Hybrid Sol–Gel Superhydrophobic Coatings Based on Alkyl Silane-Modified Nanosilica
Abstract
1. Introduction
2. Experimental
2.1. Materials and Reagents
2.2. Pretreatment of the Substrates
2.3. Synthesis and Coating Process
2.4. Characterization
3. Results and Discussion
3.1. Wettability of the Treated Surfaces
3.2. Morphology of the Coatings
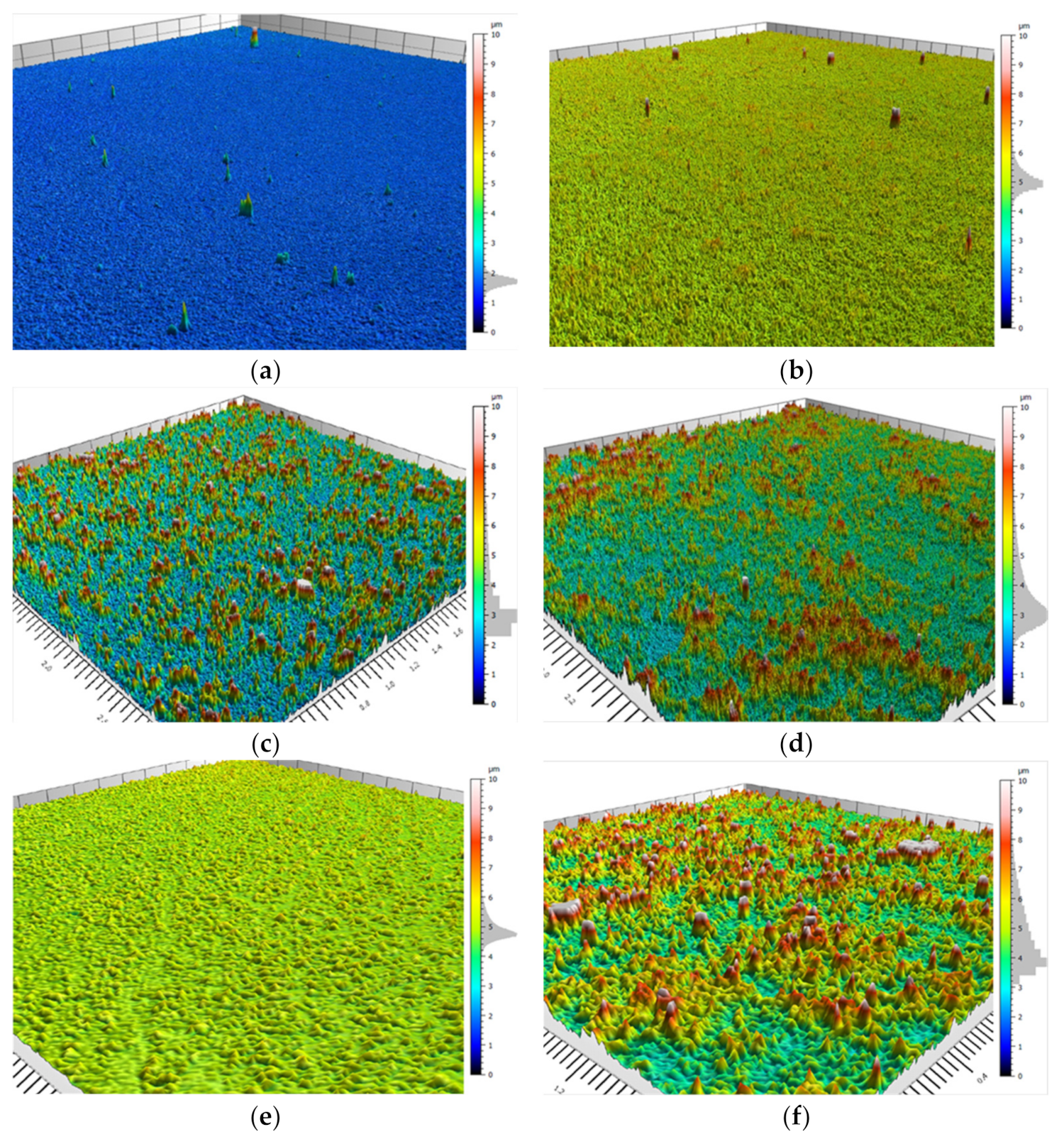
3.3. Confocal Morphology Analysis
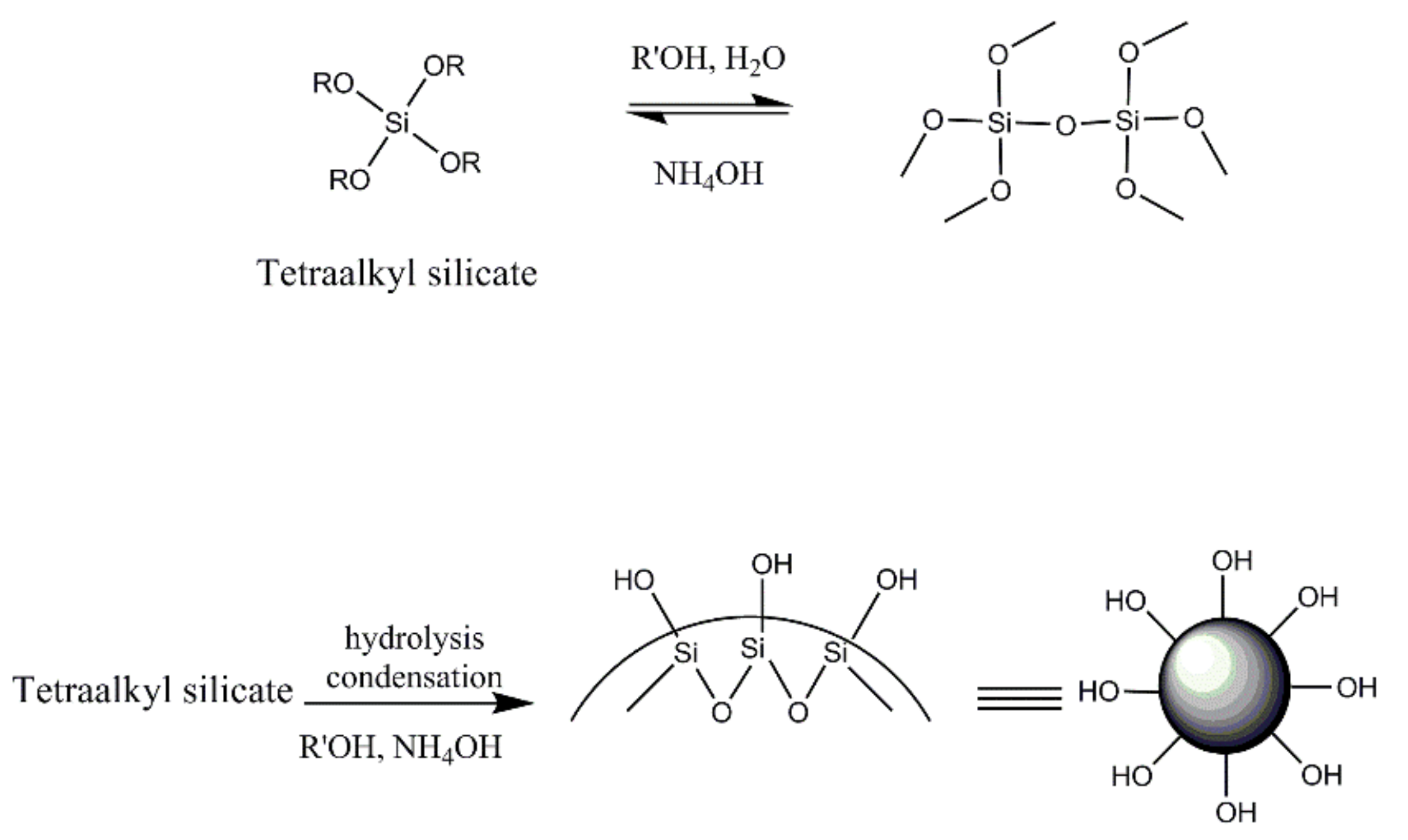
3.4. Reaction Mechanisms
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brinker, C.; Jeffrey, S.G.W. Sol-Gel Science; Elsevier: Amsterdam, The Netherlands, 1990. [Google Scholar]
- Czyzyk, S.; Dotan, A.; Dodiuk, H.; Kenig, S. Easy-to-Clean Superhydrophobic Coatings Based on Sol-Gel Technology: A Critical Review. Rev. Adhes. Adhes. 2017, 5, 325–360. [Google Scholar] [CrossRef]
- Crayston, J.A. Sol-Gel. In Comprehensive Coordination Chemistry II; McCleverty, J.A., Meyer, T.J., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2003; pp. 711–730. [Google Scholar]
- Czyzyk, S.; Dotan, A.; Dodiuk, H.; Kenig, S. Durable, Transparent, Superhydrophobic Coatings Based on Hybrid Sol-Gel Prepared by Thermal, Radiation and dual Curing Process. accepted.
