatings based on PANI-ES and PANI-ES/APP for fire protection. J. Mater. Res. Technol. 2019, 8, 2832–2845. [Google Scholar] [CrossRef]
- Ding, Z.; Xu, M.-R.; Dai, J.-G.; Dong, B.-Q.; Zhang, M.-J.; Hong, S.-X.; Xing, F. Strengthening concrete using phosphate cement-based fiber-reinforced inorganic composites for improved fire resistance. Constr. Build. Mater. 2019, 212, 755–764. [Google Scholar] [CrossRef]
- Legrand, V.; TranVan, L.; Casari, P.; Jacquemin, F. Structure-properties relationships of moisturized sandwich composite materials under extreme temperature conditions (fire resistance). Compos. Struct. 2020, 235, 111774. [Google Scholar] [CrossRef]
- Contescu, C.I.; Adhikari, S.P.; Gallego, N.C.; Evans, N.D.; Biss, B.E. Activated carbons derived from high-temperature pyrolysis of lignocellulosic biomass. C J. Carbon Res. 2018, 4, 51. [Google Scholar] [CrossRef]
- Aslani, A.; Mazzuca-Sobczuk, T.; Eivazi, S.; Bekhrad, K. Analysis of bioenergy technologies development based on life cycle and adaptation trends. Renew. Energy 2018, 127, 1076–1086. [Google Scholar] [CrossRef]
- Eschenbacher, A.; Saraeian, A.; Shanks, B.H.; Mentzel, U.V.; Ahrenfeldt, J.; Henriksen, U.B.; Jensen, A.D. Counteracting rapid catalyst deactivation by concomitant temperature increase during catalytic upgrading of biomass pyrolysis vapors using solid acid catalysts. Catalysts 2020, 10, 748. [Google Scholar] [CrossRef]
- Vincent, T.; Vincent, C.; Dumazert, L.; Otazaghine, B.; Sonnier, R.; Guibal, E. Fire behavior of innovative alginate foams. Carbohydr. Polym. 2020, 250, 116910. [Google Scholar] [CrossRef]
- Morgan, A.B.; Gilman, J.W. An overview of flame retardancy of polymeric materials: Application, technology, and future directions. Fire Mater. 2013, 37, 259–279. [Google Scholar] [CrossRef]
- Camino, G.; Costa, L. Performance and mechanisms of fire retardants in polymers—A review. Polym. Degrad. Stab. 1988, 20, 271–294. [Google Scholar] [CrossRef]
- Choi, K.; Seo, S.; Kwon, H.; Kim, D.; Park, Y.T. Fire protection behavior of layer-by-layer assembled starch–clay multilayers on cotton fabric. J. Mater. Sci. 2018, 53, 11433–11443. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, J.; Wang, X.-L.; Wang, Y.-Z. Highly thermostable and durably flame-retardant unsaturated polyester modified by a novel polymeric flame retardant containing Schiff base and spirocyclic structures. Chem. Eng. J. 2018, 344, 419–430. [Google Scholar] [CrossRef]
- Geoffroy, L.; Samyn, F.; Jimenez, M.; Bourbigot, S. Intumescent polymer metal laminates for fire protection. Polymers 2018, 10, 995. [Google Scholar] [CrossRef]
- Wu, J.-N.; Chen, L.; Fu, T.; Zhao, H.-B.; Guo, D.-M.; Wang, X.-L.; Wang, Y.-Z. New application for aromatic Schiff base: High efficient flame-retardant and anti-dripping action for polyesters. Chem. Eng. J. 2018, 336, 622–632. [Google Scholar] [CrossRef]
- Feng, Y.; Hu, J.; Xue, Y.; He, C.; Zhou, X.; Xie, X.; Ye, Y.; Mai, Y.-W. Simultaneous improvement in the flame resistance and thermal conductivity of epoxy/Al2O3 composites by incorporating polymeric flame retardant-functionalized graphene. J. Mater. Chem. A 2017, 5, 13544–13556. [Google Scholar] [CrossRef]
- Mastalska-Popławska, J.; Kadac, K.; Izak, P.; Gierej, M.; Stempkowska, A.; Góral, Z. The influence of ceramic additives on intumescence and thermal activity of epoxy coatings for steel. J. Appl. Polym. Sci. 2020, 138, 49914. [Google Scholar] [CrossRef]
- Kabir, I.I.; Sorrell, C.C.; Mofarah, S.S.; Yang, W.; Yuen, A.C.Y.; Nazir, M.T.; Yeoh, G.H. Alginate/polymer-based materials for fire retardancy: Synthesis, structure, properties, and applications. Polym. Rev. 2020, 1–58. [Google Scholar] [CrossRef]
- Villamil Watson, D.A.; Schiraldi, D.A. Biomolecules as flame retardant additives for polymers: A review. Polymers 2020, 12, 849. [Google Scholar] [CrossRef] [PubMed]
- Beach, M.W.; Hull, J.W.; King, B.A.; Beulich, I.I.; Stobby, B.G.; Kram, S.L.; Gorman, D.B. Development of a new class of brominated polymeric flame retardants based on copolymers of styrene and polybutadiene. Polym. Degrad. Stab. 2017, 135, 99–110. [Google Scholar] [CrossRef]
- Vahabi, H.; Rastin, H.; Movahedifar, E.; Antoun, K.; Brosse, N.; Saeb, M.R. Flame retardancy of bio-based polyurethanes: Opportunities and challenges. Polymers 2020, 12, 1234. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wu, C.; Xu, S. Mechanical, thermal and flame retardant properties of magnesium hydroxide filled poly(vinyl chloride) composites: The effect of filler shape. Compos. Part A Appl. Sci. Manuf. 2018, 113, 1–11. [Google Scholar] [CrossRef]
- Sinha, J.; Fairbanks, B.D.; Song, H.B.; Bowman, C.N. Phosphate-based cross-linked polymers from iodo–ene photopolymerization: Tuning surface wettability through thiol–ene chemistry. Acs Macro Lett. 2019, 8, 213–217. [Google Scholar] [CrossRef]
- Tawfik, S.Y. Flame Retardants: Additives in Plastic Technology. In Polymers and Polymeric Composites: A Reference Series; Palsule, S., Ed.; Springer: Berlin, Heidelberg, 2017; pp. 1–27. [Google Scholar]
- Alaee, M.; Arias, P.; Sjödin, A.; Bergman, Å. An overview of commercially used brominated flame retardants, their applications, their use patterns in different countries/regions and possible modes of release. Environ. Int. 2003, 29, 683–689. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, Y.; Li, M.; Du, M.; Li, X.; Li, Y. A review of a class of emerging contaminants: The classification, distribution, intensity of consumption, synthesis routes, environmental effects and expectation of pollution abatement to organophosphate flame retardants (OPFRs). Int. J. Mol. Sci. 2019, 20, 2874. [Google Scholar] [CrossRef]
- van der Veen, I.; de Boer, J. Phosphorus flame retardants: Properties, production, environmental occurrence, toxicity and analysis. Chemosphere 2012, 88, 1119–1153. [Google Scholar] [CrossRef]
- Norouzi, M.; Zare, Y.; Kiany, P. Nanoparticles as effective flame retardants for natural and synthetic textile polymers: Application, mechanism, and optimization. Polym. Rev. 2015, 55, 531–560. [Google Scholar] [CrossRef]
- Avelino, F.; Silva, K.T.; Mazzetto, S.E.; Lomonaco, D. Tailor-made organosolv lignins from coconut wastes: Effects of green solvents in microwave-assisted processes upon their structure and antioxidant activities. Bioresour. Technol. Rep. 2019, 7, 100219. [Google Scholar] [CrossRef]
- Hetterich, M.; Butschkau, D. Flame-retardant thermoplastic elastomers with adhesion to polyamides. Adhes. Adhes. Sealants 2020, 17, 12–15. [Google Scholar] [CrossRef]
- Weinert, M.; Döring, M. N-phosphorylated iminophosphoranes based on 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide and their flame-retardant behavior in epoxy resins. J. Appl. Polym. Sci. 2020, 138, 49902. [Google Scholar] [CrossRef]
- Zhou, X.; Qiu, S.; Mu, X.; Zhou, M.; Cai, W.; Song, L.; Xing, W.; Hu, Y. Polyphosphazenes-based flame retardants: A review. Compos. Part B Eng. 2020, 202, 108397. [Google Scholar] [CrossRef]
