Recent Advances and Applications of Bacterial Cellulose in Biomedicine
Abstract
1. Introduction
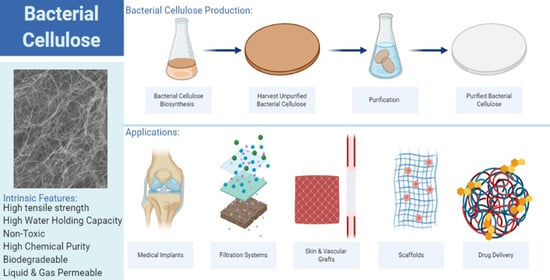
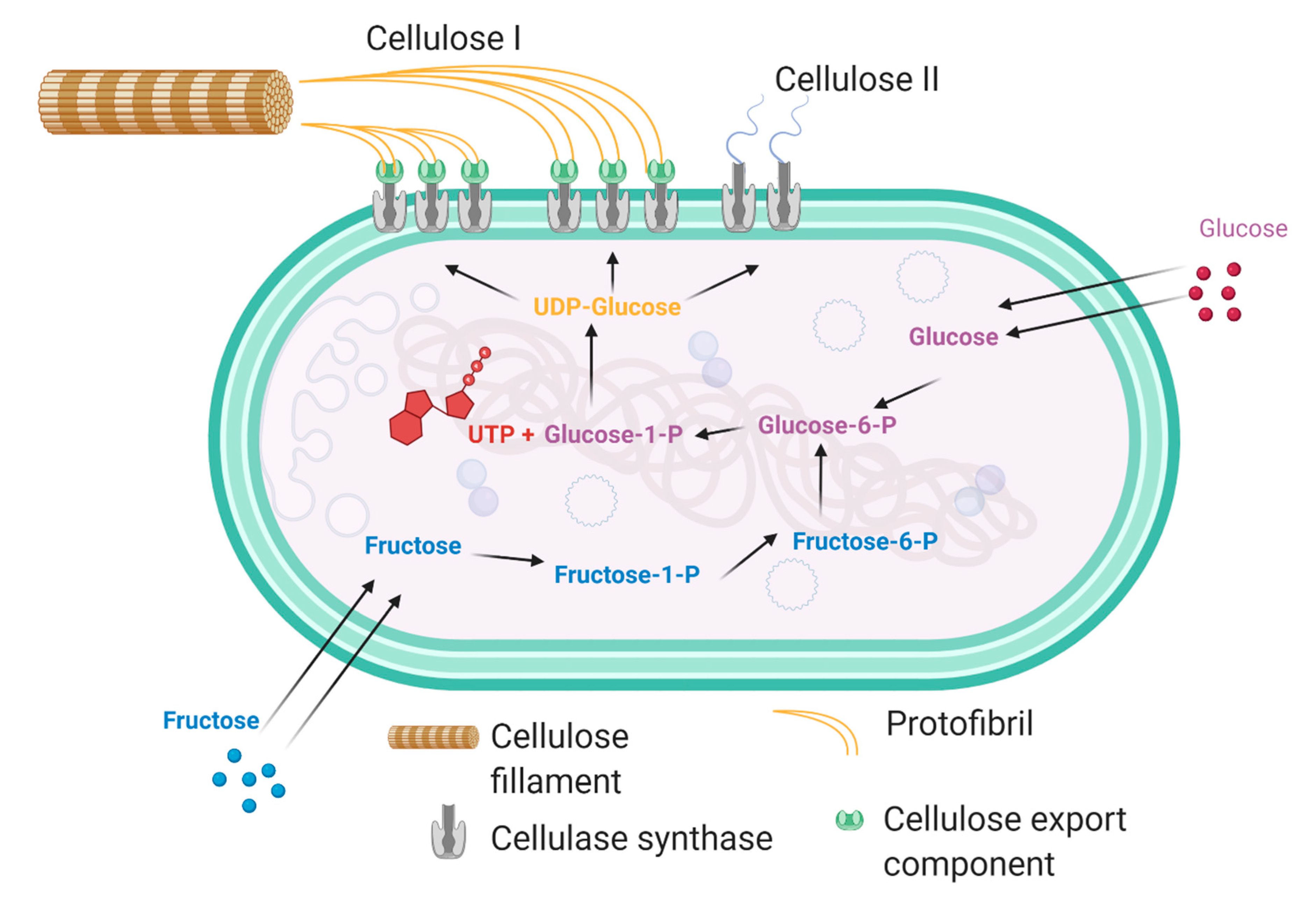
2. Production of Bacterial Cellulose
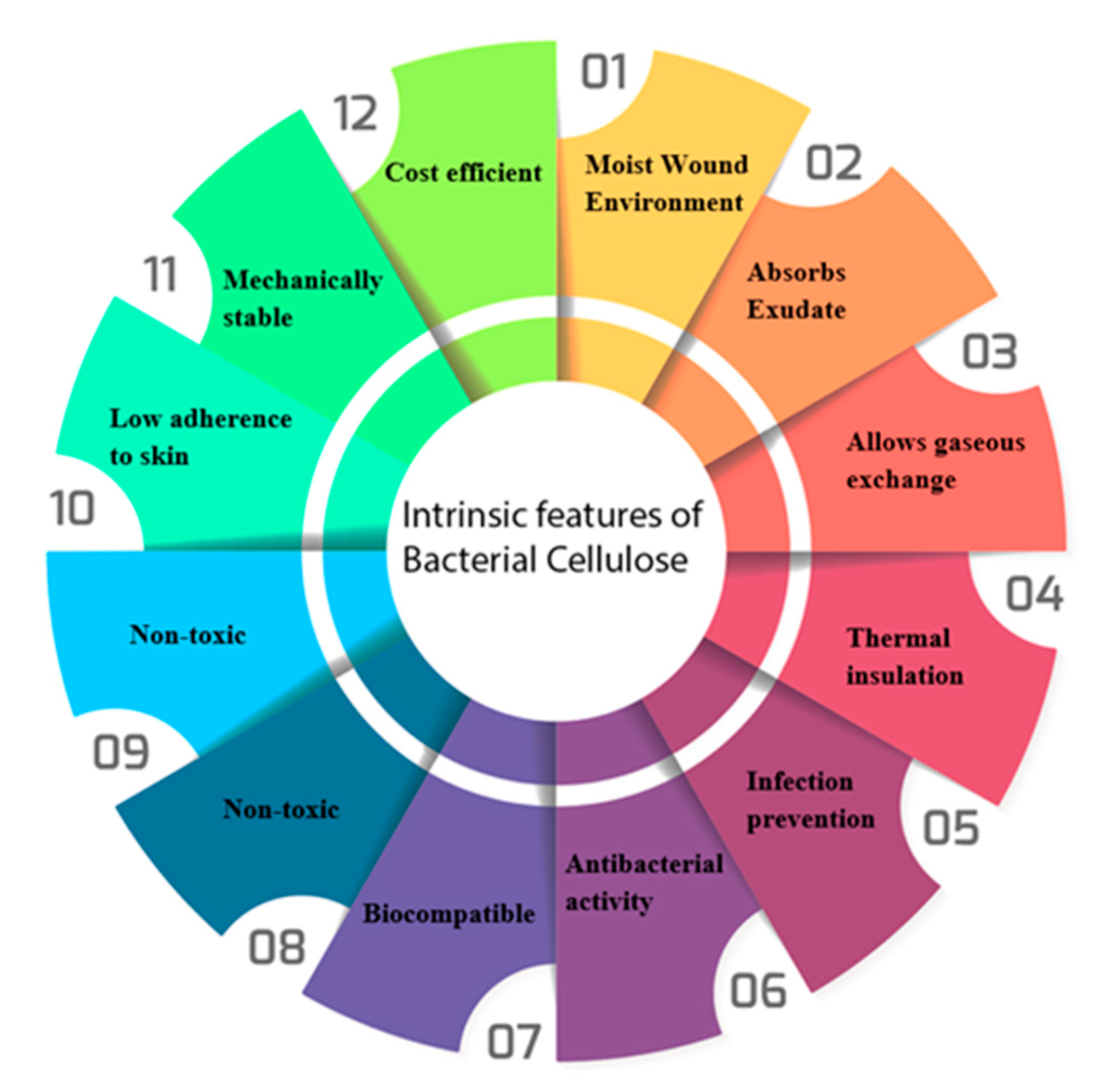
3. Intrinsic Properties of Bacterial Cellulose
3.1. Structural Properties
3.2. Mechanical Properties
3.3. Water Holding/Release Capacity
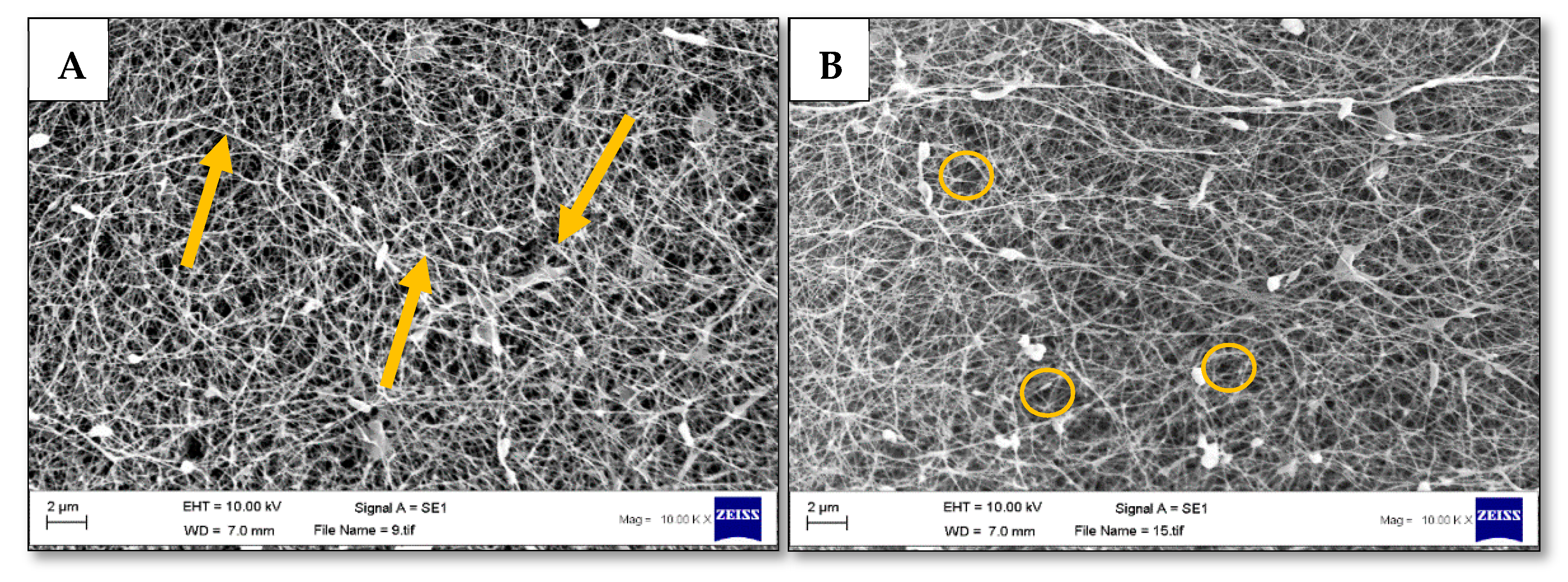
3.4. Pore Size and Fibre Morphology
3.5. Bacterial Cellulose-Based Biocompatible System
4. Bacterial Cellulose as a Biotechnological Material
4.1. Drug Delivery
4.2. Surgical Material
4.3. Tissue Regeneration
4.4. Biosensors
4.5. Immobilisation Matrix
4.6. Filtration
4.7. Electronics
4.8. Other Uses
5. Wound Treatment
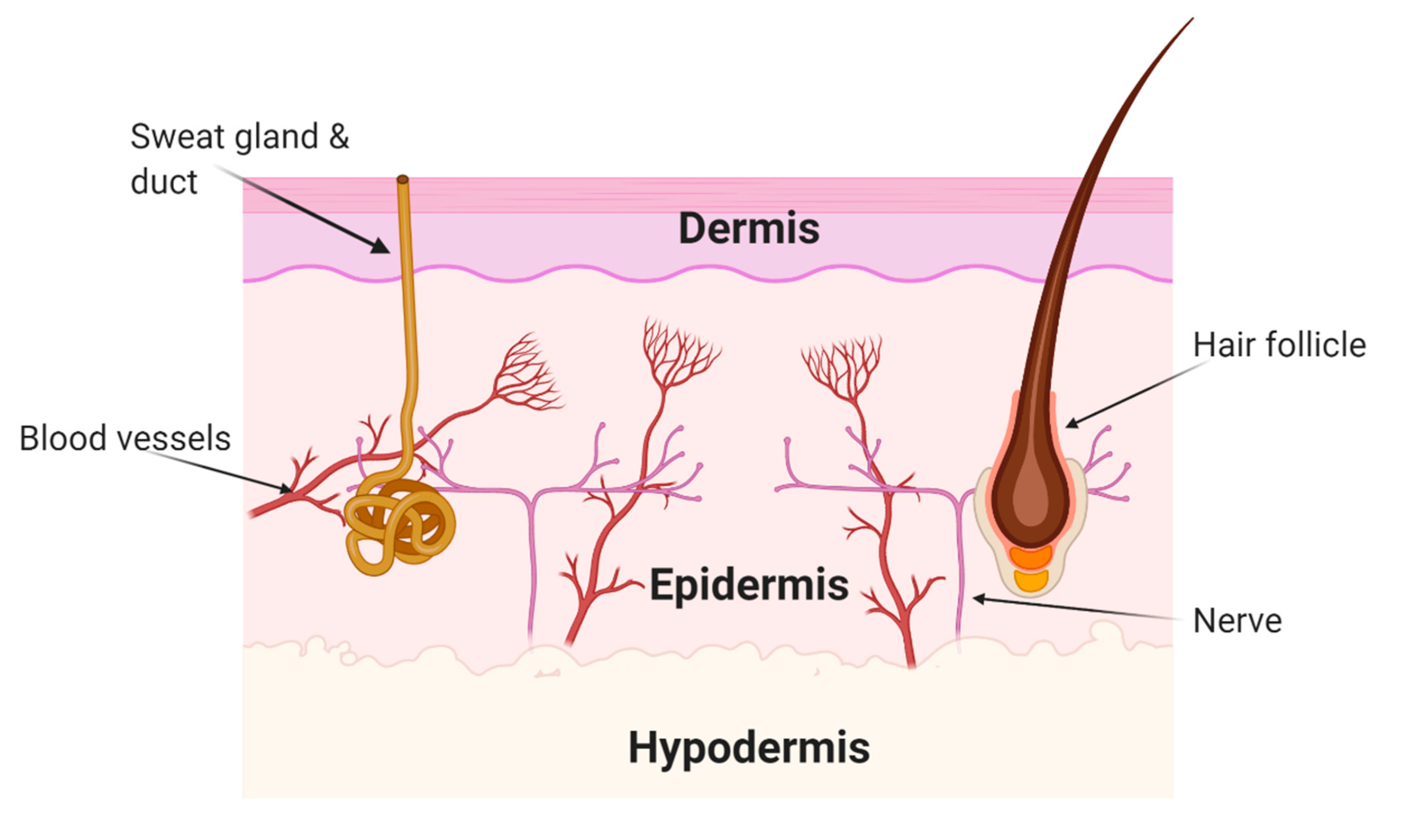
5.1. Wounds
5.2. Wound Dressings
5.3. Currently Marketed Bacterial Cellulose Wound Dressings
6. Bacterial Cellulose Composites in Wound Dressings
6.1. Polysaccahrides
6.2. Natural and Synthetic Polymers
6.3. Nanoparticles
6.4. Metal Oxides
6.5. Antimicrobials
6.6. Anaesthesia and Analgesics
6.7. Proteins, Enzymes and Amino Acids
7. Limitations and Future Advancements
8. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Park, M.-A.; Lee, S.-G.; Choi, K.-H.; Cho, B.-U. Effects of Surface Coating of Cellulose II-based MFC on Paper Properties. J. Korea Tech. Assoc. Pulp Pap. Ind. 2019, 51, 28–36. [Google Scholar] [CrossRef]
- Fernandes, S.N.; Almeida, P.L.; Monge, N.; Aguirre, L.E.; Reis, D.; De Oliveira, C.L.P.; Neto, A.M.F.; Pieranski, P.; Godinho, M.H. Cellulose Nanocrystals: Mind the Microgap in Iridescent Cellulose Nanocrystal Films. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef]
- Abba, M.; Abdullahi, M.; Nor, M.H.M.; Chong, C.S.; Ibrahim, Z. Isolation and characterisation of locally isolated Gluconacetobacter xylinus BCZM sp. with nanocellulose producing potentials. IET Nanobiotechnol. 2018, 12, 52–56. [Google Scholar] [CrossRef]
- Shpichka, A.I.; Butnaru, D.; Bezrukov, E.A.; Sukhanov, R.B.; Atalaa, A.; Burdukovskii, V.F.; Zhang, Y.; Timashev, P. Skin tissue regeneration for burn injury. Stem Cell Res. 2019, 10, 1–16. [Google Scholar] [CrossRef]
- Sajjad, W.; Khan, T.; Ul-Islam, M.; Khan, R.; Hussain, Z.; Khalid, A.; Wahid, F. Development of modified mont-morillonite-bacterial cellulose nanocomposites as a novel substitute for burn skin and tissue regeneration. Carbohydr. Polym. 2019, 206, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Singhsa, P.; Narain, R.; Manuspiya, H. Physical structure variations of bacterial cellulose produced by different Komagataeibacter xylinus strains and carbon sources in static and agitated conditions. Cellulose 2018, 25, 1571–1581. [Google Scholar] [CrossRef]
- Krasteva, P.V.; Bernal-Bayard, J.; Travier, L.; Martin, F.A.; Kaminski, P.-A.; Karimova, G.; Fronzes, R.; Ghigo, J.-M. Insights into the structure and assembly of a bacterial cellulose secretion system. Nat. Commun. 2017, 8, 1–10. [Google Scholar] [CrossRef]
- Jang, W.D.; Kim, T.Y.; Kim, H.U.; Shim, W.Y.; Ryu, J.Y.; Park, J.H.; Lee, S.Y. Genomic and metabolic analysis ofKomagataeibacter xylinusDSM 2325 producing bacterial cellulose nanofiber. Biotechnol. Bioeng. 2019, 116, 3372–3381. [Google Scholar] [CrossRef]
- He, F.; Yang, H.; Zeng, L.; Hu, H.; Hu, C. Production and characterization of bacterial cellulose obtained by Gluconacetobacter xylinus utilizing the by-products from Baijiu production. Bioprocess Biosyst. Eng. 2020, 43, 927–936. [Google Scholar] [CrossRef]
