Hypergravity-Induced Accumulation: A New, Efficient, and Simple Strategy to Improve the Thermal Conductivity of Boron Nitride Filled Polymer Composites
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of BN/SR Composites with Hypergravity Treatments
2.3. Preparation of sBN/SR Composites without Hypergravity Treatments
2.4. Preparation of BN/PEG Composite with Hypergravity Treatments
2.5. Preparation of BN/PVA Composite with Hypergravity Treatments
2.6. Samples for Viscosity Tests
2.7. Characterization
3. Results
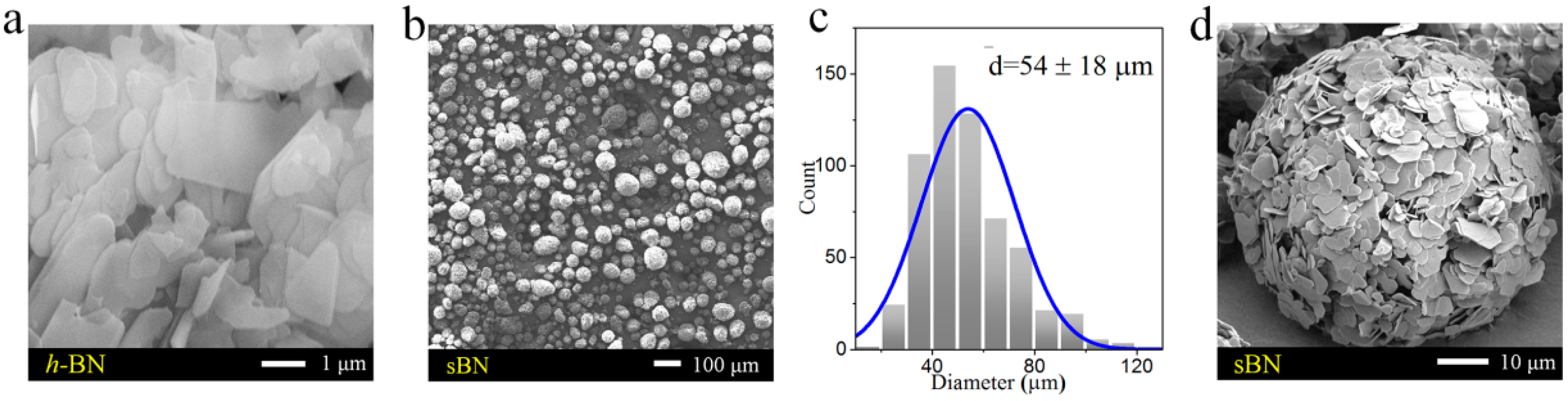
3.1. Hypergravity-Induced Accumulation of BN
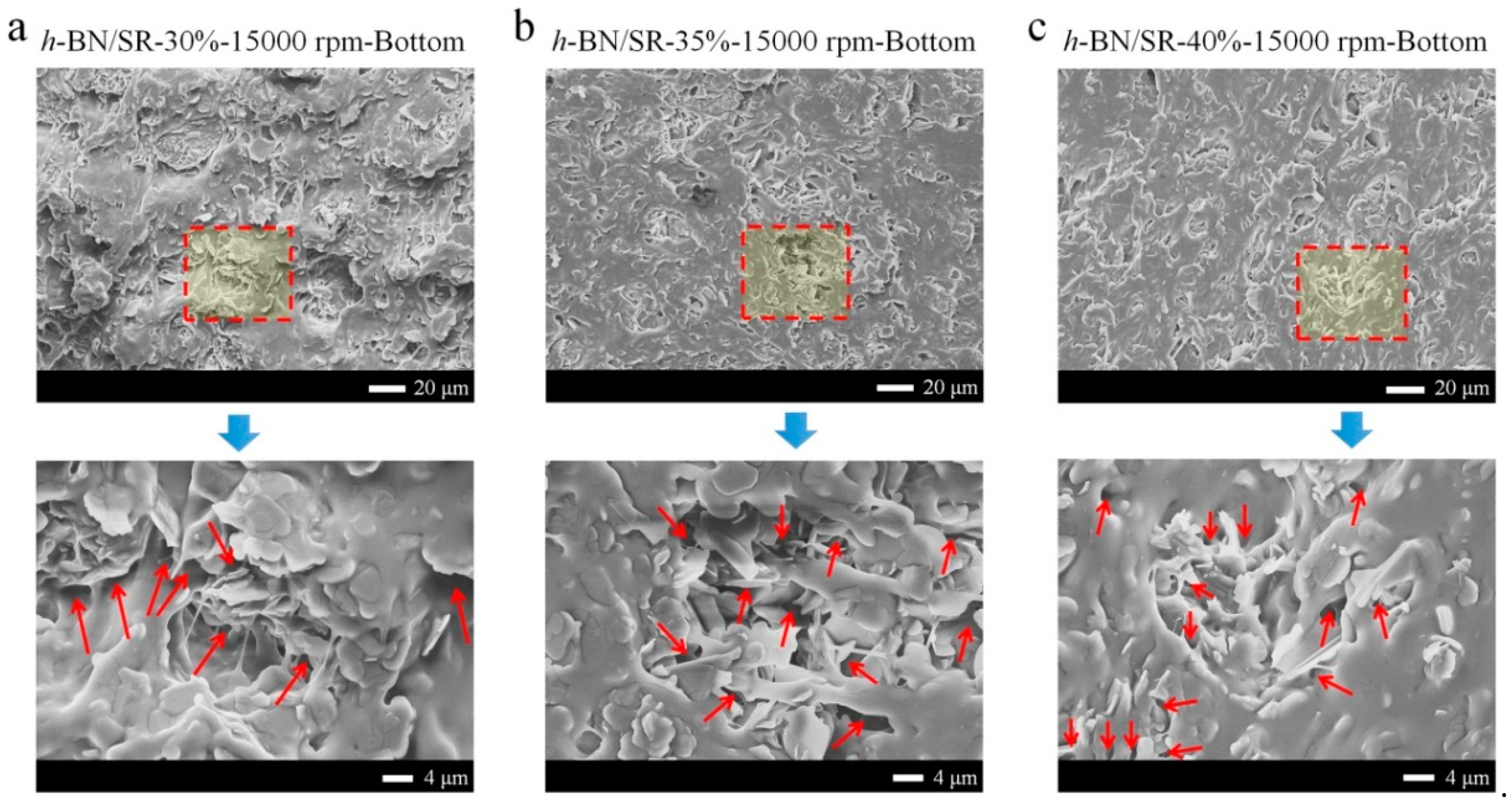
3.2. The Microstructures of BN/SR Composites
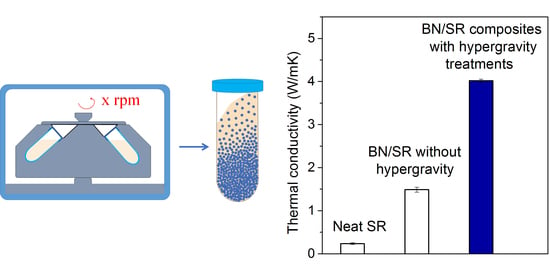
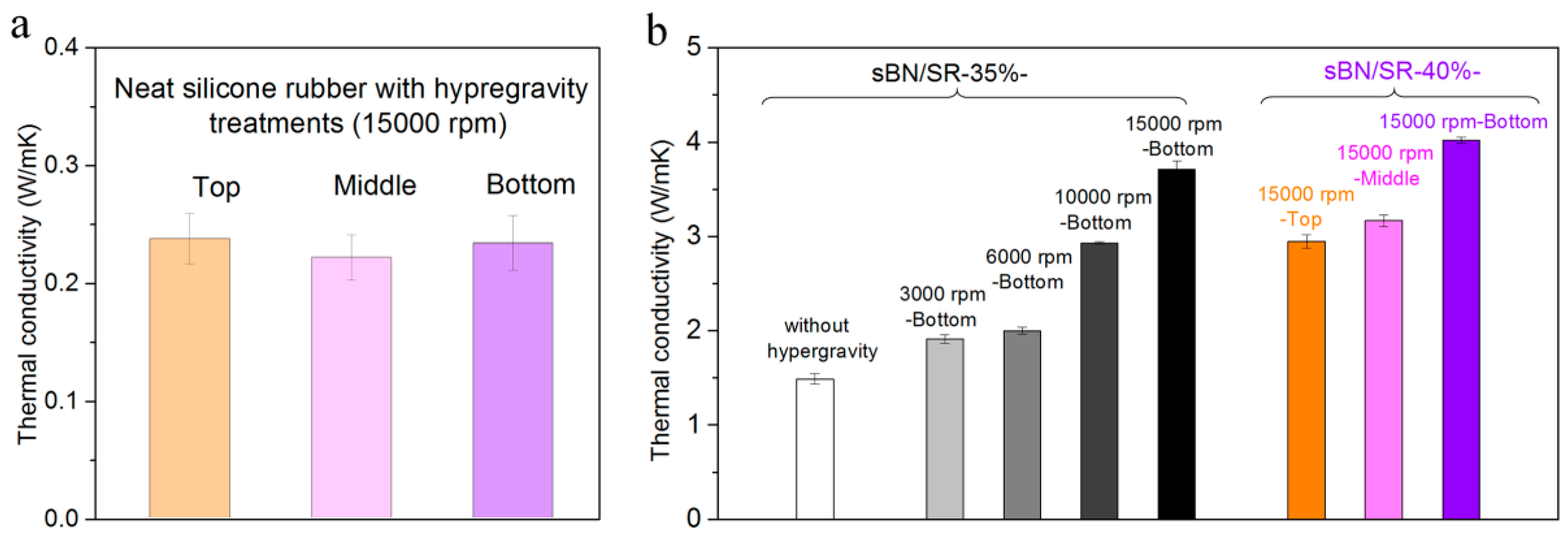
3.3. The Thermal Conductivity of BN/SR Composites
3.4. Possible Applications
4. Discussion
4.1. The High-Viscosity Problem
4.2. The Micro-Cracks in BN/SR Composites
4.3. The Thermal Conductivity of BN/SR Composites in the Literature and in This Work
4.4. Advantages and Disadvantages of the Hypergravity Accumulation Strategy
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Waldrop, M.M. The semiconductor industry will soon abandon its pursuit of moore’s law. Now things could get a lot more interesting. Nature 2016, 530, 144–147. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.L.; Shi, L. Emerging challenges and materials for thermal management of electronics. Mater. Today 2014, 17, 163–174. [Google Scholar] [CrossRef]
- Xia, G.; Cao, L.; Bi, G. A review on battery thermal management in electric vehicle application. J. Power Sources 2017, 367, 90–105. [Google Scholar] [CrossRef]
- Huang, C.; Qian, X.; Yang, R. Thermal conductivity of polymers and polymer nanocomposites. Mater. Sci. Eng. R Rep. 2018, 132, 1–22. [Google Scholar] [CrossRef]
- Leung, S.N. Thermally conductive polymer composites and nanocomposites: Processing-structure-property relationships. Compos. B Eng. 2018, 150, 78–92. [Google Scholar] [CrossRef]
- Guo, Y.Q.; Ruan, K.P.; Shi, X.T.; Yang, X.T.; Gu, J.W. Factors affecting thermal conductivities of the polymers and polymer composites: A review. Compos. Sci. Technol. 2020, 193, 108134. [Google Scholar] [CrossRef]
- Hansson, J.; Nilsson, T.M.J.; Ye, L.; Liu, J. Novel nanostructured thermal interface materials: A review. Int. Mater. Rev. 2018, 63, 22–45. [Google Scholar] [CrossRef]
