Evaluating Antibacterial Efficacy and Biocompatibility of PAN Nanofibers Loaded with Diclofenac Sodium Salt
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PAN/Diclofenac Electrospinning Solution
2.3. Electrospinning Process
2.4. Surface Morphology Characterizations
2.5. Physicochemical Characterizations
2.6. XRD Characterizations
2.7. The In-Vitro Release Behavior of DLF from the PAN Nanofibrous Mats
2.8. Antibacterial Efficacy
2.9. Cell Viability and Adhesion
2.10. Statistical Analysis
3. Results and Discussion
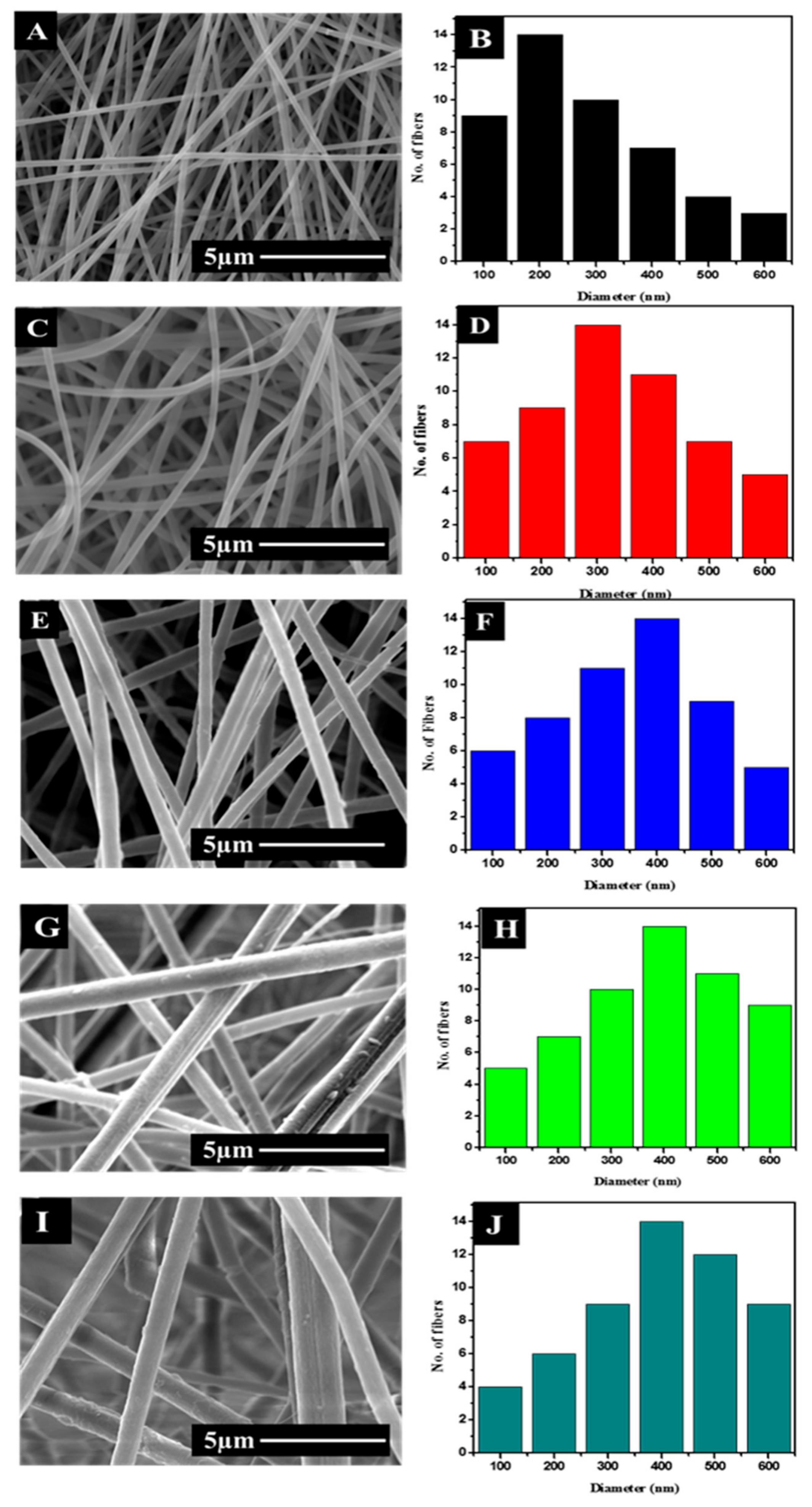
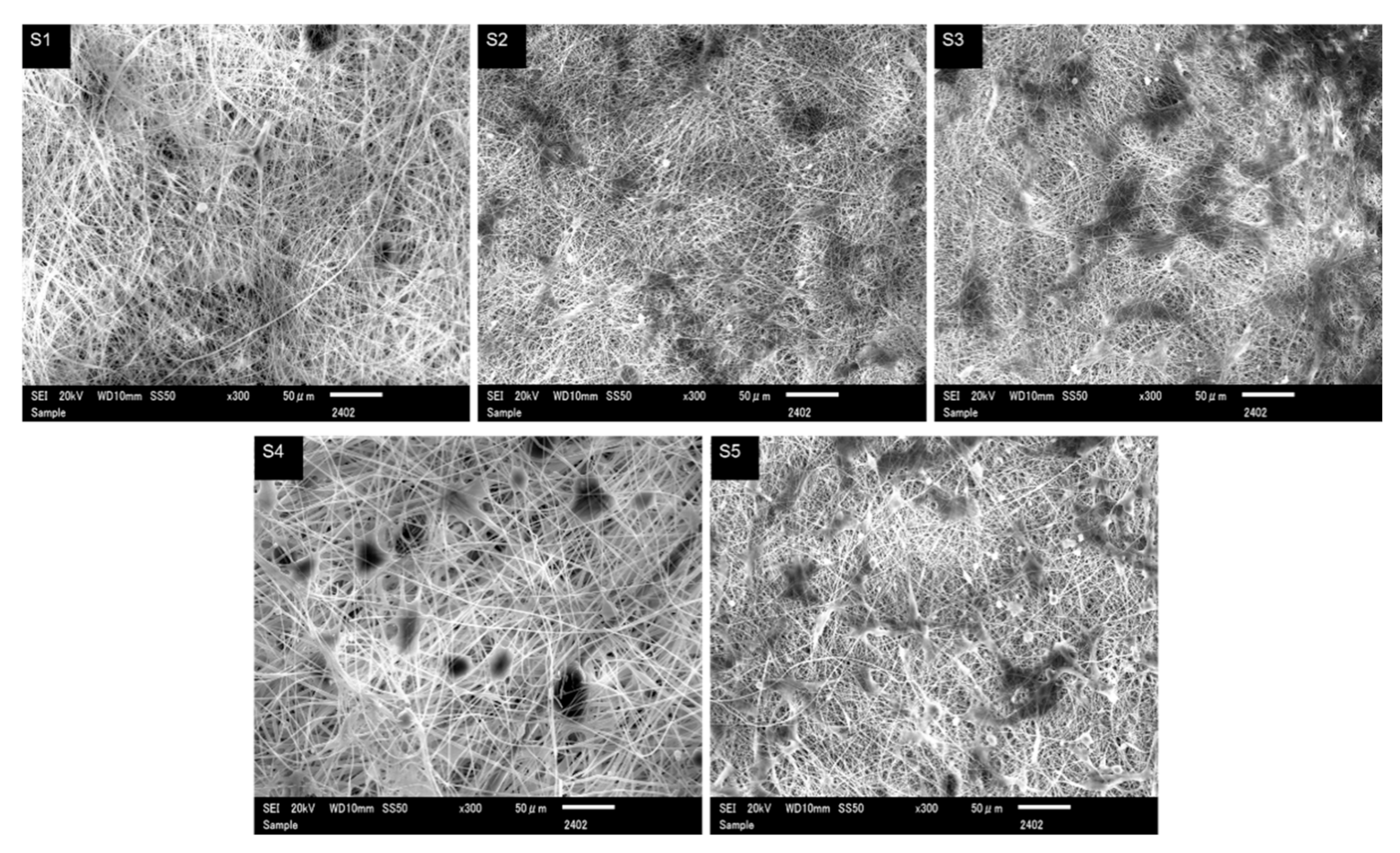
3.1. Morphology and Diameter Analysis
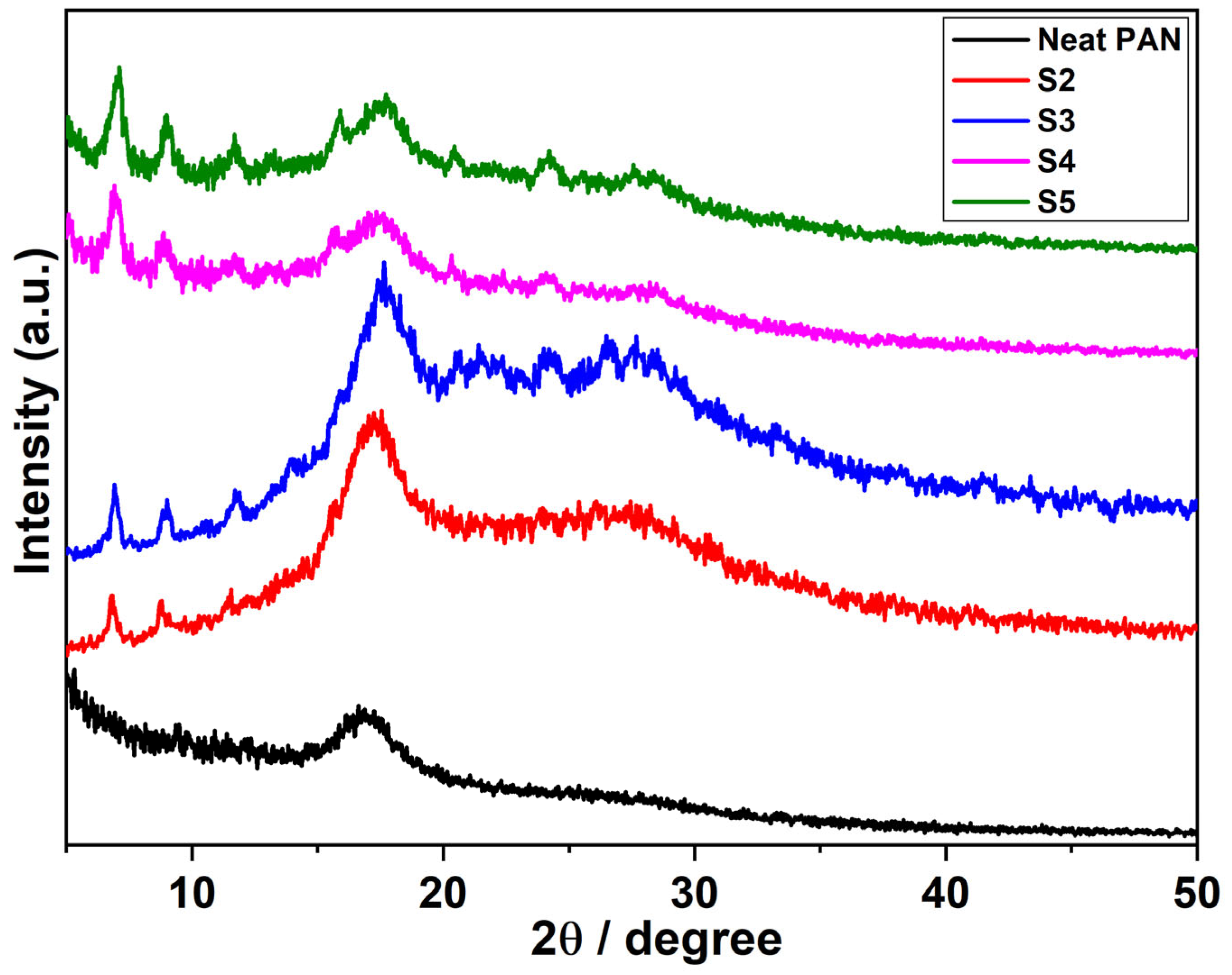
3.2. Study of XRD Spectra
3.3. Physicochemical Analysis
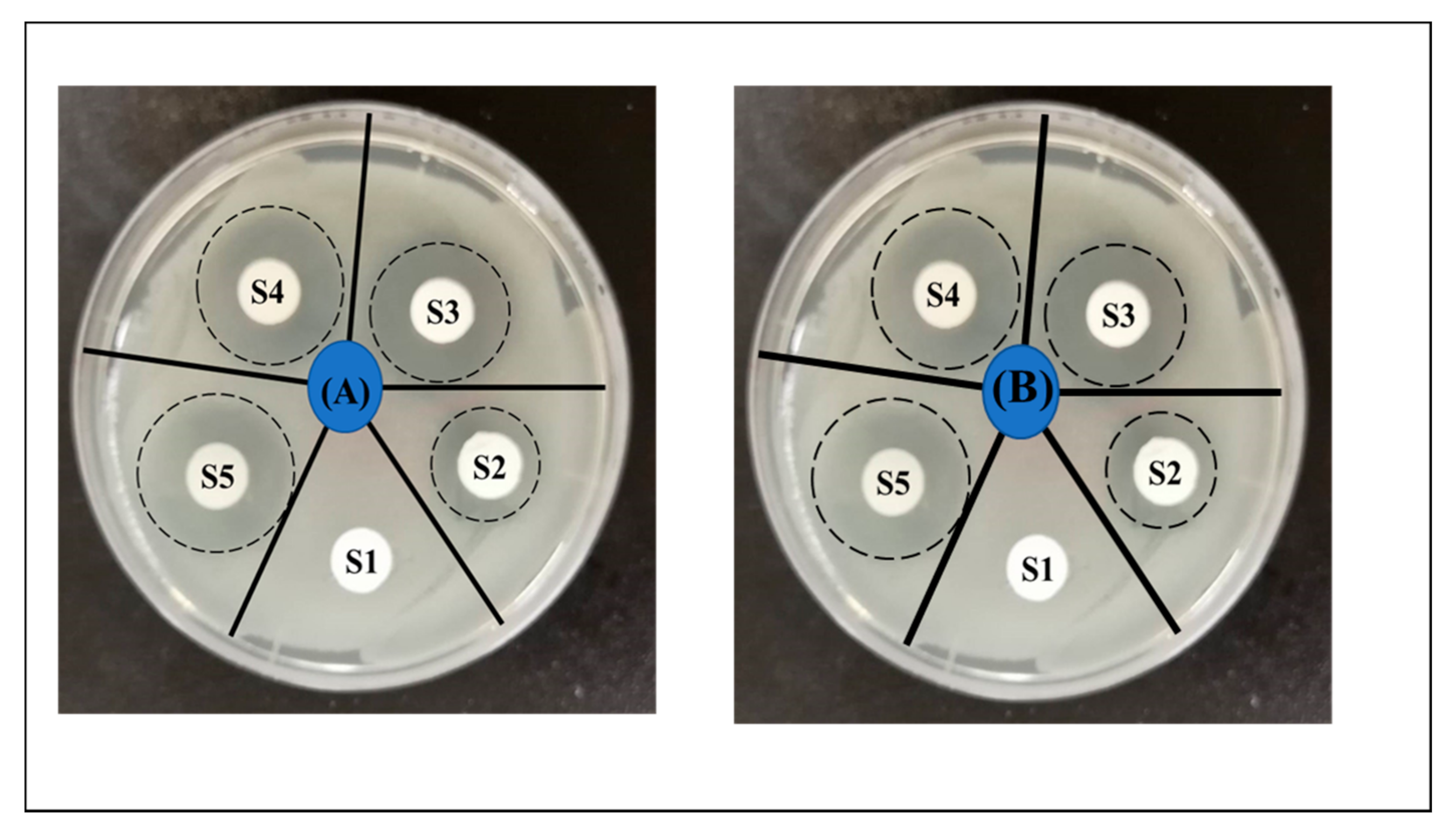
3.4. Antibacterial Activity
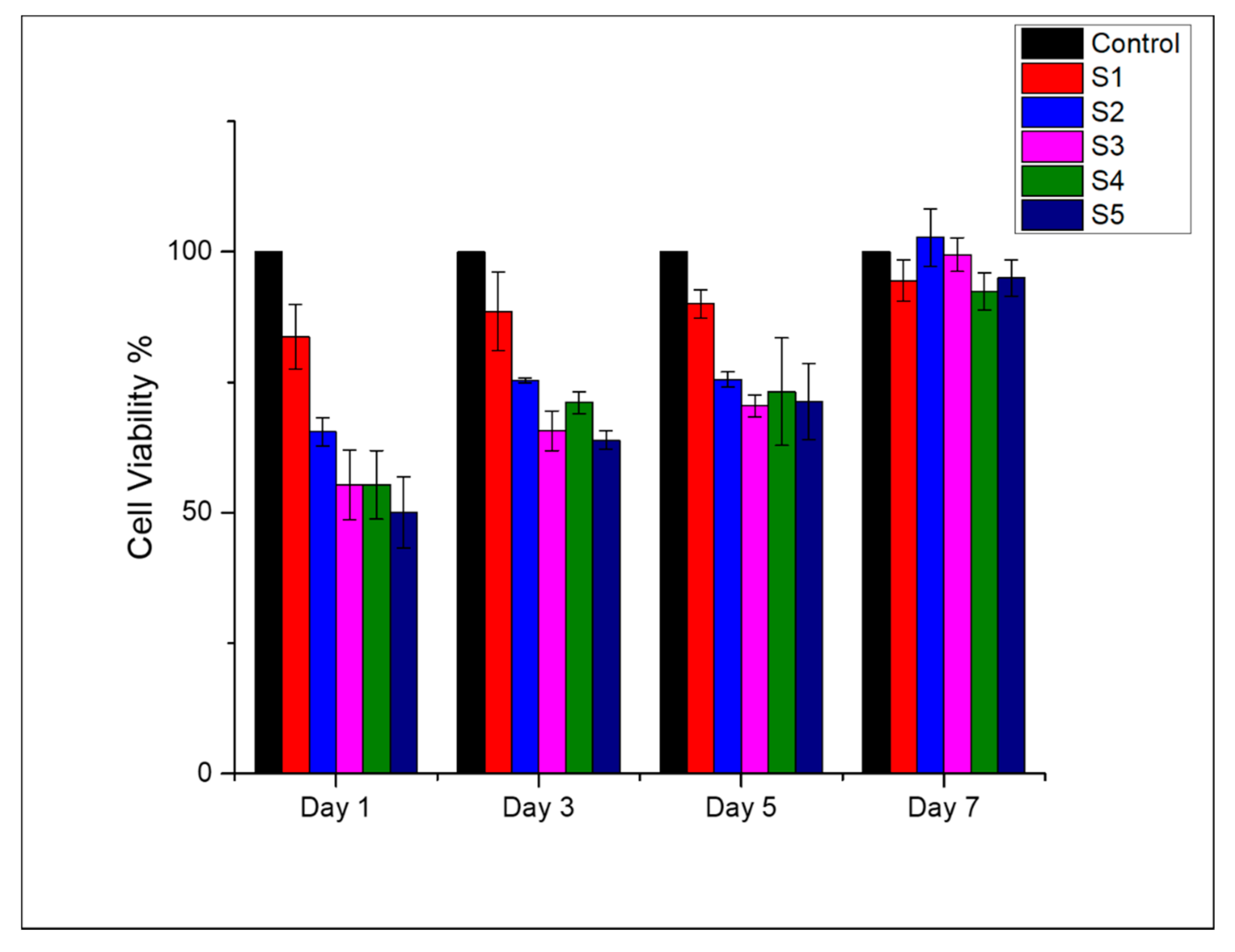
3.5. Cell Viability and Cell Adhesion
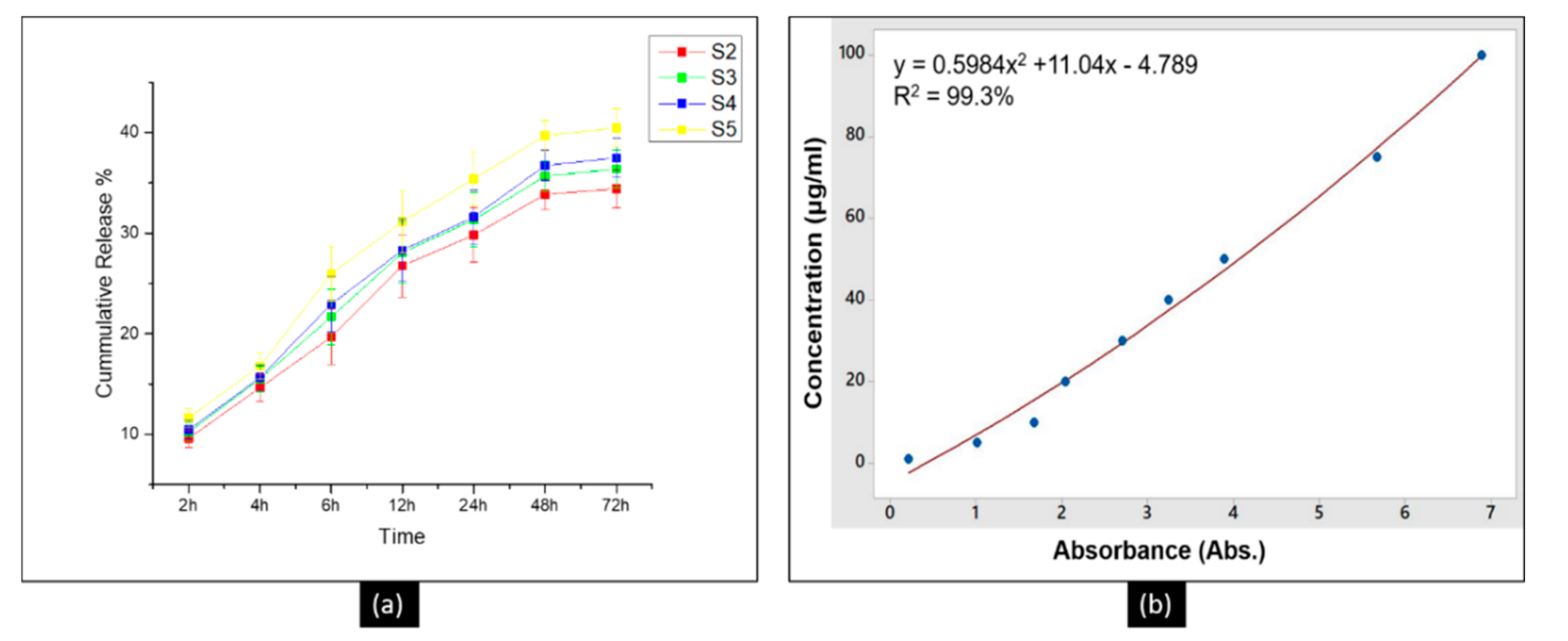
3.6. The Release Profile of DLF in PTM
3.7. Practical Implications and Future Perspective
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hussain, T.; Masood, R.; Umar, M.; Areeb, T.; Ullah, A. Development and characterization of alginate-chitosan-hyaluronic acid (ACH) composite fibers for medical applications. Fibers Polym. 2016, 17, 1749–1756. [Google Scholar] [CrossRef]
- Masood, R.; Hussain, T.; Umar, M.; Ullah, A.; Areeb, T.; Riaz, S. In situ development and application of natural coatings on non absorbable sutures to reduce incision site infections. J. Wound Care 2017, 26, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Masood, R.; Hussain, T.; Miraftab, M.; Ullah, A.; Raza, Z.A.; Areeb, T.; Umar, M. Novel alginate, chitosan, and psyllium composite fiber for wound-care applications. J. Ind. Text. 2017, 47, 20–37. [Google Scholar] [CrossRef]