- Römling, U.; Galperin, M.Y. Bacterial cellulose biosynthesis: Diversity of operons, subunits, products, and functions. Trends Microbiol. 2015, 23, 545–557. [Google Scholar] [CrossRef]
- Badshah, M.; Ullah, H.; Khan, A.R.; Khan, S.; Park, J.K.; Khan, T. Surface modification and evaluation of bacterial cellulose for drug delivery. Int. J. Biol. Macromol. 2018, 113, 526–533. [Google Scholar] [CrossRef] [PubMed]
- Thiruvengadam, V.; Vitta, S. Bacterial cellulose based flexible multifunctional nanocomposite sheets. Cellulose 2017, 24, 3341–3351. [Google Scholar] [CrossRef]
- Prosvirnikov, D.B.; Safin, R.G.; Zakirov, S. Microcrystalline Cellulose Based on Cellulose Containing Raw Material Modified by Steam Explosion Treatment. Solid State Phenom. 2018, 284, 773–778. [Google Scholar] [CrossRef]
- Volova, T.; Prudnikova, S.V.; Sukovatyi, A.G.; Shishatskaya, E.I. Production and properties of bacterial cellulose by the strain Komagataeibacter xylinus B-12068. Appl. Microbiol. Biotechnol. 2018, 102, 7417–7428. [Google Scholar] [CrossRef] [PubMed]
- Gorgieva, S. Bacterial Cellulose as a Versatile Platform for Research and Development of Biomedical Materi-als. Processes 2020, 8, 624. [Google Scholar] [CrossRef]
- Negut, I.; Grumezescu, V.; Grumezescu, A.M. Treatment Strategies for Infected Wounds. Molecules 2018, 23, 2392. [Google Scholar] [CrossRef]
- Rahman, M.; Netravali, A. Aligned Bacterial Cellulose Arrays As “Green” Nanofibers for Composite Materials. ACS Macro Lett. 2016, 5, 1070–1074. [Google Scholar] [CrossRef]
- Czaja, W.; Romanovicz, D.; Brown, R.M. Structural investigations of microbial cellulose produced in stationary and agitated culture. Cellulose 2004, 11, 403–411. [Google Scholar] [CrossRef]
- Thygesen, A.; Oddershede, J.; Lilholt, H.; Thomsen, A.; Ståhl, K. On the determination of crystallinity and Cel-lulose content in plant fibres. Cellulose 2005, 12, 563–576. [Google Scholar] [CrossRef]
- Ruan, C.; Zhu, Y.; Zhou, X.; Abidi, N.; Hu, Y.; Catchmark, J. Effect of cellulose crystallinity on Bacterial Cellu-lose assembly. Cellulose 2016, 23, 3417–3427. [Google Scholar] [CrossRef]
- Fernandes, M.; Gama, M.; Dourado, F.; Souto, A. Development of novel bacterial cellulose composites for the tex-tile and shoe industry. Microb. Biotechnol. 2019, 12, 650–661. [Google Scholar] [CrossRef] [PubMed]
- Du, R.; Wang, Y.; Zhao, F.; Qiao, X.; Song, Q.; Li, S.; Kim, R.-C.; Pan, L.; Han, Y.; Xiao, H.; et al. Production, Optimization and Partial Characterization of Bacterial Cellulose from Gluconacetobacter xylinus TJU-D2. Waste Biomass Valorization 2020, 11, 1681–1690. [Google Scholar] [CrossRef]
- Huang, Y.; Zhu, C.; Yang, J.; Nie, Y.; Chen, C.; Sun, D. Recent advances in bacterial cellulose. Cellulose 2014, 21, 1–30. [Google Scholar] [CrossRef]
- Revin, V.; Pestov, N.; Shchankin, M.; Mishkin, V.; Platonov, V.; Uglanov, D. A Study of The Physical and Me-chanical Properties of Aerogels Obtained from Bacterial Cellulose. Biomacromolecules 2019, 20, 1401–1411. [Google Scholar] [CrossRef] [PubMed]
- Ul-Islam, M.; Khan, T.; Park, J.K. Water holding and release properties of bacterial cellulose obtained by in situ and ex situ modification. Carbohydr. Polym. 2012, 88, 596–603. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.; Saha, N.; Sáha, P. Characterization of Bacterial Cellulose Produced using Media Containing Waste Apple Juice. Appl. Biochem. Microbiol. 2018, 54, 649–657. [Google Scholar] [CrossRef]
