Greener, Faster, Stronger: The Benefits of Deep Eutectic Solvents in Polymer and Materials Science
Abstract
1. Introduction
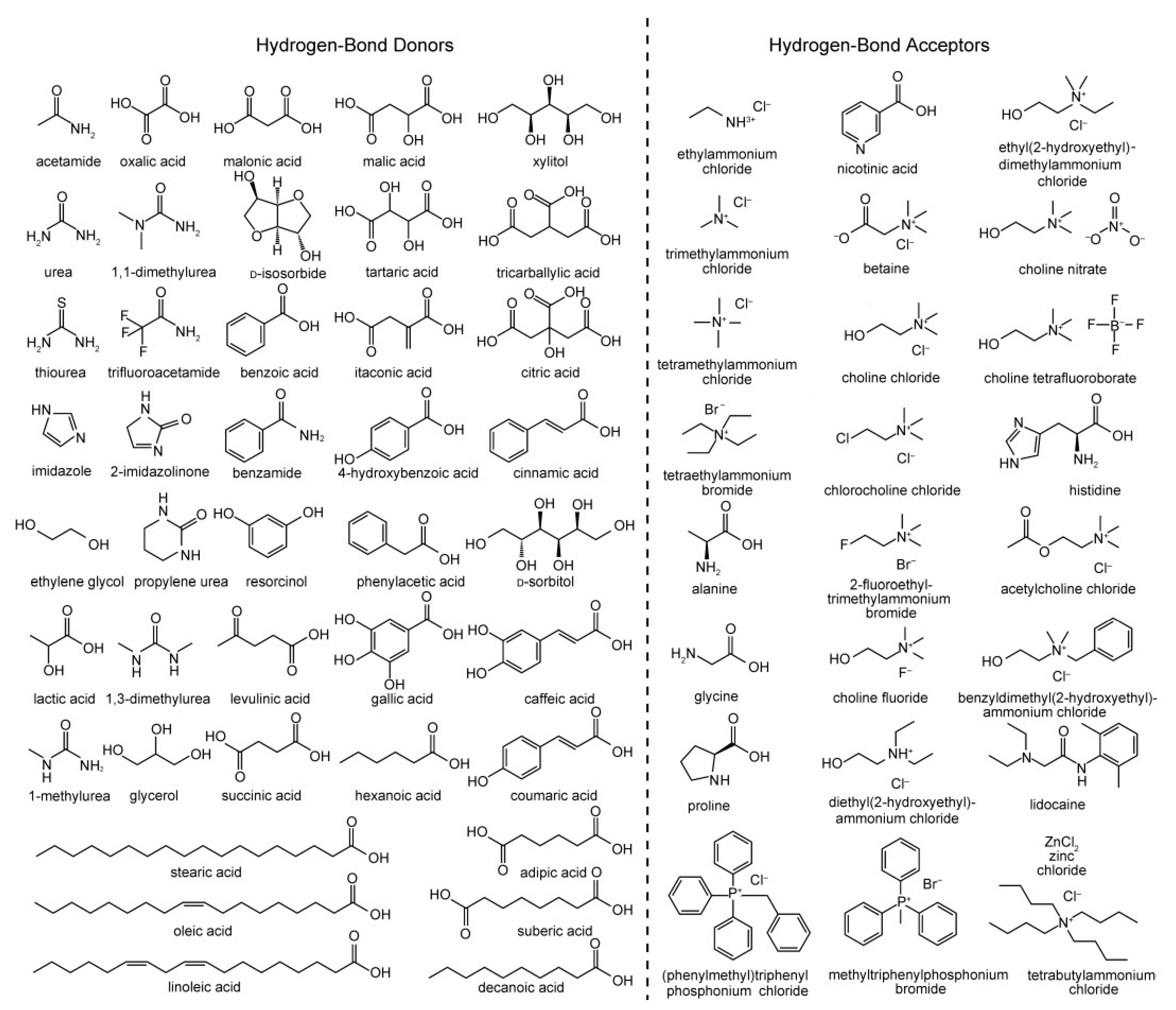
1.1. Preparation and Classification of DES Systems
1.2. Physical and Chemical Properties of DES Systems
2. DES Systems in Polymer and Materials Science
2.1. Mechanisms of Polymerization
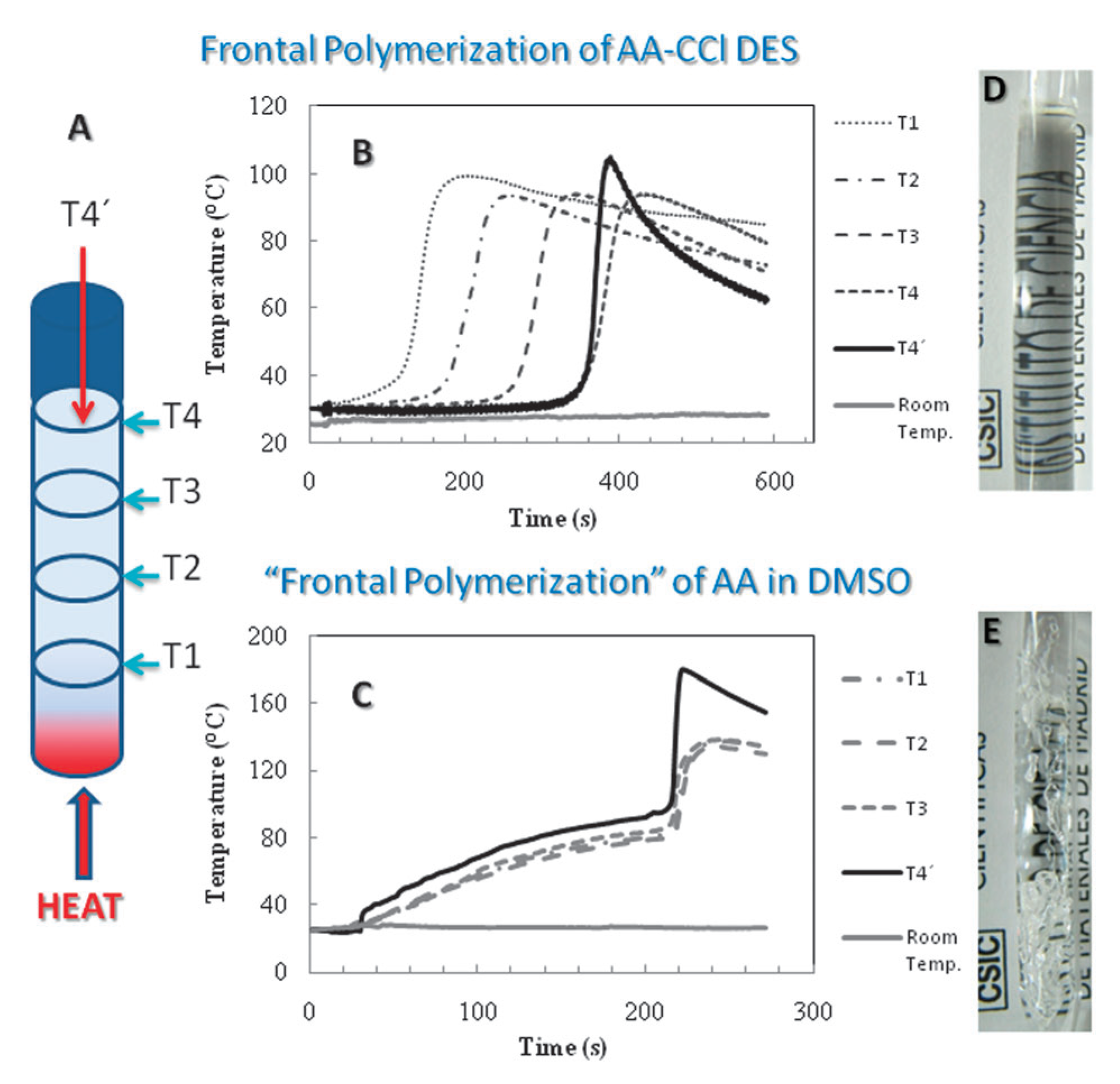
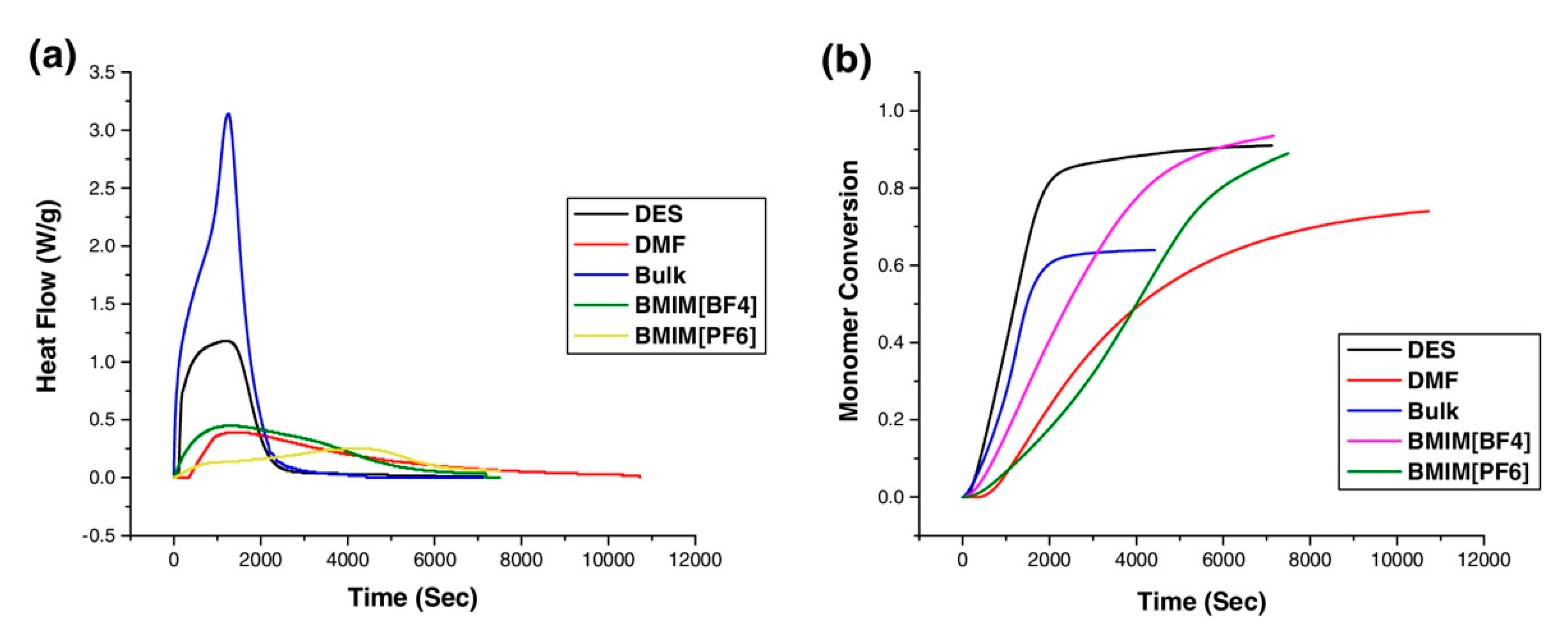
2.1.1. Free-Radical Polymerization
2.1.2. Reversible Deactivation Radical Polymerization (RDRP)
2.1.3. Anionic Polymerization
2.1.4. Ring Opening Polymerization (ROP)
2.1.5. Polycondensation
2.1.6. Electrochemical Polymerization
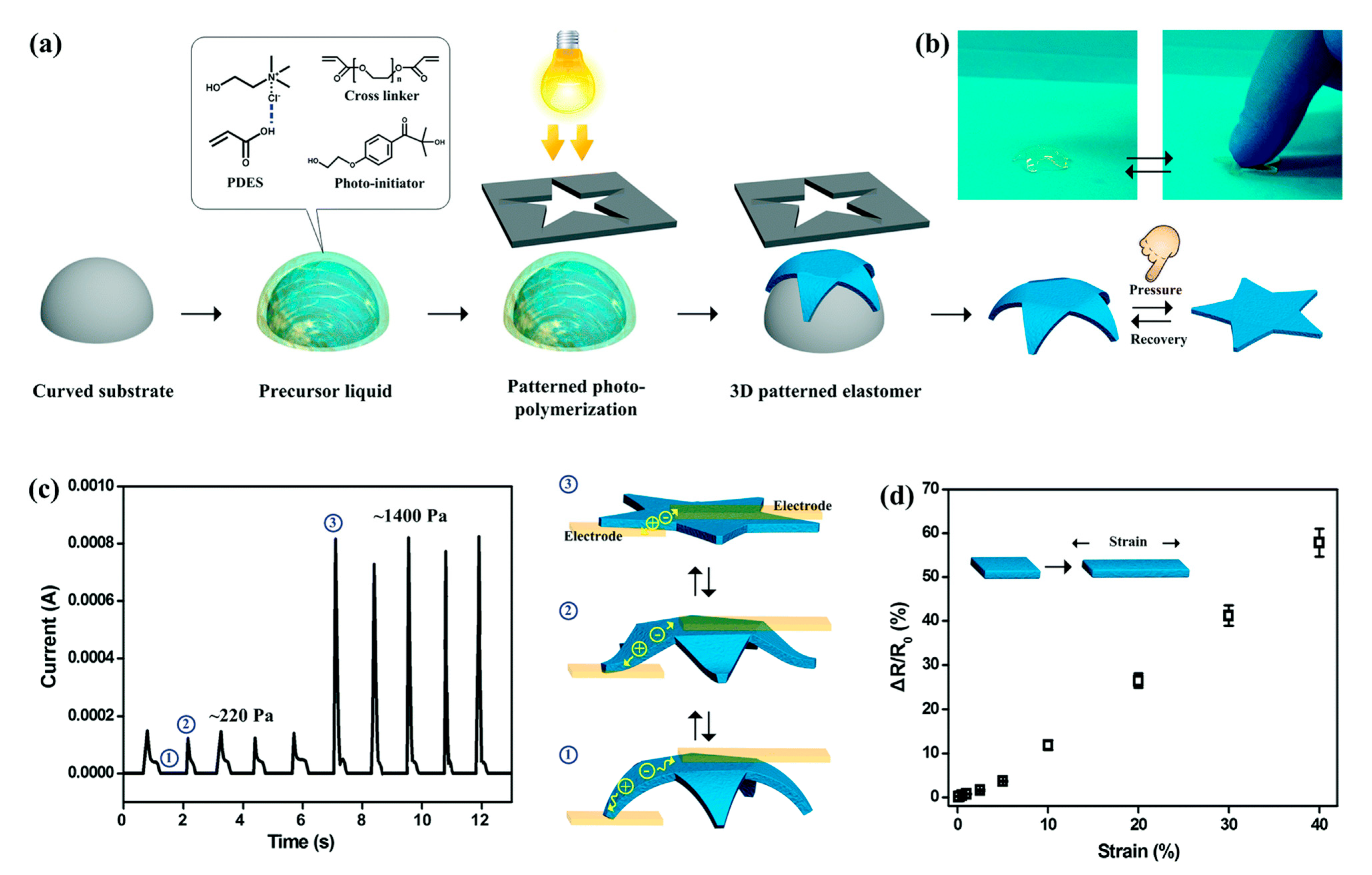
2.2. Preparation of Hydrogels
2.2.1. Polymer Hydrogels
2.2.2. Self-Assembled/Supramolecular Gels
2.3. Porous Polymer Monoliths
2.3.1. Synthesis via Porogens
2.3.2. Polymerized High Internal Phase Emulsions (PolyHIPEs)
2.4. Molecularly Imprinted Polymers (MIPs)
3. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dubé, M.A.; Salehpour, S. Applying the principles of green chemistry to polymer production technology. Macromol. React. Eng. 2014, 8, 7–28. [Google Scholar] [CrossRef]
- Sherwood, J.; De Bruyn, M.; Constantinou, A.; Moity, L.; McElroy, C.R.; Farmer, T.J.; Duncan, T.; Raverty, W.; Hunt, A.J.; Clark, J.H. Dihydrolevoglucosenone (Cyrene) as a bio-based alternative for dipolar aprotic solvents. Chem. Commun. 2014, 50, 9650–9652. [Google Scholar] [CrossRef]
- Cseri, L.; Szekely, G. Towards cleaner PolarClean: Efficient synthesis and extended applications of the polar aprotic solvent methyl 5-(dimethylamino)-2-methyl-5-oxopentanoate. Green Chem. 2019, 21, 4178–4188. [Google Scholar] [CrossRef]
- Ferlin, F.; Luciani, L.; Viteritti, O.; Brunori, F.; Piermatti, O.; Santoro, S.; Vaccaro, L. Polarclean as a sustainable reaction medium for the waste minimized synthesis of heterocyclic compounds. Front. Chem. 2019, 6, 659. [Google Scholar] [CrossRef] [PubMed]
- Mudraboyina, B.P.; Farag, S.; Banerjee, A.; Chaouki, J.; Jessop, P.G. Supercritical fluid rectification of lignin pyrolysis oil methyl ether (LOME) and its use as a bio-derived aprotic solvent. Green Chem. 2016, 18, 2089–2094. [Google Scholar] [CrossRef]
- Delolo, F.G.; dos Santos, E.N.; Gusevskaya, E.V. Anisole: A further step to sustainable hydroformylation. Green Chem. 2019, 21, 1091–1098. [Google Scholar] [CrossRef]
- Tobiszewski, M.; Bystrzanowska, M. Monetary values estimates of solvent emissions. Green Chem. 2020, 22, 7983–7988. [Google Scholar] [CrossRef]
- Guthrie, F. On eutexia. Proc. Phys. Soc. Lond. 1884, 6, 124. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 70–71. [Google Scholar] [CrossRef]
- Francisco, M.; van den Bruinhorst, A.; Kroon, M.C. Low-transition-temperature mixtures (LTTMs): A new generation of designer solvents. Angew. Chem. Int. Ed. Engl. 2013, 52, 3074–3085. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep eutectic solvents (DESs) and their applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef] [PubMed]
- Jeong, K.M.; Lee, M.S.; Nam, M.W.; Zhao, J.; Jin, Y.; Lee, D.-K.; Kwon, S.W.; Jeong, J.H.; Lee, J. Tailoring and recycling of deep eutectic solvents as sustainable and efficient extraction media. J. Chromatogr. A 2015, 1424, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Wang, Y.; Yu, W.; Wang, C.; Li, F.; Tan, Z. Temperature-responsive deep eutectic solvents as green and recyclable media for the efficient extraction of polysaccharides from Ganoderma lucidum. J. Clean. Prod. 2020, 274, 123047. [Google Scholar] [CrossRef]
- Cui, Y.; Li, C.; Bao, M. Deep eutectic solvents (DESs) as powerful and recyclable catalysts and solvents for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones. Green Process. Synth. 2019, 8, 568–576. [Google Scholar] [CrossRef]
- Hayyan, M.; Hashim, M.A.; Hayyan, A.; Al-Saadi, M.A.; AlNashef, I.M.; Mirghani, M.E.; Saheed, O.K. Are deep eutectic solvents benign or toxic? Chemosphere 2013, 90, 2193–2195. [Google Scholar] [CrossRef]
- Jung, D.; Jung, J.; Kang, S.; Li, K.; Hwang, I.; Jeong, J.H.; Kim, H.S.; Lee, J. Toxico-metabolomics study of a deep eutectic solvent comprising choline chloride and urea suggests in vivo toxicity involving oxidative stress and ammonia stress. Green Chem. 2021. [Google Scholar] [CrossRef]
