Effects of Alkyl-Substituted Polybenzoxazines on Tribological Properties of Non-Asbestos Composite Friction Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Benzoxazine Resins
2.3. Preparation of Polybenzoxazine Composites
2.4. Characterizations
3. Results and Discussion
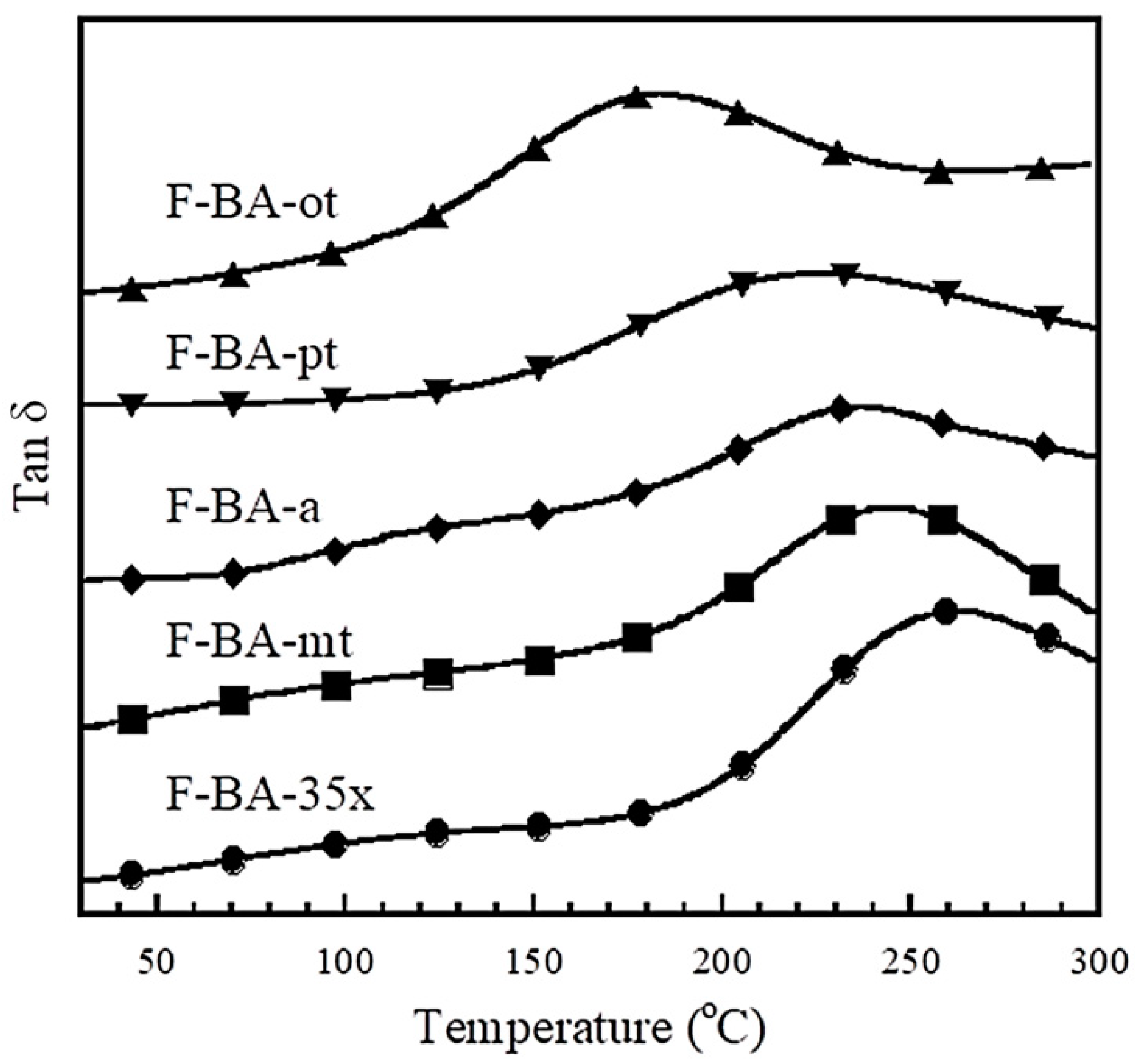
3.1. Thermomechanical Properties of Polybenzoxazine Composites
3.2. Thermal Stability of Polybenzoxazine Composites
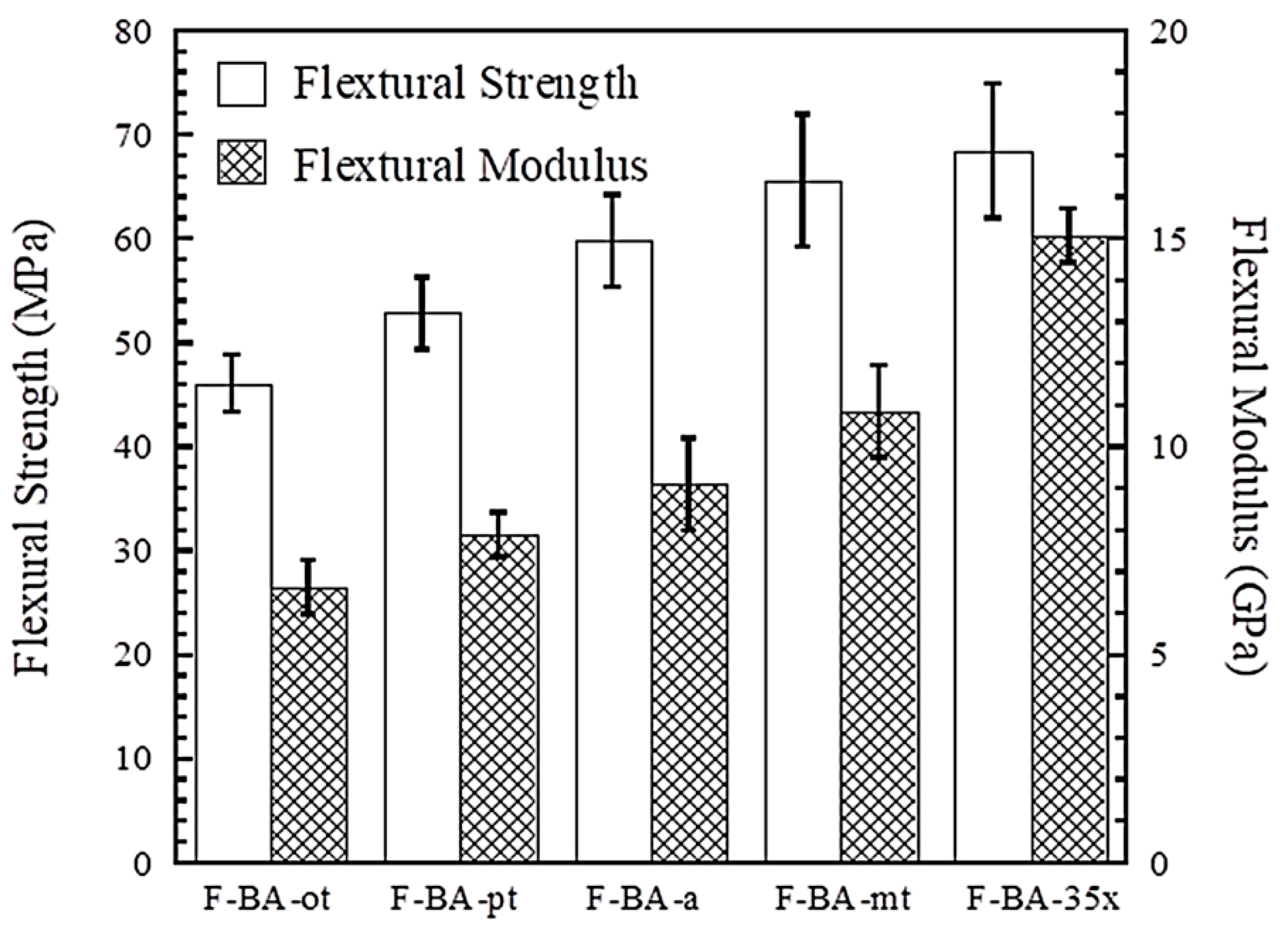
3.3. Flexural Property of Polybenzoxazine Composites
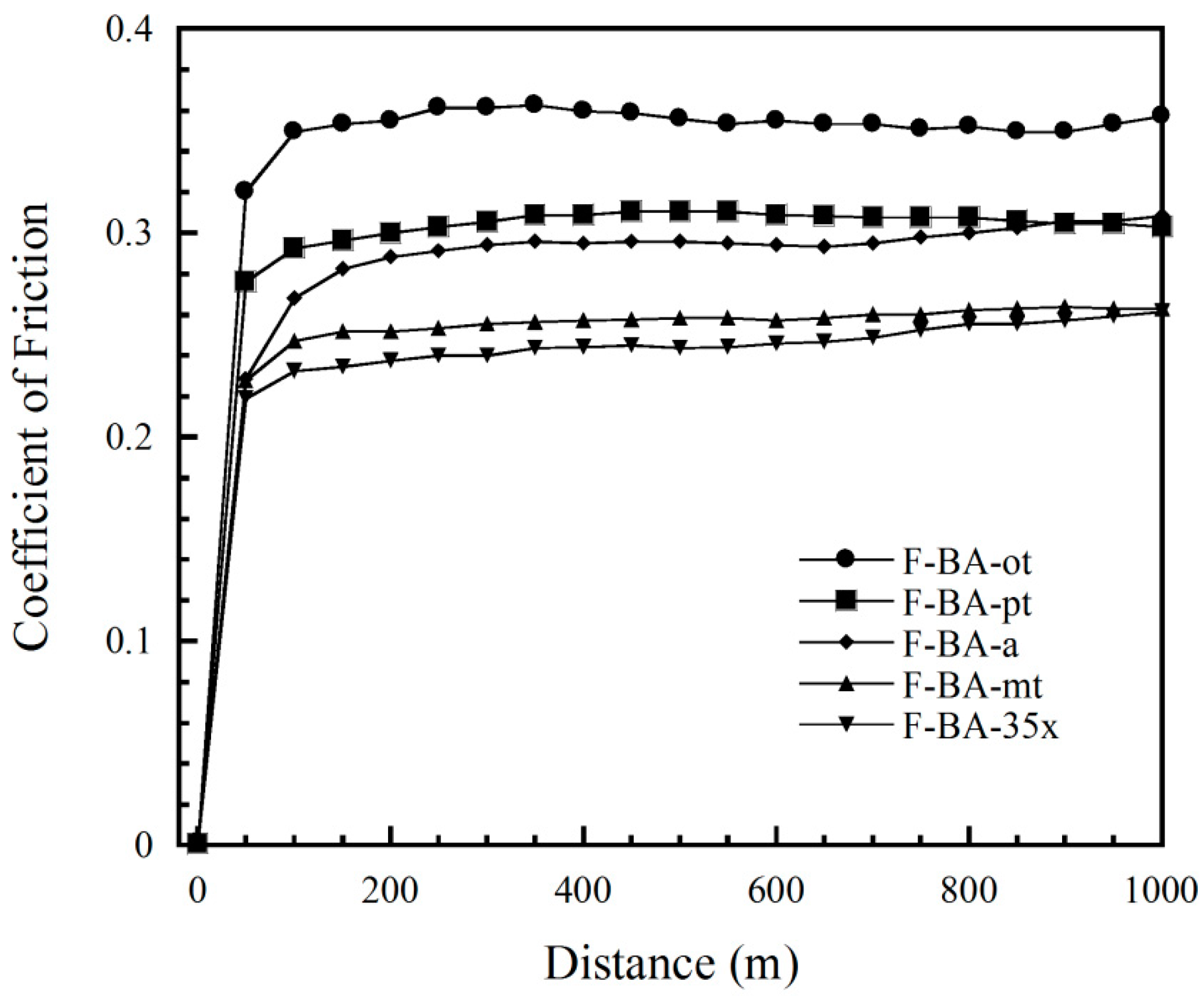
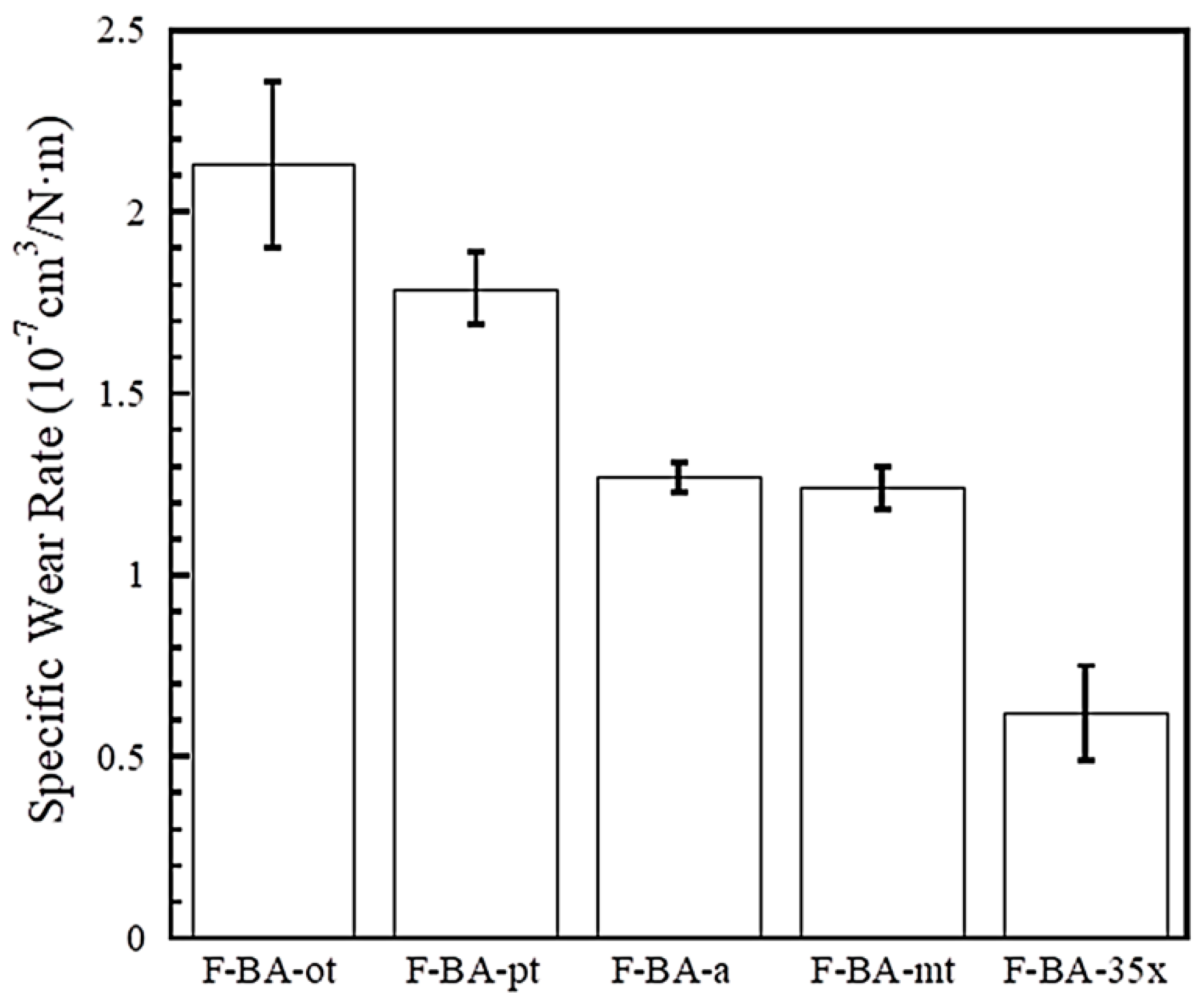
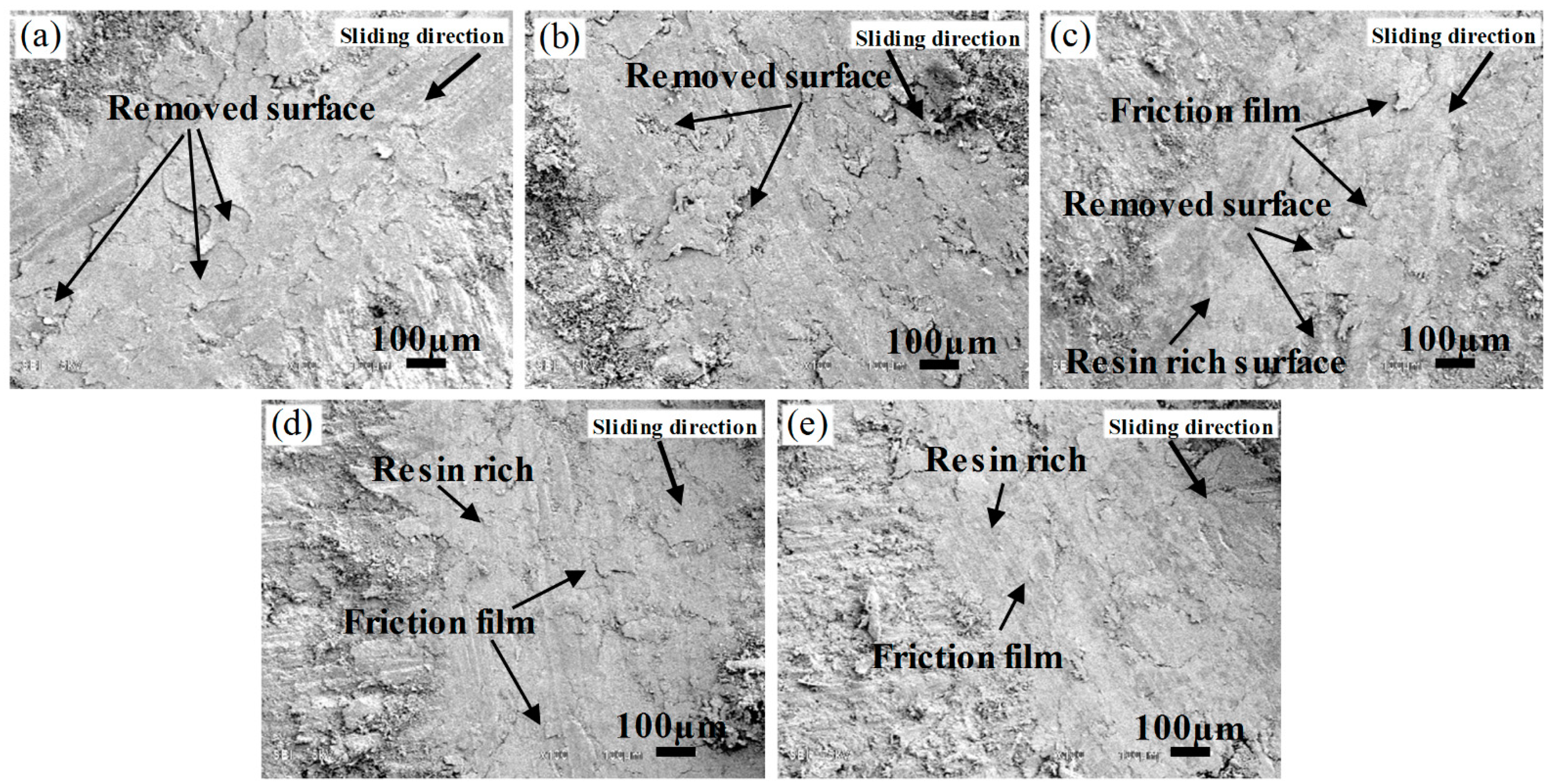
3.4. Tribological Properties of Polybenzoxazine Composites
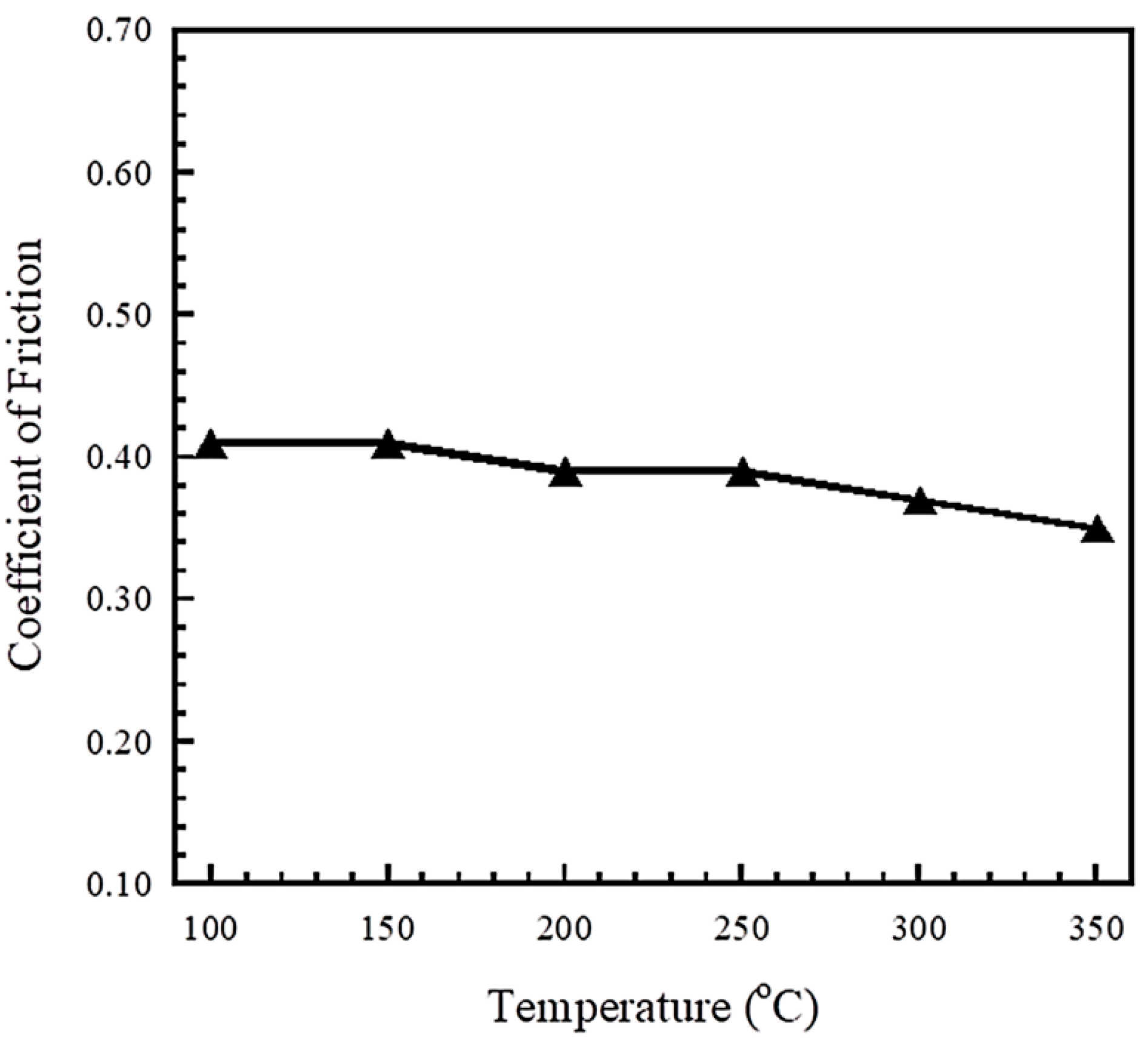
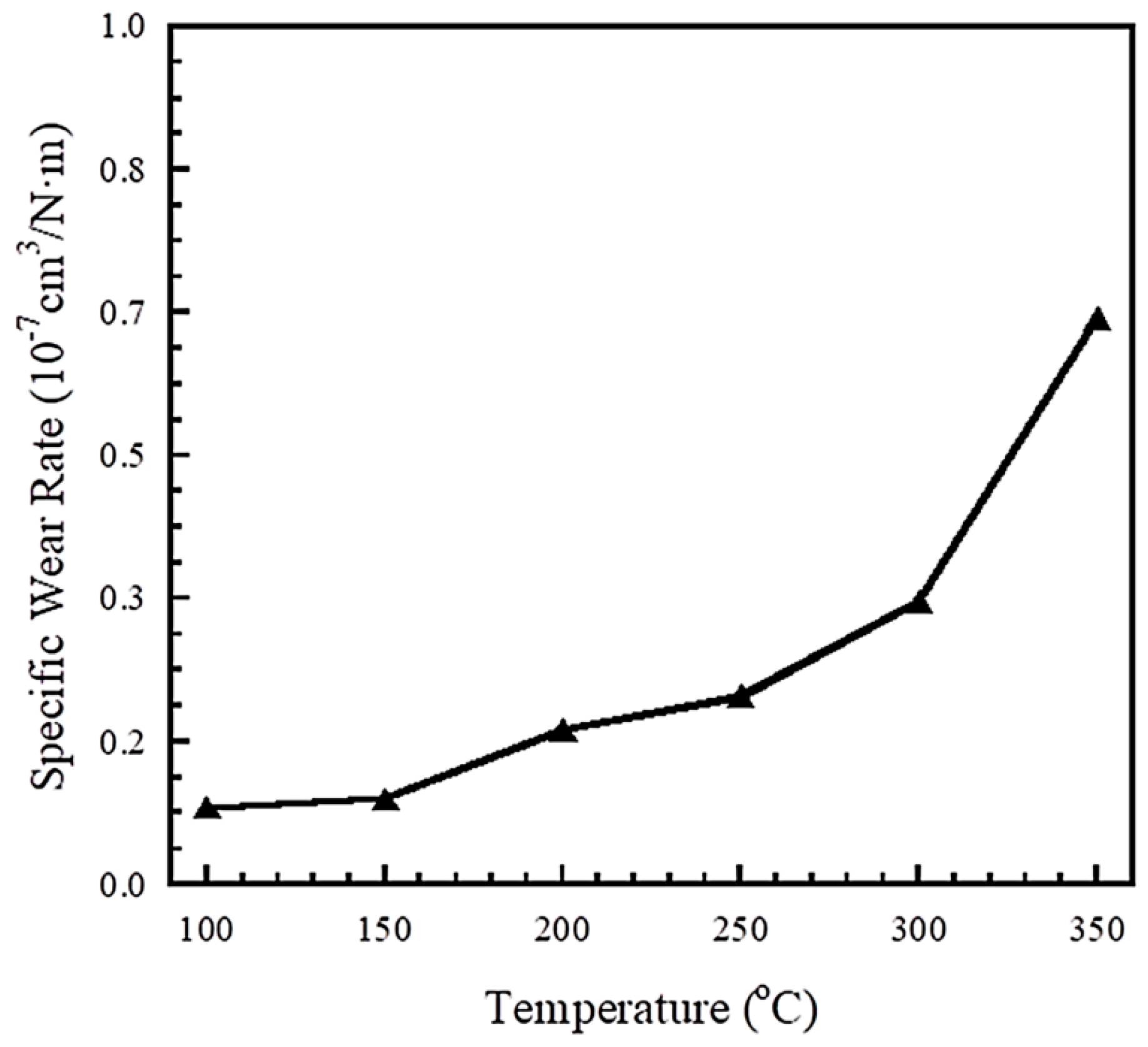
3.5. Tribological Properties of Polybenzoxazine Composites at 100–300 °C
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Owen, C.E. The Automotive Brake Systems; Thomson-Delmar Learning: Boston, MA, USA, 2003. [Google Scholar]
- Halderman, J.D. Automotive Chassis Systems, 7th ed.; Pearson: New York, NY, USA, 2017. [Google Scholar]