- Liu, D.; Cao, Y.; Qu, R.; Gao, G.; Chen, S.; Zhang, Y.; Wu, M.; Ma, T.; Li, G. Production of bacterial cellulose hy-drogels with tailored crystallinity from Enterobacter sp. FY-07 by the controlled expression of colanic acid synthetic genes. Carbohydr. Polym. 2019, 207, 563–570. [Google Scholar] [CrossRef]
- Rühs, P.; Storz, F.; López Gómez, Y.; Haug, M.; Fischer, P. 3D bacterial cellulose biofilms formed by foam templat-ing. npj Biofilms Microbiomes 2018, 4, 1–6. [Google Scholar] [CrossRef]
- Zhai, X.; Lin, D.; Li, W.; Yang, X. Improved characterization of nanofibers from bacterial cellulose and its potential application in fresh-cut apples. Int. J. Biol. Macromol. 2020, 149, 178–186. [Google Scholar] [CrossRef]
- Sulaeva, I.; Henniges, U.; Rosenau, T.; Potthast, A. Bacterial cellulose as a material for wound treatment: Proper-ties and modifications. A review. Biotechnol. Adv. 2015, 33, 1547–1571. [Google Scholar] [CrossRef]
- Khan, M.U.A.; Haider, S.; Haider, A.; Razak, S.I.A.; Kadir, M.R.A.; A Shah, S.; Javed, A.; Shakir, I.; Al-Zahrani, A.A. Development of porous, antibacterial and biocompatible GO/n-HAp/bacterial cellulose/β-glucan biocomposite scaffold for bone tissue engineering. Arab. J. Chem. 2021, 14, 102924. [Google Scholar] [CrossRef]
- Roman, M.; Haring, A.P.; Bertucio, T.J. The growing merits and dwindling limitations of bacterial cellulose-based tissue engineering scaffolds. Curr. Opin. Chem. Eng. 2019, 24, 98–106. [Google Scholar] [CrossRef]
- Alavi, M. Modifications of microcrystalline cellulose (MCC), nanofibrillated cellulose (NFC), and nanocrystalline cellulose (NCC) for antimicrobial and wound healing applications. e-Polymers 2019, 19, 103–119. [Google Scholar] [CrossRef]
- Cavalcanti, L.M.; Pinto, F.C.M.; De Oliveira, G.M.; Lima, S.V.C.; Aguiar, J.L.D.A.; Lins, E.M. Efficacy of bacterial cellulose membrane for the treatment of lower limbs chronic varicose ulcers: A randomized and controlled trial. Rev. Do Colégio Bras. De Cir. 2017, 44, 72–80. [Google Scholar] [CrossRef]
- Grela, E.; Kozłowska, J.; Grabowiecka, A. Current methodology of MTT assay in bacteria—A review. Acta Histochem. 2018, 120, 303–311. [Google Scholar] [CrossRef]
- Silva, W.N.; Leonel, C.; Prazeres, P.H.D.M.; Sena, I.F.G.; Guerra, D.A.P.; Heller, D.; Diniz, I.M.A.; Fortuna, V.; Mintz, A.; Birbrair, A. Role of Schwann cells in cutaneous wound healing. Wound Repair Regen. 2018, 26, 392–397. [Google Scholar] [CrossRef]
- Bayazidi, P.; Almasi, H.; Asl, A.K. Immobilization of lysozyme on bacterial cellulose nanofibers: Characteristics, antimicrobial activity and morphological properties. Int. J. Biol. Macromol. 2018, 107, 2544–2551. [Google Scholar] [CrossRef]
- Cacicedo, M.L.; Pacheco, G.; Islan, G.A.; Alvarez, V.A.; Barud, H.S.; Castro, G.R. Chitosan-bacterial cellulose patch of ciprofloxacin for wound dressing: Preparation and characterization studies. Int. J. Biol. Macromol. 2020, 147, 1136–1145. [Google Scholar] [CrossRef]
- Volova, T.; Shumilova, A.; Nikolaeva, E.; Kirichenko, A.; Shishatskaya, E. Biotechnological wound dressings based on bacterial cellulose and degradable copolymer P(3HB/4HB). Int. J. Biol. Macromol. 2019, 131, 230–240. [Google Scholar] [CrossRef]
