1. Introduction
Skin wound, either acute or chronic, is a major healthcare burden around the world. This is clear with the size of the global advanced wound care market with USD 10.43 billion in 2019 and this is projected to reach USD 15.59 billion in 2027 [
1]. Currently, the increasing global Muslim populations have brought along a great demand in halal-based products such as food, supplements and medical devices. As a result, efforts toward developing the niche market of halal wound care products are also underway [
2].
Specific to the management of full-thickness wounds, the loss of the dermis layer results in the inability of the keratinocytes from the native epidermis layer to re-epithelialise. Hence, any loss of full-thickness skin of more than 4 cm requires a skin graft in order for it to regenerate efficiently [
3]. However, a skin graft commonly presents with complications such as secondary infection and pain, particularly at the donor site [
4].
An alternative to a skin graft is the use of skin substitutes made from various biomaterials [
5]. Instead of only covering the wound, these skin substitutes interact with the wound environment to facilitate healing [
6]. These interactions are usually achieved via utilisation of bioactive polymers as raw materials, fabrication of a microstructure that mimics the extracellular matrix (ECM) and incorporation of soluble bioactive molecules into the bioscaffolds. In essence, skin substitutes can act as both a provisional scaffold for cell migration and an advocator for the wound to be healed [
7].
The ECM of the skin is comprised mainly of collagen [
8]. Accordingly, collagen is widely used in the skin substitute application such as Integra
®, Apligraft, Excellagen
®, CollaWound and many more [
9]. The challenge in the production of collagen-based skin substitutes is the extraction of collagen from its natural source. In order to obtain intact collagen from specific animals’ tissue, extra precaution needs to be taken to prevent denaturation of the collagen conformation [
10]. Consequently, the extraction of collagen can be complicated and may translate to a higher product cost. Down the line, the final product may be expensive and will be less accessible to the patient [
11].
An alternative denatured collagen product known as gelatin can be effectively extracted from an animal by-product [
12]. Gelatin shares many chemical and biological properties with undenatured collagen [
13]. As a result, gelatin can serve as an alternative collagen with lower production costs. Moreover, manufacturing of halal gelatin can further improve the accessibility of the final product to the global Muslim population [
2]. Furthermore, gelatin has been widely used in clinical settings including wound dressings, implantable antibiotic carriers, vascular stent modifying material and neurosurgical applications with desired biocompatibility, biodegradability and non-immunogenicity [
14].
Nevertheless, a challenge ensues in utilizing both materials as skin substitute due to instability. This limits their applications in clinical settings [
15]. As a result, crosslinking treatments are required to achieve the necessary stability via physical or chemical intervention. Physical crosslinking methods include dehydrothermal (DHT) treatment and exposure to ultraviolet radiation. The primary advantage of the physical methods is that they maintain the chemical composition of the scaffold and have rarely been reported to be toxic to the cell. However, the limitation of these methods is that they are less efficient for obtaining the desired amount of crosslinking [
16].
Alternatively, many chemicals such as formaldehyde, glutaraldehyde, polyepoxy compounds, tannic acid, dimethyl suberimidate, carbodiimide and acyl azide have been used to chemically modify the gelatin for biomedical applications. Chemical crosslinkers provide more stable hydrolysis resistance and thermal stability; however, these synthetic crosslinking reagents are relatively toxic to the cell, hence indicating they are the least efficient in terms of biocompatibility [
17]. The choice of crosslinking method in fabricating skin substitute requires a balance between the efficiency of the crosslinking and the potential cytotoxic effect to the resident cell in the wound site [
18].
The key determining factors for developing a skin substitute that can facilitate the healing of a full-thickness wound is its physicochemical properties that are adaptive to the wound environment [
19]. As a temporary scaffold, where the resident cells in the wound bed migrate and form newly formed skin at the injury site, it is important for the skin substitute to be degraded when the healing is complete [
20]. Failure to degrade on time will result in further complications such as pain, infection and inflammation when the newly formed skin is aggregated in the scaffold [
21]. Hence, biodegradability is a crucial physical property needed for skin substitute [
19].
The freeze-drying method is widely used to fabricate 3D porous scaffolds, where the porosity of the scaffolds is achieved by the rapid freezing of polymer solution which results in the formation of ice crystals [
22]. The microstructure of the scaffold influences the water absorption ability of the scaffold. The interconnected channels in a porous scaffold facilitate the movement of water [
23]. On the other hand, a lesser degree of these interconnected channels creates resistance against water movement throughout the scaffold [
24]. As a result, the scaffold has the ability to retain water. A wound that has high exudation requires high absorption ability in the skin substitute [
25]. Alternatively, a low exudation wound requires the skin substitute to be able to retain water. Furthermore, the migration of local cells from the wound bed into the scaffold is also dependent on its porosity.
Fortunately, the crosslinking treatment of biomaterial such as gelatin allows tunability. The biodegradability and microstructure of the gelatin scaffold (GS) can be adjusted according to its crosslinking degree [
14]. The modification of the crosslinking degree can be achieved by selection of different types of crosslinker or adjustment of the crosslinker’s concentration. In turn, the crosslinking degree will determine the biodegradability rate of the GS [
26]. Moreover, the crosslinking degree also determines the architecture of the scaffold, particularly its porosity. The scaffold porosity then determines the water absorbance or water retention ability of the scaffold.