- Laoutid, F.; Bonnaud, L.; Alexandre, M.; Lopez-Cuesta, J.M.; Dubois, P. New prospects in flame retardant polymer materials: From fundamentals to nanocomposites. Mater. Sci. Eng. R Rep. 2009, 63, 100–125. [Google Scholar] [CrossRef]
- Bourbigot, S.; Duquesne, S. Fire retardant polymers: Recent developments and opportunities. J. Mater. Chem. 2007, 17, 2283–2300. [Google Scholar] [CrossRef]
- Sun, Z.; Hou, Y.; Hu, Y.; Hu, W. Effect of additive phosphorus-nitrogen containing flame retardant on char formation and flame retardancy of epoxy resin. Mater. Chem. Phys. 2018, 214, 154–164. [Google Scholar] [CrossRef]
- Zabihi, O.; Ahmadi, M.; Khayyam, H.; Naebe, M. Fish DNA-modified clays: Towards highly flame retardant polymer nanocomposite with improved interfacial and mechanical performance. Sci. Rep. 2016, 6, 38194. [Google Scholar] [CrossRef]
- Lazar, S.T.; Kolibaba, T.J.; Grunlan, J.C. Flame-retardant surface treatments. Nat. Rev. Mater. 2020, 5, 259–275. [Google Scholar] [CrossRef]
- Costes, L.; Laoutid, F.; Aguedo, M.; Richel, A.; Brohez, S.; Delvosalle, C.; Dubois, P. Phosphorus and nitrogen derivatization as efficient route for improvement of lignin flame retardant action in PLA. Eur. Polym. J. 2016, 84, 652–667. [Google Scholar] [CrossRef]
- Niu, L.; Xu, J.-L.; Yang, W.-L.; Kang, C.-H.; Ma, J.-Q.; Su, J.-Q. Synergistic effect between nano-Sb2O3 and brominated epoxy resin on the flame retardancy of poly(butylene terephthalate). Sci. Adv. Mater. 2019, 11, 466–475. [Google Scholar] [CrossRef]
- Mihajlović, I. Recent Development of Phosphorus Flame Retardants in Thermoplastic Blends and Nanocomposites. In Flame Retardants: Polymer Blends, Composites and Nanocomposites; Visakh, P.M., Arao, Y., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 79–114. [Google Scholar]
- Zilberman, J.; Yoffe, D.; Piotrowski, A.; Singh, M.P.; Suryadevara, K.; Levchik, S. Comparative study of reactive flame retardants based on 9,10-dihydro-9-oxa-10-phosphaphenanthrene 10-oxide. J. Fire Sci. 2017, 35, 235–256. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, Z.; Fang, Z. Fabrication of 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide-decorated fullerene to improve the anti-oxidative and flame-retardant properties of polypropylene. Compos. Part B Eng. 2020, 183, 107672. [Google Scholar] [CrossRef]
- Wang, J. Kinetic evaluation of 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide as a flame retardant for epoxy resins. J. Macromol. Sci. Part B 2020, 59, 542–550. [Google Scholar] [CrossRef]
- Yan, W.; Yu, J.; Zhang, M.; Long, L.; Wang, T.; Qin, S.; Huang, W. Novel flame retardancy effect of phenethyl-bridged DOPO derivative on epoxy resin. High Perform. Polym. 2017, 30, 667–676. [Google Scholar] [CrossRef]
- Liu, M.; Yin, H.; Chen, X.; Yang, J.; Liang, Y.; Zhang, J.; Yang, F.; Deng, Y.; Lu, S. Preliminary ecotoxicity hazard evaluation of DOPO-HQ as a potential alternative to halogenated flame retardants. Chemosphere 2018, 193, 126–133. [Google Scholar] [CrossRef]
- Krivoshiev, B.V.; Beemster, G.T.S.; Sprangers, K.; Cuypers, B.; Laukens, K.; Blust, R.; Husson, S.J. Transcriptome profiling of HepG2 cells exposed to the flame retardant 9,10-dihydro-9-oxa-10-phosphaphenanthrene 10-oxide (DOPO). Toxicol. Res. 2018, 7, 492–502. [Google Scholar] [CrossRef]
- Yeon, D.; Lee, Y.; Ryou, M.-H.; Lee, Y.M. New flame-retardant composite separators based on metal hydroxides for lithium-ion batteries. Electrochim. Acta 2015, 157, 282–289. [Google Scholar] [CrossRef]
- Artabe, A.E.; Cunha-Silva, H.; Barranco, A. Enzymatic assays for the assessment of toxic effects of halogenated organic contaminants in water and food. A review. Food Chem. Toxicol. 2020, 145, 111677. [Google Scholar] [CrossRef] [PubMed]
- Mello, F.V.; Kasper, D.; Alonso, M.B.; Torres, J.P.M. Halogenated natural products in birds associated with the marine environment: A review. Sci. Total Environ. 2020, 717, 137000. [Google Scholar] [CrossRef] [PubMed]
- De Miranda, B.R.; Greenamyre, J.T. Trichloroethylene, a ubiquitous environmental contaminant in the risk for Parkinson’s disease. Environ. Sci. Process. Impacts 2020, 22, 543–554. [Google Scholar] [CrossRef] [PubMed]
- Luyt, A.S.; Malik, S.S.; Gasmi, S.A.; Porfyris, A.; Andronopoulou, A.; Korres, D.; Vouyiouka, S.; Grosshauser, M.; Pfaendner, R.; Brüll, R.; et al. Halogen-free flame-retardant compounds. Thermal decomposition and flammability behavior for alternative polyethylene grades. Polymers 2019, 11, 1479. [Google Scholar] [CrossRef] [PubMed]
- Horrocks, A.R. The potential for bio-sustainable organobromine-containing flame retardant formulations for textile applications—a review. Polymers 2020, 12, 2160. [Google Scholar] [CrossRef]
- Kausar, A.; Rafique, I.; Anwar, Z.; Muhammad, B. Recent developments in different types of flame retardants and effect on fire retardancy of epoxy composite. Polym. Plast. Technol. Eng. 2016, 55, 1512–1535. [Google Scholar] [CrossRef]
- Zhang, Q.-Y.; Liu, X.-H.; Ren, Y.-L.; Zhang, Y.-G.; Cheng, B.-W. Fabrication of a high phosphorus–nitrogen content modifier with star structure for effectively enhancing flame retardancy of lyocell fibers. Cellulose 2020, 27, 8369–8383. [Google Scholar] [CrossRef]
- Burger, D.; Winter, A.; Subbiahdoss, G.; Oberlerchner, J.T.; Beaumont, M.; Tamada, Y.; Rosenau, T. Partial amorphization of cellulose through zinc chloride treatment: A facile and sustainable pathway to functional cellulose nanofibers with flame-retardant and catalytic properties. Acs Sustain. Chem. Eng. 2020, 8, 13576–13582. [Google Scholar] [CrossRef]
- Ahmed, L.; Zhang, B.; Hatanaka, L.C.; Mannan, M.S. Application of polymer nanocomposites in the flame retardancy study. J. Loss Prev. Process Ind. 2018, 55, 381–391. [Google Scholar] [CrossRef]
- Ahn, K.-J.; Lee, Y.; Choi, H.; Kim, M.-S.; Im, K.; Noh, S.; Yoon, H. Surfactant-templated synthesis of polypyrrole nanocages as redox mediators for efficient energy storage. Sci. Rep. 2015, 5, 14097. [Google Scholar] [CrossRef]
- Choi, H.; Ahn, K.-J.; Lee, Y.; Noh, S.; Yoon, H. Free-standing, multilayered graphene/polyaniline-glue/graphene nanostructures for flexible, solid-state electrochemical capacitor application. Adv. Mater. Interfaces 2015, 2, 1500117. [Google Scholar] [CrossRef]
- Kim, M.-S.; Kim, S.; Kong, H.J.; Kwon, O.S.; Yoon, H. Tunable electrical-sensing performance of random-alternating layered graphene/polyaniline nanoarchitectures. J. Phys. Chem. C 2016, 120, 18289–18295. [Google Scholar] [CrossRef]
- Kim, S.; Le, T.-H.; Park, C.S.; Park, G.; Kim, K.H.; Kim, S.; Kwon, O.S.; Lim, G.T.; Yoon, H. A solution-processable, nanostructured, and conductive graphene/polyaniline hybrid coating for metal-corrosion protection and monitoring. Sci. Rep. 2017, 7, 15184. [Google Scholar] [CrossRef] [PubMed]
- Noh, S.; Le, T.-H.; Park, C.S.; Kim, S.; Kim, Y.; Park, J.-J.; Yoon, H. Physical exfoliation of graphene and molybdenum disulfide sheets using conductive polyaniline: An efficient route for synthesizing unique, random-layered 3D ternary electrode materials. New J. Chem. 2018, 42, 17379–17388. [Google Scholar] [CrossRef]