- Razeeb, K.M.; Dalton, E.; Cross, G.L.W.; Robinson, A.J. Present and future thermal interface materials for electronic devices. Int. Mater. Rev. 2018, 63, 1–21. [Google Scholar] [CrossRef]
- Ma, H.Q.; Gao, B.; Wang, M.Y.; Yuan, Z.Y.; Shen, J.B.; Zhao, J.Q.; Feng, Y.K. Strategies for enhancing thermal conductivity of polymer-based thermal interface materials: A review. J. Mater. Sci. 2021, 56, 1064–1086. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, L.C.; Fischer, A.; Zhong, Y.H.; Drummer, D.; Wu, W. Enhanced thermal conductivity and flame retardancy of polyamide 6/flame retardant composites with hexagonal boron nitride. J. Polym. Eng. 2018, 38, 767–774. [Google Scholar] [CrossRef]
- Fu, X.; Guo, Y.; Du, Q.; Guan, L.; He, S. Improved dielectric stability of epoxy composites with ultralow boron nitride loading. RSC Adv. 2019, 9, 4344–4350. [Google Scholar] [CrossRef]
- Guo, Y.Y.; Cao, C.L.; Fb, L.; Huang, B.Q.; Xiao, L.R.; Qian, Q.R.; Chen, Q.H. Largely enhanced thermal conductivity and thermal stability of ultra high molecular weight polyethylene composites via BN/CNT synergy. RSC Adv. 2019, 9, 40800–40809. [Google Scholar] [CrossRef]
- Feng, C.-P.; Wan, S.-S.; Wu, W.-C.; Bai, L.; Bao, R.-Y.; Liu, Z.-Y.; Yang, M.-B.; Chen, J.; Yang, W. Electrically insulating, layer structured SiR/GNPs/BN thermal management materials with enhanced thermal conductivity and breakdown voltage. Compos. Sci. Technol. 2018, 167, 456–462. [Google Scholar] [CrossRef]
- Kim, K.; Oh, H.; Kim, J. Fabrication of covalently linked exfoliated boron nitride nanosheet/multi-walled carbon nanotube hybrid particles for thermal conductive composite materials. RSC Adv. 2018, 8, 33506–33515. [Google Scholar] [CrossRef]
- Zhou, Y.C.; Liu, F. High-performance polyimide nanocomposites with core-shell AgNWs@BN for electronic packagings. Appl. Phys. Lett. 2016, 109, 082901. [Google Scholar] [CrossRef]
- Tong, J.; Huang, H.X.; Wu, M. Simultaneously facilitating dispersion and thermal reduction of graphene oxide to enhance thermal conductivity of poly(vinylidene fluoride)/graphene nanocomposites by water in continuous extrusion. Chem. Eng. J. 2018, 348, 693–703. [Google Scholar] [CrossRef]