- Piasecka-Zelga, J.; Zelga, P.; Szulc, J.; Wietecha, J.; Ciechańska, D. An in vivo biocompatibility study of surgical meshes made from bacterial cellulose modified with chitosan. Int. J. Biol. Macromol. 2018, 116, 1119–1127. [Google Scholar] [CrossRef]
- Cazón, P.; Velazquez, G.; Vázquez, M. Characterization of bacterial cellulose films combined with chitosan and polyvinyl alcohol: Evaluation of mechanical and barrier properties. Carbohydr. Polym. 2019, 216, 72–85. [Google Scholar] [CrossRef] [PubMed]
- Van Zyl, E.; Coburn, J. Hierarchical Structure of Bacterial-Derived Cellulose and Its Impact on Biomedical Ap-plications. Curr. Opin. Chem. Eng. 2019, 24, 122–130. [Google Scholar] [CrossRef]
- Ye, S.; Jiang, L.; Su, C.; Zhu, Z.; Wen, Y.; Shao, W. Development of gelatin/bacterial cellulose composite sponges as potential natural wound dressings. Int. J. Biol. Macromol. 2019, 133, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, N.; Loh, E.Y.X.; Fauzi, M.B.; Ng, M.H.; Amin, M.C.I.M. In vivo evaluation of bacterial cellulose/acrylic acid wound dressing hydrogel containing keratinocytes and fibroblasts for burn wounds. Drug Deliv. Transl. Res. 2018, 9, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Keyes, B.E.; Liu, S.; Asare, A.; Naik, S.; Levorse, J.; Polak, L.; Lu, C.P.; Nikolova, M.; Pasolli, H.A.; Fuchs, E. Impaired Epidermal to Dendritic T Cell Signaling Slows Wound Repair in Aged Skin. Cell 2016, 167, 1323–1338.e4. [Google Scholar] [CrossRef]
- Swingler, S.; Gupta, A.; Heaselgrave, W.; Kowalczuk, M.; Radecka, I. An investigation into the anti-microbial properties of bacterial cellulose wound dressings loaded with curcumin:hydroxypropyl-β-cyclodextrin supramolecular inclusion complex An investigation into the anti-microbial properties of bacterial cellulose wound dressings loaded with curcumin:hydroxypropyl-β-cyclodextrin supramolecular inclusion complex. Access Microbiol. 2019, 1, 19. [Google Scholar] [CrossRef]
- Shao, W.; Wang, S.; Liu, X.; Liu, H.; Wu, J.; Zhang, R.; Min, H.; Huang, M. Tetracycline hydrochloride loaded regenerated cellulose composite membranes with controlled release and efficient antibacterial performance. RSC Adv. 2015, 6, 3068–3073. [Google Scholar] [CrossRef]
- De Mattos, I.B.; Nischwitz, S.P.; Tuca, A.-C.; Groeber-Becker, F.; Funk, M.; Birngruber, T.; Mautner, S.I.; Kamolz, L.-P.; Holzer, J.C. Delivery of antiseptic solutions by a bacterial cellulose wound dressing: Uptake, release and antibacterial efficacy of octenidine and povidone-iodine. Burn 2020, 46, 918–927. [Google Scholar] [CrossRef]
- Junka, A.; Bartoszewicz, M.; Dziadas, M.; Szymczyk, P.; Dydak, K.; Żywicka, A.; Owczarek, A.; Bil-Lula, I.; Czajkowska, J.; Fijałkowski, K. Application of Bacterial Cellulose Experimental Dressings Saturated with Gentamycin for Man-agement of Bone Biofilm In Vitro And Ex Vivo. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 108, 30–37. [Google Scholar] [CrossRef]
- El-Wakil, N.; Hassan, E.; Hassan, M.; Abd El-Salam, S. Bacterial Cellulose/Phytochemical’s Extracts Biocomposites For Potential Active Wound Dressings. Environ. Sci. Pollut. Res. 2019, 26, 26529–26541. [Google Scholar] [CrossRef]
- Ao, H.; Jiang, W.; Nie, Y.; Zhou, C.; Zong, J.; Liu, M.; Liu, X.; Wan, Y.-Z. Engineering quaternized chitosan in the 3D bacterial cellulose structure for antibacterial wound dressings. Polym. Test. 2020, 86, 106490. [Google Scholar] [CrossRef]
