Nature of Carbon Black Reinforcement of Rubber: Perspective on the Original Polymer Nanocomposite
Abstract
1. Introduction
2. Regions of Reinforcement
- Payne effect,
- Minimum/transition,
- Stress upturn,
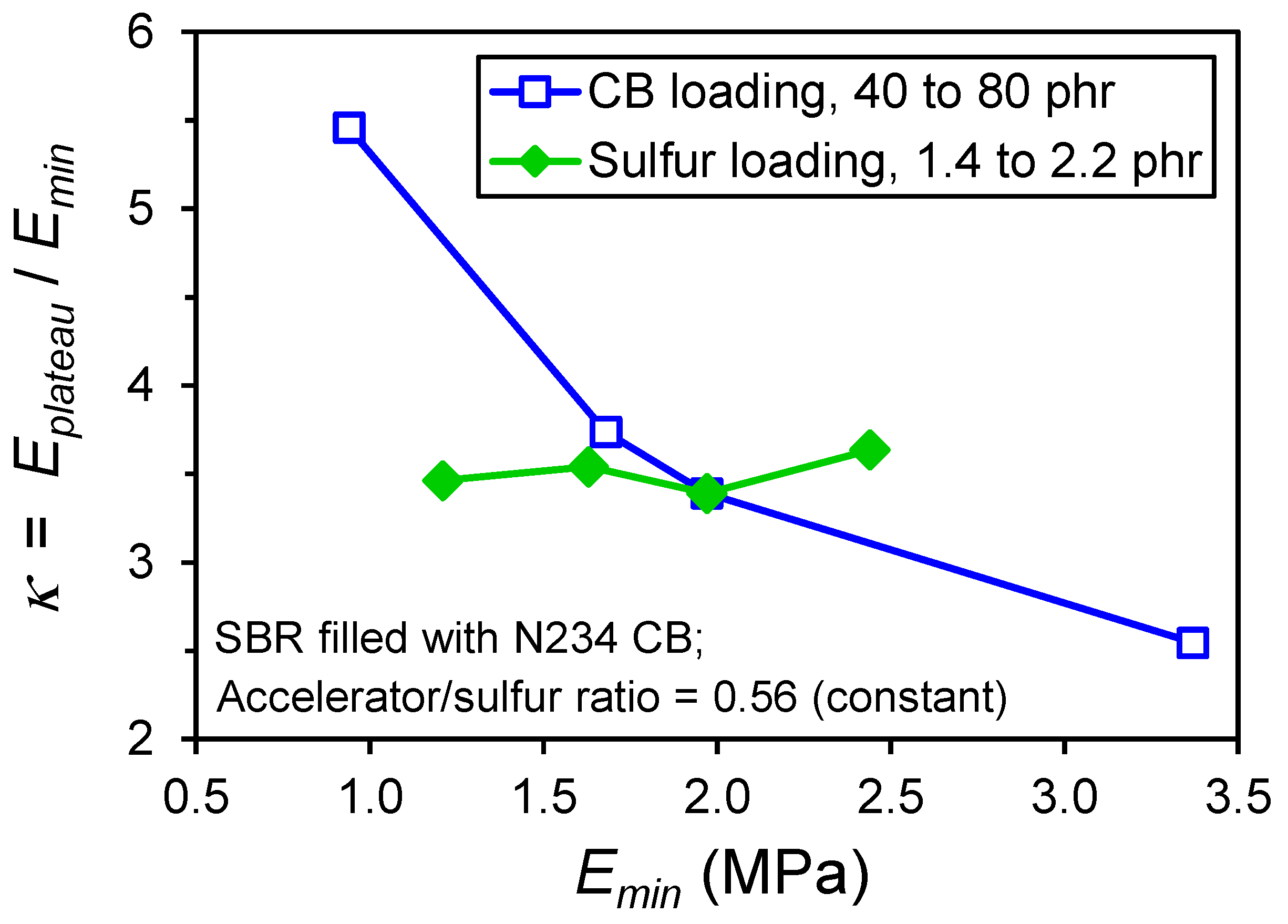
- Modulus plateau, and
- Ultimate softening and break.
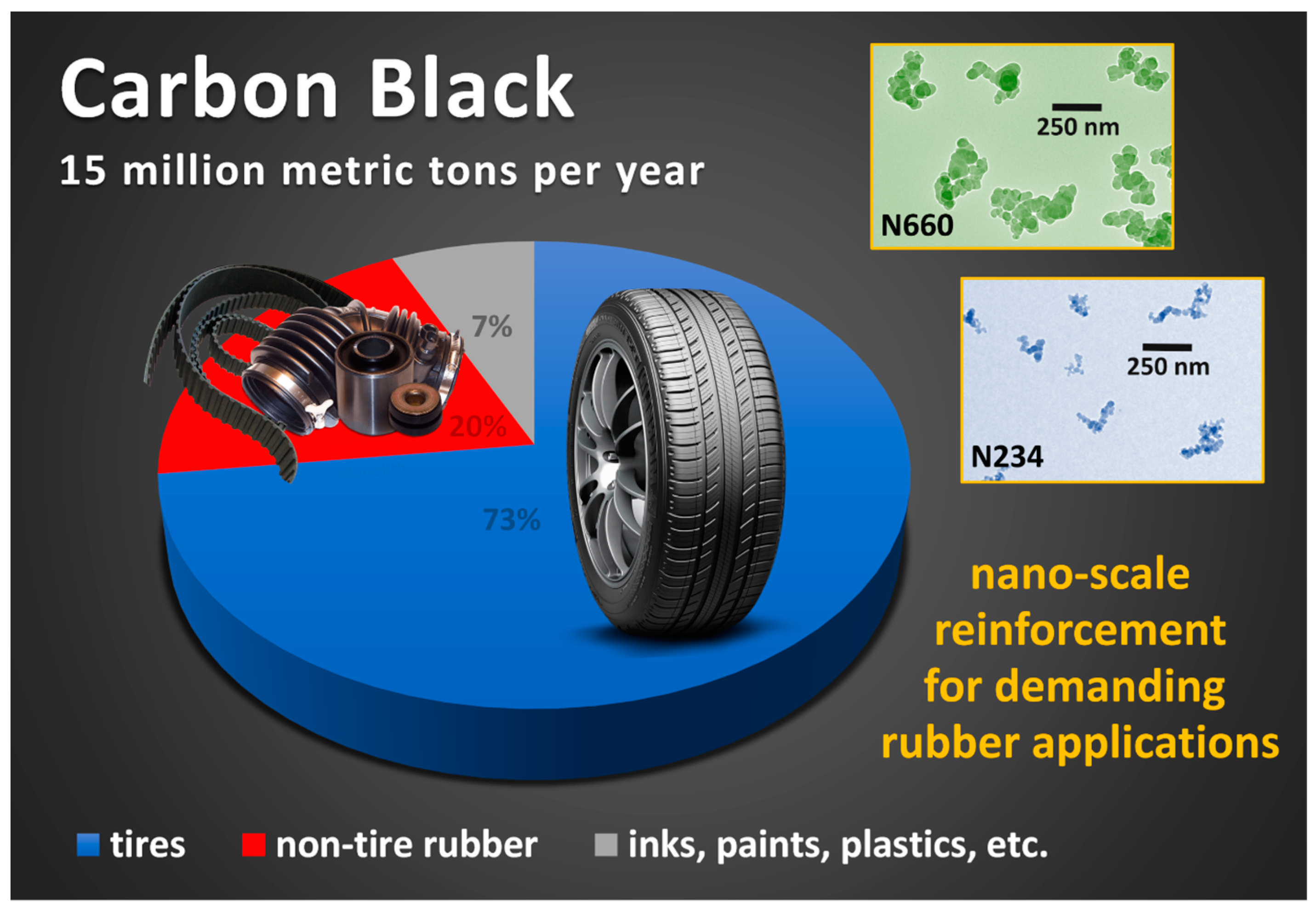
3. Structural and Chemical Characteristics of Carbon Black
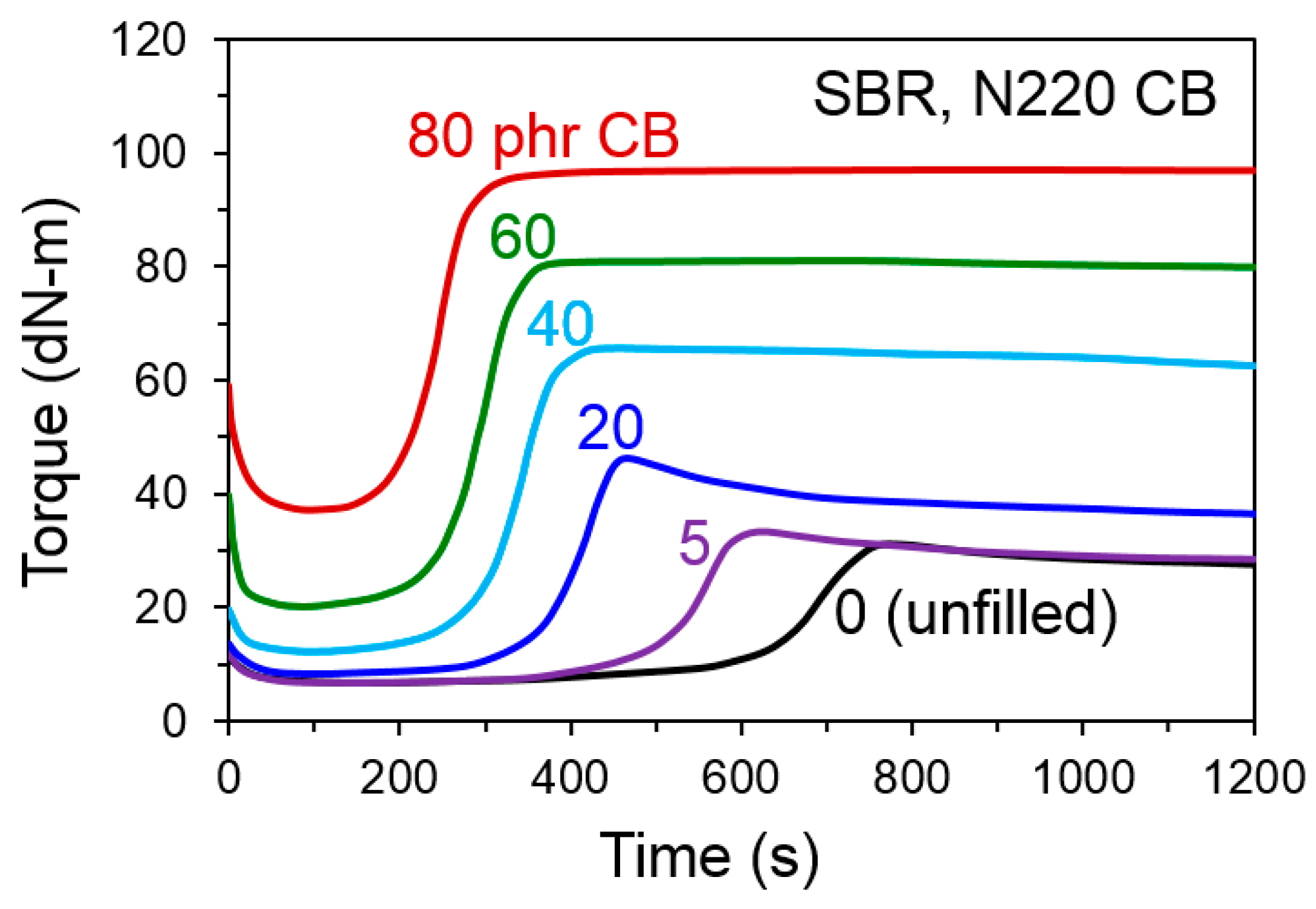
4. Processing Effects on Reinforcement
5. Chemical Modification Effects
6. Influences of Carbon Black on Crosslinking
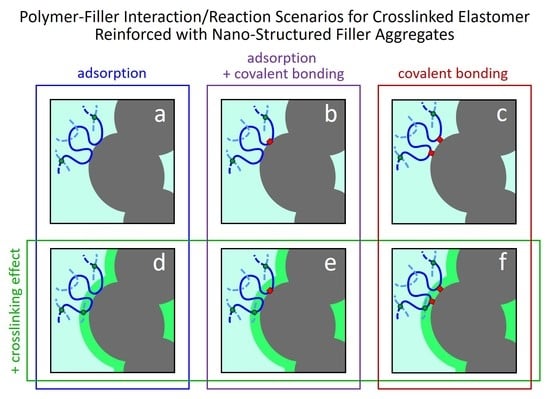
7. Possible Polymer–Filler Interactions
8. Reinforcement Models
9. Final Comments
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Srivastava, S.K.; Mishra, Y.K. Nanocarbon Reinforced Rubber Nanocomposites: Detailed Insights about Mechanical, Dynamical Mechanical Properties, Payne, and Mullin Effects. Nanomaterials 2018, 8, 945. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Kasaliwal, G.R.; Jurk, R.; Boldt, R.; Fischer, D.; Stöckelhuber, K.W.; Heinrich, G. Rubber composites based on graphene nanoplatelets, expanded graphite, carbon nanotubes and their combination: A comparative study. Compos. Sci. Technol. 2012, 72, 1961–1967. [Google Scholar] [CrossRef]
- Galimberti, M.; Infortuna, G.; Guerra, S.; Barbera, V.; Agnelli, S.; Pandini, S. sp2 carbon allotropes in elastomer matrix: From master curves for the mechanical reinforcement to lightweight materials. eXPRESS Polym. Lett. 2018, 12, 265–283. [Google Scholar] [CrossRef]
- Dierkes, W.; Blume, A. Silica Reinforcement. In Encyclopedia of Polymeric Nanomaterials; Kobayashi, S., Müllen, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar] [CrossRef]
- Carbon Black Industry, Strong Potential for Supernormal Profitability? Available online: https://www.televisory.com/blogs/-/blogs/carbon-black-industry-strong-potential-for-supernormal-profitability- (accessed on 8 February 2021).