- Bhushan, B.; Jung, Y.C. Natural and biomimetic artificial surfaces for superhydrophobicity, self-cleaning, low adhesion, and drag reduction. Prog. Mater. Sci. 2011, 56, 1–108. [Google Scholar] [CrossRef]
- Dodiuk, H.; Kenig, S.; Dotan, A. Do self-cleaning surfaces repel ice? J. Adhes. Sci. Technol. 2012, 26, 701–714. [Google Scholar] [CrossRef]
- Patankar, N.A. Mimicking the lotus effect: Influence of double roughness structures and slender pillars. Langmuir 2004, 20, 8209–8213. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Li, S.; Li, Y.; Li, H.; Zhang, L.; Zhai, J.; Song, Y.; Liu, B.; Jiang, L.; Zhu, D. Super-hydrophobic surfaces: From natural to artificial. Adv. Mater. 2002, 14, 1857–1860. [Google Scholar] [CrossRef]
- Barthlott, W.; Neinhuis, C. Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta 1997, 202, 1–8. [Google Scholar] [CrossRef]
- Fürstner, R.; Barthlott, W.; Neinhuis, C.; Walzel, P. Wetting and self-cleaning properties of artificial superhydrophobic surfaces. Langmuir 2005, 21, 956–961. [Google Scholar] [CrossRef] [PubMed]
- Koch, K.; Bhushan, B.; Barthlott, W. Diversity of structure, morphology and wetting of plant surfaces. Soft Matter 2008, 4, 1943–1963. [Google Scholar] [CrossRef]
- Bixler, G.D.; Bhushan, B. Fluid drag reduction and efficient self-cleaning with rice leaf and butterfly wing bioinspired surfaces. Nanoscale 2013, 5, 7685–7710. [Google Scholar] [CrossRef] [PubMed]
- Rios, P.F.; Dodiuk, H.; Kenig, S.; McCarthy, S.; Dotan, A. Transparent ultra-hydrophobic surfaces. J. Adhes. Sci. Technol. 2007, 21, 399–408. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, Z. Biomimetic self-slippery and transferable transparent lubricant-infused functional surfaces. Nanoscale 2018, 10, 19879–19889. [Google Scholar] [CrossRef]
- Yu, S.; Guo, Z.; Liu, W. Biomimetic transparent and superhydrophobic coatings: From nature and beyond nature. Chem. Commun. 2015, 51, 1775–1794. [Google Scholar] [CrossRef]
- Rios, P.F.; Dodiuk, H.; Kenig, S.; Mccarthy, S.; Dotan, A. The effects of nanostructure and composition on the hydrophobic properties of solid surfaces. J. Adhes. Sci. Technol. 2006, 20, 563–587. [Google Scholar] [CrossRef]
- Bhushan, B.; Jung, Y.C. Micro- and nanoscale characterization of hydrophobic and hydrophilic leaf surfaces. Nanotechnology 2006, 17, 2758–2772. [Google Scholar] [CrossRef]
- Nishino, T.; Meguro, M.; Nakamae, K.; Matsushita, M.; Ueda, Y. The lowest surface free energy based on -CF3 alignment. Langmuir 1999, 15, 4321–4323. [Google Scholar] [CrossRef]