- Masood, R.; Hussain, T.; Miraftab, M.; Ali Raza, Z.; Ullah, A.; Areeb, T.; Umar, M.; Riaz, R. Development of tri-component antibacterial hybrid fibres for potential use in wound care. J. Wound Care 2018, 27, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Ullah, S.; Areeb, T.; Umar, M.; Nam, P.D.; Masood, R.; Park, S.; Kim, I.S. An Experimental Study on Modelling the Physical Properties of Composite Psyllium, Alginate and Chitosan Fibers Using Box-Behnken Technique. Fibers Polym. 2020, 21, 2494–2504. [Google Scholar] [CrossRef]
- Maurer, N.; Fenske, D.B.; Cullis, P.R. Developments in liposomal drug delivery systems. Expert Opin. Biol. Ther. 2001, 1, 923–947. [Google Scholar] [CrossRef] [PubMed]
- Woranuch, S.; Yoksan, R. Eugenol-loaded chitosan nanoparticles: I. Thermal stability improvement of eugenol through encapsulation. Carbohydr. Polym. 2013, 96, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.A.; Park, H.J.; Hwang, S.J.; Park, J.B.; Lee, J.S. Preparation and characterization of chitosan microparticles intended for controlled drug delivery. Int. J. Pharm. 2002, 249, 165–174. [Google Scholar] [CrossRef]
- Goonoo, N.; Bhaw-Luximon, A.; Jhurry, D. Drug loading and release from electrospun biodegradable nanofibers. J. Biomed. Nanotechnol. 2014, 10, 2173–2199. [Google Scholar] [CrossRef]
- Phan, D.N.; Khan, M.Q.; Nguyen, N.T.; Phan, T.T.; Ullah, A.; Khatri, M.; Kien, N.N.; Kim, I.S. A review on the fabrication of several carbohydrate polymers into nanofibrous structures using electrospinning for removal of metal ions and dyes. Carbohydr. Polym. 2021, 252, 117175. [Google Scholar] [CrossRef]