- Paiva, A.; Craveiro, R.; Aroso, I.; Martins, M.; Reis, R.L.; Duarte, A.R.C. Natural deep eutectic solvents—Solvents for the 21st century. ACS Sustain. Chem. Eng. 2014, 2, 1063–1071. [Google Scholar] [CrossRef]
- Durand, E.; Lecomte, J.; Villeneuve, P. From green chemistry to nature: The versatile role of low transition temperature mixtures. Biochimie 2016, 120, 119–123. [Google Scholar] [CrossRef]
- Liao, H.G.; Jiang, Y.X.; Zhou, Z.Y.; Chen, S.P.; Sun, S.G. Shape-controlled synthesis of gold nanoparticles in deep eutectic solvents for studies of structure-functionality relationships in electrocatalysis. Angew. Chem. Int. Ed. Engl. 2008, 47, 9100–9103. [Google Scholar] [CrossRef]
- Zhu, A.; Jiang, T.; Han, B.; Zhang, J.; Xie, Y.; Ma, X. Supported choline chloride/urea as a heterogeneous catalyst for chemical fixation of carbon dioxide to cyclic carbonates. Green Chem. 2007, 9, 169–172. [Google Scholar] [CrossRef]
- Abbott, A.P.; Bell, T.J.; Handa, S.; Stoddart, B. Cationic functionalisation of cellulose using a choline based ionic liquid analogue. Green Chem. 2006, 8. [Google Scholar] [CrossRef]
- Nguyen, H.V.D.; De Vries, R.; Stoyanov, S.D. Natural deep eutectics as a “Green” cellulose cosolvent. ACS Sustain. Chem. Eng. 2020, 8, 14166–14178. [Google Scholar] [CrossRef]
- Mamajanov, I.; Engelhart, A.E.; Bean, H.D.; Hud, N.V. DNA and RNA in anhydrous media: Duplex, triplex, and G-quadruplex secondary structures in a deep eutectic solvent. Angew. Chem. Int. Ed. Engl. 2010, 49, 6310–6314. [Google Scholar] [CrossRef] [PubMed]
- Vatanpour, V.; Dehqan, A.; Harifi-Mood, A.R. Ethaline deep eutectic solvent as a hydrophilic additive in modification of polyethersulfone membrane for antifouling and separation improvement. J. Membr. Sci. 2020, 614, 118528. [Google Scholar] [CrossRef]
- Jiang, B.; Zhou, J.; Xu, M.; Dou, H.; Zhang, H.; Yang, N.; Zhang, L. Multifunctional ternary deep eutectic solvent-based membranes for the cost-effective ethylene/ethane separation. J. Membr. Sci. 2020, 610, 118243. [Google Scholar] [CrossRef]
- Karimi, M.B.; Mohammadi, F.; Hooshyari, K. Effect of deep eutectic solvents hydrogen bond acceptor on the anhydrous proton conductivity of Nafion membrane for fuel cell applications. J. Membr. Sci. 2020, 605, 118116. [Google Scholar] [CrossRef]
- Karimi, M.B.; Mohammadi, F.; Hooshyari, K. Potential use of deep eutectic solvents (DESs) to enhance anhydrous proton conductivity of Nafion 115® membrane for fuel cell applications. J. Membr. Sci. 2020, 611, 118217. [Google Scholar] [CrossRef]
- Feng, M.; Lu, X.; Zhang, J.; Li, Y.; Shi, C.; Lu, L.; Zhang, S. Direct conversion of shrimp shells to O-acylated chitin with antibacterial and anti-tumor effects by natural deep eutectic solvents. Green Chem. 2019, 21, 87–98. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Quaternary ammonium zinc- or tin-containing ionic liquids: Water insensitive, recyclable catalysts for Diels-Alder reactions. Green Chem. 2002, 4, 24–26. [Google Scholar] [CrossRef]
- Singh, B.; Lobo, H.; Shankarling, G. Selective N-alkylation of aromatic primary amines catalyzed by bio-catalyst or deep eutectic solvent. Catal. Lett. 2010, 141, 178–182. [Google Scholar] [CrossRef]
- Maugeri, Z.; Leitner, W.; Domínguez de María, P. Chymotrypsin-catalyzed peptide synthesis in deep eutectic solvents. Eur. J. Org. Chem. 2013, 2013, 4223–4228. [Google Scholar] [CrossRef]
- Nejrotti, S.; Iannicelli, M.; Jamil, S.S.; Arnodo, D.; Blangetti, M.; Prandi, C. Natural deep eutectic solvents as an efficient and reusable active system for the Nazarov cyclization. Green Chem. 2020, 22, 110–117. [Google Scholar] [CrossRef]
- Zhao, H.; Baker, G.A. Ionic liquids and deep eutectic solvents for biodiesel synthesis: A review. J. Chem. Technol. Biotechnol. 2013, 88, 3–12. [Google Scholar] [CrossRef]
- Durand, E.; Lecomte, J.; Villeneuve, P. Deep eutectic solvents: Synthesis, application, and focus on lipase-catalyzed reactions. Eur. J. Lipid Sci. Technol. 2013, 115, 379–385. [Google Scholar] [CrossRef]
- Tomé, L.I.N.; Baião, V.; da Silva, W.; Brett, C.M.A. Deep eutectic solvents for the production and application of new materials. Appl. Mater. Today 2018, 10, 30–50. [Google Scholar] [CrossRef]