In recent years, increasing demand for a crosslinking agent that can form a stable and biocompatible crosslink product without causing cytotoxicity has brought attention to the natural crosslinking agent, known as genipin (GNP). GNP is a natural compound derived from gardenia fruit (Gardenia jasminoides ELLIS). GNP has gained increasing attention as a crosslinking agent following its excellent biocompatibility and ability to improve mechanical properties [
27,
28]. When gelatin is crosslinked with genipin, two mechanisms of reactions occur. The first reaction involves the amine group (lysine) of gelatin executing a nucleophilic charge ensuring heterocyclic linking of genipin to the gelatin amine. In the subsequent response, the ester group of the genipin undergoes nucleophilic exchange by the amine group of the second gelatin fragment, marking the beginning of the crosslinking [
28]. From the occurrence of the reaction, long-range intermolecular crosslinks are made among the gelatin molecules (
Figure 1A) [
29].
Most of the known synthetic chemical crosslinkers stabilize a polymer by forming intermolecular bridges. This introduction of foreign molecules within the polymeric network typically results cytotoxicity in the crosslinked product [
20]. Like GNP, the synthetic zero-length crosslinking agent, 1-ethyl-3-(3-dimethylaminopropyl) (EDC), can also form a stable and biocompatible product without causing cytotoxicity. This is due to the activation of EDC carboxylic acid residues conjugating with the adjacent amino groups instead of introducing foreign molecules into the network. As a result, EDC form intramolecular crosslinks within a gelatin molecule or short-range intermolecular crosslinks between two adjacent gelatin molecules (
Figure 1B) [
29].
Mechanical strength is a paramount consideration in the fabrication of a skin substitute. When the wound contracts, adequate mechanical strength is required to prevent the skin substitute from breaking and exposing the underlying tissue to external contamination [
30]. A combination of GNP and EDC, the two crosslinkers with good biocompatibility and a distinct crosslinking mechanism, which may complement each other, is a crosslinking approach to fabricate GS with better mechanical strength (
Figure 1C) [
29]. Fortification of a genipin-crosslinked gelatin sponge with EDC may produce a skin substitute with better mechanical strength without jeopardizing its biocompatibility.
This study aims to develop a versatile gelatin-based sponge scaffold as a skin substitute for the treatment of full-thickness cutaneous wounds. The effect of GS fabricated via the freeze-drying method and crosslinked by GNP with or without fortification by EDC is investigated. Physicochemical, biomechanical and cellular biocompatibility and cell interaction with the fabricated GS, respective to each crosslinking method, were evaluated.
2. Materials and Methods
This research was approved by Universiti Kebangsaan Malaysia (UKM) Research Ethics Committee (Code No. JEP-2018-0618) under the University Grant (Code No. GUP-2017-117).
2.1. Gelatin
Buffalo gelatin originally manufactured at a Nitta-Gelatin facility in Cochin, India was obtained from Nitta-Gelatin Ltd. (Osaka, Japan). A gelatin particle is negatively charged in physiological pH. The gelatin was extracted from buffalo raw bone materials and has halal certification from different regions. It came in a high-grade quality powder with a low endotoxin unit < 3000 that is essential to reduce immune rejection post implantation.
2.2. Fabrication of GS
Gelatin powder was dissolved in distilled water for 30 min at 40 °C to achieve a final concentration of 5% (w/v). The gelatin solution was cast in a particular mould for further analysis. The solution was pre-frozen at −80 °C for 6 h before being transferred into the freeze-dryer for lyophilisation processing (Ilshin, Korea) for 48 h.
Two types of chemical crosslinker were then prepared: Genipin (GNP; CBC, Touliu, Taiwan) and 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC; Thermofisher, Rockford, IL, USA). The genipin solution was prepared by diluting genipin powder using 70% ethanol at two concentration: 0.1% (w/v) and 0.5% (w/v). The EDC solution was prepared by diluting EDC powder using distilled water and two concentrations were made: 15 mM and 30 mM. The fabricated GS was divided into five group.
The first two were soaked into genipin solution at 0.1% (w/v) and 0.5% (w/v). The crosslinking process was stopped after 6 h, at which point the GS was removed and washed using phosphate-buffered saline (PBS). The GS was pre-frozen and lyophilized back for further analysis.
The other two-group crosslinking approach was a repetition of the previous procedure but it underwent a second crosslinked process using an EDC solution. GS with 0.1% (w/v) genipin was crosslinked again with 15 mM EDC, while GS with 0.5% (w/v) genipin was crosslinked with 30 mM EDC. All GS soaked with EDC solution underwent a 6 h crosslinking process before being removed and washed with 2-(N-morpholino)- ethanesulfonic acid (MES; pH 5.5). The GS was pre-frozen and lyophilized back for further analysis.