- Kim, S.; Le, T.-H.; Choi, Y.; Lee, H.; Heo, E.; Lee, U.; Kim, S.; Chae, S.; Kim, Y.A.; Yoon, H. Electrical monitoring of photoisomerization of block copolymers intercalated into graphene sheets. Nat. Commun. 2020, 11, 1324. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, S.; Noh, S.; Kim, S.; Park, G.; Le, T.-H.; Han, H.; Kim, Y.A.; Yoon, H. Single-walled carbon nanotube-mediated physical gelation of binary polymer blends: An efficient route to versatile porous carbon electrode materials. Chem. Eng. J. 2018, 353, 849–857. [Google Scholar] [CrossRef]
- Le, T.-H.; Yoon, H. Strategies for fabricating versatile carbon nanomaterials from polymer precursors. Carbon 2019, 152, 796–817. [Google Scholar] [CrossRef]
- Lee, J.E.; Shim, H.W.; Kwon, O.S.; Huh, Y.-I.; Yoon, H. Real-time detection of metal ions using conjugated polymer composite papers. Analyst 2014, 139, 4466–4475. [Google Scholar] [CrossRef]
- Lyu, W.; Cui, Y.; Zhang, X.; Yuan, J.; Zhang, W. Fire and thermal properties of PA 66 resin treated with poly-N-aniline-phenyl phosphamide as a flame retardant. Fire Mater. 2017, 41, 349–361. [Google Scholar] [CrossRef]
- Kundu, C.K.; Gangireddy, C.S.R.; Song, L.; Hu, Y. Flame retardant treatments for polyamide 66 textiles: Analysis the role of phosphorus compounds. Polym. Degrad. Stab. 2020, 182, 109376. [Google Scholar] [CrossRef]
- Wang, X.; Wang, W.; Wang, S.; Yang, Y.; Li, H.; Sun, J.; Gu, X.; Zhang, S. Self-intumescent polyelectrolyte for flame retardant poly (lactic acid) nonwovens. J. Clean. Prod. 2020, 282, 124497. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.; Sharma, V.; Basak, S.; Ali, S.W. Sodium lignin sulfonate: A bio-macromolecule for making fire retardant cotton fabric. Cellulose 2019, 26, 8191–8208. [Google Scholar] [CrossRef]
- Salmeia, K.A.; Fage, J.; Liang, S.; Gaan, S. An overview of mode of action and analytical methods for evaluation of gas phase activities of flame retardants. Polymers 2015, 7, 504–526. [Google Scholar] [CrossRef]
- Schartel, B. Phosphorus-based Flame Retardancy Mechanisms—Old Hat or a Starting Point for Future Development? Materials 2010, 3, 4710–4745. [Google Scholar] [CrossRef]
- Maddalena, L.; Carosio, F.; Gomez, J.; Saracco, G.; Fina, A. Layer-by-layer assembly of efficient flame retardant coatings based on high aspect ratio graphene oxide and chitosan capable of preventing ignition of PU foam. Polym. Degrad. Stab. 2018, 152, 1–9. [Google Scholar] [CrossRef]
- Sinha Ray, S.; Kuruma, M. Halogen-Free Flame-Retardant Polymers, Next-Generation Fillers for Polymer Nanocomposite Applications; Springer International Publishing: Cham, Switzerland, 2020; Volume 294. [Google Scholar]
- Qiao, Y.; Wang, Y.; Zou, M.; Xu, D.; Pan, Y.; Luo, Z.; Wang, B. One-step synthesis of highly efficient oligo(phenylphosphonic dihydroxypropyl silicone oil) flame retardant for polycarbonate. Polymers 2019, 11, 1977. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, D.; Xu, M.; Li, B. Synthesis of a novel phosphorus and nitrogen-containing flame retardant and its application in rigid polyurethane foam with expandable graphite. Polym. Degrad. Stab. 2020, 173, 109077. [Google Scholar] [CrossRef]
- Shao, L.; Xu, B.; Ma, W.; Wang, J.; Liu, Y.; Qian, L. Flame retardant application of a hypophosphite/cyclotetrasiloxane bigroup compound on polycarbonate. J. Appl. Polym. Sci. 2020, 137, 48699. [Google Scholar] [CrossRef]
- Wu, K.; Wang, X.; Xu, Y.; Guo, W. Flame retardant efficiency of modified para-aramid fiber synergizing with ammonium polyphosphate on PP/EPDM. Polym. Degrad. Stab. 2020, 172, 109065. [Google Scholar] [CrossRef]
- He, W.; Song, P.; Yu, B.; Fang, Z.; Wang, H. Flame retardant polymeric nanocomposites through the combination of nanomaterials and conventional flame retardants. Prog. Mater. Sci. 2020, 114, 100687. [Google Scholar] [CrossRef]
- Qu, L.; Sui, Y.; Zhang, C.; Dai, X.; Li, P.; Sun, G.; Xu, B.; Fang, D. Improved flame retardancy of epoxy resin composites modified with a low additive content of silica-microencapsulated phosphazene flame retardant. React. Funct. Polym. 2020, 148, 104485. [Google Scholar] [CrossRef]
- Liu, C.; Xing, T.; Wei, B.; Chen, G. Synergistic effects and mechanism of modified silica sol flame retardant systems on silk fabric. Materials 2018, 11, 1842. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, Y.; Zhao, Y.; Liu, S.; Zhao, J. Flame–retarded epoxy resin with high glass transition temperature cured by DOPO-containing H-benzimidazole. High Perform. Polym. 2016, 29, 94–103. [Google Scholar] [CrossRef]
- Chen, R.; Gong, J.; Jiang, Y.; Wang, Q.; Xi, Z.; Xie, H. Halogen-free flame retarded cold-mix epoxy asphalt binders: Rheological, thermal and mechanical characterization. Constr. Build. Mater. 2018, 186, 863–870. [Google Scholar] [CrossRef]
- Xu, Y.-J.; Chen, L.; Rao, W.-H.; Qi, M.; Guo, D.-M.; Liao, W.; Wang, Y.-Z. Latent curing epoxy system with excellent thermal stability, flame retardance and dielectric property. Chem. Eng. J. 2018, 347, 223–232. [Google Scholar] [CrossRef]
- Pourchet, S.; Sonnier, R.; Ben-Abdelkader, M.; Gaillard, Y.; Ruiz, Q.; Placet, V.; Plasseraud, L.; Boni, G. New reactive isoeugenol based phosphate flame retardant: Toward green epoxy resins. Acs Sustain. Chem. Eng. 2019, 7, 14074–14088. [Google Scholar] [CrossRef]
- Zhang, Y.-C.; Xu, G.-L.; Liang, Y.; Yang, J.; Hu, J. Preparation of flame retarded epoxy resins containing DOPO group. Thermochim. Acta 2016, 643, 33–40. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, G.; Li, J.; Jing, Z.; Qin, J.; Feng, Y. The flame retardancy and thermal stability properties of flame-retarded epoxy resins based on α-hydroxyphosphonate cyclotriphosphazene. J. Therm. Anal. Calorim. 2017, 129, 1667–1678. [Google Scholar] [CrossRef]
- Jain, P.; Choudhary, V.; Varma, I.K. Flame retarding epoxies with phosphorus. J. Macromol. Sci. Part C 2002, 42, 139–183. [Google Scholar] [CrossRef]
- Levchik, S.V.; Weil, E.D. Thermal decomposition, combustion and flame-retardancy of epoxy resins—a review of the recent literature. Polym. Int. 2004, 53, 1901–1929. [Google Scholar] [CrossRef]
- Rakotomalala, M.; Wagner, S.; Döring, M. Recent developments in halogen free flame retardants for epoxy resins for electrical and electronic applications. Materials 2010, 3, 4300–4327. [Google Scholar] [CrossRef]
- Marosi, G.; Szolnoki, B.; Bocz, K.; Toldy, A. Chapter 5—Reactive and Additive Phosphorus-based Flame Retardants of Reduced Environmental Impact. In Polymer Green Flame Retardants; Papaspyrides, C.D., Kiliaris, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 181–220. [Google Scholar]
- Howell, B.A.; Lienhart, G.W.; Livingstone, V.J.; Aulakh, D. 1-Dopyl-1,2-(4-hydroxyphenyl)ethene: A flame retardant hardner for epoxy resin. Polym. Degrad. Stab. 2020, 175, 109110. [Google Scholar] [CrossRef]
- Velencoso, M.M.; Battig, A.; Markwart, J.C.; Schartel, B.; Wurm, F.R. Molecular firefighting—How modern phosphorus chemistry can help solve the challenge of flame retardancy. Angew. Chem. Int. Ed. 2018, 57, 10450–10467. [Google Scholar] [CrossRef] [PubMed]