- Alkhatib, Y.; Dewaldt, M.; Moritz, S.; Nitzsche, R.; Kralisch, D.; Fischer, D. Controlled Extended Octenidine Re-lease From A Bacterial Nanocellulose/Poloxamer Hybrid System. Eur. J. Pharm. Biopharm. 2017, 112, 164–176. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Keddie, D.; Kannappan, V.; Gibson, H.; Khalil, I.; Kowalczuk, M.; Martin, C.; Shuai, X.; Radecka, I. Production and characterisation of bacterial cellulose hydrogels loaded with curcumin encapsulated in cyclodextrins as wound dressings. Eur. Polym. J. 2019, 118, 437–450. [Google Scholar] [CrossRef]
- Gupta, A.; Briffa, S.M.; Swingler, S.; Gibson, H.; Kannappan, V.; Adamus, G.; Kowalczuk, M.; Martin, C.; Radecka, I. Synthesis of Silver Nanoparticles Using Curcumin-Cyclodextrins Loaded into Bacterial Cellulose-Based Hydrogels for Wound Dressing Applications. Biomacromolecules 2020, 21, 1802–1811. [Google Scholar] [CrossRef]
- Shao, W.; Liu, H.; Wang, S.; Wu, J.; Huang, M.; Min, H.; Liu, X. Controlled release and antibacterial activity of tetracycline hydrochloride-loaded bacterial cellulose composite membranes. Carbohydr. Polym. 2016, 145, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Maver, U.; Xhanari, K.; Žižek, M.; Gradišnik, L.; Repnik, K.; Potočnik, U.; Finšgar, M. Carboxymethyl cellu-lose/diclofenac bioactive coatings on AISI 316LVM for controlled drug delivery, and improved osteogenic poten-tial. Carbohydr. Polym. 2020, 230, 115612. [Google Scholar] [CrossRef]
- Silva, N.H.; Rodrigues, A.F.; Almeida, I.F.; Costa, P.; Rosado, C.F.; Neto, C.P.; Silvestre, A.J.D.; Freire, C.S.R. Bacterial cellulose membranes as transdermal delivery systems for diclofenac: In vitro dissolution and permeation studies. Carbohydr. Polym. 2014, 106, 264–269. [Google Scholar] [CrossRef]
- Hoshi, T.; Yamazaki, K.; Sato, Y.; Shida, T.; Aoyagi, T. Production of hollow-type spherical bacterial cellulose as a controlled release device by newly designed floating cultivation. Heliyon 2018, 4, e00873. [Google Scholar] [CrossRef]
- Zmejkoski, D.; Spasojević, D.; Orlovska, I.; Kozyrovska, N.; Soković, M.D.; Glamočlija, J.; Dmitrović, S.; Matovic, B.; Tasić, N.; Maksimović, V.; et al. Bacterial cellulose-lignin composite hydrogel as a promising agent in chronic wound healing. Int. J. Biol. Macromol. 2018, 118, 494–503. [Google Scholar] [CrossRef]
- Bäckdahl, H.; Esguerra, M.; Delbro, D.; Risberg, B.; Gatenholm, P. Engineering Microporosity in Bacterial Cellu-lose Scaffolds. J. Tissue Eng. Regen. Med. 2008, 2, 320–330. [Google Scholar] [CrossRef]
- Leitão, A.F.; Faria, M.; Faustino, A.M.R.; Moreira, R.; Mela, P.; Loureiro, L.; Silva, I.; Gama, M. A Novel Small-Caliber Bacterial Cellulose Vascular Prosthesis: Production, Characterization, and Preliminary In Vivo Testing. Macromol. Biosci. 2015, 16, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Pour, H.S.-R.; Khajavi, R.; Yazdanshenas, M.E.; Zahedi, P.; Mirjalili, M. Cellulose acetate/poly(vinyl alcohol) hybrid fibrous mat containing tetracycline hydrochloride and phenytoin sodium: Morphology, drug release, antibacterial, and cell culture studies. J. Bioact. Compat. Polym. 2018, 33, 597–611. [Google Scholar] [CrossRef]