- Umar, M.; Ullah, A.; Nawaz, H.; Areeb, T.; Hashmi, M.; Kharaghani, D.; Oh, K.; Soo, I. Wet-spun bi-component alginate based hydrogel fi bers: Development and in-vitro evaluation as a potential moist wound care dressing. Int. J. Biol. Macromol. 2021, 168, 601–610. [Google Scholar] [CrossRef]
- Greiner, A.; Wendorff, J.H. Electrospinning: A fascinating method for the preparation of ultrathin fibers. Angew. Chem. Int. Ed. 2007, 46, 5670–5703. [Google Scholar] [CrossRef]
- Ahmadi Majd, S.; Rabbani Khorasgani, M.; Moshtaghian, S.J.; Talebi, A.; Khezri, M. Application of Chitosan/PVA Nano fiber as a potential wound dressing for streptozotocin-induced diabetic rats. Int. J. Biol. Macromol. 2016, 92, 1162–1168. [Google Scholar] [CrossRef]
- Abdelgawad, A.M.; Hudson, S.M.; Rojas, O.J. Antimicrobial wound dressing nanofiber mats from multicomponent (chitosan/silver-NPs/polyvinyl alcohol) systems. Carbohydr. Polym. 2014, 100, 166–178. [Google Scholar] [CrossRef]
- Haider, K.; Ullah, A.; Sarwar, M.N.; Saito, Y.; Sun, L.; Park, S.; Kim, I.S. Lignin-mediated in-situ synthesis of CuO nanoparticles on cellulose nanofibers: A potential wound dressing material. Int. J. Biol. Macromol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Phan, D.N.; Dorjjugder, N.; Saito, Y.; Khan, M.Q.; Ullah, A.; Bie, X.; Taguchi, G.; Kim, I.S. Antibacterial mechanisms of various copper species incorporated in polymeric nanofibers against bacteria. Mater. Today Commun. 2020, 25, 101377. [Google Scholar] [CrossRef]