The last GS group was physically crosslinked using the dehydrothermal (DHT) method at 140 °C for 72 h. The crosslinked GS were then freeze dried again for 48 h for further analysis. A non-crosslinked GS was used as an experimental control.
2.3. Crosslinking Degree Evaluation
The crosslinking degree of fabricated GS was determined using a ninhydrin assay (Sigma-Aldrich, Saint Louis, MO, USA). The ninhydrin assay was used to determine the percentage of free amino groups in the gelatin samples, which can be calculated to the degree of crosslinking by comparing to uncrosslinked gelatin. The test samples were first lyophilized for 24 h and then weighed. Subsequently, the test sample was boiled with ninhydrin solution for 2 min at 100 °C following the manufacturer’s instructions. The amount of free amino groups in the test sample was determined using optical absorbance at 570 nm (Abs
570) recorded with a spectrophotometer (BioTek, PowerWave XS, Highland Park, IL, USA). In addition, different concentrations (1.0, 2.0, 3.0, 4.0 and 5.0 mg/mL) of glycine were used as standards. The amount of free amino groups was proportional to the value of Abs
570, whereas glycine at various known concentrations was used to create a standard curve of glycine concentration vs absorbance. The degree of crosslinking of the sample was then calculated following the equation below:
where
Amino0 is the free NH
2 concentration in non-cross-linked samples, and
Aminoc is the free NH
2 concentration in the cross-linked sample.
2.4. Enzymatic Biodegradation
The enzymatic hydrolysis resistance of GS was determined via incubation of each sample in 0.0006% (w/v) collagenase type I (Worthington, Lakewood, NJ, USA) at 37 °C for 14 h. A stop solution of 0.2 M ethylenediaminetetraacetic acid (EDTA) was added into the reaction followed by rapid cooling at 4 °C to terminate the enzymatic reaction. The GS was then subjected to the freeze-drying step followed by weighing of the dry weight. The dry weight of the GS was recorded every hour until the degradation was complete.
2.5. Thermal Stability
Thermal stability evaluation was measured by thermogravimetric analysis (TGA) that allows the measurement of the mass change of a sample similarly to the temperature changes in a controlled environment. The loss of weight of gelatin depends on its stability at different temperatures. Thermal stability of samples was measured with a thermogravimetric analyser. All tests were performed in a nitrogen-contained environment at a heating rate of 10 °C/min between 50 °C and 800 °C. The initial weight of each selected sample was approximately 10 mg.
2.6. Surface Topography and Microstructure Observation
The surface topography and cross-sectional microstructure of gelatin scaffolds were observed with scanning electron microscopy (SEM; FEI, Hillsboro, OR, USA). At days 1 and 7, the construct was fixed with 4% glutaraldehyde (Sigma, Saint Louis, MO, USA) and dehydrated through serial dilutions of ethanol and dried in a critical point dryer (Quorum, Q150R Plus, Laughton, UK). The samples were sputter-coated with gold and observed by scanning electron microscope (SEM) (SUPRA 55VP, Zeiss, Jena, Germany).
2.7. Pore Size and Porosity Evaluation
A microstructure analysis includes an evaluation of the pore size and porosity as described by Fauzi et al. (2019) [
20]. The pore size of the scaffolds was arbitrarily quantified using Phenom Pro X integrated software (Phenom, Eindhoven, The Netherlands). The solvent replacement method was used to determine the porosity of the GS. Lyophilised GS were weighed (M
1), immersed overnight in absolute ethanol (Merck, Darmstadt, Germany) and blotted with tissue paper to remove excess ethanol from the surface before weighing (
M2). The dimension of the GS was determined using a Vernier caliper. Porosity was calculated using the following equation:
where
M1 and
M2 = masses of GS before and after immersion in absolute ethanol, respectively,
ρ = density of absolute ethanol and
V = volume of the GS.
2.8. Swelling Ratio
The swelling ratio analysis was performed as described by Fauzi et al. (2019) [
20]. Dry gelatin scaffolds were weighed (
W1) and rehydrated in PBS for 2 h at room temperature. The excess buffer on the GS surface was blotted with tissue paper carefully and the wet weight (
W2) was measured. The swelling ratios were calculated as follows:
2.9. Contact Angle
Lyophilised gelatin scaffolds that were left at 20–24 °C overnight were used to determine the contact angle; 10 µL of distilled water was carefully dropped onto the surface of the gelatin scaffold, and images were captured using a digital camera with continuous shooting mode (Sony A6000, Tokyo, Japan). The contact angle was measured using Axio Vision LE image analysis software (Carl Zeiss, Dublin, CA, USA).
2.10. Water Vapour Transmission Rate
The water vapour transmission rate (
WVTR) of the hydrogel was carried out as described by Mohamad et al. (2016) [
31]. Prior to analysis, gelatin scaffolds were cut into disc-like shapes with 1.1 cm diameter and placed on the mouth of the glass vials containing 20 mL of distilled water. The vials were then placed in a humidity chamber at 37 °C with 84% humidity. Evaporation of water through the test membrane was monitored by weighing the vials at specific time intervals. Loss of water from the gelatin scaffold was determined by measuring the decrease in the weight. For analysis purposes, the data of reduced weight was plotted against time for each sample. Finally, the WVTR was determined by dividing the mean weight reduction of water by the area of the vial surface.