- Patti, A.; Russo, P.; Acierno, D.; Acierno, S. The effect of filler functionalization on dispersion and thermal conductivity of polypropylene/multi wall carbon nanotubes composites. Compos. B Eng. 2016, 94, 350–359. [Google Scholar] [CrossRef]
- Hong, J.; Lee, J.; Hong, C.K.; Shim, S.E. Effect of dispersion state of carbon nanotube on the thermal conductivity of poly(dimethyl siloxane) composites. Curr. Appl. Phys. 2010, 10, 359–363. [Google Scholar] [CrossRef]
- Hamidinejad, S.M.; Chu, R.K.M.; Zhao, B.; Park, C.B.; Filleter, T. Enhanced thermal conductivity of graphene nanoplatelet-polymer nanocomposites fabricated via supercritical fluid-assisted in situ exfoliation. ACS Appl. Mater. Interfaces 2018, 10, 1225–1236. [Google Scholar] [CrossRef]
- Wang, X.; Wu, P. Aqueous phase exfoliation of two-dimensional materials assisted by thermoresponsive polymeric ionic liquid and their applications in stimuli-responsive hydrogels and highly thermally conductive films. ACS Appl. Mater. Interfaces 2018, 10, 2504–2514. [Google Scholar] [CrossRef]
- Ho, Q.B.; Osazuwa, O.; Modler, R.; Daymond, M.; Gallerneault, M.T.; Kontopoulou, M. Exfoliation of graphite and expanded graphite by melt compounding to prepare reinforced, thermally and electrically conducting polyamide composites. Compos. Sci. Technol. 2019, 176, 111–120. [Google Scholar] [CrossRef]
- Lule, Z.; Kim, J. Surface modification of aluminum nitride to fabricate thermally conductive poly(butylene succinate) nanocomposite. Polymers 2019, 11, 148. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.C.; Wu, K.; Luo, F.B.; Lu, M.P.; Xiao, F.; Du, X.X.; Zhang, S.H.; Liang, L.Y.; Lu, M.G. Significantly enhanced thermal conductivity in polyvinyl alcohol composites enabled by dopamine modified graphene nanoplatelets. Compos. Part A Appl. Sci. Manuf. 2019, 117, 134–143. [Google Scholar] [CrossRef]
- Chen, W.L.; Wu, K.; Liu, Q.; Lu, M.G. Functionalization of graphite via Diels-Alder reaction to fabricate poly (vinyl alcohol) composite with enhanced thermal conductivity. Polymer 2020, 186, 122075. [Google Scholar] [CrossRef]
- Saeidijavash, M.; Garg, J.; Grady, B.; Smith, B.; Li, Z.L.; Young, R.J.; Tarannum, F.; Bekri, N.B. High thermal conductivity through simultaneously aligned polyethylene lamellae and graphene nanoplatelets. Nanoscale 2017, 9, 12867–12873. [Google Scholar] [CrossRef]