- Phan, D.N.; Dorjjugder, N.; Saito, Y.; Taguchi, G.; Ullah, A.; Kharaghani, D.; Kim, I.S. The synthesis of silver-nanoparticle-anchored electrospun polyacrylonitrile nanofibers and a comparison with as-spun silver/polyacrylonitrile nanocomposite membranes upon antibacterial activity. Polym. Bull. 2019. [Google Scholar] [CrossRef]
- Kim, M.O.; Khan, M.Q.; Ullah, A.; Duy, N.P.; Zhu, C.; Lee, J.S.; Kim, I.S. Development of VOCs gas sensor with high sensitivity using colorimetric polymer nanofiber: A unique sensing method. Mater. Res. Express 2019, 6. [Google Scholar] [CrossRef]
- Nawaz, H.; Umar, M.; Ullah, A.; Razzaq, H.; Zia, K.M.; Liu, X. Polyvinylidene fluoride nanocomposite super hydrophilic membrane integrated with Polyaniline-Graphene oxide nano fillers for treatment of textile effluents. J. Hazard. Mater. 2021, 403, 123587. [Google Scholar] [CrossRef] [PubMed]
- Ulbrich, K.; Holá, K.; Šubr, V.; Bakandritsos, A.; Tuček, J.; Zbořil, R. Targeted Drug Delivery with Polymers and Magnetic Nanoparticles: Covalent and Noncovalent Approaches, Release Control, and Clinical Studies. Chem. Rev. 2016, 116, 5338–5431. [Google Scholar] [CrossRef] [PubMed]
- Goyal, R.; Macri, L.K.; Kaplan, H.M.; Kohn, J. Nanoparticles and nanofibers for topical drug delivery. J. Control. Release 2016, 240, 77–92. [Google Scholar] [CrossRef]
- Pchelova, N.V.; Budkute, I.A.; Shcherbina, L.A.; Ustinov, K.Y. Polyacrylonitrile Fiber Modified by a Quaternary Ammonium Salt. Fibre Chem. 2014, 46, 97–100. [Google Scholar] [CrossRef]
- Wu, Y.; Gao, R.; Gao, S.; Li, M. Poly(vinylidene fluoride)–polyacrylonitrile blend flat-sheet membranes reinforced with carbon nanotubes for wastewater treatment. J. Appl. Polym. Sci. 2018, 135. [Google Scholar] [CrossRef]