- Satapathy, B.K.; Bijwe, J. Fade and Recovery Behavior of Non-Asbestos Organic (NAO) Composite Friction Materials based on Combinations of Rock Fibers and Organic Fibers. J. Reinf. Plast. Compos. 2005, 24, 563–577. [Google Scholar] [CrossRef]
- Mahale, V.; Bijwe, J.; Sinha, S. Efforts towards green friction materials. Tribol. Int. 2019, 136, 196–206. [Google Scholar] [CrossRef]
- Riva, G. A Multi-Scale Simulation Approach to InvestigateLocal Contact Temperatures for Commercial Cu-Fulland Cu-Free Brake Pads. Lubricants 2019, 7, 80. [Google Scholar] [CrossRef]
- Biczó, R.; Kalácska, G.; Mankovits, T. Micromechanical Model and Thermal Properties of Dry-Friction Hybrid Polymer Composite Clutch Facings. Materials 2020, 13, 4508. [Google Scholar] [CrossRef]
- Padhan, M.; Marathe, U.; Bijwe, J. Surface topography modification, Film transfer and Wear mechanism for fibre reinforced polymer composites—An Overview. Surf. Topogr. Metrol. Prop. 2020, 8, 043002. [Google Scholar] [CrossRef]
- Bhatt, B.; Kalel, N.; Abdel-Latif, M.; Bijwe, J. Influence of Increasing Amount of Attapulgite on the Performance Properties of Cu-Free Brake-Pads. SAE Tech. Pap. 2020. [Google Scholar] [CrossRef]