- Han, Y.; Li, C.; Cai, Q.; Bao, X.; Tang, L.; Ao, H.; Liu, J.; Jin, M.; Zhou, Y.; Wan, Y.; et al. Studies on Bacterial Cellulose/Poly(Vinyl Alcohol) Hydrogel Composites as Tissue-Engineered Corneal Stroma. Biomed. Mater. 2020, 15, 035022. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Catchmark, J. Integration of cellulases into bacterial cellulose: Toward bioabsorbable cellulose compo-sites. J. Biomed. Mater. Res. Part B Appl. Biomater. 2011, 97, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Inoue, B.S.; Streit, S.; Schneider, A.L.D.S.; Meier, M.M. Bioactive bacterial cellulose membrane with prolonged release of chlorhexidine for dental medical application. Int. J. Biol. Macromol. 2020, 148, 1098–1108. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Patel, D.; Lim, K. Functional Cellulose-Based Hydrogels as Extracellular Matrices for Tissue Engineering. J. Biol. Eng. 2019, 13, 1–9. [Google Scholar] [CrossRef]
- Hu, Y.; Catchmark, J.M.; Zhu, Y.; Abidi, N.; Zhou, X.; Wang, J.; Liang, N. Engineering of porous bacterial cellulose toward human fibroblasts ingrowth for tissue engineering. J. Mater. Res. 2014, 29, 2682–2693. [Google Scholar] [CrossRef]
- Torgbo, S.; Sukyai, P. Fabrication of Microporous Bacterial Cellulose Embedded with Magnetite and Hydroxyap-atite Nanocomposite Scaffold For Bone Tissue Engineering. Mater. Chem. Phys. 2019, 237, 121868. [Google Scholar] [CrossRef]
- Wu, J.; Yin, N.; Chen, S.; Weibel, D.B.; Wang, H. Simultaneous 3D cell distribution and bioactivity enhancement of bacterial cellulose (BC) scaffold for articular cartilage tissue engineering. Cellulose 2019, 26, 2513–2528. [Google Scholar] [CrossRef]
- Pang, M.; Huang, Y.; Meng, F.; Zhuang, Y.; Liu, H.; Du, M.; Ma, Q.; Wang, Q.; Chen, Z.; Chen, L.; et al. Application of bacterial cellulose in skin and bone tissue engineering. Eur. Polym. J. 2020, 122, 109365. [Google Scholar] [CrossRef]
- Qiao, H.; Guo, T.; Zheng, Y.; Zhao, L.; Sun, Y.; Liu, Y.; Xie, Y. A Novel Microporous Oxidized Bacterial Cellu-lose/Arginine Composite and Its Effect on Behavior of Fibroblast/Endothelial Cell. Carbohydr. Polym. 2018, 184, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, N.F.; Andrade, F.K.; Vieira, L.D.A.P.; Vieira, R.S.; Vaz, J.M.; Chevallier, P.; Mantovani, D.; Borges, M.D.F.; Rosa, M.D.F. Oxidized bacterial cellulose membrane as support for enzyme immobilization: Properties and morphological features. Cellulose 2020, 27, 3055–3083. [Google Scholar] [CrossRef]
- Mandour, Y.; Mohammed, S.; Menem, M. Bacterial cellulose graft versus fat graft in closure of tympanic mem-brane perforation. Am. J. Otolaryngol. 2019, 40, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, S.W.; Park, S.; Lim, K.-T.; Seonwoo, H.; Kim, Y.; Hong, B.H.; Choung, Y.-H.; Chung, J.H. Bacterial Cellulose Nanofibrillar Patch as a Wound Healing Platform of Tympanic Membrane Perforation. Adv. Healthc. Mater. 2013, 2, 1525–1531. [Google Scholar] [CrossRef] [PubMed]
- Rebelo, A.; Liu, C.; Schäfer, K.; Saumer, M.; Yang, G.; Liu, Y. Poly(4-Vinylaniline)/Polyaniline Bi-layer-Functionalized Bacterial Cellulose for Flexible Electrochemical Biosensors. Langmuir 2019, 35, 10354–10366. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wei, Q.; Wang, Q.; Lv, P.; Wei, Q. Preparation of Pd/Bacterial Cellulose Hybrid Nanofibers for Dopamine Detection. Molecules 2016, 21, 618. [Google Scholar] [CrossRef]
- Yu, S.; Ding, L.; Lin, H.; Wu, W.; Huang, J. A novel optical fiber glucose biosensor based on carbon quantum dots-glucose oxidase/cellulose acetate complex sensitive film. Biosens. Bioelectron. 2019, 146, 111760. [Google Scholar] [CrossRef]
- Rao, A.; Sathiavelu, A.; Mythili, S. Mini review on nanoimmobilization of lipase and cellulase for biofuel production. Biofuels 2017, 11, 191–200. [Google Scholar] [CrossRef]