- Medalia, A.I.; Kraus, G. Reinforcement of Elastomers by Particulate Fillers, Chapter 8. In Science and Technology of Rubber, 2nd ed.; Mark, J.E., Erman, B., Eirich, F.R., Eds.; Academic Press: San Diego, CA, USA, 1994; pp. 387–418. [Google Scholar]
- Brennan, J.J.; Jermyn, T.E.; Boonstra, B.B. Carbon Black-Polymer Interaction: A Measure of Reinforcement. J. Appl. Polym. Sci. 1964, 8, 2687–2706. [Google Scholar] [CrossRef]
- Klüppel, M. The Role of Disorder in Filler Reinforcement of Elastomers on Various Length Scales. Adv. Polym. Sci. 2003, 164, 1–86. [Google Scholar]
- Hosseini, S.M.; Razzaghi-Kashani, M. Catalytic and networking effects of carbon black on the kinetics and conversion of sulfur vulcanization in styrene butadiene rubber. Soft Matter 2018, 14, 9194–9208. [Google Scholar] [CrossRef]
- Pal, P.K.; Bhowmick, A.K.; De, S.K. The Effects of Carbon Black-Vulcanization System Interactions on Natural Rubber Network Structures and Properties. Rubber Chem. Technol. 1982, 55, 23–40. [Google Scholar] [CrossRef]
- Warasitthinon, N.; Robertson, C.G. Interpretation of the tanδ Peak Height for Particle-Filled Rubber and Polymer Nanocomposites with Relevance to Tire Tread Performance Balance. Rubber Chem. Technol. 2018, 91, 577–594. [Google Scholar] [CrossRef]
- Payne, A.R. The dynamic properties of carbon black-loaded natural rubber vulcanizates. Part I. J. Appl. Polym. Sci. 1962, 6, 57–63. [Google Scholar] [CrossRef]
- Payne, A.R. Strainwork dependence of filler-loaded vulcanizates. J. Appl. Polym. Sci. 1964, 8, 2661–2686. [Google Scholar] [CrossRef]
- Fletcher, W.P.; Gent, A.N. Nonlinearity in the Dynamic Properties of Vulcanized Rubber Compounds. Trans. Inst. Rub. Ind. 1953, 29, 266–280. [Google Scholar] [CrossRef]
- Tunnicliffe, L.B.; Busfield, J.J.C. Reinforcement of Rubber and Filler Network Dynamics at Small Strains. In Designing of Elastomer Nanocomposites: From Theory to Applications; Stöckelhuber, K., Das, A., Klüppel, M., Eds.; Springer: Cham, Switzerland, 2016; Volume 275. [Google Scholar] [CrossRef]
- Heinrich, G.; Klüppel, M. Recent advances in the theory of filler networking in elastomers. Adv. Polm. Sci. 2002, 160, 1–44. [Google Scholar]
- Robertson, C.G. Dynamic Mechanical Properties. In Encyclopedia of Polymeric Nanomaterials; Kobayashi, S., Müllen, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 647–654. [Google Scholar]
- Dillon, J.H.; Prettyman, I.B.; Hall, G.L. Hysteretic and Elastic Properties of Rubberlike Materials Under Dynamic Shear Stresses. J. Appl. Phys. 1944, 15, 309–323. [Google Scholar] [CrossRef]
- Smith, S.M.; Simmons, D.S. Horizons for Design of Filled Rubber Informed by Molecular Dynamics Simulation. Rubber Chem. Technol. 2017, 90, 238–263. [Google Scholar] [CrossRef]
- Warasitthinon, N.; Genix, A.-C.; Sztucki, M.; Oberdisse, J.; Robertson, C.G. The Payne effect: Primarily polymer-related or filler-related phenomenon? Rubber Chem. Technol. 2019, 92, 599–611. [Google Scholar] [CrossRef]
- Kraus, G. Mechanical Losses in Carbon-Black-Filled Rubbers. Appl. Polym. Symp. 1984, 39, 75–92. [Google Scholar]
- Hentschke, R. The Payne effect revisited. Express Polym. Lett. 2017, 11, 278–292. [Google Scholar] [CrossRef]
- Mujtaba, A.; Keller, M.; Ilisch, S.; Radusch, H.J.; Beiner, M.; Thurn-Albrecht, T.; Saalwächter, K. Detection of Surface-Immobilized Components and Their Role in Viscoelastic Reinforcement of Rubber–Silica Nanocomposites. ACS Macro Lett. 2014, 3, 481–485. [Google Scholar] [CrossRef]
- Berriot, J.; Lequeux, F.; Monnerie, L.; Montes, H.; Long, D.; Sotta, P. Filler-Elastomer Interaction in Model Filled Rubbers, a H-1 NMR Study. J. Non-Cryst. Solids 2002, 307, 719–724. [Google Scholar] [CrossRef]
- Xiong, W.; Wang, X. Linear-nonlinear dichotomy of rheological responses in particle-filled polymer melts. J. Rheol. 2018, 62, 171–181. [Google Scholar] [CrossRef]
- Hohenberger, T.W.; Windslow, R.J.; Pugno, N.M.; Busfield, J.J.C. A Constitutive Model for Both Low and High Strain Nonlinearities in Highly Filled Elastomers and Implementation with User-Defined Material Subroutines in Abaqus. Rubber Chem. Technol. 2019, 92, 653–686. [Google Scholar] [CrossRef]
- Mark, J.E.; Erman, B. Rubberlike Elasticity: A Molecular Primer, 2nd ed.; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Gent, A.N. Extensibility of rubber under different types of deformation. J. Rheol. 2005, 49, 271–275. [Google Scholar] [CrossRef]
- Smith, T.L.; Dickie, R.A. Effect of finite extensibility on the viscoelastic properties of a styrene–butadiene rubber vulcanizate in simple tensile deformations up to rupture. J. Polym. Sci. Part A-2 Polym. Phys. 1969, 7, 635–658. [Google Scholar] [CrossRef]
- Sainumsai, W.; Toki, S.; Amnuaypornsri, S.; Nimpaiboon, A.; Sakdapipanich, J.; Rong, L.; Hsiao, B.; Suchiva, K. Dependence of the onset of strain-induced crystallization of natural rubber and its synthetic analogue on crosslink and entanglement by using synchrotron X-ray. Rubber Chem. Technol. 2017, 90, 728–742. [Google Scholar] [CrossRef]