- Yu, D.-G.; Branford-White, C.; Li, L.; Wu, X.-M.; Zhu, L.-M. The compatibility of acyclovir with polyacrylonitrile in the electrospun drug-loaded nanofibers. J. Appl. Polym. Sci. 2010. [Google Scholar] [CrossRef]
- Kedem, S.; Schmidt, J.; Paz, Y.; Cohen, Y. Composite polymer nanofibers with carbon nanotubes and titanium dioxide particles. Langmuir 2005, 21, 5600–5604. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Li, Y.; Yang, S.; Du, J.; Wang, S.; Zhang, C.; Yang, Q.; Chen, X. Synthesis of AgCl/PAN composite nanofibres using an electrospinning method. Nanotechnology 2007, 18. [Google Scholar] [CrossRef]
- Pawar, H.V.; Tetteh, J.; Boateng, J.S. Preparation, optimisation and characterisation of novel wound healing film dressings loaded with streptomycin and diclofenac. Colloids Surf. B Biointerfaces 2013, 102, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, G.S.; Diane, M.; Knighton, D.R.; David, J.; Rodeheaver, G.; Robson, M.C. Definitions and Guidelines fro Assesment of Wounds ad Evaluation of Healing. Arch Dermatol. 1994, 130, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Yu, D.; Zhu, L.; Branford-White, C.; White, K.; Chatterton, N.P. Electrospun diclofenac sodium loaded Eudragit® L 100-55 nanofibers for colon-targeted drug delivery. Int. J. Pharm. 2011, 408, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Huh, B.K.; Kim, B.H.; Kim, S.N.; Park, C.G.; Lee, S.H.; Kim, K.R.; Heo, C.Y.; Choy, Y. Bin Surgical suture braided with a diclofenac-loaded strand of poly(lactic-co-glycolic acid) for local, sustained pain mitigation. Mater. Sci. Eng. C 2017, 79, 209–215. [Google Scholar] [CrossRef]