- Galdino, C.J.S.; Maia, A.D.; Meira, H.M.; Souza, T.C.; Amorim, J.D.; Almeida, F.C.; Costa, A.F.; Sarubbo, L.A. Use of a bacterial cellulose filter for the removal of oil from wastewater. Process. Biochem. 2020, 91, 288–296. [Google Scholar] [CrossRef]
- Pirsa, S.; Shamusi, T.; Kia, E.M. Smart films based on bacterial cellulose nanofibers modified by conductive polypyrrole and zinc oxide nanoparticles. J. Appl. Polym. Sci. 2018, 135, 46617. [Google Scholar] [CrossRef]
- Lizundia, E.; Rincón-Iglesias, M.; Lanceros-Méndez, S. Combining cobalt ferrite and graphite with cellulose nanocrystals for magnetically active and electrically conducting mesoporous nanohybrids. Carbohydr. Polym. 2020, 236, 116001. [Google Scholar] [CrossRef]
- Chen, X.; Yuan, F.; Zhang, H.; Huang, Y.; Yang, J.; Sun, D. Recent approaches and future prospects of bacterial cellulose-based electroconductive materials. J. Mater. Sci. 2016, 51, 5573–5588. [Google Scholar] [CrossRef]
- Lay, M.; González, I.; Tarrés, J.A.; Pellicer, N.; Bun, K.N.; Vilaseca, F. High electrical and electro-chemical properties in bacterial cellulose/polypyrrole membranes. Eur. Polym. J. 2017, 91, 1–9. [Google Scholar] [CrossRef]
- Dutta, H.; Paul, S.K. Kombucha Drink: Production, Quality, and Safety Aspects. In Production and Management of Beverages; Elsevier BV: Amsterdam, The Netherlands, 2019; pp. 259–288. [Google Scholar]
- Dourado, F.; Gama, M.; Rodrigues, A. A Review on The Toxicology and Dietetic Role of Bacterial Cellu-lose. Toxicol. Rep. 2017, 4, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Lin, D.; Liu, D.; Yang, X. Emulsions stabilized by nanofibers from bacterial cellulose: New potential food-grade Pickering emulsions. Food Res. Int. 2018, 103, 12–20. [Google Scholar] [CrossRef]
- Amnuaikit, T.; Chusuit, T.; Raknam, P.; Boonme, P. Effects of A Cellulose Mask Synthesized by A Bacterium on Facial Skin Characteristics and User Satisfaction. Med. Devices Evid. Res. 2011, 4, 77. [Google Scholar]
- Maculotti, D.; Dassenno, D. Management of Peristomal Skin Complications with Negative Pressure Wound Therapy: A Case Study. Anat. Physiol. 2016, 6. [Google Scholar] [CrossRef]
- Yokouchi, M.; Kubo, A. Maintenance of tight junction barrier integrity in cell turnover and skin diseases. Exp. Derm. 2018, 27, 876–883. [Google Scholar] [CrossRef]
- Ganesan, A.; Shaikh, F.; Bradley, W.; Blyth, D.; Bennett, D.; Petfield, J.; Carson, M.; Wells, J.; Tribble, D. Classifica-tion of Trauma-Associated Invasive Fungal Infections to Support Wound Treatment Decisions. Emerg. Infect. Dis. 2019, 25, 1639. [Google Scholar] [CrossRef]
- Ribeiro, D.M.L.; Júnior, A.R.C.; De Macedo, G.H.R.V.; Chagas, V.L.; Silva, L.D.S.; Cutrim, B.D.S.; Santos, D.M.; Soares, B.L.L.; Zagmignan, A.; Miranda, R.d.C.M.D.; et al. Polysaccharide-Based Formulations for Healing of Skin-Related Wound Infections: Lessons from Animal Models and Clinical Trials. Biomolecules 2019, 10, 63. [Google Scholar] [CrossRef]
- Fisher, M.C.; Gurr, S.J.; Cuomo, C.A.; Blehert, D.S.; Jin, H.; Stukenbrock, E.H.; Stajich, J.E.; Kahmann, R.; Boone, C.; Denning, D.W.; et al. Threats Posed by the Fungal Kingdom to Humans, Wildlife, and Agriculture. mBio 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Ameriburn.Org. Burn Incidence Fact Sheet—American Burn Association. 2019. Available online: https://Ameriburn.Org/Who-We-Are/Media/Burn-Incidence-Fact-Sheet/ (accessed on 17 July 2019).