2.11. Energy Dispersive X-Ray
Energy dispersive X-ray spectrometry (EDS) analysis was performed using a Phenom Pro X SEM_EDX microscope (Phenom, Eindhoven, The Netherlands) to analyse the presence of elements in the scaffolds. Commercially available gelatin was used as a control.
2.12. Fourier Transform Infrared Spectroscopy
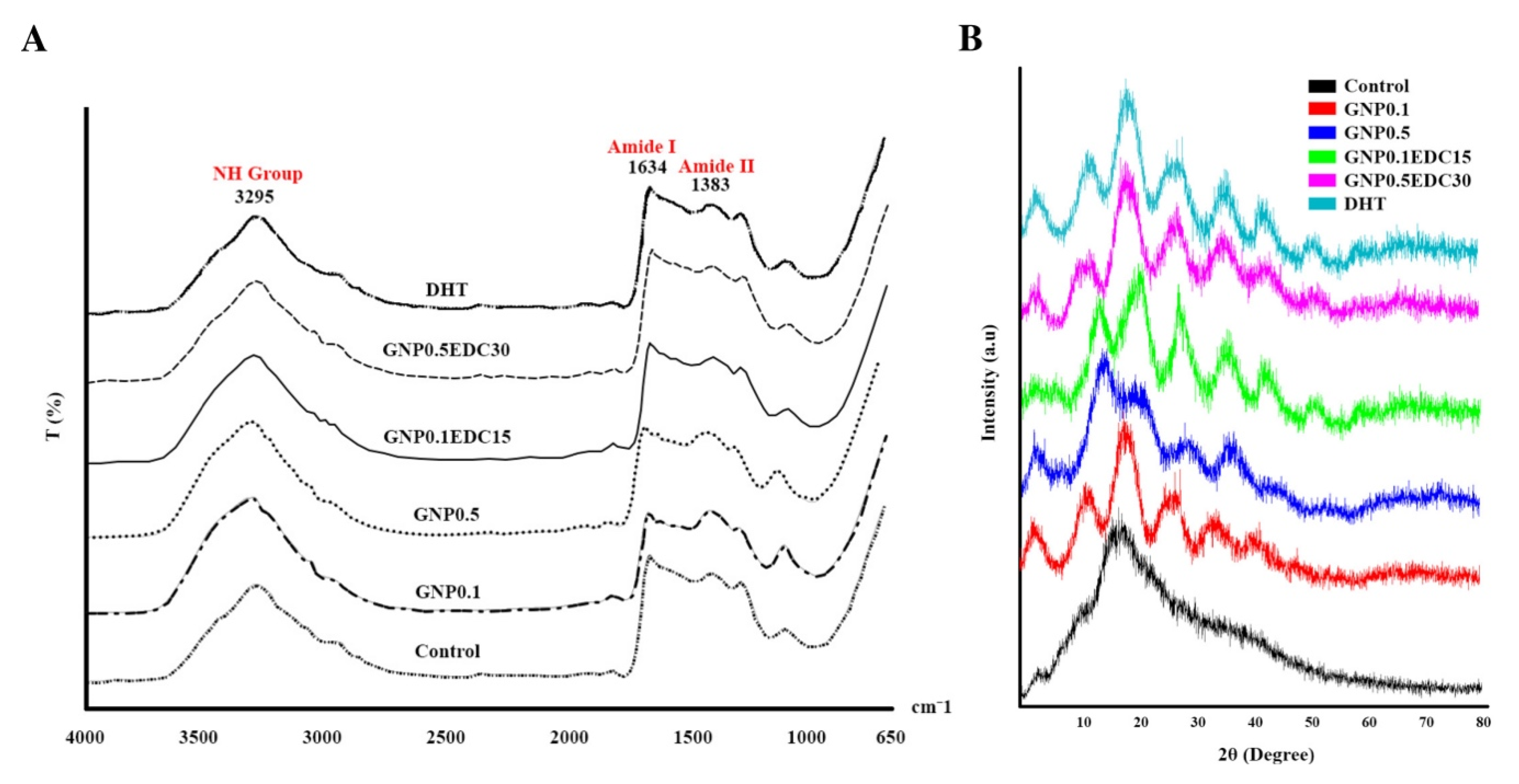
The chemical characterisation of gelatin scaffolds was performed using Fourier transform infrared spectroscopy (FTIR). One mm3 of gelatin scaffolds was analysed, and the spectral data were recorded using a PE Spectrum 100 FTIR spectrometer (PE, Waltham, MA, USA) at a wavelength range of 700 to 4000 cm−1. The absorbance peaks were analysed to identify the chemical structure and changes resulting from the crosslinking.
2.13. X-Ray Diffraction Study
The X-ray diffraction (XRD) characterisation of the gelatin was performed using radiation at room temperature in the –2 scan mode. The diffraction pattern was recorded with an XRD analyser using CuKα radiation (λ = 1.542 Å) at 35 kV and 10 mA. The samples were scanned with 2θ (where θ is the Bragg angle) varying from 10° to 70° in a continuous mode. The result obtained was analysed using integrated software to identify the specific peaks.