- Jubsilp, C.; Rimdusit, S. Polybenzoxazine-Based Self-Lubricating and Friction Materials. In Advanced and Emerging Polybenzoxazine Science and Technology; Elsevier: Amsterdam, The Netherlands, 2017; pp. 945–974. [Google Scholar] [CrossRef]
- Gurunath, P.V.; Bijwe, J. Friction and wear studies on brake-pad materials based on newly developed resin. Wear 2007, 263, 1212–1219. [Google Scholar] [CrossRef]
- Chan, D.; Stachowiak, G.W. Review of Automotive Brake Friction Materials. Proc. Inst. Mech. Eng. Part D J. Automob. Eng. 2004, 218, 953–966. [Google Scholar] [CrossRef]
- Zheng, K.; Gao, C.; He, F.; Lin, Y. The Role of Rare Earth Lanthanum Oxide in Polymeric Matrix Brake Composites to Replace Copper. Polymers 2018, 10, 1027. [Google Scholar] [CrossRef]
- Bijwe, J. Multifunctionality of Nonasbestos Organic Brake Materials. In Multifunctionality of Polymer Composites; Elsevier: Amsterdam, The Netherlands, 2015; pp. 551–572. [Google Scholar]
- Kim, S.J.; Jang, H. Friction and wear of friction materials containing two different phenolic resins reinforced with aramid pulp. Tribol. Int. 2000, 33, 477–484. [Google Scholar] [CrossRef]