- Mbous, Y.P.; Hayyan, M.; Hayyan, A.; Wong, W.F.; Hashim, M.A.; Looi, C.Y. Applications of deep eutectic solvents in biotechnology and bioengineering-Promises and challenges. Biotechnol. Adv. 2017, 35, 105–134. [Google Scholar] [CrossRef]
- Shishov, A.; Bulatov, A.; Locatelli, M.; Carradori, S.; Andruch, V. Application of deep eutectic solvents in analytical chemistry. A review. Microchem. J. 2017, 135, 33–38. [Google Scholar] [CrossRef]
- Satlewal, A.; Agrawal, R.; Bhagia, S.; Sangoro, J.; Ragauskas, A.J. Natural deep eutectic solvents for lignocellulosic biomass pretreatment: Recent developments, challenges and novel opportunities. Biotechnol. Adv. 2018, 36, 2032–2050. [Google Scholar] [CrossRef]
- Gotor-Fernandez, V.; Paul, C.E. Deep eutectic solvents for redox biocatalysis. J. Biotechnol. 2019, 293, 24–35. [Google Scholar] [CrossRef]
- Genies, E.M.; Tsintavis, C. Redox mechanism and electrochemical behaviour of polyaniline deposits. J. Electroanal. Chem. 1985, 195, 109. [Google Scholar] [CrossRef]
- Mota-Morales, J.D.; Sánchez-Leija, R.J.; Carranza, A.; Pojman, J.A.; del Monte, F.; Luna-Bárcenas, G. Free-radical polymerizations of and in deep eutectic solvents: Green synthesis of functional materials. Prog. Polym. Sci. 2018, 78, 139–153. [Google Scholar] [CrossRef]
- Abbott, A.P.; Boothby, D.; Capper, G.; Davies, D.L.; Rasheed, R.K. Deep eutectic solvents formed between choline chloride and carboxylic acid: Versatile alternatives to ionic liquids. J. Am. Chem. Soc. 2004, 126, 9142–9147. [Google Scholar] [CrossRef] [PubMed]
- Florindo, C.; Oliveira, F.S.; Rebelo, L.P.N.; Fernandes, A.M.; Marrucho, I.M. Insights into the synthesis and properties of deep eutectic solvents based on cholinium chloride and carboxylic acids. ACS Sustain. Chem. Eng. 2014, 2, 2416–2425. [Google Scholar] [CrossRef]
- Yiin, C.L.; Quitain, A.T.; Yusup, S.; Sasaki, M.; Uemura, Y.; Kida, T. Characterization of natural low transition temperature mixtures (LTTMs): Green solvents for biomass delignification. Bioresour. Technol. 2016, 199, 258–264. [Google Scholar] [CrossRef]
- Gutierrez, M.C.; Ferrer, M.L.; Mateo, C.R.; del Monte, F. Freeze-drying of aqueous solutions of deep eutectic solvents: A suitable approach to deep eutectic suspensions of self-assembled structures. Langmuir 2009, 25, 5509–5515. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Munro, H.L.; Rasheed, R.K.; Tambyrajah, V. Preparation of novel, moisture-stable, Lewis-acidic ionic liquids containing quaternary ammonium salts with functional side chains. Chem. Commun. 2001, 2010–2011. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K. Ionic liquid analogues formed from hydrated metal salts. Chem. Eur. J. 2004, 10, 3769–3774. [Google Scholar] [CrossRef]
- Gurkan, B.; Squire, H.; Pentzer, E. Metal-free deep eutectic solvents: Preparation, physical properties, and significance. J. Phys. Chem. Lett. 2019, 10, 7956–7964. [Google Scholar] [CrossRef]
- Ndizeye, N.; Suriyanarayanan, S.; Nicholls, I.A. Polymer synthesis in non-ionic deep eutectic solvents. Polym. Chem. 2019, 10, 5289–5295. [Google Scholar] [CrossRef]
- Zhang, Q.; De Oliveira Vigier, K.; Royer, S.; Jerome, F. Deep eutectic solvents: Syntheses, properties and applications. Chem. Soc. Rev. 2012, 41, 7108–7146. [Google Scholar] [CrossRef]
- Ruggeri, S.; Poletti, F.; Zanardi, C.; Pigani, L.; Zanfrognini, B.; Corsi, E.; Dossi, N.; Salomäki, M.; Kivelä, H.; Lukkari, J.; et al. Chemical and electrochemical properties of a hydrophobic deep eutectic solvent. Electrochim. Acta 2019, 295, 124–129. [Google Scholar] [CrossRef]
- Pereira, V.A.; Rezende, T.C.; Mendonça, P.V.; Coelho, J.F.J.; Serra, A.C. Homogeneous polymerization of hydrophobic monomers in a bio-based dl-menthol/1-tetradecanol eutectic mixture by ATRP and RAFT polymerization. Green Chem. 2020, 22, 6827–6835. [Google Scholar] [CrossRef]
- Florindo, C.; Celia-Silva, L.G.; Martins, L.F.G.; Branco, L.C.; Marrucho, I.M. Supramolecular hydrogel based on a sodium deep eutectic solvent. Chem. Commun. 2018, 54, 7527–7530. [Google Scholar] [CrossRef] [PubMed]
- Abbott, A.P.; El Ttaib, K.; Frisch, G.; Ryder, K.S.; Weston, D. The electrodeposition of silver composites using deep eutectic solvents. Phys. Chem. Chem. Phys. 2012, 14, 2443–2449. [Google Scholar] [CrossRef] [PubMed]
- Wagle, D.V.; Zhao, H.; Baker, G.A. Deep eutectic solvents: Sustainable media for nanoscale and functional materials. Acc. Chem. Res. 2014, 47, 2299–2308. [Google Scholar] [CrossRef] [PubMed]