- Ghalei, S.; Asadi, H.; Ghalei, B. Zein nanoparticle-embedded electrospun PVA nanofibers as wound dressing for topical delivery of anti-inflammatory diclofenac. J. Appl. Polym. Sci. 2018, 135, 46643. [Google Scholar] [CrossRef]
- Saudi, S.; Bhattarai, S.R.; Adhikari, U.; Khanal, S.; Sankar, J.; Aravamudhan, S.; Bhattarai, N. Nanonet-nano fiber electrospun mesh of PCL–chitosan for controlled and extended release of diclofenac sodium. Nanoscale 2020, 12. [Google Scholar] [CrossRef]
- Semnani, K.; Shams-Ghahfarokhi, M.; Afrashi, M.; Fakhrali, A.; Semnani, D. Antifungal Activity of Eugenol Loaded Electrospun PAN Nanofiber Mats Against Candida Albicans. Curr. Drug Deliv. 2018, 15, 860–866. [Google Scholar] [CrossRef]
- Thonemann, B.; Schmalz, G.; Hiller, K.A.; Schweikl, H. Responses of L929 mouse fibroblasts, primary and immortalized bovine dental papilla-derived cell lines to dental resin components. Dent. Mater. 2002, 18, 318–323. [Google Scholar] [CrossRef]
- Hussain, T.; Ullah, A.; Umar, M.; Areeb, T.; Zubair, Z.; Masood, R.; Zia, Q. Modelling the properties of pigment-printed polypropylene nonwoven fabric using the Box-Behnken technique. Color. Technol. 2015, 131, 474–480. [Google Scholar] [CrossRef]
- Ullah, A.; Saito, Y.; Ullah, S.; Haider, M.K.; Nawaz, H.; Duy-Nam, P.; Kharaghani, D.; Kim, I.S. Bioactive Sambong oil-loaded electrospun cellulose acetate nanofibers: Preparation, characterization, and in-vitro biocompatibility. Int. J. Biol. Macromol. 2020, 166, 1009–1021. [Google Scholar] [CrossRef] [PubMed]