- Bijwe, J.; Nidhi, N.; Majumdar, N.; Satapathy, B.K. Influence of modified phenolic resins on the fade and recovery behavior of friction materials. Wear 2005, 259, 1068–1078. [Google Scholar] [CrossRef]
- Lamport, R.A.; Biermann-Weaver, J.M.; Jain, V.K.; Shih, P.T. Resin Mixture for Friction Materials. U.S. Patent 5,753,018, 19 May 1998. [Google Scholar]
- Gupta, M.K.; Hindersinn, R.R. Shelf life of phenolic resole resins. Polym. Eng. Sci. 1987, 27, 976–978. [Google Scholar] [CrossRef]
- Kurihara, S.; Idei, H.; Aoyagi, Y.; Kuroe, M. Binder Resin for Friction Material. U.S. Patent 8,227,390 B2, 24 July 2012. [Google Scholar]
- Hong, U.S.; Jung, S.L.; Cho, K.H.; Cho, M.H.; Kim, S.J.; Jang, H. Wear mechanism of multiphase friction materials with different phenolic resin matrices. Wear 2009, 266, 739–744. [Google Scholar] [CrossRef]
- Ishida, H.; Sanders, D.P. Improved Thermal and Mechanical Properties of Polybenzoxazines Based on Alkyl-Substituted Aromatic Amines. J. Polym. Sci. B Polym. Phys. 2000, 38, 3289–3301. [Google Scholar] [CrossRef]