- Zhang, D.-L.; Zha, J.-W.; Li, W.-K.; Li, C.-Q.; Wang, S.-J.; Wen, Y.; Dang, Z.-M. Enhanced thermal conductivity and mechanical property through boron nitride hot string in polyvinylidene fluoride fibers by electrospinning. Compos. Sci. Technol. 2018, 156, 1–7. [Google Scholar] [CrossRef]
- Xue, Y.; Li, X.; Wang, H.; Zhao, F.; Zhang, D.; Chen, Y. Improvement in thermal conductivity of through-plane aligned boron nitride/silicone rubber composites. Mater. Des. 2019, 165, 107580. [Google Scholar] [CrossRef]
- An, F.; Li, X.; Min, P.; Li, H.; Dai, Z.; Yu, Z.-Z. Highly anisotropic graphene/boron nitride hybrid aerogels with long-range ordered architecture and moderate density for highly thermally conductive composites. Carbon 2018, 126, 119–127. [Google Scholar] [CrossRef]
- Tian, Z.L.; Sun, J.J.; Wang, S.G.; Zeng, X.L.; Zhou, S.; Bai, S.L.; Zhao, N.; Wong, C.P. A thermal interface material based on foam-templated three-dimensional hierarchical porous boron nitride. J. Mater. Chem. A 2018, 6, 17540–17547. [Google Scholar] [CrossRef]
- Wang, X.; Wu, P. Melamine foam-supported 3D interconnected boron nitride nanosheets network encapsulated in epoxy to achieve significant thermal conductivity enhancement at an ultralow filler loading. Chem. Eng. J. 2018, 348, 723–731. [Google Scholar] [CrossRef]
- Feng, Y.; Li, X.; Zhao, X.; Ye, Y.; Zhou, X.; Liu, H.; Liu, C.; Xie, X. Synergetic improvement in thermal conductivity and flame retardancy of epoxy/silver nanowires composites by incorporating “branch-like” flame-retardant functionalized graphene. ACS Appl. Mater. Interfaces 2018, 10, 21628–21641. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.W.; Lu, H.; Ni, H.Y.; Chen, J. Structure, thermal conductive, dielectric and electrical insulating properties of UHMWPE/BN composites with a segregated structure. J. Polym. Res. 2017, 25, 6. [Google Scholar] [CrossRef]
- Su, K.-H.; Su, C.-Y.; Cho, C.-T.; Lin, C.-H.; Jhou, G.-F.; Chang, C.-C. Development of thermally conductive polyurethane composite by low filler loading of spherical BN/PMMA composite powder. Sci. Rep. 2019, 9, 14397. [Google Scholar] [CrossRef] [PubMed]
- Golberg, D.; Bando, Y.; Huang, Y.; Terao, T.; Mitome, M.; Tang, C.C.; Zhi, C.Y. Boron nitride nanotubes and nanosheets. ACS Nano 2010, 4, 2979–2993. [Google Scholar] [CrossRef] [PubMed]