- Hasan, J.; Webb, H.K.; Truong, V.K.; Watson, G.S.; Watson, J.A.; Tobin, M.J.; Gervinskas, G.; Juodkazis, S.; Wang, J.Y.; Crawford, R.J.; et al. Spatial variations and temporal metastability of the self-cleaning and superhydrophobic properties of damselfly wings. Langmuir 2012, 28, 17404–17409. [Google Scholar] [CrossRef] [PubMed]
- Nahum, T.; Dodiuk, H.; Kenig, S.; Panwar, A.; Barry, C.; Mead, J. The effect of composition and thermodynamics on the surface morphology of durable superhydrophobic polymer coatings. Nanotechnol. Sci. Appl. 2017, 10, 53–68. [Google Scholar] [CrossRef]
- Rios, P.F.; Dodiuk, H.; Kenig, S. Self-cleaning coatings. Surf. Eng. 2009, 25, 89–92. [Google Scholar] [CrossRef]
- Bogush, G.H.; Tracy, M.A.; Zukoski, C.F. IV Preparation of monodisperse silica particles: Control of size and mass fraction. J. Non. Cryst. Solids 1988, 104, 95–106. [Google Scholar] [CrossRef]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Meier, M.; Ungerer, J.; Klinge, M.; Nirschl, H. Synthesis of nanometric silica particles via a modified Stöber synthesis route. Colloids Surfaces A Physicochem. Eng. Asp. 2018, 538, 559–564. [Google Scholar] [CrossRef]
- Han, Y.; Lu, Z.; Teng, Z.; Liang, J.; Guo, Z.; Wang, D.; Han, M.Y.; Yang, W. Unraveling the growth mechanism of silica particles in the stöber method: In situ seeded growth model. Langmuir 2017, 33, 5879–5890. [Google Scholar] [CrossRef] [PubMed]
- Greasley, S.L.; Page, S.J.; Sirovica, S.; Chen, S.; Martin, R.A.; Riveiro, A.; Hanna, J.V.; Porter, A.E.; Jones, J.R. Controlling particle size in the Stöber process and incorporation of calcium. J. Colloid Interface Sci. 2016, 469, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Green, D.L.; Lin, J.S.; Lam, Y.F.; Hu, M.Z.C.; Schaefer, D.W.; Harris, M.T. Size, volume fraction, and nucleation of Stober silica nanoparticles. J. Colloid Interface Sci. 2003, 266, 346–358. [Google Scholar] [CrossRef]
- Binyamini, R.B.S.; Boguslavsky, Y.; Laux, E.; Keppner, H.; Lellouche, J.P.M. A simple one-step approach to the decoration of parylene C coatings using functional silica-based NPs. Surf. Coat. Technol. 2015, 263, 36–43. [Google Scholar] [CrossRef]
- Adam, J.; Roscher, C.; Eger, C.; Adebahr, T.; Wieczorreck, R.; Pylik, M. Silicon Dioxide Dispersion. U.S. Patent 9,376,544, 28 June 2016. [Google Scholar]
- Rahman, I.A.; Padavettan, V. Synthesis of Silica Nanoparticles by Sol-Gel: Size-Dependent Properties, Surface Modification, and Applications in Silica-Polymer Nanocomposites—A Review. J. Nanomater. 2012, 2012. [Google Scholar] [CrossRef]