- Taha, E.I.; Shazly, G.A.G.; Harisa, G.I.; Barakat, N.S.; Al-Enazi, F.K.; Elbagory, I.M. Formulation and in vivo evaluation of diclofenac sodium sustained release matrix tablet: Effect of compression force. Pak. J. Pharm. Sci. 2015, 28, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.Y.; Kim, M.H.; Jeong, Y.G.; Yoon, Y. Il; Park, W.H. Effect of alkaline hydrolysis on cyclization reaction of PAN nanofibers. Mater. Des. 2017, 124, 69–77. [Google Scholar] [CrossRef]
- Patel, S.; Konar, M.; Sahoo, H.K.; Hota, G. Surface functionalization of electrospun PAN nanofibers with ZnO-Ag heterostructure nanoparticles: Synthesis and antibacterial study. Nanotechnology 2019, 30, 205704. [Google Scholar] [CrossRef]
- Fini, A.; Cavallari, C.; Ospitali, F. Diclofenac salts. V. examples of polymorphism among diclofenac Salts with Alkyl-hydroxy Amines Studied by DSC and HSM. Pharmaceutics 2010, 2, 136–158. [Google Scholar] [CrossRef]
- Alavarse, A.C.; de Oliveira Silva, F.W.; Colque, J.T.; da Silva, V.M.; Prieto, T.; Venancio, E.C.; Bonvent, J.J. Tetracycline hydrochloride-loaded electrospun nanofibers mats based on PVA and chitosan for wound dressing. Mater. Sci. Eng. C 2017, 77, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Dastidar, S.G.; Ganguly, K.; Chaudhuri, K.; Chakrabarty, A.N. The anti-bacterial action of diclofenac shown by inhibition of DNA synthesis. Int. J. Antimicrob. Agents 2000, 14, 249–251. [Google Scholar] [CrossRef]
- Salem-Milani, A.; Balaei-Gajan, E.; Rahimi, S.; Moosavi, Z.; Abdollahi, A.; Zakeri-Milani, P.; Bolourian, M. Antibacterial Effect of Diclofenac Sodium on Enterococcus faecalis. J. Dent. (Tehran) 2013, 10, 16–22. [Google Scholar]