2.14. Mechanical Properties Analysis
The mechanical strength of the gelatin scaffolds was measured in dry conditions at room temperature using Instron 8874 Tabletop Axial-Torsion Systems (Instron, Norwood, MA, USA) fitted with 50 N of load transducer at a crosshead velocity of 0.05 mm/min. Six scaffolds of 3 cm2 from each group were tested for evaluation using Young’s modulus and tensile strain.
2.15. Cell Isolation and Culture
Redundant skin samples were obtained from consenting healthy patients undergoing abdominoplasty or circumcision. Sample collection was approved by Universiti Kebangsaan Malaysia (UKM) Research Ethics Committee (Code No. FF-2018-429). In brief, the skin sample (3 cm2) was cleaned of unwanted fragments such as fat, hair and debris, and minced into small pieces (approximately 2 mm2). Next, the skin was digested with 0.6% collagenase type I (Worthington, Lakewood, NJ, USA) for 5 to 6 h in a 37 °C incubator shaker followed by cell dissociation using 0.05% trypsin-EDTA (Gibco, Carlsbad, CA, USA) for 8–10 min.
The digested skin containing both HEK and HDF was re-suspended in a co-culture medium at a 1:1 ratio (a mixture of HEK growth medium; Epilife® (Gibco/BRL, Grand Island, NY, USA) and HDF growth medium; F-12:Dulbecco’s Modified Eagle Medium supplemented with 10% fetal bovine serum (FBS; Biowest, Riverside, MO, USA)) and seeded into three wells (surface area of 9.6 cm2/well) of a six-well culture plate (Greiner Bio-One, Monroe, NC, USA) at 37 °C with 5% CO2. The culture medium was replaced every two days until the cells reached the desired confluency.
When the cells reached 70–80% confluency, differential trypsinisation was performed by using 0.05% Trypsin-EDTA for 3 to 5 min to dissociate the HDF from the culture surface. HEK and HDF were sub-cultured separately until the required number of cells was obtained, with the medium being changed every 48 h. Cells at passage 2 to 3 were used in all experiments.
2.16. Cell Seeding and In Vitro Analyses
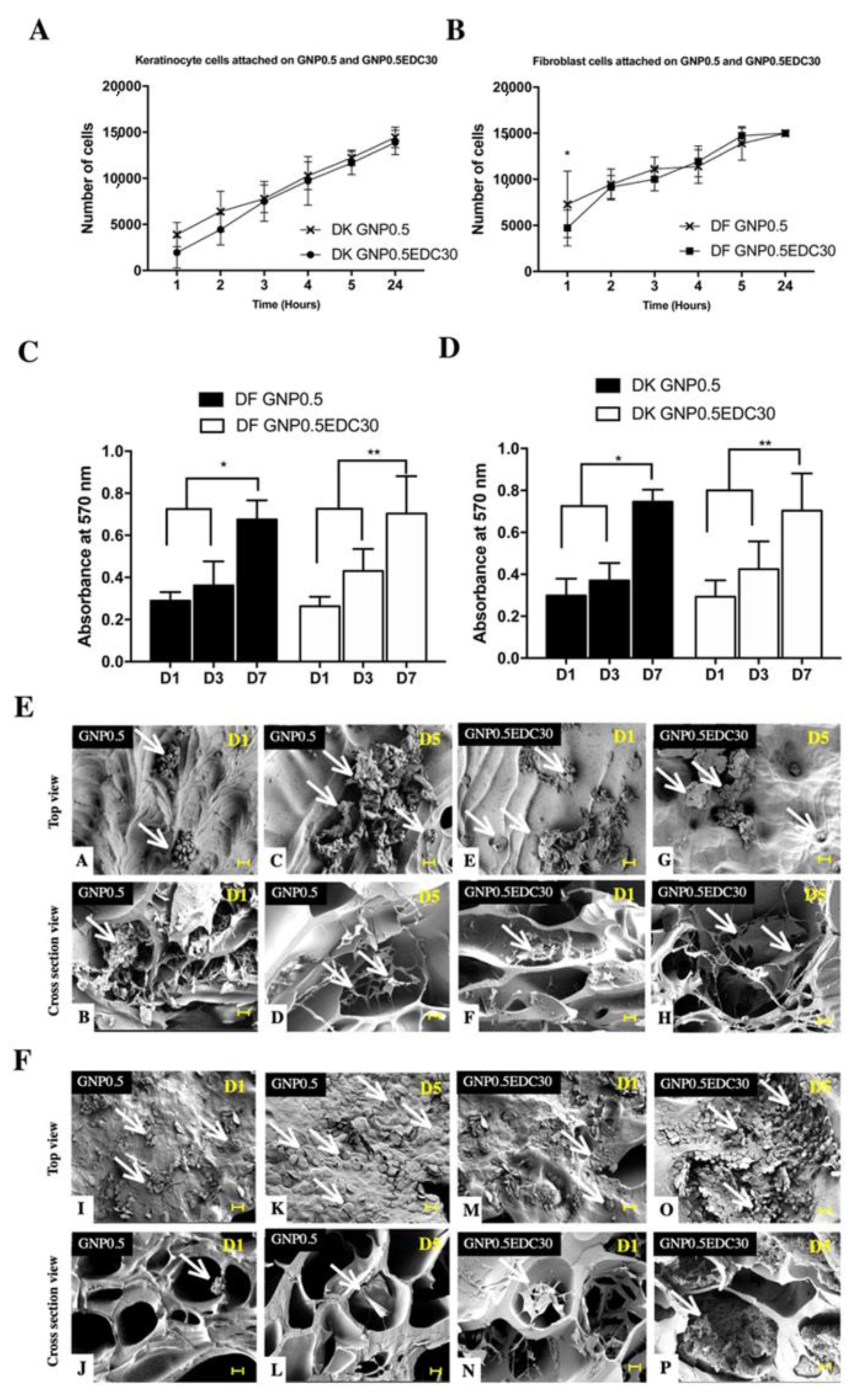
Biocompatibility was evaluated via attachment, proliferation and cytotoxicity of HEK and HDF on GS. For cell attachment and growth rate analysis, HEK and HDF at passage 2 were seeded on the gelatin scaffolds at a density of 6 × 10
4 cells/cm
3 and 4 × 10
4 cells/cm
3, respectively. Unattached cells in the culture medium were quantified every hour for the first 6 h and 24 h after seeding using a trypan blue exclusion test. The percentage of cell attachment on gelatin scaffolds was measured using the following equation:
The morphological features of the cells on gelatin scaffolds were observed using SEM. The proliferation of HEK and HDF on the gelatin scaffolds was analysed using a 3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay kit (Thermo Fisher Scientific, Waltham, MA, USA). Cells were cultured on GS, and the MTT assay was performed according to the manufacturer’s recommendations on days 1 and 7 to evaluate the growth rate. In brief, the culture medium was changed to 100 μL of fresh medium for each well; 10 μL of 12 mM MTT reagent (prepared with 1 mL of sterile PBS to one 5 mg vial of MTT) was added to the wells and incubated for 4 h at 37 °C. Then, 100 μL of dissolution reagents (10 mL of 0.01 M HCl to one tube containing 1 g of SDS) was added, followed by incubation for 4 h at 37 °C. The absorbance was measured using a spectrophotometer at 565 nm wavelength.
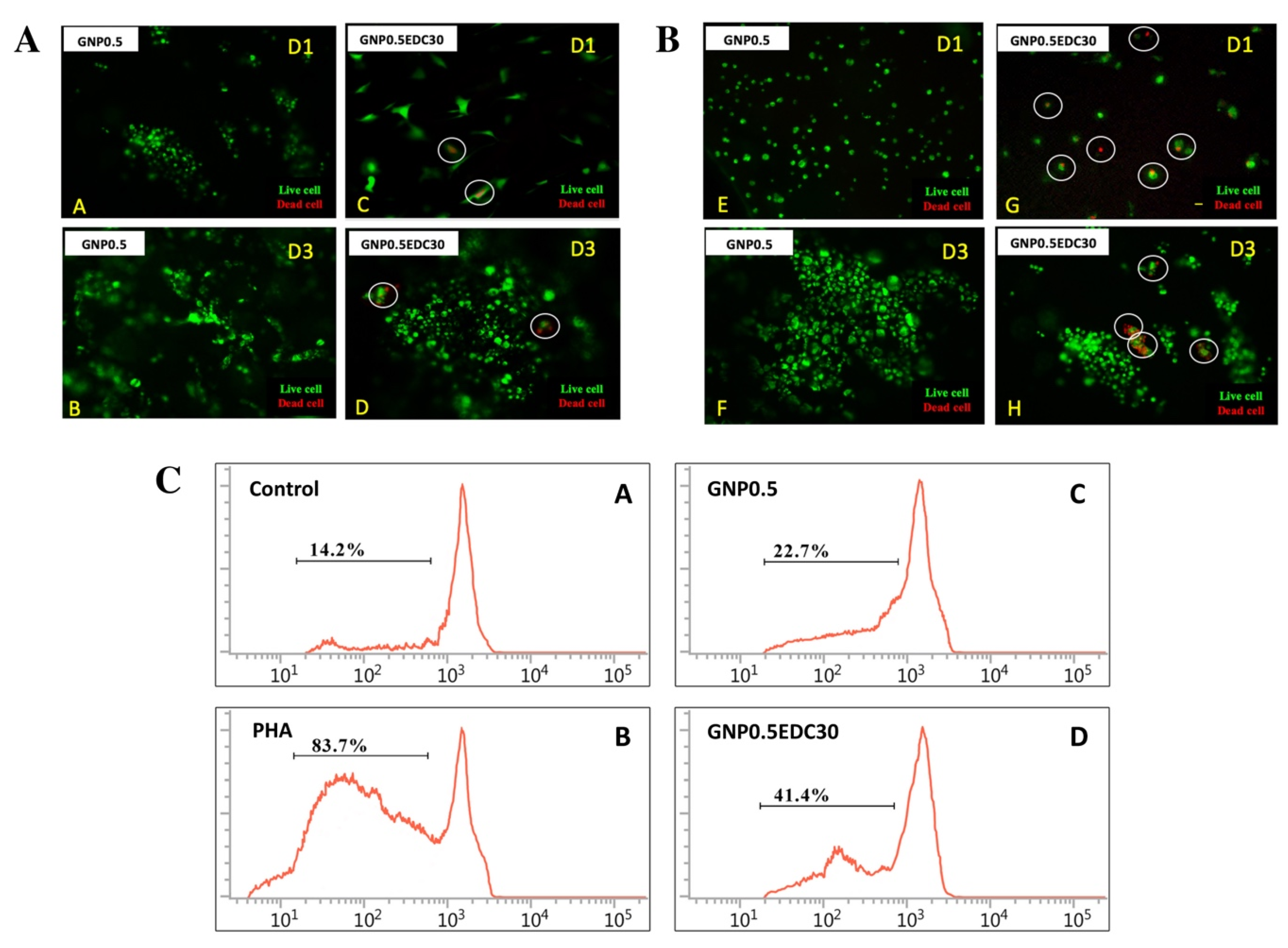
The LIVE/DEAD® Cell Viability Assay (Life Technologies, Carlsbad, CA, USA) was used to analyse the cytotoxic effect of gelatin scaffolds on HEK and HDF according to the manufacturer’s protocol. Briefly, HEK and HDF cultured on the gelatin scaffolds were incubated with 2 mM calcein AM and 4 mM EthD-1 in PBS for 30 min and washed with PBS prior to observation using Nikon A1R-A1 confocal laser scanning microscopy (CLSM; Nikon, Tokyo, Japan).
2.17. In Vitro Immunogenicity
Peripheral blood mononuclear cells (PBMCs) were obtained from healthy donors. The cells were separated by centrifugation in a Ficoll-Paque (GE, Marlborough, MA, USA) density gradient. PBMCs were stained with carboxyfluorescein succinimidyl ester (CFSE) as per the manufacturer’s instructions (BioLegend, San Diego, CA, USA). The cells were then resuspended in RPMI1640 (Invitrogen, Grand Island, NY, USA) containing 10% FBS. Subsequently, the cells were seeded at 2.5 × 105 cells/cm2 and layered with 2 mm discs of gelatin scaffolds. The proliferative response of PBMCs was evaluated by assessing the incorporation of tritiated thymidine (3H-TdR; Perkin Elmer, Boston, MA, USA); 3H-TdR (0.037 MBq/well (0.5 μCi/well)) was added to the wells and incubated at 37 °C for 96 h. A total of 100 μL of cell suspension was transferred to a 96-well plate and exposed to a freeze/thaw cycle at −20 °C to lyse the cells, followed by harvesting onto a filter mat by using an automated cell harvester (Harvester Mach III M; TOMTEC, Hamden, CT, USA). Thymidine incorporation was measured in counts per minute via liquid scintillation spectroscopy on a beta counter (MicroBeta® TriLux; Perkin Elmer, Waltham, MA, USA) after the addition of scintillation fluid (OptiPhase Super-Mix Cocktail; Perkin Elmer, Waltham, MA, USA). The cultures, activated with 1 μg/mL phytohemagglutinin (PHA), were used as a positive control.