- Wu, Y.; Zeng, M.; Xu, Q.; Hou, S.; Jin, H.; Fan, L. Effects of glass-to-rubber transition of thermosetting resin matrix on the friction and wear properties of friction materials. Tribol. Int. 2012, 54, 51–57. [Google Scholar] [CrossRef]
- Ishida, H.; Heights, S. Process for Preparetion of Other Publications Benzoxazine. U.S. Patent 5,543,516, 6 August 1996. [Google Scholar]
- Karger-Kocsis, J.; Mousa, A.; Major, Z.; Békési, N. Dry friction and sliding wear of EPDM rubbers against steel as a function of carbon black content. Wear 2008, 264, 359–367. [Google Scholar] [CrossRef]
- Landel, R.F.; Nielsen, L.E. Mechanical Properties of Polymers and Composites, 2nd ed.; CRC Press: Boca Raton, FL, USA, 1993. [Google Scholar]
- Singh, T.; Patnaik, A. Performance assessment of lapinus-aramid based brake pad hybrid phenolic composites in friction braking. Arch. Civ. Mech. Eng. 2015, 15, 151–161. [Google Scholar] [CrossRef]
- Lertwassana, W.; Parnklang, T.; Mora, P.; Jubsilp, C.; Rimdusit, S. High performance aramid pulp/carbon fiber-reinforced polybenzoxazine composites as friction materials. Compos. Part B Eng. 2019, 177. [Google Scholar] [CrossRef]