- Jung, H.S.; Moon, D.S.; Lee, J.K. Quantitative analysis and efficient surface modification of silica nanoparticles. J. Nanomater. 2012, 2012. [Google Scholar] [CrossRef]
- Miranda, M.S.L.; da Silveira, N.P.; Frost, R.L.; dos Santos, J.H.Z.; Pires, G.P.; Brambilla, R. Spherical and lamellar octadecylsilane hybrid silicas. J. Non. Cryst. Solids 2008, 354, 5033–5040. [Google Scholar] [CrossRef]
- Brambilla, R.; Pires, G.P.; dos Santos, J.H.Z.; Lacerda Miranda, M.S. Octadecylsilane hybrid silicas prepared by the sol-gel method: Morphological and textural aspects. J. Colloid Interface Sci. 2007, 312, 326–332. [Google Scholar] [CrossRef]
- Choi, H.; Chen, I.W. Surface-modified silica colloid for diagnostic imaging. J. Colloid Interface Sci. 2003. [Google Scholar] [CrossRef]
- Venkatathri, N. Preparation of silica nanoparticle through coating with octyldecyltrimethoxy silane. Indian J. Chem. Sect. A Inorg. Phys. Theor. Anal. Chem. 2007, 46, 1955–1958. [Google Scholar]
- Barrera, E.G.; Livotto, P.R.; dos Santos, J.H.Z. Hybrid silica bearing different organosilanes produced by the modified Stöber method. Powder Technol. 2016, 301, 486–492. [Google Scholar] [CrossRef]
- Wang, H.; Fang, J.; Cheng, T.; Ding, J.; Qu, L.; Dai, L.; Wang, X.; Lin, T. One-step coating of fluoro-containing silica nanoparticles for universal generation of surface superhydrophobicity. Chem. Commun. 2008, 877–879. [Google Scholar] [CrossRef]
- Wang, H.; Ding, J.; Xue, Y.; Wang, X.; Lin, T. Superhydrophobic fabrics from hybrid silica sol-gel coatings: Structural effect of precursors on wettability and washing durability. J. Mater. Res. 2010, 25, 1336–1343. [Google Scholar] [CrossRef]
- Rahman, I.A.; Jafarzadeh, M.; Sipaut, C.S. Synthesis of organo-functionalized nanosilica via a co-condensation modification using γ-aminopropyltriethoxysilane (APTES). Ceram. Int. 2009, 35, 1883–1888. [Google Scholar] [CrossRef]
- Chen, S.; Osaka, A.; Hayakawa, S.; Tsuru, K.; Fujii, E.; Kawabata, K. Novel one-pot sol-gel preparation of amino-functionalized silica nanopartieles. Chem. Lett. 2008, 37, 1170–1171. [Google Scholar] [CrossRef]
- Kobler, J.; Bein, T. Porous thin films of functionalized mesoporous silica nanoparticles. ACS Nano 2008, 2, 2324–2330. [Google Scholar] [CrossRef]
- Branda, F.; Silvestri, B.; Luciani, G.; Costantini, A. The effect of mixing alkoxides on the Stöber particles size. Colloids Surfaces A Physicochem. Eng. Asp. 2007, 299, 252–255. [Google Scholar] [CrossRef]