- Toki, S.; Che, J.; Rong, L.; Hsiao, B.S.; Amnuaypornsri, S.; Nimpaiboon, A.; Sakdapipanich, J. Entanglements and Networks to Strain-Induced Crystallization and Stress–Strain Relations in Natural Rubber and Synthetic Polyisoprene at Various Temperatures. Macromolecules 2013, 46, 5238–5248. [Google Scholar] [CrossRef]
- Heinrich, G.; Vilgis, T.A. Contribution of entanglements to the mechanical properties of carbon black-filled polymer networks. Macromolecules 1993, 26, 1109–1119. [Google Scholar] [CrossRef]
- Lee, D.J.; Donovan, J.A. Microstructural Changes in the Crack Tip Region of Carbon-Black-Filled Natural Rubber. Rubber Chem. Technol. 1987, 60, 910–923. [Google Scholar] [CrossRef]
- Einstein, A. Eine neue Bestimmung der Moleküldimensionen. Annalen Der Physik 1906, 324, 289–306. [Google Scholar] [CrossRef]
- Guth, E. Theory of Filler Reinforcement. Rubber Chem. Technol. 1945, 18, 596–604. [Google Scholar] [CrossRef]
- Kohls, D.J.; Beaucage, G. Rational design of reinforced rubber. Curr. Opin. Solid State Mater. Sci. 2002, 6, 183–194. [Google Scholar] [CrossRef]
- Fukahori, Y.; Hon, A.A.; Jha, V.; Busfield, J.J.C. Modified Guth-Gold Equation for Carbon Black-Filled Rubbers. Rubber Chem. Technol. 2013, 86, 218–232. [Google Scholar] [CrossRef]
- Smith, R.W. Vacuole Formation and the Mullins Effect in SBR Blends with Polybutadiene. Rubber Chem. Technol. 1967, 40, 350–358. [Google Scholar] [CrossRef]
- Hess, W.H.; Lyon, F.; Burgess, K.A. Einfluss der Adhäsion zwischen Ruß und Kautschuk auf die Eigenschaften der Vulkanisate. Kautsch. Gummi Kunstst. 1967, 20, 135. [Google Scholar]
- Robertson, C.G.; Tunnicliffe, L.B.; Maciag, L.; Bauman, M.A.; Miller, K.; Herd, C.R.; Mars, W.V. Characterizing Distributions of Tensile Strength and Crack Precursor Size to Evaluate Filler Dispersion Effects and Reliability of Rubber. Polymers 2020, 12, 203. [Google Scholar] [CrossRef] [PubMed]
- Watson, J.W. Chemical Aspects of Reinforcement. Rubber Chem. Technol. 1957, 30, 987–999. [Google Scholar] [CrossRef]
- Ghosh, P.; Katare, S.; Patkar, P.; Caruthers, J.; Venkatasubramanian, V.; Walker, K. Sulfur Vulcanization of Natural Rubber for Benzothiazole accelerated formulations: From reaction mechanism to a rational kinetic model. Rubber Chem. Technol. 2003, 76, 592–693. [Google Scholar] [CrossRef]
- Nieuwenhuizen, P.J.; Reedijk, J.; van Duin, M.; Mcgill, W.J. Thiuram- and dithiocarbamate- accelerated sulfur vulcanization from the chemist’s perspective; Methods, materials, and mechanisms reviewed. Rubber Chem. Technol. 1997, 70, 368–429. [Google Scholar] [CrossRef]
- Donnet, J.-B.; Bansal, R.C.; Wang, M.-J. Carbon Black: Science and Technology, 2nd ed.; Marcel Dekker Inc., (CRC Press): New York, NY, USA, 1993. [Google Scholar]
- Magee, R.W. Evaluation of the External Surface Area of Carbon Black by Nitrogen Adsorption. Rubber Chem. Technol. 1995, 68, 590–600. [Google Scholar] [CrossRef]
- Janzen, J.; Kraus, G. Specific Surface Area Measurements on Carbon Black. Rubber Chem. Technol. 1971, 44, 1287–1296. [Google Scholar] [CrossRef]
- Tunnicliffe, L.B. Thixotropic Flocculation Effects in Carbon Black Reinforced Rubber: Kinetics and Thermal Activation. Rubber Chem. Technol. 2021, 94. accepted for publication. [Google Scholar]
- Heidenreich, R.D.; Hess, W.M.; Ban, L.L. A test object and criteria for high resolution electron microscopy. J. Appl. Cryst. 1968, 1, 1–19. [Google Scholar] [CrossRef]
- Boehm, H.P. Some aspects of the surface chemistry of carbon blacks and other carbons. Carbon 1994, 32, 759–769. [Google Scholar] [CrossRef]
- Oickle, A.; Goertzen, S.; Hopper, K.; Abdalla, Y.; Andreas, H. Standardization of the Boehm titration: Part II. Method of agitation effect of filtering dilute titrant. Carbon 2010, 48, 3313–3322. [Google Scholar] [CrossRef]
- Schroeder, A.; Klüppel, M.; Schuster, R.H.; Heidberg, J. Surface energy distribution of carbon black measured by static gas adsorption. Carbon 2002, 40, 207–210. [Google Scholar] [CrossRef]
- Schroeder, A. Charakterisierung Verschiedener Russtypen Durch Systematische Statische Gas Adsorption. Ph.D. Dissertation, Leibniz Universitat, Hannover, Germany, 2000. [Google Scholar]
- Whitesides, R.; Kollias, A.C.; Domin, D.; Lester, W.A., Jr.; Frenklach, M. Graphene layer growth: Collision of migrating five-member rings. Proc. Combust. Inst. 2007, 31, 539–546. [Google Scholar] [CrossRef]
- Stone, A.J.; Wales, D.J. Theoretical studies of icosahedral C60 and some related structures. Chem. Phys. Lett. 1986, 128, 501–503. [Google Scholar] [CrossRef]
- Banhart, F.; Kotakoski, J.; Krasheninnikov, A.V. Structural Defects in Graphene. ACS Nano 2011, 5, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Bekyarova, E.; Niyogi, S.; Haddon, R.C. Diels-Alder Chemistry of Graphite and Graphene: Graphene as Diene and Dienophile. J. Amer. Chem. Soc. 2011, 133, 3324–3327. [Google Scholar] [CrossRef]
- Liu, H.; Ryu, S.; Chen, Z.; Steigerwald, M.L.; Nuckolls, C.; Brus, L.E. Photochemical reactivity of graphene. J. Amer. Chem. Soc. 2009, 131, 17099–17101. [Google Scholar] [CrossRef] [PubMed]