2.18. Statistical Analysis
Data were shown as mean ± SD. One-way analysis of variance (ANOVA) was used to compare the means of more than two groups. The comparison of the mean between the two groups was assessed with Student’s paired t-test. A p-value ≤ 0.05 was considered significantly different.
4. Discussion
This study demonstrated the fabrication of versatile sponge scaffold using gelatin for the treatment of full-thickness skin wounds. The Gelatin sponge (GS) biodegradability, thermal stability, mechanical strength, porosity, water retention ability, wettability and water absorption ability can be manipulated to suit the requirement of wound healing via the selection of crosslinking agents and the concentration. Fortification of genipin (GNP) crosslinking with 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) demonstrated the improvement of mechanical strength compared to crosslinking with GNP alone.
Both GNP and EDC crosslink the amine group within gelatin to either the other amine group or the carboxyl group. Thus, detection of the amount concerning the free amine group with a colorimetric indicator such as ninhydrin provides the information needed on how much of the polymeric network has been crosslinked in a bioscaffold [
26]. This parameter, known as degree of crosslinking, is important as the higher the crosslink, the higher the ability of the scaffold to maintain its structure. As expected, an increasing trend for the degree of crosslinking was observed in this study following an increased number of crosslinking agents and their concentration. The degree of crosslinking can serve as a predictor towards all the favorable physical parameters required in the fabrication of the best skin substitute.
Crosslinking stabilizes the structure of a polymer and prevents it from degradation [
17]. The stability of fabricated gelatin bioscaffolds is dependent to the crosslinking degree. In the current study, the highest concentration of double crosslink scaffolds unraveled higher enzymatic hydrolysis resistance and thermal stability compared to the remaining crosslinked group. This translates to a slower biodegradation rate when the skin substitute being implanted into the wound bed [
32]. Lower degradation of double crosslinked scaffolds could be useful for cell growth and tissue regeneration [
33]. In general, wound healing requires 3–4 weeks to be completed [
34]. By manipulating the crosslinking process, the skin substitute can be fabricated to degrade according to the requirement of the specific individual skin wound [
20].