| Sample No. | Weight Ratio (%) | |
|---|---|---|
| PAN | DLF | |
| S1 | 100 | 0 |
| S2 | 97 | 3 |
| S3 | 96 | 4 |
| S4 | 95 | 5 |
| S5 | 94 | 6 |
| Samples Labels | Zone of Inhibition (mm) | |
|---|---|---|
| E. coli | S. aureus | |
| S1(neat PAN) | 0 | 0 |
| S2(3% DLF) | 7 ± 0.15 | 8 ± 0.17 |
| S3(4% DLF) | 10.5 ± 0.19 | 12 ± 0.27 |
| S4(5% DLF) | 14 ± 0.38 | 15 ± 0.59 |
| S5(6% DLF) | 16 ± 0.46 | 15.5 ± 0.28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarwar, M.N.; Ullah, A.; Haider, M.K.; Hussain, N.; Ullah, S.; Hashmi, M.; Khan, M.Q.; Kim, I.S. Evaluating Antibacterial Efficacy and Biocompatibility of PAN Nanofibers Loaded with Diclofenac Sodium Salt. Polymers 2021, 13, 510. https://doi.org/10.3390/polym13040510
Sarwar MN, Ullah A, Haider MK, Hussain N, Ullah S, Hashmi M, Khan MQ, Kim IS. Evaluating Antibacterial Efficacy and Biocompatibility of PAN Nanofibers Loaded with Diclofenac Sodium Salt. Polymers. 2021; 13(4):510. https://doi.org/10.3390/polym13040510
Chicago/Turabian StyleSarwar, Muhammad Nauman, Azeem Ullah, Md. Kaiser Haider, Nadir Hussain, Sana Ullah, Motahira Hashmi, Muhammad Qamar Khan, and Ick Soo Kim. 2021. "Evaluating Antibacterial Efficacy and Biocompatibility of PAN Nanofibers Loaded with Diclofenac Sodium Salt" Polymers 13, no. 4: 510. https://doi.org/10.3390/polym13040510
APA StyleSarwar, M. N., Ullah, A., Haider, M. K., Hussain, N., Ullah, S., Hashmi, M., Khan, M. Q., & Kim, I. S. (2021). Evaluating Antibacterial Efficacy and Biocompatibility of PAN Nanofibers Loaded with Diclofenac Sodium Salt. Polymers, 13(4), 510. https://doi.org/10.3390/polym13040510
