- Cai, P.; Wang, Y.; Wang, T.; Wang, Q. Effect of resins on thermal, mechanical and tribological properties of friction materials. Tribol. Int. 2015, 87, 1–10. [Google Scholar] [CrossRef]
- Mathur, R.; Thiyagarajan, P.; Dhami, T.L. Controlling the Hardness and Tribological Behaviour of Non-asbestos Brake Lining Materials for Automobiles. Carbon Lett. 2004, 5, 6–11. [Google Scholar]
- Jubsilp, C.; Taewattana, R.; Takeichi, T.; Rimdusit, S. Investigation on Rubber-Modified Polybenzoxazine Composites for Lubricating Material Applications. J. Mater. Eng. Perform. 2015, 24, 3958–3968. [Google Scholar] [CrossRef]
- Jubsilp, C.; Singto, J.; Yamo, W.; Rimdusit, S. Effect of Graphite Particle Size on Tribological and Mechanical Properties of Polybenzoxazine Composites. Chem. Eng. Trans. 2017, 57, 1351–1356. [Google Scholar]
- Thailand Industrial Standard (TIS97-2557). Available online: http://www.mratchakitcha.soc.go.th/alert.html (accessed on 12 January 2020).
- Wang, F.; Liu, Y. Mechanical and tribological properties of ceramic-matrix friction materials with steel fiber and mullite fiber. Mater. Des. 2014, 57, 449–455. [Google Scholar] [CrossRef]