- Meng, W.J.; Huang, Y.; Fu, Y.Q.; Wang, Z.F.; Zhi, C.Y. Polymer composites of boron nitride nanotubes and nanosheets. J. Mater. Chem. C 2014, 2, 10049–10061. [Google Scholar] [CrossRef]
- Yu, C.; Zhang, J.; Tian, W.; Fan, X.; Yao, Y. Polymer composites based on hexagonal boron nitride and their application in thermally conductive composites. RSC Adv. 2018, 8, 21948–21967. [Google Scholar] [CrossRef]
- Guerra, V.; Wan, C.Y.; McNally, T. Thermal conductivity of 2D nano-structured boron nitride (BN) and its composites with polymers. Prog. Mater. Sci. 2019, 100, 170–186. [Google Scholar] [CrossRef]
- Sun, C.; Qiu, X.; Qin, F.; Ding, Z.; Fang, Z. Research progress in liquid phase exfoliation of boron nitride and their applications in thermal management of electronic devices. J. Mater. Eng. 2019, 47, 21–32. [Google Scholar]
- Morishita, T.; Okamoto, H. Facile exfoliation and noncovalent superacid functionalization of boron nitride nanosheets and their use for highly thermally conductive and electrically insulating polymer nanocomposites. ACS Appl. Mater. Interfaces 2016, 8, 27064–27073. [Google Scholar] [CrossRef]
- Yu, B.; Xing, W.Y.; Guo, W.W.; Qiu, S.L.; Wang, X.; Lo, S.M.; Hu, Y. Thermal exfoliation of hexagonal boron nitride for effective enhancements on thermal stability, flame retardancy and smoke suppression of epoxy resin nanocomposites via sol-gel process. J. Mater. Chem. A 2016, 4, 7330–7340. [Google Scholar] [CrossRef]
- Ryu, S.; Oh, H.; Kim, J. Facile liquid-exfoliation process of boron nitride nanosheets for thermal conductive polyphthalamide composite. Polymers 2019, 11, 1628. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Lyu, Z.; Yang, X.; Lu, Y.; Ruan, K.; Wu, Y.; Kong, J.; Gu, J. Enhanced thermal conductivities and decreased thermal resistances of functionalized boron nitride/polyimide composites. Compos. B Eng. 2019, 164, 732–739. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Q.; Chen, Z.; Li, H.; Zheng, S.; Tang, X. Functionalization of boron nitride nanosheets by diazonium salt for preparation of nanocomposites with high-density polyethylene. Polym. Compos. 2019, 40, 2346–2356. [Google Scholar] [CrossRef]
- Zhang, J.; Li, C.; Yu, C.; Wang, X.; Li, Q.; Lu, H.; Zhang, Q.; Zhao, J.; Songfeng, E.; Hu, M.; et al. Large improvement of thermal transport and mechanical performance of polyvinyl alcohol composites based on interface enhanced by SiO2 nanoparticle-modified-hexagonal boron nitride. Compos. Sci. Technol. 2019, 169, 167–175. [Google Scholar] [CrossRef]