- Parkinson, D. The reinforcement of rubber by carbon black. Br. J. Appl. Phys. 1951, 2, 273. [Google Scholar] [CrossRef]
- Kraus, G.; Collins, R.L. Odd Electrons in Rubber Reinforcing Carbon Blacks. Rubber Chem. Technol. 1959, 32, 107–117. [Google Scholar] [CrossRef]
- Collins, R.L.; Bell, M.D.; Kraus, G. Unpaired electrons in carbon blacks. Rubber Chem. Technol. 1960, 33, 993–1004. [Google Scholar] [CrossRef]
- Bunce, N.J. Introduction to the interpretation of electron spin resonance spectra of organic radicals. J. Chem. Educ. 1987, 64, 907–914. [Google Scholar] [CrossRef]
- Kawakubo, M.; Tsunoda, D.; Yajima, H.; Ishii, T.; Kaidou, H. Reactions of Radicals in Filled Rubber Compounds: I. Detection of free radicals in rubbers by ESR. Rubber Chem. Technol. 2005, 78, 644–658. [Google Scholar] [CrossRef]
- Herd, C.R.; McDonald, G.C.; Smith, R.E.; Hess, W.M. The use of skeletonization for the shape and classification of carbon black aggregates. Rubber Chem. Technol. 1993, 66, 491–509. [Google Scholar] [CrossRef]
- Collins, R.L. Activated Carbon Black. U.S. Patent 3,029,576; (to Phillips Petroleum Company), 17 April 1962. [Google Scholar]
- Dannenberg, E.M. The Effects of Surface Chemical Interactions on the Properties of Filler-Reinforced Rubbers. Rubber Chem. Technol. 1975, 48, 410–444. [Google Scholar] [CrossRef]
- Gessler, A.M. Effect of Mechanical Shear on the Structure of Carbon Black in Reinforced Elastomers. Rubber Chem. Technol. 1970, 43, 943–959. [Google Scholar] [CrossRef]
- Kraus, G. A Carbon Black Structure-Concentration Equivalence Principle. Application to Stress-Strain Relationships of Filled Rubbers. Rubber Chem. Technol. 1971, 44, 199–213. [Google Scholar] [CrossRef]
- Dollinger, R.E.; Kallenberger, R.H.; Studebaker, M.L. Effect of Carbon Black Densification on Structure Measurements. Rubber Chem. Technol. 1967, 40, 1311–1318. [Google Scholar] [CrossRef]
- Herd, C.R.; McDonald, G.C.; Hess, W.M. Morphology of Carbon-Black Aggregates: Fractal Versus Euclidean Geometry. Rubber Chem. Technol. 1992, 65, 107–129. [Google Scholar] [CrossRef]
- McDonald, G.C.; Hess, W.M. Carbon Black Morphology in Rubber. Rubber Chem. Technol. 1977, 50, 842–862. [Google Scholar] [CrossRef]
- Standard Test Method for Carbon Black—Oil Absorption Number of Compressed Sample (COAN); Designation: D3493-19a; ASTM International: West Conshohocken, PA, USA, 2020; pp. 1–4.
- Coulais, C.; Seguin, A.; Dauchot, O. Shear Modulus and Dilatancy Softening in Granular Packings above Jamming. Phys. Rev. Lett. 2014, 113, 198001. [Google Scholar] [CrossRef]
- Majmudar, T.S.; Behringer, R.P. Contact force measurements and stress-induced anisotropy in granular materials. Nature 2005, 435, 1079–1082. [Google Scholar] [CrossRef] [PubMed]
- Hunt, J.F. Mixing of Rubber; Smithers Rapra Technology Limited: Shawbury, UK, 2009. [Google Scholar]
- Bin Hamzah, M. Flow of Rubber in an Internal Mixer. J. Nat. Rubber Res. 1987, 2, 55–65. [Google Scholar]
- Cheng, J.J.; Manas-Zloczower, I. Flow Field Characterization in a Banbury Mixer. Int. Polym. Proc. 1990, 5, 178–183. [Google Scholar] [CrossRef]
- Kiparissides, C.; Vlachopoulos, J. Finite Element Analysis of Calendering. Polym. Eng. Sci. 1976, 16, 712–719. [Google Scholar] [CrossRef]
- Yao, C.-H.; Manas-Zloczower, I. Study of Mixing Efficiency in Roll-Mills. Polym. Eng. Sci. 1996, 36, 305–310. [Google Scholar] [CrossRef]
- Leblanc, J.L. Rubber-filler interactions and rheological properties in filled compounds. Prog. Polym. Sci. 2002, 27, 627–687. [Google Scholar] [CrossRef]
- Watson, W.F. Combination of Rubber and Carbon Black on Cold Milling. Ind. Eng. Chem. 1955, 47, 1281–1286. [Google Scholar] [CrossRef]
- Medalia, A.I. Filler Aggregates and Their Effect on Reinforcement. Rubber Chem. Technol. 1974, 47, 411–433. [Google Scholar] [CrossRef]
- Hess, W.M.; Chirico, V.E.; Burgess, K.A. Carbon Black Morphology in Rubber. Kautsch. Gummi Kunstst. 1973, 26, 344. [Google Scholar]
- Berry, J.P.; Cayré, P.J. The interaction between styrene-butadiene rubber and carbon black on heating. J. Appl. Polym. Sci. 1960, 3, 213–223. [Google Scholar] [CrossRef]
- Dannenberg, E.M.; Collyer, H.J. Channel and Furnace Blacks in Low Temperature GR-S. Ind. Eng. Chem. 1949, 41, 1607–1616. [Google Scholar] [CrossRef]
- Welsh, F.E.; Richmond, B.R.; Keach, C.B.; Emerson, R.J. Paper no. 59. In Proceedings of the Meeting of the Rubber Division, ACS, Philadelphia, PA, USA, 2–5 May 1995. [Google Scholar]
- Wang, M.-J. Effect of Polymer-Filler and Filler-Filler Interactions on Dynamic Properties of Filled Vulcanizates. Rubber Chem. Technol. 1998, 71, 520–589. [Google Scholar] [CrossRef]
- Huneau, B.; Masquelier, I.; Marco, Y.; Le Saux, V.; Noizet, S.; Schiel, C.; Charrier, P. Fatigue Crack Initiation in a Carbon Black-Filled Natural Rubber. Rubber Chem. Technol. 2016, 89, 126–141. [Google Scholar] [CrossRef]
- Hess, W.M.; Swor, R.A.; Micek, E.J. The Influence of Carbon Black, Mixing, and Compounding Variables on Dispersion. Rubber Chem. Technol. 1984, 57, 959–1000. [Google Scholar] [CrossRef]
- Fukushima, Y.; Hergenrother, W.L.; Koch, R.W. Polymer-Filler Coupling Additives. U.S. Patent 8,080,605; (to Bridgestone Corp.), 20 December 2011. [Google Scholar]