- Shen, R.; Hatanaka, L.C.; Ahmed, L.; Agnew, R.J.; Mannan, M.S.; Wang, Q. Cone calorimeter analysis of flame retardant poly (methyl methacrylate)-silica nanocomposites. J. Therm. Anal. Calorim. 2017, 128, 1443–1451. [Google Scholar] [CrossRef]
- Manfredi, A.; Carosio, F.; Ferruti, P.; Alongi, J.; Ranucci, E. Disulfide-containing polyamidoamines with remarkable flame retardant activity for cotton fabrics. Polym. Degrad. Stab. 2018, 156, 1–13. [Google Scholar] [CrossRef]
- Battegazzore, D.; Frache, A.; Carosio, F. Layer-by-layer nanostructured interphase produces mechanically strong and flame retardant bio-composites. Compos. Part B Eng. 2020, 200, 108310. [Google Scholar] [CrossRef]
- Gu, L.; Qiu, C.; Qiu, J.; Yao, Y.; Sakai, E.; Yang, L. Preparation and characterization of DOPO-functionalized MWCNT and its high flame-retardant performance in epoxy nanocomposites. Polymers 2020, 12, 613. [Google Scholar] [CrossRef]
- Fina, A.; Camino, G.; Bocchini, S. Chapter 14—Comprehensive Approach to Flame-retardancy Evaluation of Layered Silicate Nanocomposites. In Polymer Green Flame Retardants; Papaspyrides, C.D., Kiliaris, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 441–459. [Google Scholar]
- Koncar, V. Smart Textiles for Monitoring and Measurement Applications. In Smart Textiles for In Situ Monitoring of Composites; Koncar, V., Ed.; Woodhead Publishing: Duxford, UK, 2019; pp. 1–151. [Google Scholar]
- He, S.; Hu, Y.; Song, L.; Tang, Y. Fire safety assessment of halogen-free flame retardant polypropylene based on cone calorimeter. J. Fire Sci. 2007, 25, 109–118. [Google Scholar] [CrossRef]
- Wang, X.; Kalali, E.N.; Wan, J.-T.; Wang, D.-Y. Carbon-family materials for flame retardant polymeric materials. Prog. Polym. Sci. 2017, 69, 22–46. [Google Scholar] [CrossRef]
- Reis Bernardes, F.; Jakeline Cunha Rezende, M.; de Oliveira Rodrigues, V.; Sandra Veiga Nascimento, R.; Pereira da Silva Ribeiro, S. Synthesis and application of H-ZSM-5 zeolites with different levels of acidity as synergistic agents in flame retardant polymeric materials. Polymers 2019, 11, 2110. [Google Scholar] [CrossRef]
- Fenimore, C.P.; Martin, F.J. Flammability of polymers. Combust. Flame 1966, 10, 135–139. [Google Scholar] [CrossRef]
- Prabhakar, M.; Shah, A.u.R.; Song, J.-I. A review on the flammability and flame retardant properties of natural fibers and polymer matrix based composites. Compos. Res. 2015, 28, 29–39. [Google Scholar] [CrossRef]
- Zhuge, J.; Chen, X.; Ks, A.; Manica, D.P. Microscale combustion calorimeter—application and limitation. Fire Mater. 2016, 40, 987–998. [Google Scholar] [CrossRef]
- Afzal, A.; Tariq, A.; Shakir, F.; Satti, A.N.; Taimoor, M.; Ghani, U.; Jaffer, U.; Rashid, I.A.; Khaliq, Z. Development and characterization of multifunctional carbon fabric-reinforced polymer composites incorporated with inorganic flame retardants. Polym. Compos. 2020, 41, 3043–3051. [Google Scholar] [CrossRef]
- Mincheva, R.; Guemiza, H.; Hidan, C.; Moins, S.; Coulembier, O.; Dubois, P.; Laoutid, F. Development of inherently flame—retardant phosphorylated PLA by combination of ring-opening polymerization and reactive extrusion. Materials 2020, 13, 13. [Google Scholar] [CrossRef] [PubMed]
- Holdsworth, A.F.; Horrocks, A.R.; Kandola, B.K. Potential synergism between novel metal complexes and polymeric brominated flame retardants in polyamide 6.6. Polymers 2020, 12, 1543. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhao, Z.; Chen, T.; Zhu, Y.; Lv, Z.; Gong, X.; Niu, Y.; Ma, B. Preparation of highly dispersed expandable graphite/polystyrene composite foam via suspension polymerization with enhanced fire retardation. Carbon 2019, 146, 503–512. [Google Scholar] [CrossRef]
- Bachtiar, E.V.; Kurkowiak, K.; Yan, L.; Kasal, B.; Kolb, T. Thermal stability, fire performance, and mechanical properties of natural fibre fabric-reinforced polymer composites with different fire retardants. Polymers 2019, 11, 699. [Google Scholar] [CrossRef]
- Zhu, J.; Uhl, F.M.; Morgan, A.B.; Wilkie, C.A. Studies on the mechanism by which the formation of nanocomposites enhances thermal stability. Chem. Mater. 2001, 13, 4649–4654. [Google Scholar] [CrossRef]
- Gilman, J.W.; Jackson, C.L.; Morgan, A.B.; Harris, R.; Manias, E.; Giannelis, E.P.; Wuthenow, M.; Hilton, D.; Phillips, S.H. Flammability properties of polymer−layered-silicate nanocomposites. Polypropylene and polystyrene nanocomposites. Chem. Mater. 2000, 12, 1866–1873. [Google Scholar]
- Gilman, J.W.; Harris, R.H., Jr.; Shields, J.R.; Kashiwagi, T.; Morgan, A.B. A study of the flammability reduction mechanism of polystyrene-layered silicate nanocomposite: Layered silicate reinforced carbonaceous char. Polym. Adv. Technol. 2006, 17, 263–271. [Google Scholar] [CrossRef]
- Zhang, Y.-L.; Zang, C.-G.; Jiao, Q.-J. Electrical, thermal, and mechanical properties of silicone foam composites filled with carbon-based nanofillers. J. Appl. Polym. Sci. 2020, 137, 49191. [Google Scholar] [CrossRef]
- Li, L.; Shao, X.; Zhao, Z.; Liu, X.; Jiang, L.; Huang, K.; Zhao, S. Synergistic Fire Hazard Effect of a Multifunctional Flame Retardant in Building Insulation Expandable Polystyrene through a Simple Surface-Coating Method. Acs Omega 2020, 5, 799–807. [Google Scholar] [CrossRef]
- Bacakova, L.; Pajorova, J.; Tomkova, M.; Matejka, R.; Broz, A.; Stepanovska, J.; Prazak, S.; Skogberg, A.; Siljander, S.; Kallio, P. Applications of Nanocellulose/Nanocarbon Composites: Focus on Biotechnology and Medicine. Nanomaterials 2020, 10, 196. [Google Scholar] [CrossRef] [PubMed]
- Cabello-Alvarado, C.; Reyes-Rodríguez, P.; Andrade-Guel, M.; Cadenas-Pliego, G.; Pérez-Alvarez, M.; Cruz-Delgado, V.J.; Melo-López, L.; Quiñones-Jurado, Z.V.; Ávila-Orta, C.A. Melt-mixed thermoplastic nanocomposite containing carbon nanotubes and titanium dioxide for flame retardancy applications. Polymers 2019, 11, 1204. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Pan, Y.-T.; Zhang, Z.; Zhang, W.; Li, X.; Yang, R. Nickle nanocrystals decorated on graphitic nanotubes with broad channels for fire hazard reduction of epoxy resin. J. Hazard. Mater. 2021, 402, 123880. [Google Scholar] [CrossRef]
- Han, H.; Noh, S.; Chae, S.; Kim, S.; Choi, Y.; Le, T.-H.; Chang, M.; Kim, H.; Yoon, H. Pine cone mold: A toolbox for fabricating unique metal/carbon nanohybrid electrocatalysts. Nanoscale 2019, 11, 23241–23250. [Google Scholar] [CrossRef]
- Han, H.; Park, G.; Kim, S.; Choi, Y.; Park, C.S.; Le, T.-H.; Chae, S.; Kim, Y.A.; Yoon, H. Selective incorporation of aqueous-phase SWNTs into Pine Cones: A Unique route to creating versatile carbon precursors for electrode materials. Acs Sustain. Chem. Eng. 2018, 6, 12426–12435. [Google Scholar] [CrossRef]
- Choi, H.; Yoon, H. Nanostructured electrode materials for electrochemical capacitor applications. Nanomaterials 2015, 5, 906–936. [Google Scholar] [CrossRef]
- Kong, H.J.; Kim, S.; Le, T.-H.; Kim, Y.; Park, G.; Park, C.S.; Kwon, O.S.; Yoon, H. Nanostructured mesophase electrode materials: Modulating charge-storage behavior by thermal treatment. Nanoscale 2017, 9, 17450–17458. [Google Scholar] [CrossRef] [PubMed]