- Dureja, N.; Bijwe, J.; Gurunath, P.V. Role of Type and Amount of Resin on Performance Behavior of Non-asbestos Organic (NAO) Friction Materials. J. Reinf. Plast. Compos. 2008, 28, 489–497. [Google Scholar] [CrossRef]



| Ingredient | wt% |
|---|---|
| Benzoxazine resin | 10 |
| Aramid pulp | 3.75 |
| Carbon fiber | 1.25 |
| Glass fiber | 10 |
| Steel fiber | 25 |
| Barium sulfate | 10 |
| Nitrile butadiene rubber (NBR) | 4 |
| Cashew dust | 4 |
| Graphite | 10 |
| Zirconium silicate | 25 |
| Benzoxazine Composite | Storage Modulus at Glassy State (GPa) | Storage Modulus at Rubbery State (GPa) | Crosslink Density (10−3 mol/cm3) |
|---|---|---|---|
| F-BA-ot | 5.23 | 1.10 | 8.72 |
| F-BA-pt | 5.35 | 1.61 | 9.28 |
| F-BA-a | 6.31 | 1.67 | 9.34 |
| F-BA-mt | 6.53 | 1.87 | 9.51 |
| F-BA-35x | 6.83 | 2.37 | 9.86 |
| Polybenzoxazine Friction Composite | Td5 (°C) | Residual Weight at 1000 °C (%) |
|---|---|---|
| F-BA-ot | 401 | 68.5 |
| F-BA-pt | 405 | 69.5 |
| F-BA-a | 409 | 70.2 |
| F-BA-mt | 411 | 70.2 |