- Fukushima, Y.; Koch, R.W.; Hergenrother, W.L.; Araki, S. Polymer-Filler Coupling Additives. U.S. Patent 7,186,845; (to Bridgestone Corp.), 6 March 2007. [Google Scholar]
- Raut, P.; Swanson, N.; Kulkarni, A.; Pugh, C.; Jana, S.C. Exploiting arene-perfluoroarene interactions for dispersion of carbon black in rubber compounds. Polymer 2018, 148, 247–258. [Google Scholar] [CrossRef]
- Han, S.; Kim, W.-S.; Mun, D.-Y.; Ahn, B.; Kim, W. Effect of coupling agents on the vulcanizate structure of carbon black filled natural rubber. Compos. Interfaces 2020, 27, 355–370. [Google Scholar] [CrossRef]
- Klásek, A.; Filipovičová, E.; Špaček, J. Efficiency of some dinitrodiamines and dinitrodiamides in improving dynamic properties of vulcanized rubber. J. Appl. Polym. Sci. 2001, 81, 1439–1443. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Kurimoto, I.; Ohashi, K.; Okita, T. Novel carbon black/rubber coupling agent. Kautsch. Gummi Kunstst. 1989, 42, 403–409. [Google Scholar]
- Shirakawa, Y.; Masuda, T. Coupling Agent for Rubber/Carbon Black, and Rubber Composition Containing Same for Use in Tires. U.S. Patent 9,200,145; (to Shikoku Chemicals Corp.), 1 December 2015. [Google Scholar]
- Wolff, S.; Görl, U. The influence of modified carbon blacks on viscoelastic compound properties. Kautsch. Gummi Kunstst. 1991, 44, 941–947. [Google Scholar]
- Choi, S.-S.; Kim, J.-C.; Ko, J.-E.; Cho, Y.S.; Shin, W.G. Influence of Coupling Agent on Properties of Carbon Black-Reinforced SBR and NR/SBR Vulcanizates. J. Ind. Eng. Chem. 2007, 13, 1017–1022. [Google Scholar]
- Lin, C.J.; Hogan, T.E.; Hergenrother, W.L. On the Filler Flocculation in Silica and Carbon Black Filled Rubbers: Part II. Filler Flocculation and Polymer-Filler Interaction. Rubber Chem. Technol. 2004, 77, 90–114. [Google Scholar] [CrossRef]
- Quirk, R.P. Anionic Polymerization and Chain-End Functionalization Chemistry. Rubber Chem. Technol. 2020, 93, 1–21. [Google Scholar] [CrossRef]
- Thiele, S.; Kiesekamp, J.; Rulhoff, S.; Bellgardt, D. Modified Synthetic Rubber for Silica & Carbon Black Containing Tire Treads. Kautsch. Gummi Kunstst. 2011, 64, 36–41. [Google Scholar]
- Escobar Barrios, V.A.; Garcia-Ramirez, M. Effect of Functionalization on SBR’s Interaction with Carbon Black and Silica. Int. J. Polym. Mater. 2003, 52, 985–998. [Google Scholar] [CrossRef]
- Tsutsumi, F.; Sakakibara, M.; Oshima, N. Structure and Dynamic Properties of Solution SBR Coupled with Tin Compounds. Rubber Chem. Technol. 1990, 63, 8–22. [Google Scholar] [CrossRef]
- Ulmer, J.D.; Hergenrother, W.L.; Lawson, D.F. Hysteresis Contributions in Carbon Black-Filled Rubbers Containing Conventional and Tin End-Modified Polymers. Rubber Chem. Technol. 1998, 71, 637–667. [Google Scholar] [CrossRef]
- Randall, A.M.; Robertson, C.G. Linear-Nonlinear Dichotomy of the Rheological Response of Particle-Filled Polymers. J. Appl. Polym. Sci. 2014, 131, 40818. [Google Scholar] [CrossRef]
- Park, S.J.; Cho, K.S.; Zaborski, M.; Slusarski, L. Filler-elastomer interactions. 10. Ozone treatment on interfacial adhesion of carbon Blacks/NBR compounds. Elastomers Compos. 2003, 38, 139. [Google Scholar]
- Park, S.-J.; Cho, K.-S.; Ryu, S.-K. Filler–elastomer interactions: Influence of oxygen plasma treatment on surface and mechanical properties of carbon black/rubber composites. Carbon 2003, 41, 1437–1442. [Google Scholar] [CrossRef]
- Serizawa, H.; Nakamura, T.; Ito, M.; Tanaka, K.; Nomura, A. Effects of Oxidation of Carbon Black Surface on the Properties of Carbon Black–Natural Rubber Systems. Polymer J. 1983, 15, 201–206. [Google Scholar] [CrossRef][Green Version]
- Roychoudhury, A.; De, P.P. Elastomer–carbon black interaction: Influence of elastomer chemical structure and carbon black surface chemistry on bound rubber formation. J. Appl. Polym. Sci. 1995, 55, 9–15. [Google Scholar] [CrossRef]
- Kang, M.-J.; Heo, Y.-J.; Jin, F.-L.; Park, S.-J. A review: Role of interfacial adhesion between carbon blacks and elastomeric materials. Carbon Lett. 2016, 18, 1–10. [Google Scholar] [CrossRef]
- Donnet, J.B. Nano and microcomposites of polymers elastomers and their reinforcement. Compos. Sci. Technol. 2003, 63, 1085–1088. [Google Scholar] [CrossRef]
- Roy, N.; Sengupta, R.; Bhowmick, A.K. Modifications of carbon for polymer composites and nanocomposites. Prog. Polym. Sci. 2012, 37, 781–819. [Google Scholar] [CrossRef]
- Jovanovic, V.; Budinski-Simendic, J.; Samardzija-Jovanovic, S.; Markovic, G.; Marinovic-Cincovic, M. The influence of carbon black on curing kinetics and thermal aging of acrylonitrile-butadiene rubber. Chem. Ind. Chem. Eng. Q. 2009, 15, 283–289. [Google Scholar] [CrossRef]
- Brennan, J.J.; Lambert, D.H. Rubber—Black Interaction Influence on Cure Level of Vulcanizates. Rubber Chem. Technol. 1972, 45, 94–105. [Google Scholar] [CrossRef]
- Blokh, G.A.; Melamed, C.L. The Interaction of Carbon Black with Sulfur, MBT and TMTD in Vulcanization. Rubber Chem. Technol. 1961, 34, 588–599. [Google Scholar] [CrossRef]