- Osterholtz, F.; Pohl, E. Kinetics of the hydrolysis and condensation of organofunctional alkoxysilanes: A review. J. Adhes. Sci. Technol. 1992, 6, 127–149. [Google Scholar] [CrossRef]
- Voronkov, M.G.; Mileshkevich, V.P.; Yuzhelevskii, Y.A. Siloxane Bond: Physical Properties and Chemical Transformations (Studies in Soviet Science: Physical Sciences), 1st ed.; Springer: Berlin/Heidelberg, Germany, 1978; Volume 196. [Google Scholar]
- Pohl, E.R. Kinetics and Mechanisms of Acid and Base-Catalyzed Hydrolysis of Alkyltrialkoxysilanes in Aqueous Solution. In Proceedings of the 38th Annual Technical Conference, Accra, Ghana, 6–10 November 2017. [Google Scholar]
- Hansen, C. Hansen Solubility Parameters—A User’s Handbook, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2007; Volume 8809, ISBN 9780849372483. [Google Scholar]
- Hansen, C.; Abott, S.; Yamamoto, H. HSPiP Team. Available online: https://www.hansen-solubility.com (accessed on 12 February 2021).
- Fadeev, A.Y.; McCarthy, T.J. Trialkylsilane monolayers covalently attached to silicon surfaces: Wettability studies indicating that molecular topography contributes to contact angle hysteresis. Langmuir 1999, 15, 3759–3766. [Google Scholar] [CrossRef]
- Petro, A.J. The Dipole Moment of the Carbon-Carbon Bond. J. Am. Chem. Soc. 1958, 80, 4230–4232. [Google Scholar] [CrossRef]
- Brown, M.G. Atom hybridization and bond properties. Some carbon-containing bonds. Trans. Faraday Soc. 1959, 55, 694–701. [Google Scholar] [CrossRef]
- Loy, D.A.; Baugher, B.M.; Baugher, C.R.; Schneider, D.A.; Rahimian, K. Substituent effects on the sol-gel chemistry of organotrialkoxysilanes. Chem. Mater. 2000, 12, 3624–3632. [Google Scholar] [CrossRef]
- Nahum, T.; Dodiuk, H.; Kenig, S.; Barry, C.; Mead, J. The Role of Roughness in Random Superhydrophobic Surfaces. Int. J. Nanotechnol. Nanomed. 2018, 3, 1–15. [Google Scholar] [CrossRef]
- Cohen, N.; Dotan, A.; Dodiuk, H.; Kenig, S. Thermomechanical Mechanisms of Reducing Ice Adhesion on Superhydrophobic Surfaces. Langmuir 2016, 32, 9664–9675. [Google Scholar] [CrossRef] [PubMed]
- Dodiuk, H.; Rios, P.F.; Dotan, A.; Kenig, S. Hydrophobic and self-cleaning coatings. Polym. Adv. Technol. 2007, 18, 746–750. [Google Scholar] [CrossRef]
- Semnani, D. Geometrical Characterization of Electrospun Nanofibers; Elsevier Ltd.: Amsterdam, The Netherlands, 2016; ISBN 9780081009116. [Google Scholar]
- Tayebi, N.; Polycarpou, A.A. Modeling the effect of skewness and kurtosis on the static friction coefficient of rough surfaces. Tribol. Int. 2004, 37, 491–505. [Google Scholar] [CrossRef]