It has been demonstrated that crosslinking of gelatin scaffold with GNP significantly improves mechanical strength in comparison to the non-crosslinked gelatin scaffold. This is due to the intermolecular connectivity that is created by the crosslinking process [
35]. As expected, the addition of EDC crosslinking resulted in greater mechanical strength compared to GNP alone due to the higher intermolecular bridge formation produced by the two crosslinkers [
20]. Sufficient mechanical strength is paramount for a skin substitute. This is to prevent the skin substitute from breaking and exposing the underlying tissue to external contamination when the wound contracts [
30].
Fabrication of GS using the freeze-drying method was effective in producing a highly porous bioscaffold that is suitable to be used for formation of tissue substitutes. High porosity ensures cell penetration, adequate diffusion of nutrient and oxygen that leads to increased cell proliferation and vascularization during skin tissue regeneration [
23]. However, without crosslinking, the aforementioned favourable microstructure is impractical as gelatin rapidly degrades at physiological temperature.
When the GS was crosslinked, the porosity observed in the control reduced. This is due to the crosslinking method that involved a washing step to remove residual crosslinker from the sponge. The porosity reduced significantly in low concentration of GNP and DHT method due to the incomplete crosslinking of the porous GS, resulting in non-crosslinked gelatin being cleared during the washing step [
36]. However, from a recent systematic review that examines the different methods used in preparing genipin crosslinked-gelatin scaffold, the reduced porosity level is still within the reported 60% to 95% porosity range that displayed optimum functional parameters of GS for different tissue engineering application [
37].
Freezing temperature during the freeze-drying process plays a crucial role in controlling the size of the ice crystal, and in turn pore size, porosity, interconnectivity and mechanical strength [
23]. Freezing temperature of –80 °C has been reported to produce small pore size with high open porosity in gelatin scaffolds [
28]. Consequently, the pore size observed was within the 100 µm to 300 µm range recommended to allow cell infiltration and capillary formation for tissue regeneration [
38]. In a practical context, there is no standardized pore size nor does the ratio of opened and closed porosity exist as these requirements are heavily tissue dependent [
38].
The addition of crosslinking resulted in an increase in pore size. Similar to porosity, incomplete crosslinking in the single GNP crosslink group and the physical crosslink group resulted in a large portion of non-crosslinked gelatin being washed away during the washing step. Nevertheless, the pore size in all of the crosslinked group is still within the aforementioned recommended range [
38].
The higher the porosity, the greater the water absorption capacity of a bioscaffold. Alternatively, lower porosity represents higher water retention of a bioscaffold. In this study, the increase in scaffold porosity coincides with the increase in water absorption capacity as depicted by the swelling ratio. Alternatively, water vapor transmission and wettability, two parameters that represent water retention, were reduced with a lower scaffold porosity.
Ultimately, the crosslinking process can be manipulated to fabricate a precise water absorption ability, water retention and wettability of the skin substitute depending on the requirement of the wound [
39]. For example, high concentration double crosslinking can be utilized in a non-exudating wound where moisture retention plays a big role in the wound healing process. Alternatively, less crosslinking agent with lower concentration might be more beneficial in an exudating wound.
Cellular biocompatibility of scaffolds is a top precedence to ensure the success of gelatin scaffolds in the formation of skin substitute [
19]. HEK and HDF, the two cells that reside in skin tissue, were used to evaluate the biocompatibility of GS to be used as a skin substitute. Cells seeded on the EDC crosslink scaffolds exhibited minimal cytotoxic effect as indicated by the live/dead assay. Additionally, the in vitro immunogenicity assay revealed a reduction in the cell cycle of peripheral blood mononuclear cells (PBMC) by EDC crosslinking.
The possible cytotoxicity effect of the EDC crosslinked gelatin might be attributed to the amount of urea byproduct [
40]. The urea was shown to delay the cell cycle and cause cell death; however, the mechanism involved remains unclear [
41]. Similarly, the cell cycle of PBMC was halted in EDC group treatment. Besides, it was assumed that reduction of open porosity and pore size may contribute to the retention and incomplete removal of EDC byproducts (urea) that might lead to cell death [
20].
The GNP crosslink scaffold displayed better biocompatibility compared to the EDC as indicated by the absence of dead cells in the biocompatibity assay. However, cell attachment, proliferation and migration of HEK and HDF were comparable between GNP and EDC crosslinking scaffolds [
20,
42].
Limitations in this study include the exemption of the EDC-only crosslinking groups. This is due to the objective of utilizing EDC as a secondary crosslinker to reinforce GNP crosslinking without having adverse effects such as cytotoxicity. However, fortification of GNP with EDC demonstrated a hint of cytotoxicity compared to the GNP crosslinking. Fortunately, none of the trends reported in the biocompatibility parameters was statistically significant.