- Gent, A.N.; Hartwell, J.A.; Lee, G. Effect of Carbon Black on Crosslinking. Rubber Chem. Technol. 2003, 76, 517–532. [Google Scholar]
- Valentín, J.L.; Mora-Barrantes, I.; Carretero-González, J.; López-Manchado, M.A.; Sotta, P.; Long, D.R.; Saalwächter, K. Novel Experimental Approach to Evaluate Filler−Elastomer Interactions. Macromolecules 2010, 43, 334–346. [Google Scholar] [CrossRef]
- Cotton, G.R. The Effect of Carbon Black Surface Properties and Structure on Rheometer Cure Behavior. Rubber Chem. Technol. 1972, 45, 129–144. [Google Scholar] [CrossRef]
- Wu, J.; Xing, W.; Huang, G.; Li, H.; Tang, M.; Wu, S.; Liu, Y. Vulcanization kinetics of graphene/natural rubber nanocomposites. Polymer 2013, 54, 3314–3323. [Google Scholar] [CrossRef]
- López-Manchado, M.A.; Biagiotti, J.; Valentini, L.; Kenny, J.M. Dynamic mechanical and Raman spectroscopy studies on interaction between single-walled carbon nanotubes and natural rubber. J. Appl. Polym. Sci. 2004, 92, 3394–3400. [Google Scholar] [CrossRef]
- Rumpf, F.H.; Morris, M.D.; Belmont, J.A. Modified Carbon Blacks Having Low PAH Amounts and Elastomers Containing the Same. U.S. Patent 9,175,150; (to Cabot Corporation), 3 November 2015. [Google Scholar]
- Basterra-Beroiz, B.; Rommel, R.; Kayser, F.; Westermann, S.; Valentín, J.L.; Heinrich, G. New Insights into Rubber Network Structure by a Combination of Experimental Techniques. Rubber Chem. Technol. 2017, 90, 347–366. [Google Scholar] [CrossRef]
- Robertson, C.G.; Stoček, R.; Mars, W.V. The Fatigue Threshold of Rubber and Its Characterization Using the Cutting Method; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar] [CrossRef]
- Wolff, S.; Wang, M.-J.; Tan, E.-H. Filler-Elastomer Interactions. Part VII. Study on Bound Rubber. Rubber Chem. Technol. 1993, 66, 163–177. [Google Scholar] [CrossRef]
- Hamed, G.R.; Hatfield, S. On the Role of Bound Rubber in Carbon-Black Reinforcement. Rubber Chem. Technol. 1989, 62, 143–156. [Google Scholar] [CrossRef]
- Stickney, P.B.; Falb, R.D. Carbon black-rubber interactions and bound rubber. Rubber Chem. Technol. 1964, 37, 1299–1340. [Google Scholar] [CrossRef]
- Dannenberg, E.M. Bound Rubber and Carbon Black Reinforcement. Rubber Chem. Technol. 1986, 59, 512–524. [Google Scholar] [CrossRef]
- Hoshikawa, Y.; An, B.; Kashihara, S.; Ishii, T.; Ando, M.; Fujisawa, S.; Hayakawa, K.; Hamatani, S.; Yamada, H.; Kyotani, T. Analysis of the interaction between rubber polymer and carbon black surfaces by efficient removal of physisorbed polymer from carbon-rubber composites. Carbon 2016, 99, 148–156. [Google Scholar] [CrossRef]
- Sarkawi, S.S.; Dierkes, W.K.; Noordermeer, J.W.M. Morphology of Silica-Reinforced Natural Rubber: The Effect of Silane Coupling Agent. Rubber Chem. Technol. 2015, 88, 359–372. [Google Scholar] [CrossRef]
- Nakajima, K.; Ito, M.; Nguyen, H.K.; Liang, X. Nanomechanics of the Rubber-Filler Interface. Rubber Chem. Technol. 2017, 90, 272–284. [Google Scholar] [CrossRef]
- Mujtaba, A.; Keller, M.; Ilisch, S.; Radusch, H.-J.; Thurn-Albrecht, T.; Saalwächter, K.; Beiner, M. Mechanical Properties and Cross-Link Density of Styrene−Butadiene Model Composites Containing Fillers with Bimodal Particle Size Distribution. Macromolecules 2012, 45, 6504–6515. [Google Scholar] [CrossRef]
- Mullins, L. Softening of Rubber by Deformation. Rubber Chem. Technol. 1969, 42, 339–362. [Google Scholar] [CrossRef]
- Diani, J.; Fayolle, B.; Gilormini, P. A review on the Mullins effect. Eur. Polym. J. 2009, 45, 601–612. [Google Scholar] [CrossRef]
- Blanchard, A.F.; Parkinson, D. Breakage of carbon-rubber networks by applied stress. Ind. Eng. Chem. 1952, 44, 799–812. [Google Scholar] [CrossRef]
- Bueche, F. Molecular basis for the Mullins effect. J. Appl. Polym. Sci. 1960, 4, 107–114. [Google Scholar] [CrossRef]
- Dannenberg, E.M. Molecular slippage mechanism of reinforcement. Trans. Inst. Rub. Ind. 1966, 42, 26–42. [Google Scholar]
- Rigbi, Z. Reinforcement of rubber by carbon black. Adv. Polym. Sci. 1980, 36, 21–68. [Google Scholar]
- Rigbi, Z. Reinforcement of Rubber by Carbon Black. Rubber Chem. Technol. 1982, 55, 1180–1220. [Google Scholar] [CrossRef]
- Eyring, H.; Hirschfelder, J. The Theory of the Liquid State. J. Phys. Chem. 1937, 41, 249–257. [Google Scholar] [CrossRef]
- Fukahori, Y. Generalized Concept of the Reinforcement of Elastomers. Part 1: Carbon Black Reinforcement of Rubbers. Rubber Chem. Technol. 2007, 80, 701–725. [Google Scholar] [CrossRef]
- Robertson, C.G.; Lin, C.J.; Rackaitis, M.; Roland, C.M. Influence of particle size and polymer-filler coupling on viscoelastic glass transition of particle-reinforced polymers. Macromolecules 2008, 41, 2727–2731. [Google Scholar] [CrossRef]
- Witten, T.A.; Rubinstein, M.; Colby, R.H. Reinforcement of rubber by fractal aggregates. J. Phys. II 1993, 3, 367–383. [Google Scholar] [CrossRef]
- Smith, S.M.; Simmons, D.S. Poisson ratio mismatch drives low-strain reinforcement in elastomeric nanocomposites. Soft Matter 2019, 15, 656–670. [Google Scholar] [CrossRef]