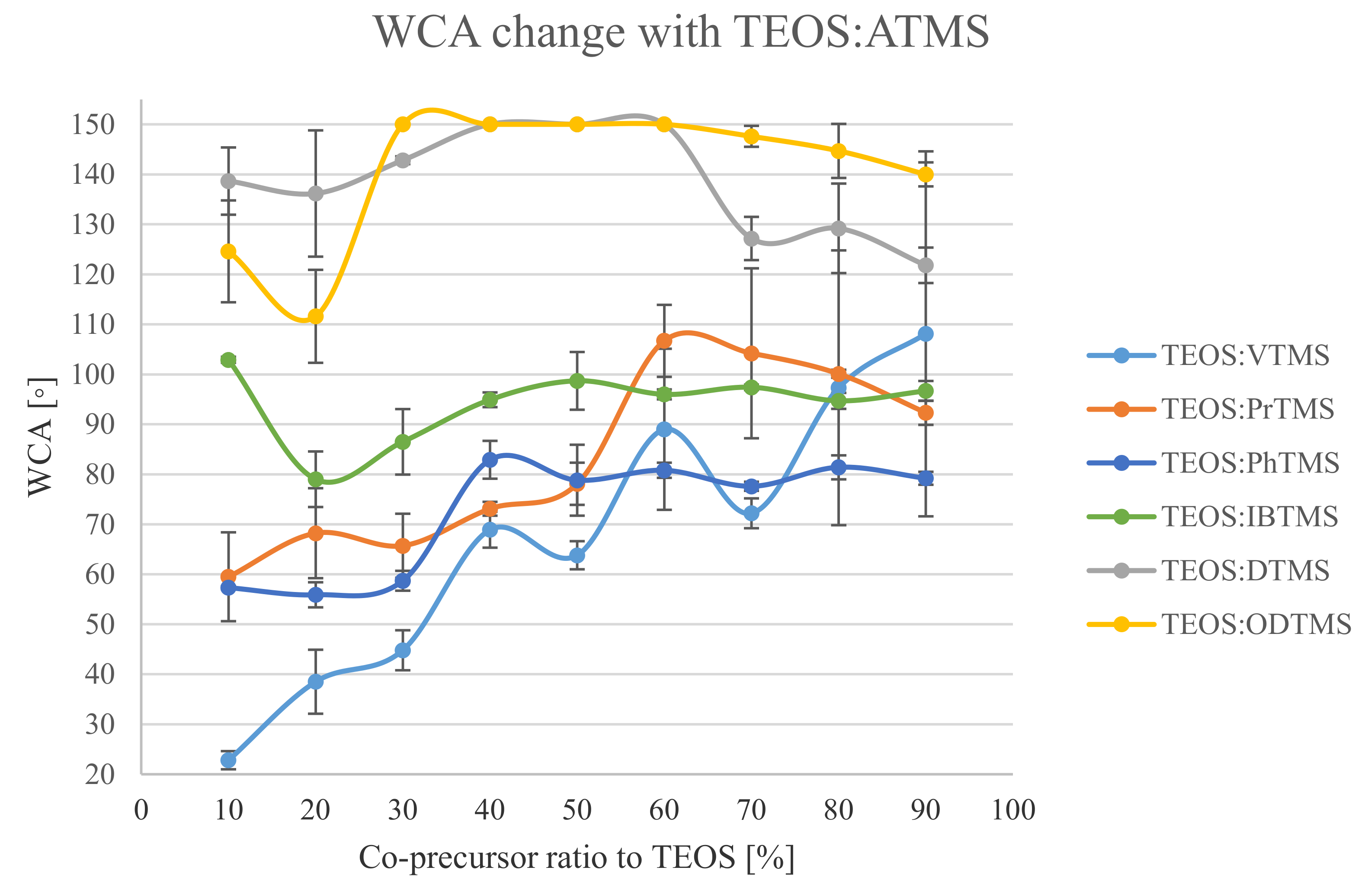




| TEOS:VTMS | WCA Avg. (°) | WSA (°) | Haze (%) | Transmittance (%) | TEOS:PrTMS | WCA Avg. (°) | WSA (°) | Haze (%) | Transmittance (%) |
|---|---|---|---|---|---|---|---|---|---|
| 9:1 | 22.8 ± 1.8 | >90.0 | 2.5 | 93.8 | 9:1 | 59.5 ± 8.9 | 90.0 | 0.2 | 93.3 |
| 8:2 | 38.5 ± 6.4 | >90.0 | 1.7 | 96.8 | 8:2 | 68.2 ± 9.0 | >90.0 | 1.6 | 93.0 |
| 7:3 | 44.8 ± 4.0 | >90.0 | 1.7 | 95.4 | 7:3 | 65.7 ± 6.4 | >90.0 | 0.7 | 93.0 |
| 6:4 | 68.9 ± 3.6 | >90.0 | 2.0 | 95.6 | 6:4 | 73.1 ± 1.4 | >90.0 | 1.4 | 93.1 |
| 5:5 | 63.8 ± 2.8 | >90.0 | 2.1 | 92.8 | 5:5 | 78.1 ± 4.2 | 90.0 | 0.3 | 92.8 |
| 4:6 | 89.0 ± 16.1 | >90.0 | 4.4 | 93.7 | 4:6 | 106.7 ± 7.2 | >90.0 | 6.0 | 91.6 |
| 3:7 | 72.2 ± 3.0 | >90.0 | 6.3 | 94.2 | 3:7 | 104.2 ± 17.0 | 40.0 | 40.0 | 88.8 |
| 2:8 | 97.3 ± 27.5 | >90.0 | 8.5 | 92.9 | 2:8 | 100.1 ± 0.8 | 50.0 | 30.8 | 91.1 |
| 1:9 | 108.1 ± 36.5 | >90.0 | 66.0 | 64.8 | 1:9 | 92.3 ± 2.4 | 27.0 | 6.7 | 92.7 |
| TEOS:DTMS | WCA Avg. (°) | WSA (°) | Haze (%) | Transmittance (%) | TEOS:ODTMS | WCA Avg. (°) | WSA (°) | Haze (%) | Transmittance (%) |
| 9:1 | 138.7 ± 6.7 | 73.0 | 36.4 | 93.7 | 9:1 | 124.6 ± 10.2 | 70.0 | 30.3 | 93.1 |
| 8:2 | 136.2 ± 12.6 | 90.0 | 25.4 | 94.2 | 8:2 | 111.6 ± 9.3 | 38.0 | 37.5 | 89.9 |
| 7:3 | 142.8 ± 0.8 | 67.0 | 22.1 | 95.1 | 7:3 | 150.0 ± 0.0 | 0.0 | 82.8 | 88.1 |
| 6:4 | 150.0 ± 0.0 | 10.0 | 49.5 | 90.1 | 6:4 | 150.0 ± 0.3 | 5.0 | 35.6 | 91.7 |
| 5:5 | 150.0 ± 0.0 | 3.0 | 82.3 | 81.4 | 5:5 | 150.0 ± 0.0 | 0.0 | 81.0 | 84.8 |
| 4:6 | 150.0 ± 0.0 | 18.0 | 95.2 | 67.2 | 4:6 | 150.0 ± 0.0 | 2.0 | 53.6 | 89.9 |
| 3:7 | 127.2 ± 4.3 | 50.0 | 66.1 | 88.8 | 3:7 | 147.6 ± 2.1 | 30.0 | 64.2 | 83.8 |
| 2:8 | 129.2 ± 8.9 | 85.0 | 65.3 | 91.9 | 2:8 | 144.7 ± 5.4 | 42.0 | 4.1 | 77.8 |
| 1:9 | 121.8 ± 3.5 | 90.0 | 76.9 | 92.8 | 1:9 | 140.0 ± 2.4 | 30.0 | 81.6 | 83.7 |
| TEOS:PhTMS | WCA Avg. (°) | WSA (°) | Haze (%) | Transmittance (%) | TEOS:IBTMS | WCA Avg. (°) | WSA (°) | Haze (%) | Transmittance (%) |
| 9:1 | 57.3 ± 0.3 | >90.0 | 2.0 | 93.2 | 9:1 | 102.9 ± 0.61 | 30.0 | 4.5 | 96.5 |
| 8:2 | 55.9 ± 2.5 | >90.0 | 2.5 | 93.4 | 8:2 | 79.0 ± 5.57 | 75.0 | 0.1 | 95.5 |
| 7:3 | 58.7 ± 2.0 | >90.0 | 1.3 | 93.4 | 7:3 | 86.5 ± 6.56 | >90.0 | 1.5 | 95.1 |
| 6:4 | 82.9 ± 3.8 | >90.0 | 0.4 | 93.5 | 6:4 | 94.9 ± 1.47 | 69.0 | 6.7 | 95.0 |
| 5:5 | 78.8 ± 7.1 | >90.0 | 3.4 | 93.8 | 5:5 | 98.7 ± 5.77 | 48.0 | 2.4 | 93.8 |
| 4:6 | 80.8 ± 1.5 | >90.0 | 0.7 | 93.2 | 4:6 | 96.0 ± 1.0 | 32.0 | 1.0 | 93.4 |