- Abbott, A.P.; Harris, R.C.; Ryder, K.S. Application of hole theory to define ionic liquids by their transport properties. J. Phys. Chem. B 2007, 111, 4910. [Google Scholar] [CrossRef]
- Kareem, M.A.; Mjalli, F.S.; Hashim, M.A.; AlNashef, I.M. Phosphonium-based ionic liquids analogues and their physical properties. J. Chem. Eng. Data 2010, 55, 4632. [Google Scholar] [CrossRef]
- Chen, W.; Xue, Z.; Wang, J.; Jiang, J.; Zhao, X.; Mu, T. Investigation on the thermal stability of deep eutectic solvents. Acta Phys. Chim. Sin. 2018, 34, 904–911. [Google Scholar] [CrossRef]
- Craveiro, R.; Aroso, I.; Flammia, V.; Carvalho, T.; Viciosa, M.T.; Dionísio, M.; Barreiros, S.; Reis, R.L.; Duarte, A.R.C.; Paiva, A. Properties and thermal behavior of natural deep eutectic solvents. J. Mol. Liq. 2016, 215, 534–540. [Google Scholar] [CrossRef]
- Fazende, K.F.; Phachansitthi, M.; Mota-Morales, J.D.; Pojman, J.A. Frontal polymerization of deep eutectic solvents composed of acrylic and methacrylic acids. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 4046–4050. [Google Scholar] [CrossRef]
- Mota-Morales, J.D.; Gutiérrez, M.C.; Ferrer, M.L.; Sanchez, I.C.; Elizalde-Peña, E.A.; Pojman, J.A.; Monte, F.D.; Luna-Bárcenas, G. Deep eutectic solvents as both active fillers and monomers for frontal polymerization. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 1767–1773. [Google Scholar] [CrossRef]
- Mota-Morales, J.D.; Gutiérrez, M.C.; Ferrer, M.L.; Jiménez, R.; Santiago, P.; Sanchez, I.C.; Terrones, M.; Del Monte, F.; Luna-Bárcenas, G. Synthesis of macroporous poly(acrylic acid)-carbon nanotube composites by frontal polymerization in deep-eutectic solvents. J. Mater. Chem. A 2013, 1, 3970. [Google Scholar] [CrossRef]
- Mota-Morales, J.D.; Gutierrez, M.C.; Sanchez, I.C.; Luna-Barcenas, G.; del Monte, F. Frontal polymerizations carried out in Deep-Eutectic mixtures providing both the monomers and the polymerization medium. Chem. Commun. 2011, 47, 5328–5330. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Leija, R.J.; Pojman, J.A.; Luna-Barcenas, G.; Mota-Morales, J.D. Controlled release of lidocaine hydrochloride from polymerized drug-based deep-eutectic solvents. J. Mater. Chem. B 2014, 2, 7495–7501. [Google Scholar] [CrossRef]
- Fazende, K.F.; Gary, D.P.; Mota-Morales, J.D.; Pojman, J.A. Kinetic studies of photopolymerization of monomer-containing deep eutectic solvents. Macromol. Chem. Phys. 2020, 221. [Google Scholar] [CrossRef]
- Isik, M.; Ruiperez, F.; Sardon, H.; Gonzalez, A.; Zulfiqar, S.; Mecerreyes, D. Innovative poly(ionic liquid)s by the polymerization of deep eutectic monomers. Macromol. Rapid Commun. 2016, 37, 1135–1142. [Google Scholar] [CrossRef]
- Ren’ai, L.; Zhang, K.; Chen, G.; Su, B.; Tian, J.; He, M.; Lu, F. Green polymerizable deep eutectic solvent (PDES) type conductive paper for origami 3D circuits. Chem. Commun. 2018, 54, 2304–2307. [Google Scholar] [CrossRef]
- Ren’ai, L.; Chen, G.; He, M.; Tian, J.; Su, B. Patternable transparent and conductive elastomers towards flexible tactile/strain sensors. J. Mater. Chem. C 2017, 5, 8475–8481. [Google Scholar] [CrossRef]
- Sánchez-Leija, R.J.; Torres-Lubián, J.R.; Reséndiz-Rubio, A.; Luna-Bárcenas, G.; Mota-Morales, J.D. Enzyme-mediated free radical polymerization of acrylamide in deep eutectic solvents. RSC Adv. 2016, 6, 13072–13079. [Google Scholar] [CrossRef]
- Nicolas, J.; Guillaneuf, Y.; Lefay, C.; Bertin, D.; Gigmes, D.; Charleux, B. Nitroxide-mediated polymerization. Prog. Polym. Sci. 2013, 38, 63–235. [Google Scholar] [CrossRef]
- Matyjaszewski, K. Atom transfer radical polymerization (ATRP): Current status and future perspectives. Macromolecules 2012, 45, 4015–4039. [Google Scholar] [CrossRef]
- Perrier, S. 50th anniversary perspective: RAFT polymerization—A user guide. Macromolecules 2017, 50, 7433–7447. [Google Scholar] [CrossRef]
- Maximiano, P.; Mendonça, P.V.; Santos, M.R.E.; Costa, J.R.C.; Guliashvili, T.; Serra, A.C.; Coelho, J.F.J. Eutectic mixtures as a green alternative for efficient catalyst recycling in atom transfer radical polymerizations. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 371–381. [Google Scholar] [CrossRef]