- Kashiwagi, T.; Du, F.; Winey, K.I.; Groth, K.M.; Shields, J.R.; Bellayer, S.P.; Kim, H.; Douglas, J.F. Flammability properties of polymer nanocomposites with single-walled carbon nanotubes: Effects of nanotube dispersion and concentration. Polymer 2005, 46, 471–481. [Google Scholar] [CrossRef]
- Lai, X.; Tang, S.; Li, H.; Zeng, X. Flame-retardant mechanism of a novel polymeric intumescent flame retardant containing caged bicyclic phosphate for polypropylene. Polym. Degrad. Stab. 2015, 113, 22–31. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Fang, Z.; Hull, T.R.; Kelarakis, A.; Stec, A.A. Mechanism of enhancement of intumescent fire retardancy by metal acetates in polypropylene. Polym. Degrad. Stab. 2017, 136, 139–145. [Google Scholar] [CrossRef]
- Zhou, X.; Mu, X.; Cai, W.; Wang, J.; Chu, F.; Xu, Z.; Song, L.; Xing, W.; Hu, Y. Design of hierarchical NiCo-LDH@PZS hollow dodecahedron architecture and application in high-performance epoxy resin with excellent fire safety. Acs Appl. Mater. Interfaces 2019, 11, 41736–41749. [Google Scholar] [CrossRef]
- Liu, L.; Xu, Y.; Xu, M.; Li, Z.; Hu, Y.; Li, B. Economical and facile synthesis of a highly efficient flame retardant for simultaneous improvement of fire retardancy, smoke suppression and moisture resistance of epoxy resins. Compos. Part B Eng. 2019, 167, 422–433. [Google Scholar] [CrossRef]
- He, L.; Song, F.; Li, D.-F.; Zhao, X.; Wang, X.-L.; Wang, Y.-Z. Strong and tough polylactic acid based composites enabled by simultaneous reinforcement and interfacial compatibilization of microfibrillated cellulose. Acs Sustain. Chem. Eng. 2020, 8, 1573–1582. [Google Scholar] [CrossRef]
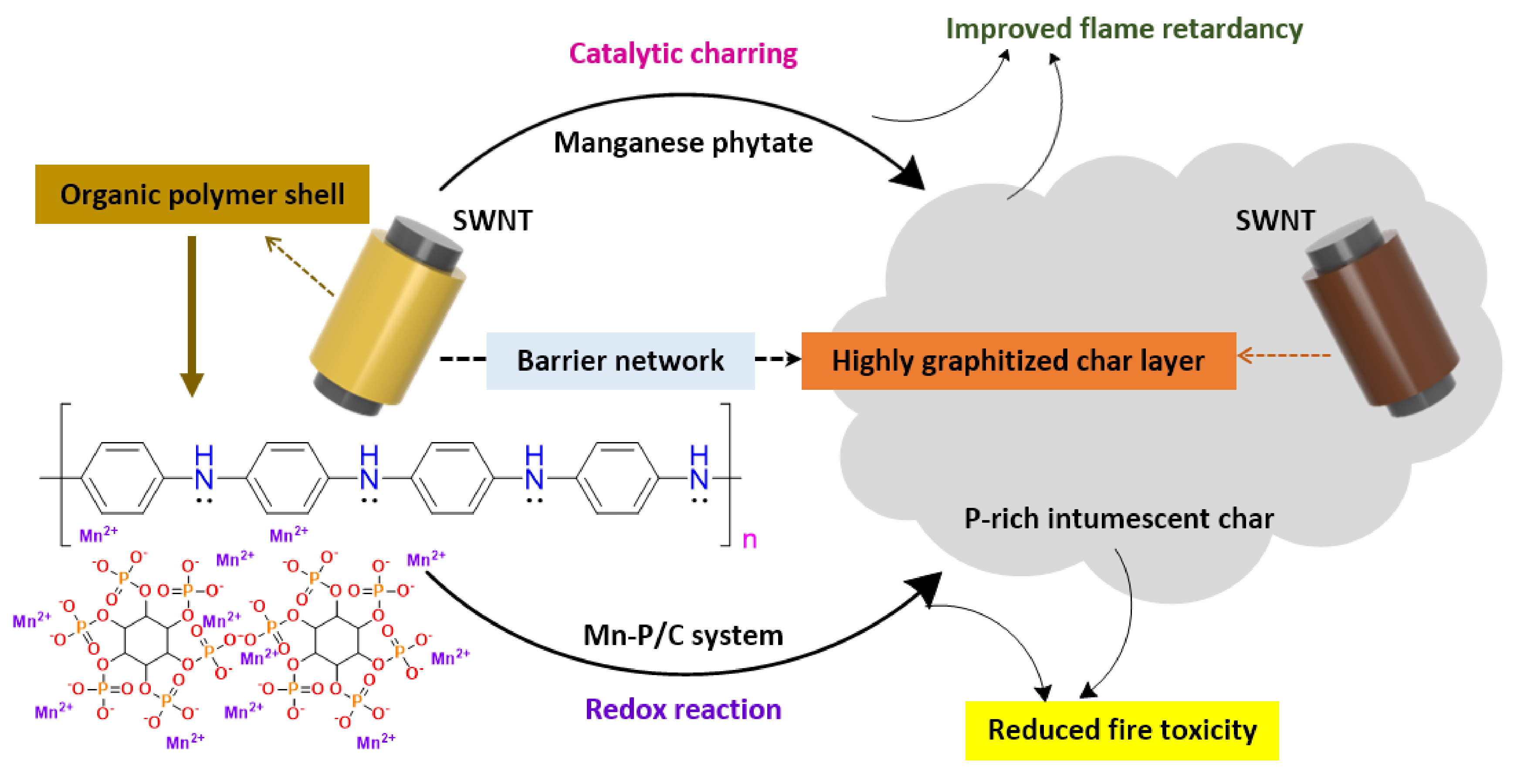
- Wang, J.; Zhan, J.; Mu, X.; Jin, X.; Chu, F.; Kan, Y.; Xing, W. Manganese phytate dotted polyaniline shell enwrapped carbon nanotube: Towards the reinforcements in fire safety and mechanical property of polymer. J. Colloid Interface Sci. 2018, 529, 345–356. [Google Scholar] [CrossRef]
- Kim, H.; Lee, S. Characterization of electrical heating textile coated by graphene nanoplatelets/PVDF-HFP composite with various high graphene nanoplatelet contents. Polymers 2019, 11, 928. [Google Scholar] [CrossRef]
- Yang, B.; Shi, Y.; Miao, J.-B.; Xia, R.; Su, L.-F.; Qian, J.-S.; Chen, P.; Zhang, Q.-L.; Liu, J.-W. Evaluation of rheological and thermal properties of polyvinylidene fluoride (PVDF)/graphene nanoplatelets (GNP) composites. Polym. Test. 2018, 67, 122–135. [Google Scholar] [CrossRef]
- Verma, M.; Chauhan, S.S.; Dhawan, S.K.; Choudhary, V. Graphene nanoplatelets/carbon nanotubes/polyurethane composites as efficient shield against electromagnetic polluting radiations. Compos. Part B Eng. 2017, 120, 118–127. [Google Scholar] [CrossRef]
- Papageorgiou, D.G.; Liu, M.; Li, Z.; Vallés, C.; Young, R.J.; Kinloch, I.A. Hybrid poly(ether ether ketone) composites reinforced with a combination of carbon fibres and graphene nanoplatelets. Compos. Sci. Technol. 2019, 175, 60–68. [Google Scholar] [CrossRef]
- Wang, C.; Wang, J.; Men, Z.; Wang, Y.; Han, Z. Thermal degradation and combustion behaviors of polyethylene/alumina trihydrate/graphene nanoplatelets. Polymers 2019, 11, 772. [Google Scholar] [CrossRef]
- Kashiwagi, T.; Grulke, E.; Hilding, J.; Groth, K.; Harris, R.; Butler, K.; Shields, J.; Kharchenko, S.; Douglas, J. Thermal and flammability properties of polypropylene/carbon nanotube nanocomposites. Polymer 2004, 45, 4227–4239. [Google Scholar] [CrossRef]
- Dittrich, B.; Wartig, K.-A.; Hofmann, D.; Mülhaupt, R.; Schartel, B. The influence of layered, spherical, and tubular carbon nanomaterials’ concentration on the flame retardancy of polypropylene. Polym. Compos. 2015, 36, 1230–1241. [Google Scholar] [CrossRef]
- Song, K.; Ganguly, I.; Eastin, I.; Dichiara, A.B. Lignin-modified carbon nanotube/graphene hybrid coating as efficient flame retardant. Int. J. Mol. Sci. 2017, 18, 2368. [Google Scholar] [CrossRef]
- Gu, L.; Qiu, J.; Yao, Y.; Sakai, E.; Yang, L. Functionalized MWCNTs modified flame retardant PLA nanocomposites and cold rolling process for improving mechanical properties. Compos. Sci. Technol. 2018, 161, 39–49. [Google Scholar] [CrossRef]
- Hua, J.; Li, Y.; Liu, X.; Li, X.; Lin, S.; Gu, J.; Cui, Z.-K.; Zhuang, Q. Graphene/MWNT/poly(p-phenylenebenzobisoxazole) multiphase nanocomposite via solution prepolymerization with superior microwave absorption properties and thermal stability. J. Phys. Chem. C 2017, 121, 1072–1081. [Google Scholar] [CrossRef]
- Liu, H.; Gu, S.; Cao, H.; Li, X.; Jiang, X.; Li, Y. Modification of MWNTs by the combination of Li-TFSI and MAPP: Novel strategy to high performance PP/MWNTs nanocomposites. Compos. Part B Eng. 2019, 176, 107268. [Google Scholar] [CrossRef]
- Mahapatra, S.S.; Yadav, S.K.; Lee, B.H.; Cho, J.W. Nanodiamond-grafted hyperbranched polymers anchored with carbon nanotubes: Mechanical, thermal, and photothermal shape-recovery properties. Polymer 2019, 160, 204–209. [Google Scholar] [CrossRef]
- Zheng, J.; Zong, Y.; Zhao, G.; Yu, Z.; Wang, M.; Zhu, C.; Li, C.; Liu, J.; Gui, D. Nematic liquid crystal 4-cyano-4′-pentylbiphenyl functionalization of MWNTs for improved thermal and mechanical properties of silicone pressure sensitive adhesives. Int. J. Adhes. Adhes. 2020, 98, 102457. [Google Scholar] [CrossRef]
- Wang, S.; Xin, F.; Chen, Y.; Qian, L.; Chen, Y. Phosphorus-nitrogen containing polymer wrapped carbon nanotubes and their flame-retardant effect on epoxy resin. Polym. Degrad. Stab. 2016, 129, 133–141. [Google Scholar] [CrossRef]
- Kingston, C.; Zepp, R.; Andrady, A.; Boverhof, D.; Fehir, R.; Hawkins, D.; Roberts, J.; Sayre, P.; Shelton, B.; Sultan, Y.; et al. Release characteristics of selected carbon nanotube polymer composites. Carbon 2014, 68, 33–57. [Google Scholar] [CrossRef]
- Köhler, A.R.; Som, C.; Helland, A.; Gottschalk, F. Studying the potential release of carbon nanotubes throughout the application life cycle. J. Clean. Prod. 2008, 16, 927–937. [Google Scholar] [CrossRef]
- Chae, S.; Le, T.-H.; Park, C.S.; Choi, Y.; Kim, S.; Lee, U.; Heo, E.; Lee, H.; Kim, Y.A.; Kwon, O.S.; et al. Anomalous restoration of sp2 hybridization in graphene functionalization. Nanoscale 2020, 12, 13351–13359. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.S.; Kim, T.; Lee, J.S.; Park, S.J.; Park, H.-W.; Kang, M.; Lee, J.E.; Jang, J.; Yoon, H. Fabrication of graphene sheets intercalated with manganese oxide/carbon nanofibers: Toward high-capacity energy storage. Small 2013, 9, 248–254. [Google Scholar] [CrossRef]
- Lee, Y.; Choi, H.; Kim, M.-S.; Noh, S.; Ahn, K.-J.; Im, K.; Kwon, O.S.; Yoon, H. Nanoparticle-mediated physical exfoliation of aqueous-phase graphene for fabrication of three-dimensionally structured hybrid electrodes. Sci. Rep. 2016, 6, 19761. [Google Scholar] [CrossRef]
- Le, T.-H.; Oh, Y.; Kim, H.; Yoon, H. Exfoliation of 2D materials for energy and environmental applications. Chem. A Eur. J. 2020, 26, 6360–6401. [Google Scholar] [CrossRef]