- Wang, T.; Wang, M.; Fu, L.; Duan, Z.; Chen, Y.; Hou, X.; Wu, Y.; Li, S.; Guo, L.; Kang, R.; et al. Enhanced thermal conductivity of polyimide composites with boron nitride nanosheets. Sci. Rep. 2018, 8, 1557. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.F.J.; Dewangga, G.R.S.; Chen, J.B. Fabrication of a thermally conductive silicone composite by incorporating surface-modified boron nitride. Text. Res. J. 2019, 89, 2637–2647. [Google Scholar] [CrossRef]
- Wang, J.H.; Zhao, D.; Zou, X.; Mao, L.; Shi, L.Y. The exfoliation and functionalization of boron nitride nanosheets and their utilization in silicone composites with improved thermal conductivity. J. Mater. Sci. Mater. Electron. 2017, 28, 12984–12994. [Google Scholar] [CrossRef]
- Zhao, L.W.; Shi, X.R.; Yin, Y.; Jiang, B.; Huang, Y.D. A self-healing silicone/BN composite with efficient healing property and improved thermal conductivities. Compos. Sci. Technol. 2020, 186, 107919. [Google Scholar] [CrossRef]
- Liu, P.; Li, L.; Wang, L.; Huang, T.; Yao, Y.; Xu, W. Effects of 2D boron nitride (BN) nanoplates filler on the thermal, electrical, mechanical and dielectric properties of high temperature vulcanized silicone rubber for composite insulators. J. Alloys Compd. 2019, 774, 396–404. [Google Scholar] [CrossRef]
- Zhong, B.; Zou, J.; An, L.; Ji, C.; Huang, X.; Liu, W.; Yu, Y.; Wang, H.; Wen, G.; Zhao, K.; et al. The effects of the hexagonal boron nitride nanoflake properties on the thermal conductivity of hexagonal boron nitride nanoflake/silicone rubber composites. Compos. Part A Appl. Sci. Manuf. 2019, 127, 105629. [Google Scholar] [CrossRef]
- Bao, C.; Zhang, H.; Wilkie, C.A.; Bi, S.; Tang, X.-Z.; Wu, J.; Yang, J. On the dispersion systems of graphene-like two-dimensional materials: From fundamental laws to engineering guidelines. Carbon 2016, 107, 774–782. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, S.; Yu, K.; Yue, C.; Liu, M.; Bao, C. Studies on the mechanism for the sudden mechanical property drops of graphene/polymer nanocomposites. Polym. Adv. Technol. 2020, 31, 786–794. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, Y.; Li, Y.; Dai, W.; Yan, Q.; Alam, F.E.; Du, S.; Wang, Z.; Nishimura, K.; Jiang, N.; et al. Graphene foam-embedded epoxy composites with significant thermal conductivity enhancement. Nanoscale 2019, 11, 17600–17606. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Zhang, C.; Zhang, Y.F. Thermal Conductivity of Graphene-Polymer Composites: Mechanisms, Properties, and Applications. Polymers 2017, 9, 437. [Google Scholar] [CrossRef]