- Mendonça, P.V.; Lima, M.S.; Guliashvili, T.; Serra, A.C.; Coelho, J.F.J. Deep eutectic solvents (DES): Excellent green solvents for rapid SARA ATRP of biorelevant hydrophilic monomers at ambient temperature. Polymer 2017, 132, 114–121. [Google Scholar] [CrossRef]
- Quirós-Montes, L.; Carriedo, G.A.; García-Álvarez, J.; Presa Soto, A. Deep eutectic solvents for Cu-catalysed ARGET ATRP under an air atmosphere: A sustainable and efficient route to poly(methyl methacrylate) using a recyclable Cu(ii) metal-organic framework. Green Chem. 2019, 21, 5865–5875. [Google Scholar] [CrossRef]
- Wang, J.; Han, J.; Khan, M.Y.; He, D.; Peng, H.; Chen, D.; Xie, X.; Xue, Z. Deep eutectic solvents for green and efficient iron-mediated ligand-free atom transfer radical polymerization. Polym. Chem. 2017, 8, 1616–1627. [Google Scholar] [CrossRef]
- Santha Kumar, A.R.S.; Singha, N.K. RAFT polymerization of 2-hydroxyethyl methacrylate in a deep eutectic solvent. J. Polym. Sci. Part A Polym. Chem. 2019, 57, 2281–2286. [Google Scholar] [CrossRef]
- Szwarc, M. Living-polymers. Nature 1956, 178, 1168. [Google Scholar] [CrossRef]
- Szwarc, M.; Levy, M.; Milkovich, R. Polymerization initiated by electron transfer to monomer. A new method of formation of block polymers1. J. Am. Chem. Soc. 1956, 78, 2656–2657. [Google Scholar] [CrossRef]
- Sanchez-Condado, A.; Carriedo, G.A.; Presa Soto, A.; Rodriguez-Alvarez, M.J.; Garcia-Alvarez, J.; Hevia, E. Organolithium-initiated polymerization of olefins in deep eutectic solvents under aerobic conditions. ChemSusChem 2019, 12, 3134–3143. [Google Scholar] [CrossRef]
- Huang, Y.-T.; Wang, W.-C.; Hsu, C.-P.; Lu, W.-Y.; Chuang, W.-J.; Chiang, M.Y.; Lai, Y.-C.; Chen, H.-Y. The ring-opening polymerization of ε-caprolactone and l-lactide using aluminum complexes bearing benzothiazole ligands as catalysts. Polym. Chem. 2016, 7, 4367–4377. [Google Scholar] [CrossRef]
- Coulembier, O.; Lemaur, V.; Josse, T.; Minoia, A.; Cornil, J.; Dubois, P. Synthesis of poly(l-lactide) and gradient copolymers from al-lactide/trimethylene carbonate eutectic melt. Chem. Sci. 2012, 3, 723–726. [Google Scholar] [CrossRef]
- Perez-Garcia, M.G.; Gutierrez, M.C.; Mota-Morales, J.D.; Luna-Barcenas, G.; Del Monte, F. Synthesis of biodegradable macroporous poly(l-lactide)/poly(epsilon-caprolactone) blend using oil-in-eutectic-mixture high-internal-phase emulsions as template. ACS Appl. Mater. Interfaces 2016, 8, 16939–16949. [Google Scholar] [CrossRef]
- García-Argüelles, S.; García, C.; Serrano, M.C.; Gutiérrez, M.C.; Ferrer, M.L.; del Monte, F. Near-to-eutectic mixtures as bifunctional catalysts in the low-temperature-ring-opening-polymerization of ε-caprolactone. Green Chem. 2015, 17, 3632–3643. [Google Scholar] [CrossRef]
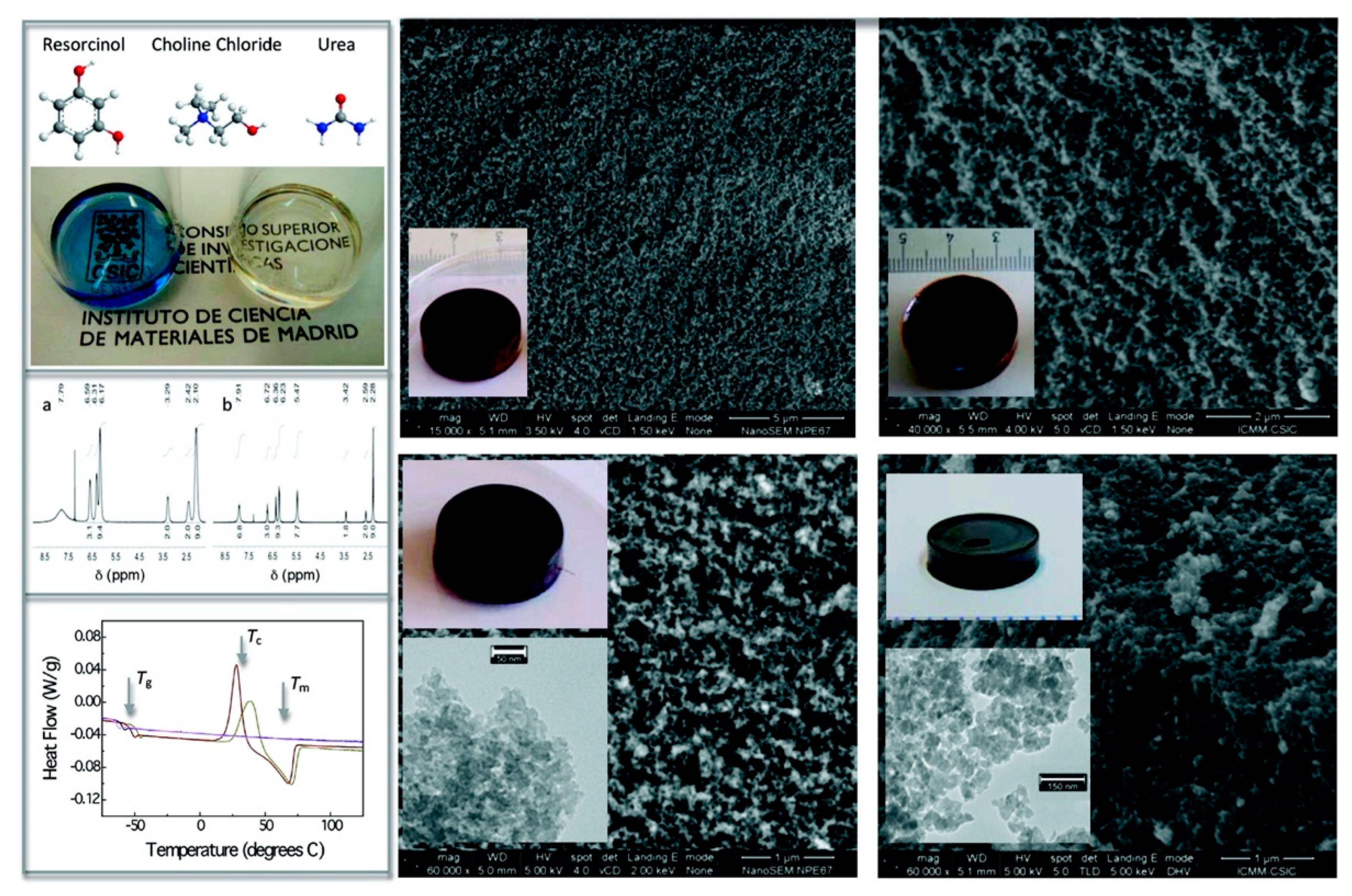
- Gutiérrez, M.C.; Rubio, F.; del Monte, F. Resorcinol-formaldehyde polycondensation in deep eutectic solvents for the preparation of carbons and carbon-carbon nanotube composites. Chem. Mater. 2010, 22, 2711–2719. [Google Scholar] [CrossRef]
- Carriazo, D.; Gutiérrez, M.C.; Ferrer, M.L.; del Monte, F. Resorcinol-based deep eutectic solvents as both carbonaceous precursors and templating agents in the synthesis of hierarchical porous carbon monoliths. Chem. Mater. 2010, 22, 6146–6152. [Google Scholar] [CrossRef]
- Gutierrez, M.C.; Carriazo, D.; Tamayo, A.; Jimenez, R.; Pico, F.; Rojo, J.M.; Ferrer, M.L.; del Monte, F. Deep-eutectic-solvent-assisted synthesis of hierarchical carbon electrodes exhibiting capacitance retention at high current densities. Chemistry 2011, 17, 10533–10537. [Google Scholar] [CrossRef]
- Gutiérrez, M.C.; Carriazo, D.; Ania, C.O.; Parra, J.B.; Ferrer, M.L.; del Monte, F. Deep eutectic solvents as both precursors and structure directing agents in the synthesis of nitrogen doped hierarchical carbons highly suitable for CO2 capture. Energy Environ. Sci. 2011, 4. [Google Scholar] [CrossRef]
- Carriazo, D.; Gutiérrez, M.C.; Jiménez, R.; Ferrer, M.L.; del Monte, F. Deep-eutectic-assisted synthesis of bimodal porous carbon monoliths with high electrical conductivities. Part. Part. Syst. Charact. 2013, 30, 316–320. [Google Scholar] [CrossRef]
- Patiño, J.; Gutiérrez, M.C.; Carriazo, D.; Ania, C.O.; Fierro, J.L.G.; Ferrer, M.L.; del Monte, F. DES assisted synthesis of hierarchical nitrogen-doped carbon molecular sieves for selective CO2versus N2 adsorption. J. Mater. Chem. A 2014, 2, 8719–8729. [Google Scholar] [CrossRef]