- Sang, B.; Li, Z.-w.; Li, X.-h.; Yu, L.-g.; Zhang, Z.-j. Graphene-based flame retardants: A review. J. Mater. Sci. 2016, 51, 8271–8295. [Google Scholar] [CrossRef]
- Huang, G.; Wang, S.; Song, P.A.; Wu, C.; Chen, S.; Wang, X. Combination effect of carbon nanotubes with graphene on intumescent flame-retardant polypropylene nanocomposites. Compos. Part A Appl. Sci. Manuf. 2014, 59, 18–25. [Google Scholar] [CrossRef]
- Zheng, X.; Cao, Y.; Liu, D.; Cai, M.; Ding, J.; Liu, X.; Wang, J.; Hu, W.; Zhong, C. Bimetallic metal–organic-framework/reduced graphene oxide composites as bifunctional electrocatalysts for rechargeable Zn–air batteries. Acs Appl. Mater. Interfaces 2019, 11, 15662–15669. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, X.; Wang, Y.; Quan, G.; Han, X.; Yan, J. Facile synthesis of magnetic nitrogen-doped porous carbon from bimetallic metal–organic frameworks for efficient norfloxacin removal. Nanomaterials 2018, 8, 664. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, M.; Li, J.; Huang, H.; Qiao, J. In situ growth of CoP nanoparticles anchored on (N,P) co-doped porous carbon engineered by MOFs as advanced bifunctional oxygen catalyst for rechargeable Zn–air battery. J. Mater. Chem. A 2020, 8, 19043–19049. [Google Scholar] [CrossRef]
- Zhao, C.-e.; Qiu, Z.; Yang, J.; Huang, Z.-D.; Shen, X.; Li, Y.; Ma, Y. Metal–organic frameworks-derived core/shell porous carbon materials interconnected by reduced graphene oxide as effective cathode catalysts for microbial fuel cells. Acs Sustain. Chem. Eng. 2020, 8, 13964–13972. [Google Scholar] [CrossRef]
- Shu, R.; Li, W.; Wu, Y.; Zhang, J.; Zhang, G. Nitrogen-doped Co-C/MWCNTs nanocomposites derived from bimetallic metal–organic frameworks for electromagnetic wave absorption in the X-band. Chem. Eng. J. 2019, 362, 513–524. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Z.; Qi, X.; Zhang, W.; Wang, D.-Y. Size tailored bimetallic metal-organic framework (MOF) on graphene oxide with sandwich-like structure as functional nano-hybrids for improving fire safety of epoxy. Compos. Part B Eng. 2020, 188, 107881. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Z.; Zhang, L.; García Molleja, J.; Wang, D.-Y. Bimetallic metal-organic frameworks and graphene oxide nano-hybrids for enhanced fire retardant epoxy composites: A novel carbonization mechanism. Carbon 2019, 153, 407–416. [Google Scholar] [CrossRef]
- Lu, F.; Zhou, M.; Zhou, Y.; Zeng, X. First-row transition metal based catalysts for the oxygen evolution reaction under alkaline conditions: Basic principles and recent advances. Small 2017, 13, 1701931. [Google Scholar] [CrossRef]
- Franco, F.; Rettenmaier, C.; Jeon, H.S.; Roldan Cuenya, B. Transition metal-based catalysts for the electrochemical CO2 reduction: From atoms and molecules to nanostructured materials. Chem. Soc. Rev. 2020, 49, 6884–6946. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Ding, X.; Zhan, Y.; Wang, Y.; Wang, X. Improving the flame retardancy of poly(lactic acid) using an efficient ternary hybrid flame retardant by dual modification of graphene oxide with phenylphosphinic acid and nano MOFs. J. Hazard. Mater. 2020, 384, 121260. [Google Scholar] [CrossRef]
- Shi, Y.; Yu, B.; Duan, L.; Gui, Z.; Wang, B.; Hu, Y.; Yuen, R.K.K. Graphitic carbon nitride/phosphorus-rich aluminum phosphinates hybrids as smoke suppressants and flame retardants for polystyrene. J. Hazard. Mater. 2017, 332, 87–96. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, L.; Fu, L.; Liu, C.; Yu, B.; Yang, F.; Hu, Y. Sodium alginate-templated synthesis of g-C3N4/carbon spheres/Cu ternary nanohybrids for fire safety application. J. Colloid Interface Sci. 2019, 539, 1–10. [Google Scholar] [CrossRef]
- Shi, Y.; Fu, L.; Chen, X.; Guo, J.; Yang, F.; Wang, J.; Zheng, Y.; Hu, Y. Hypophosphite/graphitic carbon nitride hybrids: Preparation and flame-retardant application in thermoplastic polyurethane. Nanomaterials 2017, 7, 259. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, B.; Duan, L.; Zhu, Y.; Gui, Z.; Yuen, R.K.K.; Hu, Y. Processable Dispersions of Graphitic Carbon Nitride Based Nanohybrids and Application in Polymer Nanocomposites. Ind. Eng. Chem. Res. 2016, 55, 7646–7654. [Google Scholar] [CrossRef]
- Shi, Y.; Gui, Z.; Yu, B.; Yuen, R.K.K.; Wang, B.; Hu, Y. Graphite-like carbon nitride and functionalized layered double hydroxide filled polypropylene-grafted maleic anhydride nanocomposites: Comparison in flame retardancy, and thermal, mechanical and UV-shielding properties. Compos. Part B Eng. 2015, 79, 277–284. [Google Scholar] [CrossRef]
- Shi, Y.; Xing, W.; Wang, B.; Hong, N.; Zhu, Y.; Wang, C.; Gui, Z.; Yuen, R.K.K.; Hu, Y. Synergistic effect of graphitic carbon nitride and ammonium polyphosphate for enhanced thermal and flame retardant properties of polystyrene. Mater. Chem. Phys. 2016, 177, 283–292. [Google Scholar] [CrossRef]
- Shi, Y.; Long, Z.; Yu, B.; Zhou, K.; Gui, Z.; Yuen, R.K.K.; Hu, Y. Tunable thermal, flame retardant and toxic effluent suppression properties of polystyrene based on alternating graphitic carbon nitride and multi-walled carbon nanotubes. J. Mater. Chem. A 2015, 3, 17064–17073. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, C.; Fu, L.; Yang, F.; Lv, Y.; Yu, B. Hierarchical assembly of polystyrene/graphitic carbon nitride/reduced graphene oxide nanocomposites toward high fire safety. Compos. Part B Eng. 2019, 179, 107541. [Google Scholar] [CrossRef]
- Shi, Y.; Zhu, Y.; Yu, B.; Gui, Z.; She, S.; Yuen, R.K.K.; Liu, H.; Hu, Y. Enhanced thermal stability of polystyrene by graphitic carbon nitride/spinel ZnCo2O4 nanohybrids and the catalytic mechanism investigation. Rsc Adv. 2015, 5, 41835–41838. [Google Scholar] [CrossRef]
- Shi, Y.; Yu, B.; Zhou, K.; Yuen, R.K.K.; Gui, Z.; Hu, Y.; Jiang, S. Novel CuCo2O4/graphitic carbon nitride nanohybrids: Highly effective catalysts for reducing CO generation and fire hazards of thermoplastic polyurethane nanocomposites. J. Hazard. Mater. 2015, 293, 87–96. [Google Scholar] [CrossRef]
- Gao, W.; Zhang, X.; Zhang, D.; Peng, Q.; Zhang, Q.; Dobashi, R. Flame propagation behaviours in nano-metal dust explosions. Powder Technol. 2017, 321, 154–162. [Google Scholar] [CrossRef]
- Vignes, A.; Krietsch, A.; Dufaud, O.; Santandréa, A.; Perrin, L.; Bouillard, J. Course of explosion behaviour of metallic powders—From micron to nanosize. J. Hazard. Mater. 2019, 379, 120767. [Google Scholar] [CrossRef]
- Bagaria, P.; Prasad, S.; Sun, J.; Bellair, R.; Mashuga, C. Effect of particle morphology on dust minimum ignition energy. Powder Technol. 2019, 355, 1–6. [Google Scholar] [CrossRef]
- Santandrea, A.; Vignes, A.; Krietsch, A.; Brunello, D.; Perrin, L.; Laurent, A.; Dufaud, O. Evaluating the explosion severity of nanopowders: International standards versus reality. Process Saf. Environ. Prot. 2020, 138, 279–291. [Google Scholar] [CrossRef]
- Santandrea, A.; Pacault, S.; Perrin, L.; Vignes, A.; Dufaud, O. Nanopowders explosion: Influence of the dispersion characteristics. J. Loss Prev. Process Ind. 2019, 62, 103942. [Google Scholar] [CrossRef]