- Huang, X.Y.; Zhi, C.Y.; Lin, Y.; Bao, H.; Wu, G.N.; Jiang, P.K.; Mai, Y. Thermal conductivity of graphene-based polymer nanocomposites. Mater. Sci. Eng. R Rep. 2020, 142, 100577. [Google Scholar] [CrossRef]
- Santagiuliana, G.; Picot, O.T.; Crespo, M.; Porwal, H.; Zhang, H.; Li, Y.; Rubini, L.; Colonna, S.; Fina, A.; Barbieri, E.; et al. Breaking the Nanoparticle Loading-Dispersion Dichotomy in Polymer Nanocomposites with the Art of Croissant-Making. ACS Nano 2018, 12, 9040–9050. [Google Scholar] [CrossRef]
- Ren, L.; Zeng, X.; Sun, R.; Xu, J.-B.; Wong, C.-P. Spray-assisted assembled spherical boron nitride as fillers for polymers with enhanced thermally conductivity. Chem. Eng. J. 2019, 370, 166–175. [Google Scholar] [CrossRef]







| Centrifugation Speed (rpm) | Relative Gravity Acceleration (g) |
|---|---|
| 3000 | ~800 |
| 6000 | ~3200 |
| 10,000 | ~8800 |
| 15,000 | ~20,000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, K.; Yuan, T.; Zhang, S.; Bao, C. Hypergravity-Induced Accumulation: A New, Efficient, and Simple Strategy to Improve the Thermal Conductivity of Boron Nitride Filled Polymer Composites. Polymers 2021, 13, 459. https://doi.org/10.3390/polym13030459
Yu K, Yuan T, Zhang S, Bao C. Hypergravity-Induced Accumulation: A New, Efficient, and Simple Strategy to Improve the Thermal Conductivity of Boron Nitride Filled Polymer Composites. Polymers. 2021; 13(3):459. https://doi.org/10.3390/polym13030459
Chicago/Turabian StyleYu, Kangkang, Tao Yuan, Songdi Zhang, and Chenlu Bao. 2021. "Hypergravity-Induced Accumulation: A New, Efficient, and Simple Strategy to Improve the Thermal Conductivity of Boron Nitride Filled Polymer Composites" Polymers 13, no. 3: 459. https://doi.org/10.3390/polym13030459
APA StyleYu, K., Yuan, T., Zhang, S., & Bao, C. (2021). Hypergravity-Induced Accumulation: A New, Efficient, and Simple Strategy to Improve the Thermal Conductivity of Boron Nitride Filled Polymer Composites. Polymers, 13(3), 459. https://doi.org/10.3390/polym13030459