- Patiño, J.; Gutiérrez, M.C.; Carriazo, D.; Ania, C.O.; Parra, J.B.; Ferrer, M.L.; del Monte, F. Deep eutectic assisted synthesis of carbon adsorbents highly suitable for low-pressure separation of CO2–CH4 gas mixtures. Energy Environ. Sci. 2012, 5. [Google Scholar] [CrossRef]
- Hong, S.; Sun, X.; Lian, H.; Pojman, J.A.; Mota-Morales, J.D. Zinc chloride/acetamide deep eutectic solvent-mediated fractionation of lignin produces high- and low-molecular-weight fillers for phenol-formaldehyde resins. J. Appl. Polym. Sci. 2019, 137. [Google Scholar] [CrossRef]
- Serrano, M.C.; Gutiérrez, M.C.; Jiménez, R.; Ferrer, M.L.; del Monte, F. Synthesis of novel lidocaine-releasing poly(diol-co-citrate) elastomers by using deep eutectic solvents. Chem. Commun. 2012, 48, 579–581. [Google Scholar] [CrossRef] [PubMed]
- García-Argüelles, S.; Serrano, M.C.; Gutiérrez, M.C.; Ferrer, M.L.; Yuste, L.; Rojo, F.; del Monte, F. Deep Eutectic solvent-assisted synthesis of biodegradable polyesters with antibacterial properties. Langmuir 2013, 29, 9525–9534. [Google Scholar] [CrossRef]
- Fernandes, P.M.V.; Campiña, J.M.; Pereira, N.M.; Pereira, C.M.; Silva, F. Biodegradable deep-eutectic mixtures as electrolytes for the electrochemical synthesis of conducting polymers. J. Appl. Electrochem. 2012, 42, 997–1003. [Google Scholar] [CrossRef]
- Fernandes, P.M.V.; Campiña, J.M.; Pereira, C.M.; Silva, F. Electrosynthesis of Polyaniline from choline-based deep eutectic solvents: Morphology, stability and electrochromism. J. Electrochem. Soc. 2012, 159, G97–G105. [Google Scholar] [CrossRef]
- Prathish, K.P.; Carvalho, R.C.; Brett, C.M.A. Highly sensitive poly(3,4-ethylenedioxythiophene) modified electrodes by electropolymerisation in deep eutectic solvents. Electrochem. Commun. 2014, 44, 8–11. [Google Scholar] [CrossRef]
- Prathish, K.P.; Carvalho, R.C.; Brett, C.M.A. Electrochemical characterisation of poly(3,4-ethylenedioxythiophene) film modified glassy carbon electrodes prepared in deep eutectic solvents for simultaneous sensing of biomarkers. Electrochim. Acta 2016, 187, 704–713. [Google Scholar] [CrossRef]
- Hosu, O.; Bârsan, M.M.; Cristea, C.; Săndulescu, R.; Brett, C.M.A. Nanostructured electropolymerized poly(methylene blue) films from deep eutectic solvents. Optimization and characterization. Electrochim. Acta 2017, 232, 285–295. [Google Scholar] [CrossRef]
- Jaumaux, P.; Liu, Q.; Zhou, D.; Xu, X.; Wang, T.; Wang, Y.; Kang, F.; Li, B.; Wang, G. Deep-eutectic-solvent-based self-healing polymer electrolyte for safe and long-life lithium-metal batteries. Angew. Chem. Int. Ed. Engl. 2020, 59, 9134–9142. [Google Scholar] [CrossRef]
- Bednarz, S.; Fluder, M.; Galica, M.; Bogdal, D.; Maciejaszek, I. Synthesis of hydrogels by polymerization of itaconic acid-choline chloride deep eutectic solvent. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Bednarz, S.; Półćwiartek, K.; Wityk, J.; Strachota, B.; Kredatusová, J.; Beneš, H.; Wesołowska-Piętak, A.; Kowalski, G. Polymerization-crosslinking of renewable itaconic acid in water and in deep eutectic solvents. Eur. Polym. J. 2017, 95, 241–254. [Google Scholar] [CrossRef]
- Bednarz, S.; Wesołowska, A.; Trątnowiecka, M.; Bogdał, D. Polymers from biobased-monomers: Macroporous Itaconic xerogels prepared in deep eutectic solvents. J. Renew. Mater. 2016, 4, 18–23. [Google Scholar] [CrossRef]
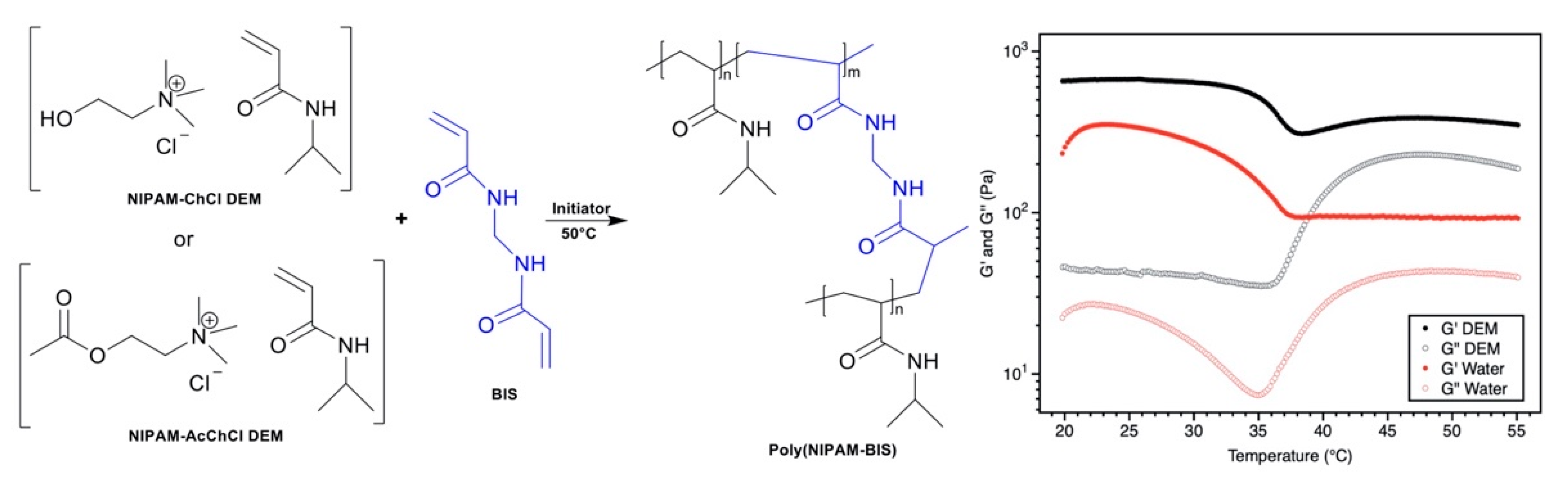
- Nahar, Y.; Horne, J.; Truong, V.; Bissember, A.C.; Thickett, S.C. Preparation of thermoresponsive hydrogels via polymerizable deep eutectic monomer solvents. Polym. Chem. 2020. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, S.; Chen, Y.; Yan, S.; Tao, M.; Wen, P. Facile and green preparation of superfast responsive macroporous polyacrylamide hydrogels by frontal polymerization of polymerizable deep eutectic monomers. Ind. Eng. Chem. Res. 2020, 59, 1526–1533. [Google Scholar] [CrossRef]
- Mukesh, C.; Gupta, R.; Srivastava, D.N.; Nataraj, S.K.; Prasad, K. Preparation of a natural deep eutectic solvent mediated self polymerized highly flexible transparent gel having super capacitive behaviour. RSC Adv. 2016, 6, 28586–28592. [Google Scholar] [CrossRef]