- Mutlu, M.; Kang, J.-H.; Raza, S.; Schoen, D.; Zheng, X.; Kik, P.G.; Brongersma, M.L. Thermoplasmonic ignition of metal nanoparticles. Nano Lett. 2018, 18, 1699–1706. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Y.-Z. A review on flame retardant technology in China. Part i: Development of flame retardants. Polym. Adv. Technol. 2010, 21, 1–26. [Google Scholar] [CrossRef]
- Maqsood, M.; Seide, G. Biodegradable flame retardants for biodegradable polymer. Biomolecules 2020, 10, 1038. [Google Scholar] [CrossRef]
- Luda, M.P.; Zanetti, M. Cyclodextrins and cyclodextrin derivatives as green char promoters in flame retardants formulations for polymeric materials. A review. Polymers 2019, 11, 664. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Yang, H.; Jiang, Y.; He, H.; Liu, H.; Huang, H.; Wan, C. Facile synthesis of a novel transparent hyperbranched phosphorous/nitrogen-containing flame retardant and its application in reducing the fire hazard of epoxy resin. J. Hazard. Mater. 2019, 379, 120793. [Google Scholar] [CrossRef] [PubMed]
- Erünal, E. Toward halogen-free Flame Retardants for Polystyrene Thermal Insulation Boards. In Environmentally-Benign Energy Solutions; Springer International Publishing: Cham, Switzerland, 2020. [Google Scholar]
- Gui, H.; Zhang, X.; Liu, Y.; Dong, W.; Wang, Q.; Gao, J.; Song, Z.; Lai, J.; Qiao, J. Effect of dispersion of nano-magnesium hydroxide on the flammability of flame retardant ternary composites. Compos. Sci. Technol. 2007, 67, 974–980. [Google Scholar] [CrossRef]
- Jeon, H.; Choi, J.; Ryou, M.-H.; Lee, Y.M. Comparative study of the adhesion properties of ceramic composite separators using a surface and interfacial cutting analysis system for lithium-ion batteries. Acs Omega 2017, 2, 2159–2164. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Liu, J.; He, C.; Li, J.; Wu, X. Composite of polyvinylidene fluoride–cellulose acetate with Al(OH)3 as a separator for high-performance lithium ion battery. J. Membr. Sci. 2017, 541, 661–667. [Google Scholar] [CrossRef]
- Gong, S.; Jeon, H.; Lee, H.; Ryou, M.-H.; Lee, Y.M. Effects of an integrated separator/electrode assembly on enhanced thermal stability and rate capability of lithium-ion batteries. Acs Appl. Mater. Interfaces 2017, 9, 17814–17821. [Google Scholar] [CrossRef]
- Shi, J.; Xia, Y.; Han, S.; Fang, L.; Pan, M.; Xu, X.; Liu, Z. Lithium ion conductive Li1.5Al0.5Ge1.5(PO4)3 based inorganic–organic composite separator with enhanced thermal stability and excellent electrochemical performances in 5 V lithium ion batteries. J. Power Sources 2015, 273, 389–395. [Google Scholar] [CrossRef]
- Kamol, S.; Limsuwan, P.; Onreabroy, W. Three-dimensional standing waves in a microwave oven. Am. J. Phys. 2010, 78, 492–495. [Google Scholar] [CrossRef]
- Oliver-Hoyo, M.; Switzer, W.L.; Robert, E. Fractional distillation of air and other demonstrations with condensed gases. J. Chem. Educ. 2005, 82, 251. [Google Scholar] [CrossRef]
- Boltinghouse, F.; Abel, K. Development of an optical relative humidity sensor. Cobalt chloride optical absorbency sensor study. Anal. Chem. 2002, 61, 1863–1866. [Google Scholar] [CrossRef]
- Ou, W.; Pan, J.; Liu, Y.; Wang, P.; Chen, Z.; Li, C. Co-catalyst MoS2-nanosheets/TiO2 nanotubes for the enhancement of photocatalytic hydrogen production. Iop Conf. Ser. Earth Environ. Sci. 2019, 358, 032008. [Google Scholar] [CrossRef]
- Liu, S.; Nie, C.; Zhou, D.; Shen, J.; Feng, S. Direct growth of vertical structure MoS2 nanosheets array film via CVD method for photodetection. Phys. E: Low-Dimens. Syst. Nanostructures 2020, 117, 113592. [Google Scholar] [CrossRef]
- Sun, L.; Zhao, Z.; Li, S.; Su, Y.; Huang, L.; Shao, N.; Liu, F.; Bu, Y.; Zhang, H.; Zhang, Z. Role of SnS2 in 2D–2D SnS2/TiO2 nanosheet heterojunctions for photocatalytic hydrogen evolution. Acs Appl. Nano Mater. 2019, 2, 2144–2151. [Google Scholar] [CrossRef]
- Zhou, X.; Qiu, S.; Cai, W.; Liu, L.; Hou, Y.; Wang, W.; Song, L.; Wang, X.; Hu, Y. Construction of hierarchical MoS2@TiO2 structure for the high performance bimaleimide system with excellent fire safety and mechanical properties. Chem. Eng. J. 2019, 369, 451–462. [Google Scholar] [CrossRef]
- Begum, J.; Hussain, Z.; Noor, T. Adsorption and kinetic study of Cr(VI) on ZIF-8 based composites. Mater. Res. Express 2020, 7, 015083. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, B.; Chen, Y.; Guo, L.; Wei, G. Carbon nanofiber-based three-dimensional nanomaterials for energy and environmental applications. Mater. Adv. 2020, 1, 2163–2181. [Google Scholar] [CrossRef]
- Mustafa, F.; Andreescu, S. Nanotechnology-based approaches for food sensing and packaging applications. Rsc Adv. 2020, 10, 19309–19336. [Google Scholar] [CrossRef]
- Eniola, J.O.; Kumar, R.; Mohamed, O.A.; Al-Rashdi, A.A.; Barakat, M.A. Synthesis and characterization of CuFe2O4/NiMgAl-LDH composite for the efficient removal of oxytetracycline antibiotic. J. Saudi Chem. Soc. 2020, 24, 139–150. [Google Scholar] [CrossRef]
- Pan, Y.-T.; Zhang, Z.; Yang, R. The rise of MOFs and their derivatives for flame retardant polymeric materials: A critical review. Compos. Part B Eng. 2020, 199, 108265. [Google Scholar] [CrossRef]
- Xie, J.; Shi, X.; Zhang, M.; Dai, X.; Wang, X. Improving the flame retardancy of polypropylene by nano metal–organic frameworks and bioethanol coproduct. Fire Mater. 2019, 43, 373–380. [Google Scholar] [CrossRef]
- Shi, X.; Dai, X.; Cao, Y.; Li, J.; Huo, C.; Wang, X. Degradable poly(lactic acid)/metal–organic framework nanocomposites exhibiting good mechanical, flame retardant, and dielectric properties for the fabrication of disposable electronics. Ind. Eng. Chem. Res. 2017, 56, 3887–3894. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, Y.; Zhan, Y.; Ding, X.; Wang, M.; Wang, X. Preparing the degradable, flame-retardant and low dielectric constant nanocomposites for flexible and miniaturized electronics with poly(lactic acid), nano ZIF-8@GO and resorcinol di(phenyl phosphate). Materials 2018, 11, 1756. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Cao, Y.; Shi, X.; Wang, X. The PLA/ZIF-8 nanocomposite membranes: The diameter and surface roughness adjustment by ZIF-8 nanoparticles, high wettability, improved mechanical property, and efficient oil/water separation. Adv. Mater. Interfaces 2016, 3, 1600725. [Google Scholar] [CrossRef]
- Wang, S.-S.; Xing, Z.-H.; Chen, G.-Y.; Cölfen, H.; Xu, A.-W. Cuboctahedral Sb2O3 mesocrystals organized from octahedral building blocks: More than self-similarity. Cryst. Growth Des. 2016, 16, 3613–3617. [Google Scholar] [CrossRef]
- Han, L.; Wu, W.; Qu, H.; Han, X.; Wang, A.; Jiao, Y.; Xu, J. Metallic ferrites as flame retardants and smoke suppressants in flexible poly(vinyl chloride). J. Therm. Anal. Calorim. 2016, 123, 293–300. [Google Scholar] [CrossRef]