| 3:7 | 77.6 ± 0.9 | >90.0 | 1.4 | 93.1 | 3:7 | 97.4 ± 0.55 | 49.0 | 0.8 | 93.1 |
| 2:8 | 81.4 ± 2.4 | >90.0 | 2.2 | 93.2 | 2:8 | 94.7 ± 1.59 | 33.0 | 0.2 | 93.3 |
| 1:9 | 79.2 ± 1.3 | >90.0 | 2.1 | 93.4 | 1:9 | 96.7 ± 1.97 | 25.0 | 1.7 | 93.1 |
| Precursor | Surface Tension (mN/m) |
|---|---|
| PrTMS | 18.1 |
| VTMS | 18.6 |
| PhTMS | 26.0 |
| DTMS | 19.2 |
| ODTMS | 19.8 |
| IBTMS | 17.1 |
| Image | Average Particle Size (nm) |
|---|---|
| Figure 5a | No particles |
| Figure 5b | 110 ± 12 |
| Figure 5c | 148 ± 18 |
| Figure 5d | 375 ± 216 |
| Figure 5e | 455 ± 267 |
| Image | Average Particle Size (nm) |
|---|---|
| Figure 6a | No particles |
| Figure 6b | 148 ± 18 |
| Figure 6c | 370 ± 75 |
| Parameter/ System | TEOS:DTMS 7:3 | TEOS:DTMS 6:4 | TEOS:DTMS 5:5 | TEOS:DTMS 4:6 | TEOS:DTMS 3:7 | TEOS:ODTMS 5:5 | TEOS:PrTMS 5:5 |
|---|---|---|---|---|---|---|---|
| Rp (µm) | 0.37 ± 0.07 | 0.90 ± 0.12 | 2.13 ± 0.16 | 1.68 ± 0.25 | 0.92 ± 0.09 | 2.21 ± 0.29 | 0.23 ± 0.08 |
| Ra (µm) | 0.12 ± 0.02 | 0.25 ± 0.03 | 0.63 ± 0.07 | 0.50 ± 0.09 | 0.25 ± 0.03 | 0.67 ± 0.08 | 0.06 ± 0.02 |
| Rq (µm) | 0.15 ± 0.02 | 0.32 ± 0.05 | 0.81 ± 0.08 | 0.63 ± 0.11 | 0.32 ± 0.03 | 0.85 ± 0.11 | 0.08 ± 0.03 |
| RSm (mm) | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.02 ± 0.01 | 0.02 ± 0.00 | 0.01 ± 0.00 | 0.02 ± 0.01 | 0.03 ± 0.03 |
| Peak density (1/cm) | 1135 ± 156 | 815 ± 121 | 480 ± 74 | 647 ± 74.1 | 719 ± 54.8 | 598 ± 118 | 483 ± 175 |
| Rsk | 0.0112 ± 0.101 | 0.54 ± 0.01 | 0.71 ± 0.11 | 0.63 ± 0.11 | 0.63 ± 0.14 | 0.51 ± 0.14 | 0.20 ± 0.26 |
| Rku | 3.10 ± 0.28 | 3.72 ± 0.24 | 3.65 ± 0.28 | 3.48 ± 0.26 | 3.97 ± 0.38 | 3.30 ± 0.32 | 5.30 ± 0.78 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heiman-Burstein, D.; Dotan, A.; Dodiuk, H.; Kenig, S. Hybrid Sol–Gel Superhydrophobic Coatings Based on Alkyl Silane-Modified Nanosilica. Polymers 2021, 13, 539. https://doi.org/10.3390/polym13040539
Heiman-Burstein D, Dotan A, Dodiuk H, Kenig S. Hybrid Sol–Gel Superhydrophobic Coatings Based on Alkyl Silane-Modified Nanosilica. Polymers. 2021; 13(4):539. https://doi.org/10.3390/polym13040539
Chicago/Turabian StyleHeiman-Burstein, Dafna, Anna Dotan, Hanna Dodiuk, and Samuel Kenig. 2021. "Hybrid Sol–Gel Superhydrophobic Coatings Based on Alkyl Silane-Modified Nanosilica" Polymers 13, no. 4: 539. https://doi.org/10.3390/polym13040539
APA StyleHeiman-Burstein, D., Dotan, A., Dodiuk, H., & Kenig, S. (2021). Hybrid Sol–Gel Superhydrophobic Coatings Based on Alkyl Silane-Modified Nanosilica. Polymers, 13(4), 539. https://doi.org/10.3390/polym13040539