- Mukesh, C.; Upadhyay, K.K.; Devkar, R.V.; Chudasama, N.A.; Raol, G.G.; Prasad, K. Preparation of a noncytotoxic hemocompatible ion gel by self-polymerization of HEMA in a green deep eutectic solvent. Macromol. Chem. Phys. 2016, 217, 1899–1906. [Google Scholar] [CrossRef]
- Qin, H.; Panzer, M.J. Chemically cross-linked poly(2-hydroxyethyl methacrylate)-supported deep eutectic solvent gel electrolytes for eco-friendly supercapacitors. ChemElectroChem 2017, 4, 2556–2562. [Google Scholar] [CrossRef]
- Okesola, B.O.; Smith, D.K. Applying low-molecular weight supramolecular gelators in an environmental setting—Self-assembled gels as smart materials for pollutant removal. Chem. Soc. Rev. 2016, 45, 4226–4251. [Google Scholar] [CrossRef]
- Noro, A.; Hayashi, M.; Ohshika, A.; Matsushita, Y. Simple preparation of supramolecular polymer gels via hydrogen bonding by blending two liquid polymers. Soft Matter 2011, 7. [Google Scholar] [CrossRef]
- Ruiz-Olles, J.; Slavik, P.; Whitelaw, N.K.; Smith, D.K. Self-assembled gels formed in deep eutectic solvents: Supramolecular eutectogels with high ionic conductivity. Angew. Chem. Int. Ed. Engl. 2019, 58, 4173–4178. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Cai, C.; Li, F.; Tan, Z.; Dong, S. Deep eutectic supramolecular polymers: Bulk supramolecular materials. Angew. Chem. Int. Ed. Engl. 2020, 59, 11871–11875. [Google Scholar] [CrossRef] [PubMed]
- Nischang, I.; Causon, T.J. Porous polymer monoliths: From their fundamental structure to analytical engineering applications. TrAC Trends Anal. Chem. 2016, 75, 108–117. [Google Scholar] [CrossRef]
- Wang, R.; Li, W.; Chen, Z. Solid phase microextraction with poly(deep eutectic solvent) monolithic column online coupled to HPLC for determination of non-steroidal anti-inflammatory drugs. Anal. Chim. Acta 2018, 1018, 111–118. [Google Scholar] [CrossRef]
- Zhang, L.S.; Gao, S.P.; Huang, Y.P.; Liu, Z.S. Green synthesis of polymer monoliths incorporated with carbon nanotubes in room temperature ionic liquid and deep eutectic solvents. Talanta 2016, 154, 335–340. [Google Scholar] [CrossRef]
- Zhang, L.S.; Zhao, Q.L.; Li, X.X.; Li, X.X.; Huang, Y.P.; Liu, Z.S. Green synthesis of mesoporous molecular sieve incorporated monoliths using room temperature ionic liquid and deep eutectic solvents. Talanta 2016, 161, 660–667. [Google Scholar] [CrossRef]
- Zhang, T.; Sanguramath, R.A.; Israel, S.; Silverstein, M.S. Emulsion templating: Porous polymers and beyond. Macromolecules 2019, 52, 5445–5479. [Google Scholar] [CrossRef]
- Cameron, N.R. High internal phase emulsion templating as a route to well-defined porous polymers. Polymer 2005, 46, 1439–1449. [Google Scholar] [CrossRef]
- Hayward, A.S.; Sano, N.; Przyborski, S.A.; Cameron, N.R. Acrylic-acid-functionalized polyHIPE scaffolds for use in 3D cell culture. Macromol. Rapid Commun. 2013, 34, 1844–1849. [Google Scholar] [CrossRef]
- Tebboth, M.; Kogelbauer, A.; Bismarck, A. Liquid-liquid extraction within emulsion templated macroporous polymers. Ind. Eng. Chem. Res. 2015, 54, 7284–7291. [Google Scholar] [CrossRef]
- Liu, H.; Wan, D.; Du, J.; Jin, M. Dendritic amphiphile mediated one-pot preparation of 3D pt nanoparticles-decorated polyHIPE as a durable and well-recyclable catalyst. ACS Appl. Mater. Interfaces 2015, 7, 20885–20892. [Google Scholar] [CrossRef] [PubMed]
- Carranza, A.; Pojman, J.A.; Mota-Morales, J.D. Deep-eutectic solvents as a support in the nonaqueous synthesis of macroporous poly(HIPEs). RSC Adv. 2014, 4. [Google Scholar] [CrossRef]
- Carranza, A.; Perez-Garcia, M.G.; Song, K.; Jeha, G.M.; Diao, Z.; Jin, R.; Bogdanchikova, N.; Soltero, A.F.; Terrones, M.; Wu, Q.; et al. Deep-eutectic solvents as MWCNT delivery vehicles in the synthesis of functional poly(HIPE) nanocomposites for applications as selective sorbents. ACS Appl. Mater. Interfaces 2016, 8, 31295–31303. [Google Scholar] [CrossRef] [PubMed]
- Pérez-García, M.G.; Carranza, A.; Puig, J.E.; Pojman, J.A.; del Monte, F.; Luna-Bárcenas, G.; Mota-Morales, J.D. Porous monoliths synthesized via polymerization of styrene and divinyl benzene in nonaqueous deep-eutectic solvent-based HIPEs. RSC Adv. 2015, 5, 23255–23260. [Google Scholar] [CrossRef]
- Li, X.; Row, K.H. Application of novel ternary deep eutectic solvents as a functional monomer in molecularly imprinted polymers for purification of levofloxacin. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 56–63. [Google Scholar] [CrossRef]