- Lorenz, H.; Klüppel, M. Microstructure-based modelling of arbitrary deformation histories of filler-reinforced elastomers. J. Mech. Phys. Solids 2012, 60, 1842–1861. [Google Scholar] [CrossRef]
- Klüppel, M. Microstructure based modelling of elastomer nano-composites. In Constitutive Models for Rubber XI; Huneau, B., Le Cam, J.-B., Marco, Y., Verron, E., Eds.; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: London, UK, 2019; pp. 98–103. [Google Scholar]
- Plagge, J.; Klüppel, M. A physically based model of stress softening and hysteresis of filled rubber including rate-and temperature dependency. Int. J. Plasticity 2017, 89, 173–196. [Google Scholar] [CrossRef]
- Plagge, J.; Ricker, A.; Kröger, N.H.; Wriggers, P.; Klüppel, M. Efficient modeling of filled rubber assuming stress-induced microscopic restructurization. Int. J. Eng. Sci. 2020, 151, 103291. [Google Scholar] [CrossRef]






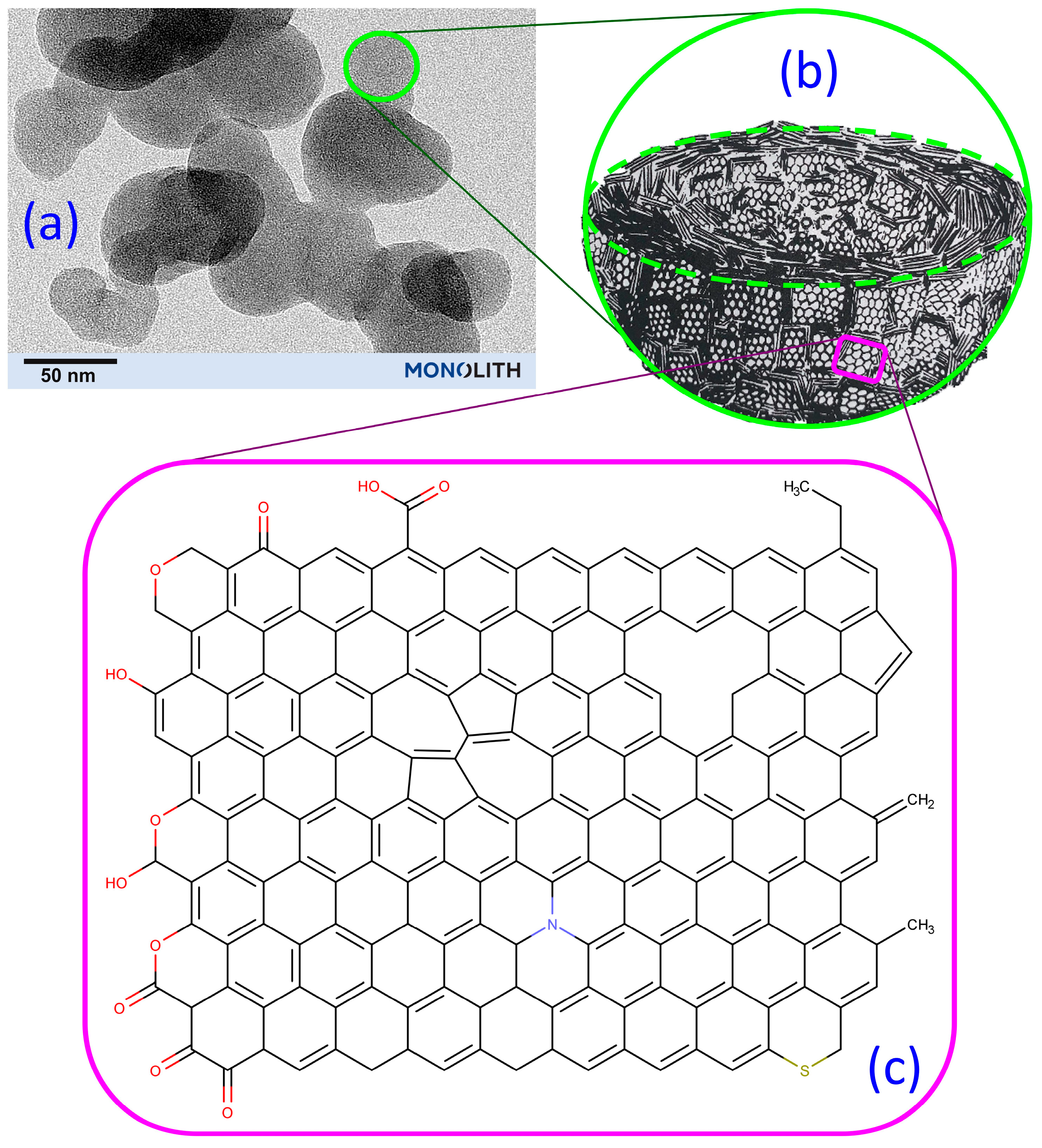


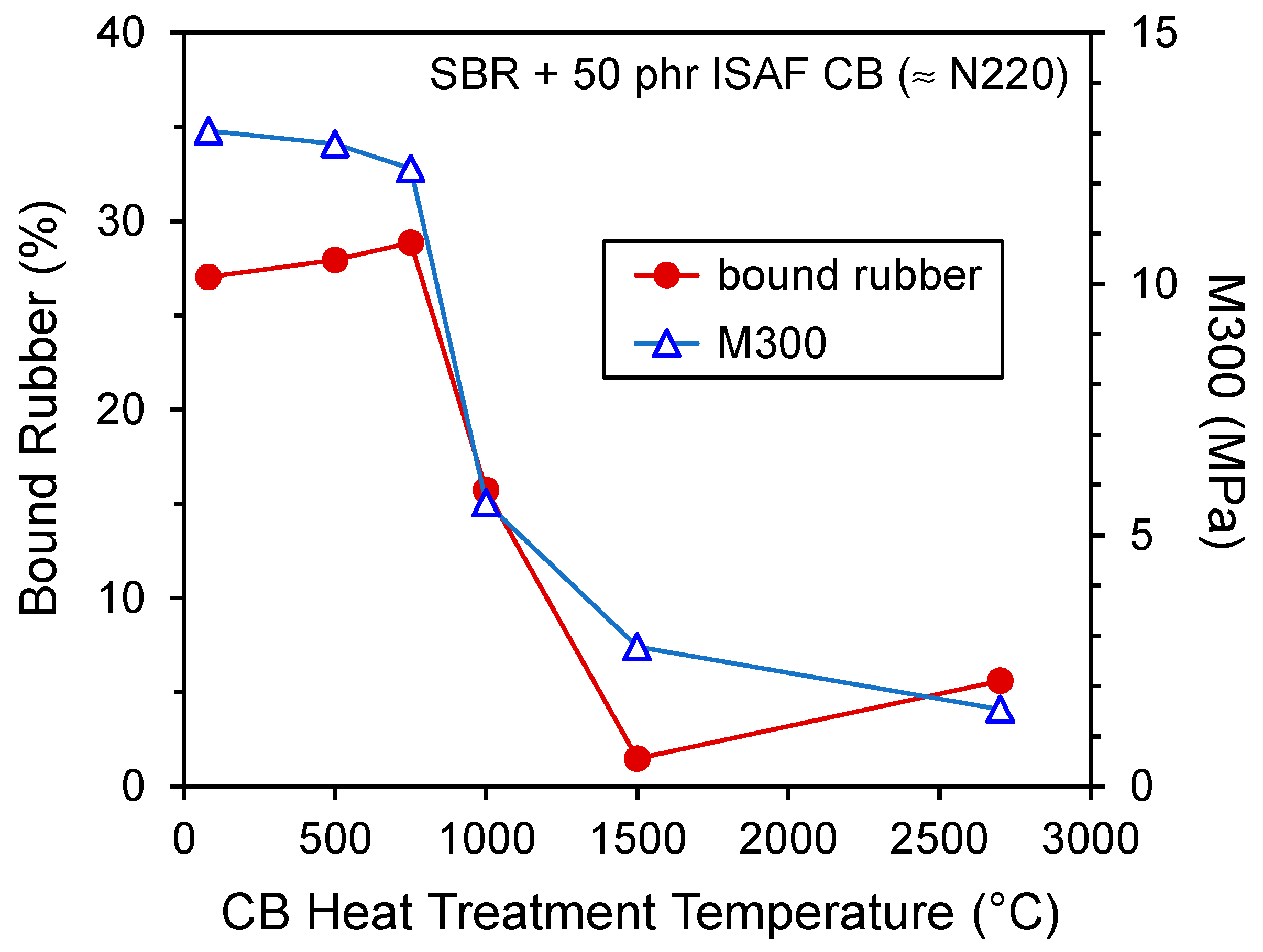

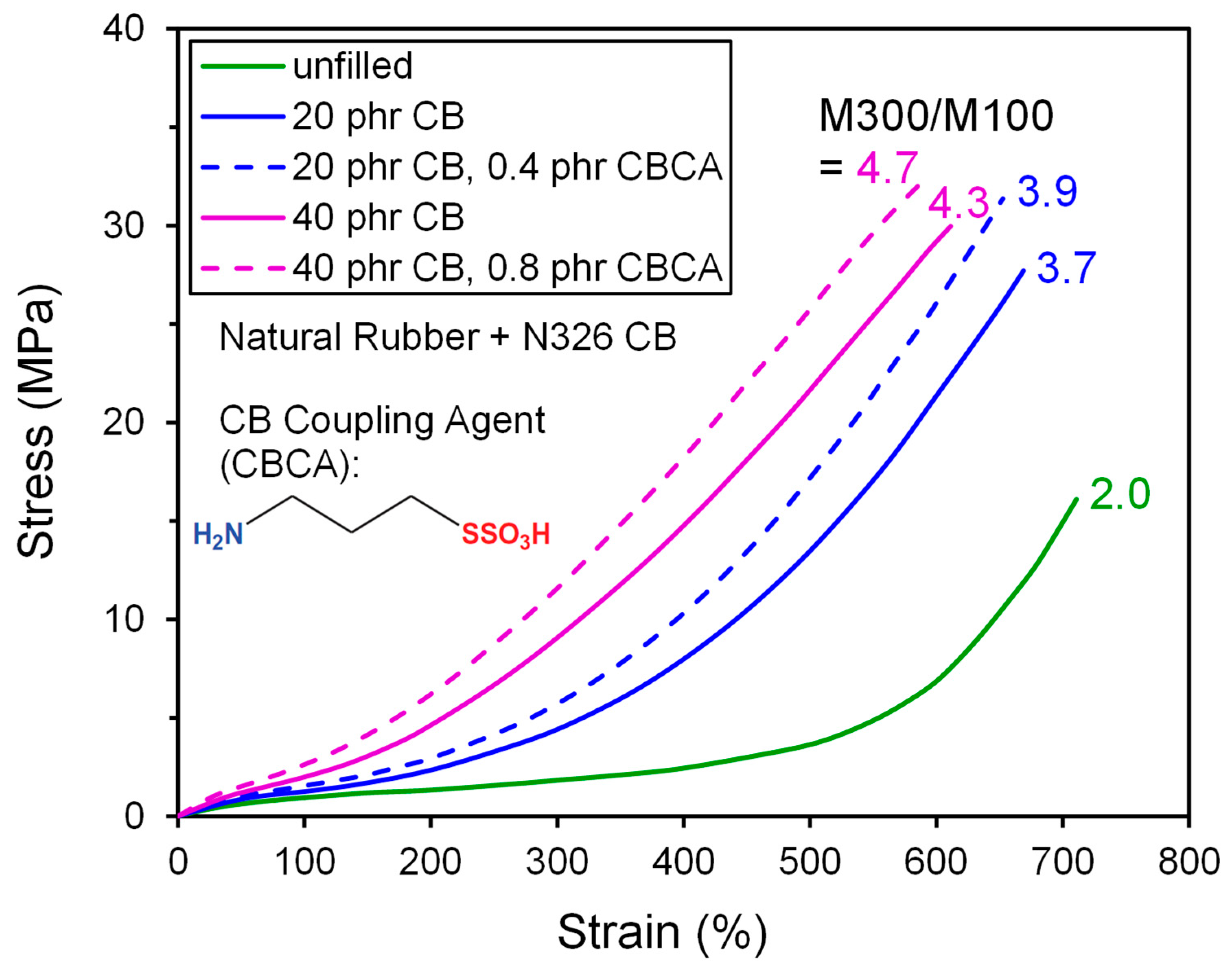

| High Structure ISAF CB (≈N234) | ISAF CB (≈N220) | |||
|---|---|---|---|---|
| Property | Original | Heat Treated (a) | Original | Heat Treated (a) |
| CB; Nitrogen Surface Area (m2/g) | 116 | 86 | 108 | 88 |
| CB; Oil Absorption (cm3/g) | 1.72 | 1.78 | 1.33 | 1.54 |
| Bound Rubber (%) | 34.4 | 5.6 | 30.6 | 5.8 |
| Mooney Viscosity at 100 °C | 83 | 87 | 73 | 76 |
| Scorch at 135 °C (min.) | 10.5 | 17 | 18 | 20 |
| Filler Macrodispersion (%) | 99 | 99 | 99 | 98.2 |
| Shore A Hardness | 73 | 68 | 68 | 65 |
| Stress at 300% Strain, M300 (MPa) | 14.5 | 3.5 | 10.3 | 2.9 |
| Tensile Strength (MPa) | 26.2 | 23.4 | 27.6 | 22.8 |
| Strain (Elongation) at Break (%) | 450 | 730 | 630 | 750 |
| Abrasion Loss (cm3/106 rev.) | 62 | 181 | 67 | 142 |
| Hysteresis | 0.204 | 0.297 | 0.238 | 0.315 |
| CB Sample | NSA (m2/g) | STSA (m2/g) | O (%) | N (%) | H (%) | S (%) | C (%) | Lc (nm) | d002 (nm) |
|---|---|---|---|---|---|---|---|---|---|
| N234, untreated | 126.4 | 120.3 | 2.21 | 0.145 | 0.337 | 0.924 | 93.7 | 1.19 | 0.365 |
| N234, 900 °C | 134.7 | 124.7 | 1.28 | 0.158 | 0.250 | 0.932 | 95.9 | 1.15 | 0.361 |
| N234, 1000 °C | 129.6 | 129.6 | 0.204 | 0.064 | 0.130 | 0.916 | 96.7 | 1.40 | 0.361 |
| N234, 1200 °C | 129.0 | 132.8 | 0.128 | 0.041 | 0.021 | 0.790 | 98.7 | 1.44 | 0.355 |
| N660, untreated | 36.4 | 35.2 | 0.576 | 0.082 | 0.339 | 1.84 | 95.9 | 1.78 | 0.352 |
| N660, 1000 °C | 36.4 | 37.3 | 0.110 | 0.056 | 0.141 | 1.78 | 96.8 | 1.59 | 0.355 |
| Carbon Black Grade | NSA (m2/g) | STSA (m2/g) | OAN (ml/100 g) | COAN (ml/100 g) | Mean Aggregate Diameter (nm) (a) |
|---|---|---|---|---|---|
| N115 | 131 | 116 | 112 | 93 | 64 |
| N134 | 140 | 129 | 125 | 104 | 63 |
| N220 | 110 | 103 | 113 | 99 | 78 |
| N234 | 116 | 110 | 126 | 104 | 67 |
| N339 | 91 | 88 | 121 | 99 | 76 |
| N330 | 76 | 76 | 102 | 89 | 84 |
| N326 | 77 | 77 | 73 | 73 | 82 |
| N550 | 38 | 38 | 121 | 83 | 179 |
| N660 | 35 | 34 | 93 | 75 | 168 |
| N772 | 32 | 31 | 69 | 62 | 169 |
| CB Sample | M100 (MPa) | M300 (MPa) | M300/M100 | Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|---|---|---|
| N234, untreated | 2.68 | 15.33 | 5.72 | 17.9 | 361 |
| N234, 900 °C | 2.77 | 15.33 | 5.54 | 19.1 | 375 |
| N234, 1000 °C | 2.11 | 11.11 | 5.27 | 22.8 | 493 |
| N234, 1200 °C | 1.78 | 7.78 | 4.36 | 21.4 | 560 |
| N660, untreated | 2.59 | 13.33 | 5.15 | 18.9 | 429 |
| N660, 1000 °C | 1.96 | 7.99 | 4.08 | 15.2 | 516 |
| Compression in the COAN Test | Rubber in Internal Mixer [74,75,76] | Rubber in Two-Roll Mill [77,78] | ||
|---|---|---|---|---|
| P (MPa) | P (MPa) | τ (MPa) | P (MPa) | τ (MPa) |
| 165 | 0.2–0.7 | 0.3–7 | 0.2–3 | 0.2–1 |
| SBR Non-Functionalized | SBR Functionalized with R3SnCl * | |
|---|---|---|
| M100 (MPa) | 4.03 | 4.01 |
| M300 (MPa) | 15.26 | 17.02 |
| M300/M100 | 3.79 | 4.24 |
| Tensile Strength (MPa) | 17.83 | 17.73 |
| Elongation at Break (%) | 378 | 346 |
| SBR/BR Compound (ϕ = 0.22) | NR Compound (ϕ = 0.19) | |||
|---|---|---|---|---|
| Carbon Black | tanδ at 60 °C | M300/M100 | tanδ at 60 °C | M300/M100 |
| CB1; Control, STSA = 75 m2/g, OAN = 102 | 0.257 | 4.12 | 0.169 | 5.31 |
| CB2; CB1 annealed for 2 h at 1400 °C | 0.329 | 2.20 | 0.192 | 3.16 |
| CB3; CB2 surface funct. with disulfide | 0.255 | 3.00 | 0.135 | 4.29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robertson, C.G.; Hardman, N.J. Nature of Carbon Black Reinforcement of Rubber: Perspective on the Original Polymer Nanocomposite. Polymers 2021, 13, 538. https://doi.org/10.3390/polym13040538
Robertson CG, Hardman NJ. Nature of Carbon Black Reinforcement of Rubber: Perspective on the Original Polymer Nanocomposite. Polymers. 2021; 13(4):538. https://doi.org/10.3390/polym13040538
Chicago/Turabian StyleRobertson, Christopher G., and Ned J. Hardman. 2021. "Nature of Carbon Black Reinforcement of Rubber: Perspective on the Original Polymer Nanocomposite" Polymers 13, no. 4: 538. https://doi.org/10.3390/polym13040538
APA StyleRobertson, C. G., & Hardman, N. J. (2021). Nature of Carbon Black Reinforcement of Rubber: Perspective on the Original Polymer Nanocomposite. Polymers, 13(4), 538. https://doi.org/10.3390/polym13040538