| F-BA-35x | 414 | 70.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wongpayakyotin, A.; Jubsilp, C.; Tiptipakorn, S.; Mora, P.; Bielawski, C.W.; Rimdusit, S. Effects of Alkyl-Substituted Polybenzoxazines on Tribological Properties of Non-Asbestos Composite Friction Materials. Polymers 2021, 13, 567. https://doi.org/10.3390/polym13040567
Wongpayakyotin A, Jubsilp C, Tiptipakorn S, Mora P, Bielawski CW, Rimdusit S. Effects of Alkyl-Substituted Polybenzoxazines on Tribological Properties of Non-Asbestos Composite Friction Materials. Polymers. 2021; 13(4):567. https://doi.org/10.3390/polym13040567
Chicago/Turabian StyleWongpayakyotin, Anun, Chanchira Jubsilp, Sunan Tiptipakorn, Phattarin Mora, Christopher W. Bielawski, and Sarawut Rimdusit. 2021. "Effects of Alkyl-Substituted Polybenzoxazines on Tribological Properties of Non-Asbestos Composite Friction Materials" Polymers 13, no. 4: 567. https://doi.org/10.3390/polym13040567
APA StyleWongpayakyotin, A., Jubsilp, C., Tiptipakorn, S., Mora, P., Bielawski, C. W., & Rimdusit, S. (2021). Effects of Alkyl-Substituted Polybenzoxazines on Tribological Properties of Non-Asbestos Composite Friction Materials. Polymers, 13(4), 567. https://doi.org/10.3390/polym13040567