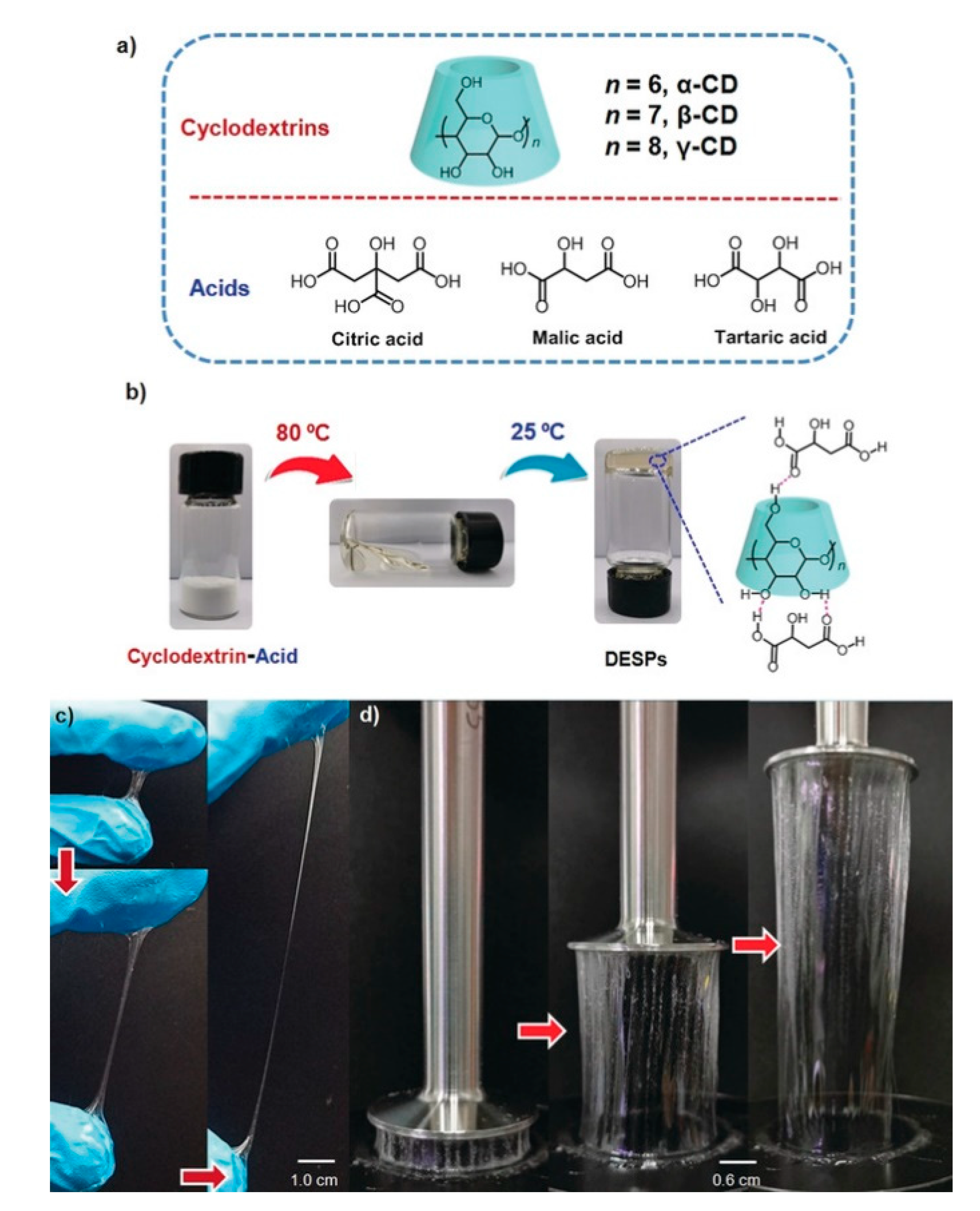
- Li, X.; Row, K.H. Development of deep eutectic solvents applied in extraction and separation. J. Sep. Sci. 2016, 39, 3505–3520. [Google Scholar] [CrossRef]
- Ma, W.; Dai, Y.; Row, K.H. Molecular imprinted polymers based on magnetic chitosan with different deep eutectic solvent monomers for the selective separation of catechins in black tea. Electrophoresis 2018. [Google Scholar] [CrossRef]
- Li, G.; Wang, W.; Wang, Q.; Zhu, T. Deep eutectic solvents modified molecular imprinted polymers for optimized purification of chlorogenic acid from honeysuckle. J. Chromatogr. Sci. 2016, 54, 271–279. [Google Scholar] [CrossRef]
- Fu, N.; Liu, X.; Li, L.; Tang, B.; Row, K.H. Ternary choline chloride/caffeic acid/ethylene glycol deep eutectic solvent as both a monomer and template in a molecularly imprinted polymer. J. Sep. Sci. 2017, 40, 2286–2291. [Google Scholar] [CrossRef]
- Li, G.; Row, K.H. Magnetic molecularly imprinted polymers for recognition and enrichment of polysaccharides from seaweed. J. Sep. Sci. 2017, 40, 4765–4772. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Dai, Q.; Zhou, Y. Magnetic deep eutectic solvents molecularly imprinted polymers for the selective recognition and separation of protein. Anal. Chim. Acta 2016, 936, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, K.; Xing, H.; Li, X.; Zhang, Q.; Han, D.; He, H.; Yan, H.; Tang, B. Deep eutectic solvents functionalized polymers for easily and efficiently promoting biocatalysis. J. Catal. 2019, 374, 306–319. [Google Scholar] [CrossRef]
- Li, S.; Han, G.; Zhang, W. Photoregulated reversible addition-fragmentation chain transfer (RAFT) polymerization. Polym. Chem. 2020, 11, 1830–1844. [Google Scholar] [CrossRef]
- Dadashi-Silab, S.; Lee, I.-H.; Anastasaki, A.; Lorandi, F.; Narupai, B.; Dolinski, N.D.; Allegrezza, M.L.; Fantin, M.; Konkolewicz, D.; Hawker, C.J.; et al. Investigating temporal control in photoinduced atom transfer radical polymerization. Macromolecules 2020, 53, 5280–5288. [Google Scholar] [CrossRef]
- Phommalysack-Lovan, J.; Chu, Y.; Boyer, C.; Xu, J. PET-RAFT polymerisation: Towards green and precision polymer manufacturing. Chem. Commun. 2018, 54, 6591–6606. [Google Scholar] [CrossRef]
- O’Dea, R.M.; Willie, J.A.; Epps, T.H. 100th anniversary of macromolecular science viewpoint: Polymers from lignocellulosic biomass. Current challenges and future opportunities. ACS Macro Lett. 2020, 9, 476–493. [Google Scholar] [CrossRef]
- Wang, S.; Shuai, L.; Saha, B.; Vlachos, D.G.; Epps, T.H. From tree to tape: Direct synthesis of pressure sensitive adhesives from depolymerized raw lignocellulosic biomass. ACS Cent. Sci. 2018, 4, 701–708. [Google Scholar] [CrossRef]
- Holmberg, A.L.; Reno, K.H.; Nguyen, N.A.; Wool, R.P.; Epps, T.H. Syringyl methacrylate, a hardwood lignin-based monomer for high-Tg polymeric materials. ACS Macro Lett. 2016, 5, 574–578. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, H.; Deng, J.; Wu, Y. High glass-transition temperature acrylate polymers derived from biomasses, syringaldehyde, and vanillin. Macromol. Chem. Phys. 2016, 217, 2402–2408. [Google Scholar] [CrossRef]
- Thickett, S.C.; Teo, G.H. Recent advances in colloidal nanocomposite design via heterogeneous polymerization techniques. Polym. Chem. 2019, 10, 2906–2924. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nahar, Y.; Thickett, S.C. Greener, Faster, Stronger: The Benefits of Deep Eutectic Solvents in Polymer and Materials Science. Polymers 2021, 13, 447. https://doi.org/10.3390/polym13030447
Nahar Y, Thickett SC. Greener, Faster, Stronger: The Benefits of Deep Eutectic Solvents in Polymer and Materials Science. Polymers. 2021; 13(3):447. https://doi.org/10.3390/polym13030447
Chicago/Turabian StyleNahar, Yeasmin, and Stuart C. Thickett. 2021. "Greener, Faster, Stronger: The Benefits of Deep Eutectic Solvents in Polymer and Materials Science" Polymers 13, no. 3: 447. https://doi.org/10.3390/polym13030447
APA StyleNahar, Y., & Thickett, S. C. (2021). Greener, Faster, Stronger: The Benefits of Deep Eutectic Solvents in Polymer and Materials Science. Polymers, 13(3), 447. https://doi.org/10.3390/polym13030447