- Kang, C.; Xu, J.; Xu, C.; Ren, S.; Zhang, J. Poly(butylene terephthalate) filled with Sb2O3 nanoparticles: Effects of particle surface treatment, particle size, particle morphology and particle loading on mechanical properties of composites. Integr. Ferroelectr. 2020, 210, 141–159. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Z.; Sun, Y.; Liang, X.; Xiang, H. Sb2O3 modified PVDF-CTFE electrospun fibrous membrane as a safe lithium-ion battery separator. J. Membr. Sci. 2019, 572, 512–519. [Google Scholar] [CrossRef]
- Mirdamadian, Z.; Ghanbari, D. Synergistic effect between Sb2O3 nanoparticles–trichloromelamine and carbon nanotube on the flame retardancy and thermal stability of the cellulose acetate. J. Clust. Sci. 2014, 25, 925–936. [Google Scholar] [CrossRef]
- Niu, L.; Xu, J.; Yang, W.; Zhao, J.; Su, J.; Guo, Y.; Liu, X. Research on nano-Sb2O3 flame retardant in char formation of PBT. Ferroelectrics 2018, 523, 14–21. [Google Scholar] [CrossRef]
- Liu, C.; Wu, W.; Shi, Y.; Yang, F.; Liu, M.; Chen, Z.; Yu, B.; Feng, Y. Creating MXene/reduced graphene oxide hybrid towards highly fire safe thermoplastic polyurethane nanocomposites. Compos. Part B Eng. 2020, 203, 108486. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, C.; Duan, Z.; Yu, B.; Liu, M.; Song, P. Interface engineering of MXene towards super-tough and strong polymer nanocomposites with high ductility and excellent fire safety. Chem. Eng. J. 2020, 399, 125829. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, C.; Liu, L.; Fu, L.; Yu, B.; Lv, Y.; Yang, F.; Song, P. Strengthening, toughing and thermally stable ultra-thin MXene nanosheets/polypropylene nanocomposites via nanoconfinement. Chem. Eng. J. 2019, 378, 122267. [Google Scholar] [CrossRef]
- Yu, B.; Yuen, A.C.Y.; Xu, X.; Zhang, Z.-C.; Yang, W.; Lu, H.; Fei, B.; Yeoh, G.H.; Song, P.; Wang, H. Engineering MXene surface with POSS for reducing fire hazards of polystyrene with enhanced thermal stability. J. Hazard. Mater. 2021, 401, 123342. [Google Scholar] [CrossRef] [PubMed]
- Mochane, M.J.; Magagula, S.I.; Sefadi, J.S.; Sadiku, E.R.; Mokhena, T.C. Morphology, thermal stability, and flammability properties of polymer-layered double hydroxide (LDH) nanocomposites: A Review. Crystals 2020, 10, 612. [Google Scholar] [CrossRef]








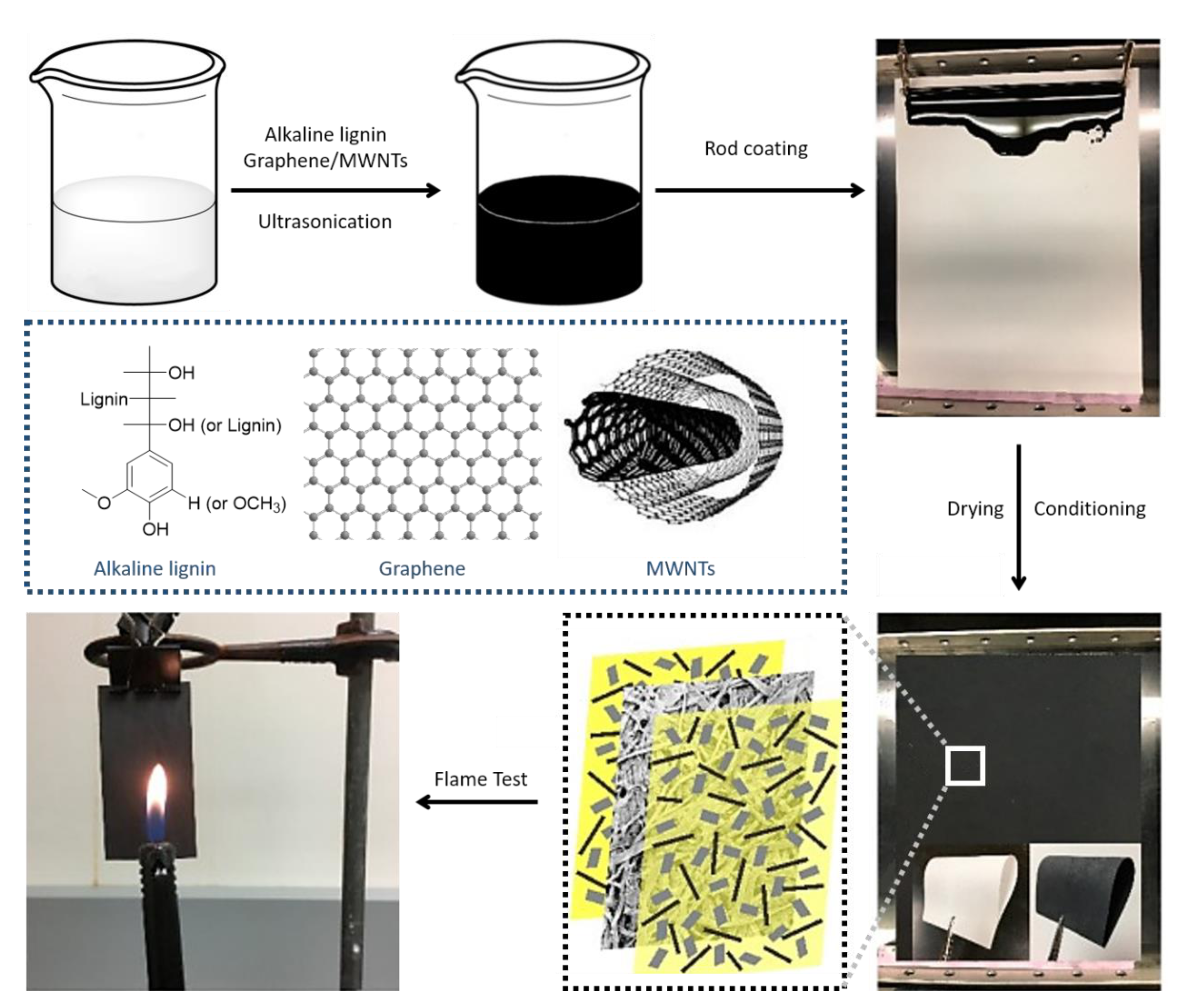


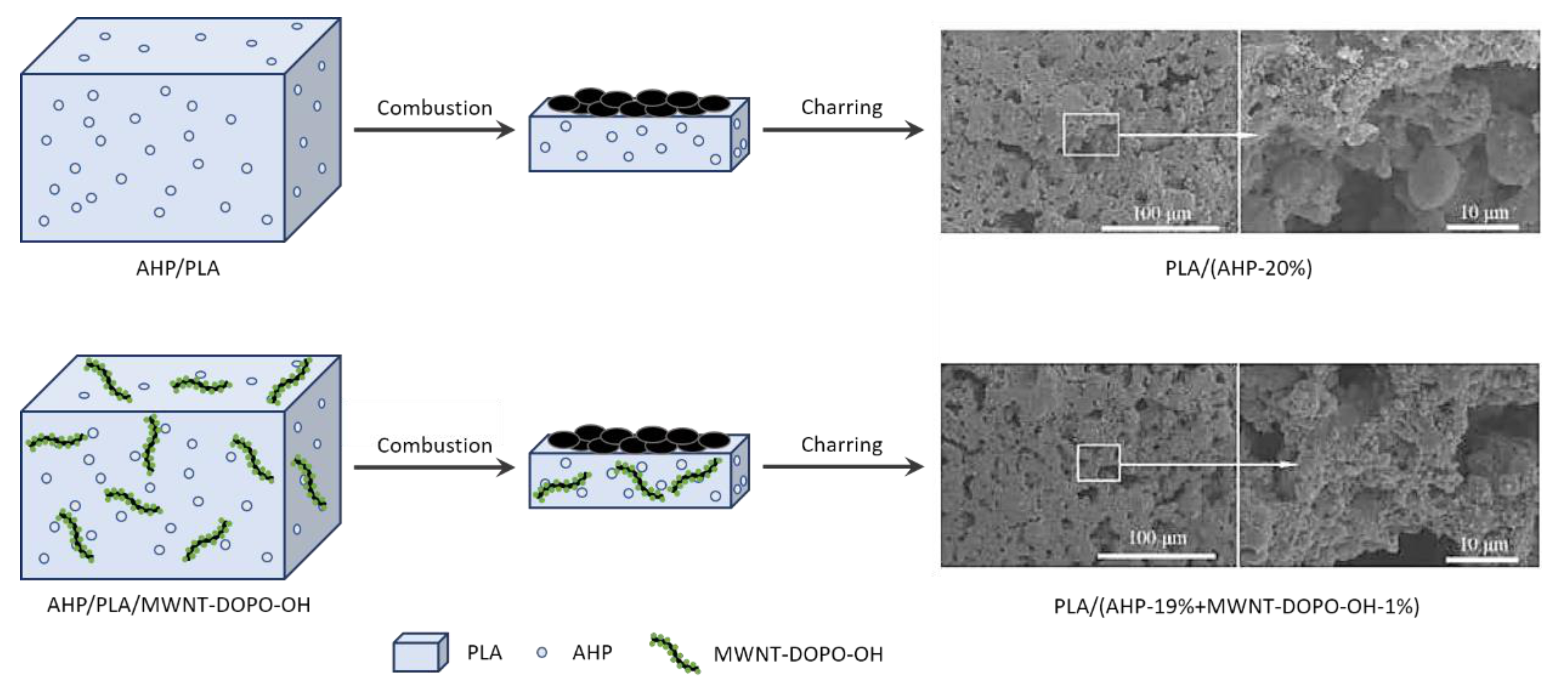
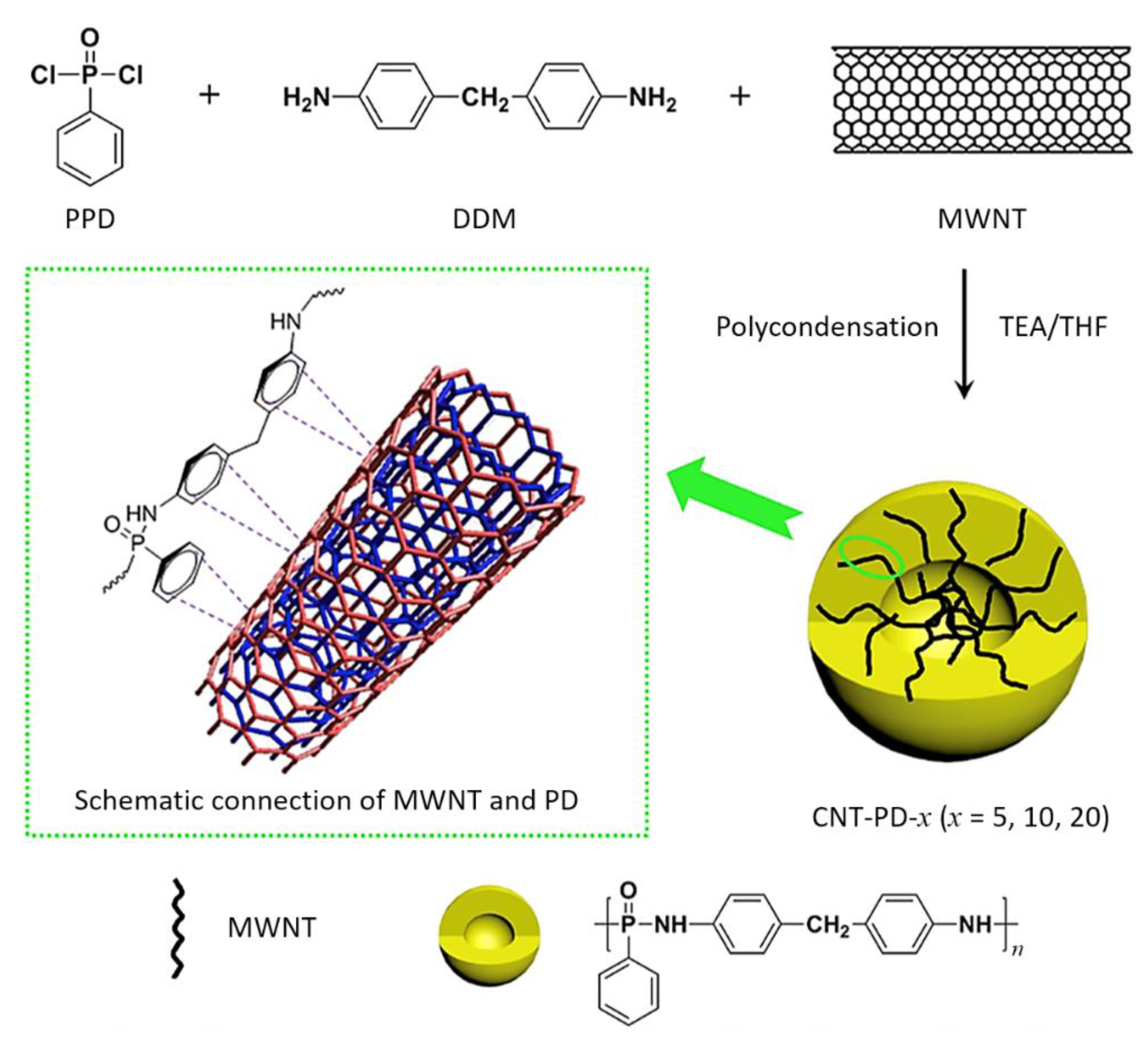

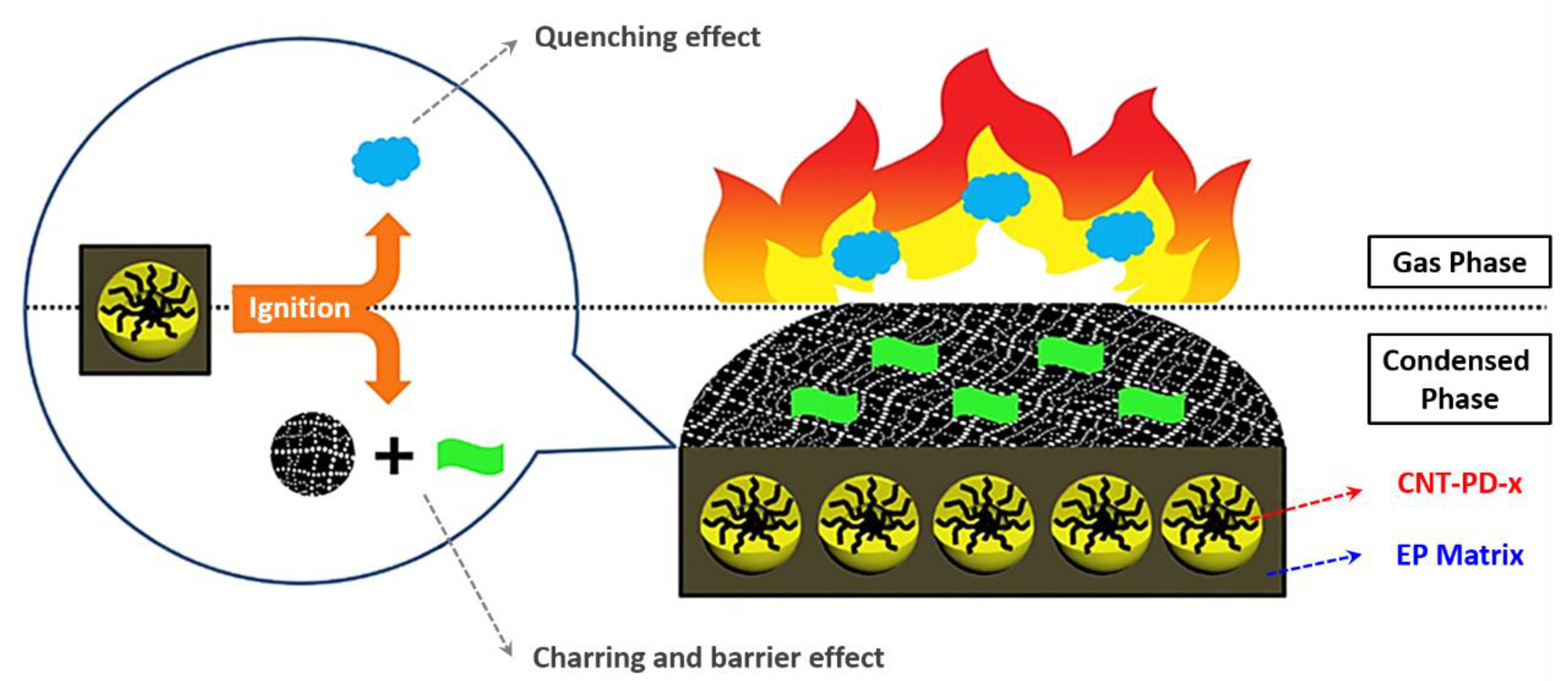






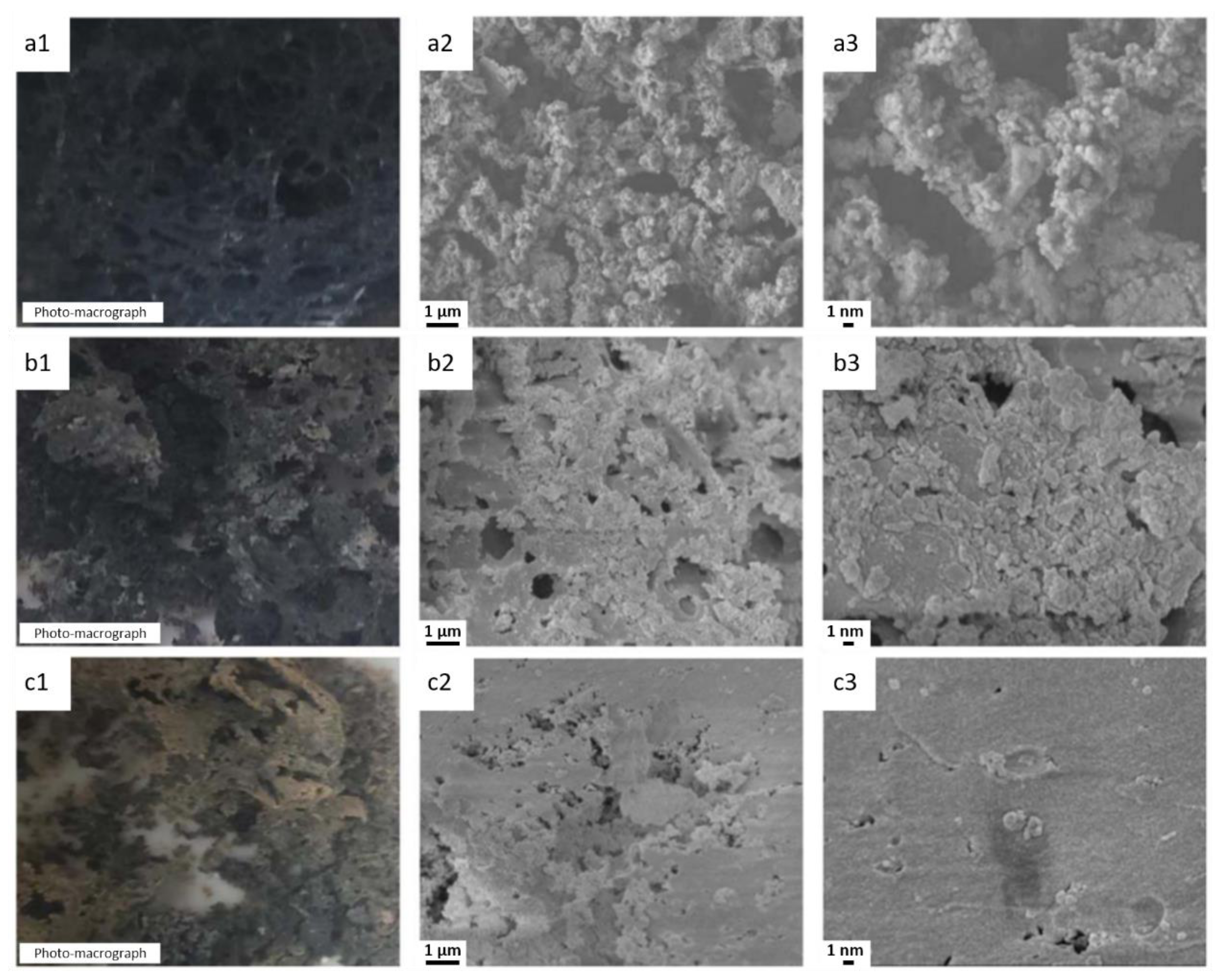
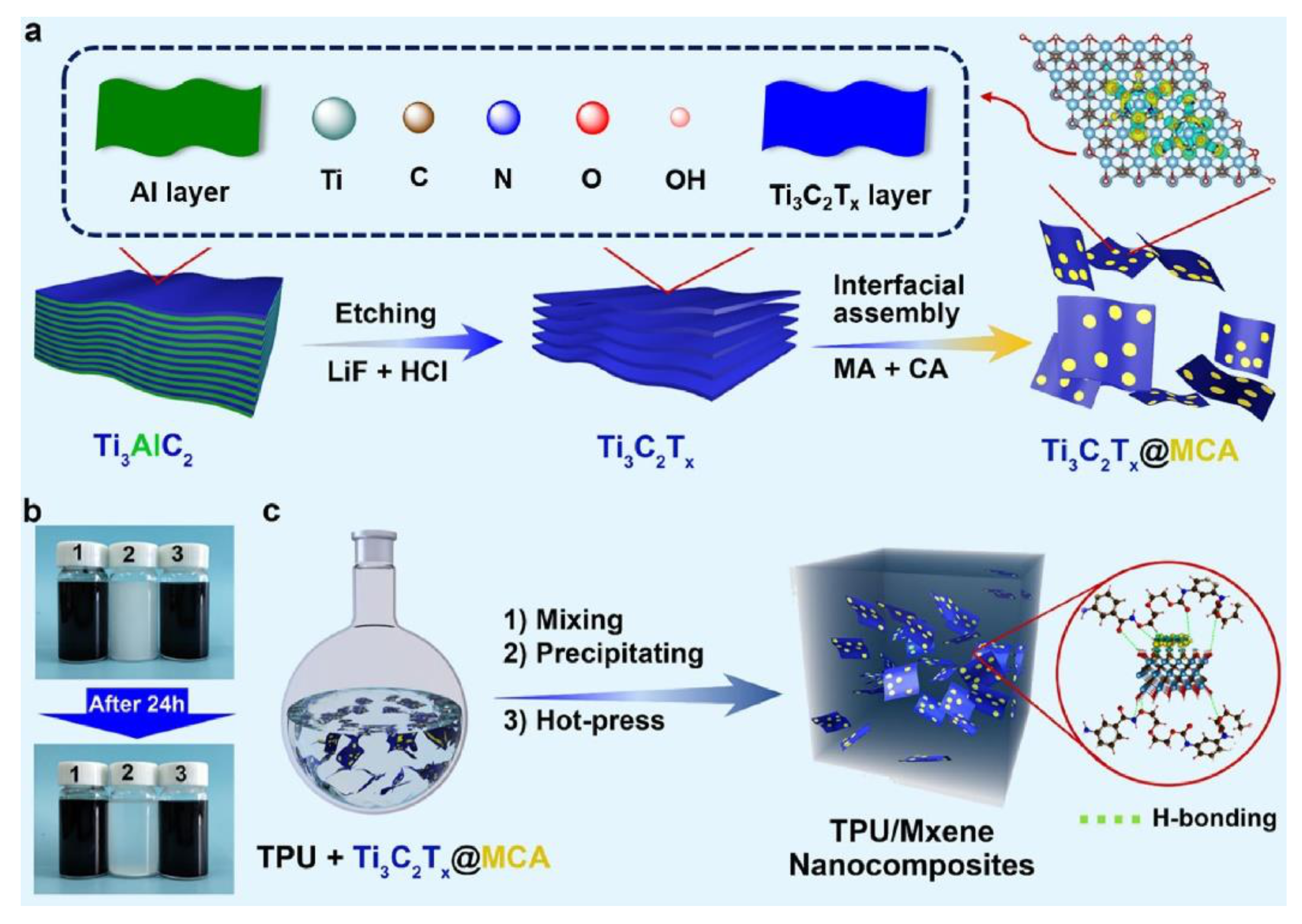
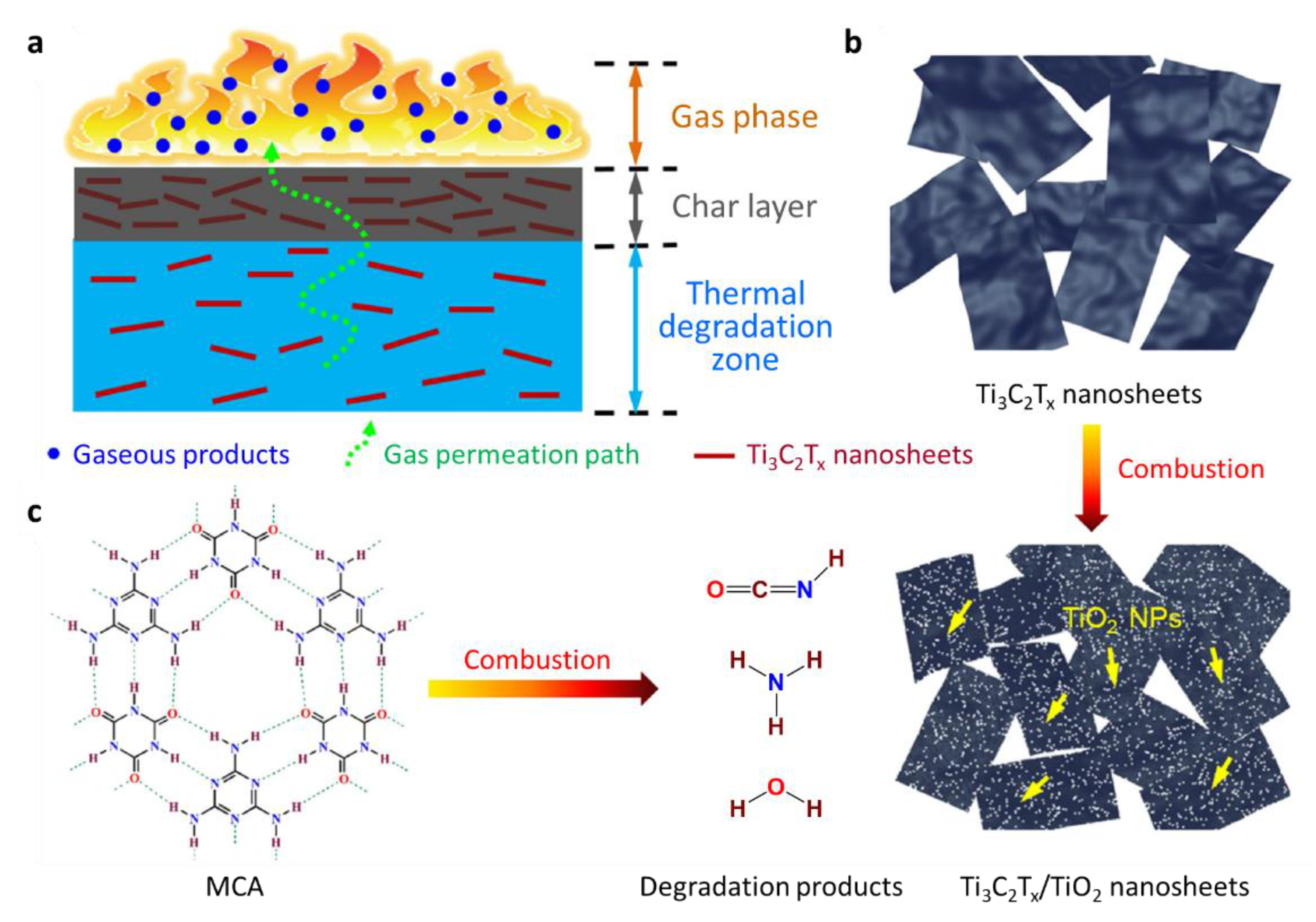

| Polymer | Heat of Combustion (ΔH, kJ/g) |
|---|---|
| Polyethylene | 46.5 |
| Polypropylene | 46.5 |
| Polybutadiene | 45.2 |
| Polystyrene | 41.5 |
| Acrylonitrile butadiene styrene copolymer | 36.0 |
| Polycarbonate | 31.0 |
| Poly(methyl methacrylate) (PMMA) | 26.1 |
| Poly(vinyl chloride) | 24.7 |
| Polyethylene terephthalate | 22.2 |
| Cotton | 17.0 |
| Cellulose | 16.7 |
| Classification by Usage | Classification by Composition | Remark | ||
| Additive | Organic | Organic | Phosphorus-based | Non-halogen |
| Nitrogen-based | ||||
| Inorganic | Phosphorus-based + halogen-based | Halogen | ||
| Halogen-based + Br or Cl compounds | ||||
| Reactive | Vinyl group Carboxyl group | Inorganic | Metal hydroxides Boron-based Antimony-based | Non-halogen |
| Hydroxyl group Epoxy group | ||||
| Polymer | LOI (%) |
|---|---|
| Polyoxymethylene | 15.7 |
| Polyurethane foam | 16.5 |
| Cotton | 16–17 |
| PMMA | 17.3 |
| Polyethylene | 17.4 |
| Polystyrene | 17.6–18.3 |
| Polycarbonate | 22.5 |
| Red oak | 23.0 |
| Nylon 6 | 25–26 |
| Poly(vinyl chloride) | 45–49 |
| Polytetrafluoroethylene | 95.0 |
| Polymer | Repeat Unit Structure | HRR (W cm−2) |
|---|---|---|
| Polypropylene |  | 150.9 |
| Polystyrene |  | 110.1 |
| Polycarbonate |  | 42.9 |
| Poly(vinyl chloride) | 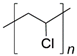 | 17.5 |
| Polyethylene |  | 140.8 |
| Polylactic acid |  | 27.2 |
| UL 94 V-0 | t1 and t2 less than 10 s for each specimen t1 + t2 less than 50 s for the 5 specimens t2 + t3 less than 30 s for each specimen No after flame or afterglow up to the holding clamp No burning drops |
| UL 94 V-1 | t1 and t2 less than 30 s for each specimen t1 + t2 less than 250 s for the 5 specimens t2 + t3 less than 60 s for each specimen No after flame or afterglow up to the holding clamp No burning drops |
| UL 94 V-2 | t1 and t2 less than 30 s for each specimen t1 + t2 less than 250 s for the 5 specimens t2 + t3 less than 60 s for each specimen No after flame or afterglow up to the holding clamp Burning drops allowed |
| Mass Fraction of SWNTs (%) | 0.0 | 0.2 | 0.5 a | 0.5 | 1.0 |
|---|---|---|---|---|---|
| Residual mass/original mass (%) | 0.0 | 0.07 ± 0.05 | 0.76 ± 0.05 | 0.99 ± 0.05 | 1.81 ± 0.05 |
| Sample | TTI (s) | PHRR (KW m−2) | ASEA (m2 kg−1) | AMLR (g s−1) | LOI (%) | UL 94 |
|---|---|---|---|---|---|---|
| PP | 42 ± 4 | 1242 ± 21 | 552 ± 14 | 0.049 ± 0.006 | 17.8 | Failed |
| PP/MWNTs | 44 ± 2 | 538 ± 12 | 511 ± 15 | 0.048 ± 0.004 | 20.6 | Failed |
| PP/RGO | 43 ± 3 | 486 ± 14 | 474 ± 12 | 0.048 ± 0.004 | 20.1 | Failed |
| PP/MWNTs/RGO | 48 ± 2 | 465 ± 12 | 439 ± 12 | 0.046 ± 0.005 | 21.0 | Failed |
| PP/IFR | 53 ± 4 | 350 ± 11 | 412 ± 13 | 0.037 ± 0.003 | 29.2 | V-1 |
| PP/IFR/MWNTs | 64 ± 3 | 278 ± 11 | 401 ± 13 | 0.035 ± 0.004 | 29. | V-1 |
| PP/IFR/RGO | 66 ± 4 | 245 ± 12 | 396 ± 14 | 0.034 ± 0.004 | 30.6 | V-1 |
| PP/IFR/MWNTs/RGO | 82 ± 3 | 212 ± 8 | 380 ± 12 | 0.032 ± 0.003 | 31.4 | V-0 |
| Sample | Formulation (wt%) | CP Ratio (wt%) | Process |
|---|---|---|---|
| A0 | PA6/CNBR = 90/40 | - | - |
| A1 | PA6/CPA = 90/100 | CNBR/nano-MDH = 40/60 | New process (illustrated in Figure 30) |
| A2 | PA6/CNBR/nano-MDH = 90/40/60 | - | Conventional process |
| LOI (%) | PHRR (kW m−2) | THR (MJ m−2) | TTI (s) | SET (s) | UL 94 | Ref. | |
|---|---|---|---|---|---|---|---|
| Functionalized MWNTs | 29.2 | V-0 | [176] | ||||
| Epoxy/MWNTs composites | 33.6 | 754 ± 31 | 102 ± 3 | - | - | [181] | |
| RGO and PP/MWNTs | 31.4 | 212 ± 8 | - | 82 ± 3 | V-0 | [189] | |
| MOGO and MOF | 29 | 702 ± 9 | [195] | ||||
| Modified GO with nano ZIF-8 | 27 | 258 ± 7 | 29.9 | 62 ± 1 | 10.2 | V-2 | [199] |
| MoS2@TiO2 structure | 314.25 ± 1.82 | 53.58 ± 0.21 | [232] | ||||
| Nano-MDH | 277 | 150 | [221] | ||||
| ATH and MDH | 7 s for ATH6 s for MDH | [85] | |||||
| Nano ZIF-8/PP composites | 25 | V-2 | [238] | ||||
| Nano ZIF-8/PLA composites | 26 | 2.9 s | V-2 | [210] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.; Lee, S.; Yoon, H. Fire-Safe Polymer Composites: Flame-Retardant Effect of Nanofillers. Polymers 2021, 13, 540. https://doi.org/10.3390/polym13040540
Kim Y, Lee S, Yoon H. Fire-Safe Polymer Composites: Flame-Retardant Effect of Nanofillers. Polymers. 2021; 13(4):540. https://doi.org/10.3390/polym13040540
Chicago/Turabian StyleKim, Yukyung, Sanghyuck Lee, and Hyeonseok Yoon. 2021. "Fire-Safe Polymer Composites: Flame-Retardant Effect of Nanofillers" Polymers 13, no. 4: 540. https://doi.org/10.3390/polym13040540
APA StyleKim, Y., Lee, S., & Yoon, H. (2021). Fire-Safe Polymer Composites: Flame-Retardant Effect of Nanofillers. Polymers, 13(4), 540. https://doi.org/10.3390/polym13040540