- Picheth, G.F.; Pirich, C.L.; Sierakowski, M.R.; Woehl, M.A.; Sakakibara, C.N.; De Souza, C.F.; Martin, A.A.; Da Silva, R.; De Freitas, R.A. Bacterial cellulose in biomedical applications: A review. Int. J. Biol. Macromol. 2017, 104, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Chu, M.; Gao, H.; Liu, S.; Wang, L.; Jia, Y.; Gao, M.; Wan, M.; Xu, C.; Ren, L. Functionalization of composite bacterial cellulose with C60nanoparticles for wound dressing and cancer therapy. RSC Adv. 2018, 8, 18197–18203. [Google Scholar] [CrossRef]
- Howell, R.S.; Gorenstein, S.; Gillette, B.M.; Digregorio, J.; Criscitelli, T.; Davitz, M.S.; Woods, J.S.; Acerra, M.; Brem, H. A Framework to Assist Providers in the Management of Patients with Chronic, Nonhealing Wounds. Adv. Ski. Wound Care 2018, 31, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Stumpf, T.R.; Yang, X.; Zhang, J.; Cao, X. In situ and ex situ modifications of bacterial cellulose for applications in tissue engineering. Mater. Sci. Eng. C 2018, 82, 372–383. [Google Scholar] [CrossRef]
- Chiaoprakobkij, N.; Seetabhawang, S.; Sanchavanakit, N.; Phisalaphong, M. Fabrication and characterization of novel bacterial cellulose/alginate/gelatin biocomposite film. J. Biomater. Sci. Polym. Ed. 2019, 30, 961–982. [Google Scholar] [CrossRef]
- Kim, H. Wound Dressing Materials: The Essentials. J. Wound Manag. Res. 2018, 14, 141–142. [Google Scholar] [CrossRef]
- Sionkowska, A.; Mężykowska, O.; Piątek, J. Bacterial nanocelullose in biomedical applications: A review. Polym. Int. 2019, 68, 1841–1847. [Google Scholar] [CrossRef]
- Tamahkar, E.; Bakhshpour, M.; Denizli, A. Molecularly imprinted composite bacterial cellulose nanofibers for antibiotic release. J. Biomater. Sci. Polym. Ed. 2019, 30, 450–461. [Google Scholar] [CrossRef]
- Weyell, P.; Beekmann, U.; Küpper, C.; Dederichs, M.; Thamm, J.; Fischer, D.; Kralisch, D. Tailor-made material characteristics of bacterial cellulose for drug delivery applications in dentistry. Carbohydr. Polym. 2019, 207, 1–10. [Google Scholar] [CrossRef]
- Hajmohammadi, K.; Zabihi, R.E.; Akbarzadeh, K.; Parizad, N. Using a combination therapy to combat scalp necrosis: A case report. J. Med. Case Rep. 2020, 14, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Schwarzkopf, R. Do Different Wound Dressings after Total Joint Arthroplasty Make a Difference? Do Different Wound Dressings After Total Joint Arthroplasty Make A Difference? Orthop. Surg. Sports Med. 2018, 1, 1–2. [Google Scholar] [CrossRef]
- Delli Santi, G.; Borgognone, A. The Use of Epiprotect®, An Advanced Wound Dressing, To Heal Paediatric Pa-tients With Burns: A Pilot Study. Burn. Open 2019, 3, 103–107. [Google Scholar] [CrossRef]
- Pooja, R.; Vadodaria, K.; Vidhya, S. Synthesis of bacterial cellulose and herbal extract for the development of wound dressing. Mater. Today Proc. 2019, 15, 284–293. [Google Scholar] [CrossRef]
- Kaviani, A.; Zebarjad, S.M.; Javadpour, S.; Ayatollahi, M.; Bazargan-Lari, R. Fabrication and characterization of low-cost freeze-gelated chitosan/collagen/hydroxyapatite hydrogel nanocomposite scaffold. Int. J. Polym. Anal. Charact. 2019, 24, 191–203. [Google Scholar] [CrossRef]
- Wichai, S.; Chuysinuan, P.; Chaiarwut, S.; Ekabutr, P.; Supaphol, P. Development of Bacterial Cellu-lose/Alginate/Chitosan Composites Incorporating Copper (II) Sulfate As An Antibacterial Wound Dressing. J. Drug Deliv. Sci. Technol. 2019, 51, 662–671. [Google Scholar] [CrossRef]
- Cielecka, I.; Szustak, M.; Kalinowska, H.; Gendaszewska-Darmach, E.; Ryngajłło, M.; Maniukiewicz, W.; Bielecki, S. Glycerol-Plasticized Bacterial Nanocellulose-Based Composites with Enhanced Flexibility And Liquid Sorption Capaci-ty. Cellulose 2019, 26, 5409–5426. [Google Scholar] [CrossRef]
- Figueiredo, A.; Silva, N.; Barros-Timmons, A.; Almeida, A.; Silvestre, A.; Freire, C. Antimicrobial Bacterial Cellu-lose Nanocomposites Prepared by In Situ Polymerization Of 2-Aminoethyl Methacrylate. Carbohydr. Polym. 2015, 123, 443–453. [Google Scholar] [CrossRef]
- Nguyen, T.; Ruksakulpiwat, C.; Ruksakulpiwat, Y. Effect of Cellulose Nanofibers from Cassava Pulp on Physi-cal Properties of Poly(Lactic Acid) Biocomposites. J. Thermoplast. Compos. Mater. 2020, 33, 1094–1108. [Google Scholar] [CrossRef]
- Aydogdu, M.O.; Altun, E.; Ahmed, J.; Gunduz, O.; Edirisinghe, M. Fiber Forming Capability of Binary and Ternary Compositions in the Polymer System: Bacterial Cellulose-Polycaprolactone-Polylactic Acid. Polymers 2019, 11, 1148. [Google Scholar] [CrossRef]
- Sun, Y.; Meng, C.; Zheng, Y.; Xie, Y.; He, W.; Wang, Y.; Qiao, K.; Yue, L. The Effects of Two Biocompatible Plas-ticizers on The Performance of Dry Bacterial Cellulose Membrane: A Comparative Study. Cellulose 2018, 25, 5893–5908. [Google Scholar] [CrossRef]
- Rubina, M.S.; Pigaleva, M.A.; Butenko, I.E.; Budnikov, A.V.; Naumkin, A.V.; Gromovykh, T.I.; Lutsenko, S.V.; Vasil’Kov, A.Y. The interaction effect of bacterial cellulose with gold nanoparticles obtained by metal-vapor synthesis. Дoклады Академии наук 2019, 488, 391–396. [Google Scholar] [CrossRef]
- Jalili Tabaii, M.; Emtiazi, G. Transparent Nontoxic Antibacterial Wound Dressing Based on Silver Nano Parti-cle/Bacterial Cellulose Nano Composite Synthesized in The Presence of Tripolyphosphate. J. Drug Deliv. Sci. Technol. 2018, 44, 244–253. [Google Scholar] [CrossRef]
- Mohseni, M.; Shamloo, A.; Aghababaei, Z.; Vossoughi, M.; Moravvej, H. Antimicrobial Wound Dressing Con-taining Silver Sulfadiazine with High Biocompatibility: In Vitro Study. Artif. Organs 2016, 40, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Mathew, S.; Snigdha, S.; Mathew, J.; Krishnankutty, R.E. Poly(vinyl alcohol): Montmorillonite: Boiled rice water (starch) blend film reinforced with silver nanoparticles; characterization and antibacterial properties. Appl. Clay Sci. 2018, 161, 464–473. [Google Scholar] [CrossRef]
- Aboelnaga, A.; Elmasry, M.; Adly, O.; Elbadawy, M.; Abbas, A.; Abdelrahman, I.; Salah, O.; Steinvall, I. Microbi-al Cellulose Dressing Compared with Silver Sulphadiazine for The Treatment Of Partial Thickness Burns: A Prospective, Randomised, Clinical Trial. Burns 2018, 44, 1982–1988. [Google Scholar] [CrossRef]
- Hosseini, H.; Zirakjou, A.; Goodarzi, V.; Mousavi, S.; Khonakdar, H.; Zamanlui, S. Lightweight aerogels based on bacterial cellulose/silver nanoparticles/polyaniline with tuning morphology of polyaniline and application in soft tissue engi-neering. Int. J. Biol. Macromol. 2020, 152, 57–67. [Google Scholar] [CrossRef]
- Ma, B.; Chaudhary, J.; Zhu, J.; Sun, B.; Chen, C.; Sun, D. Construction of silver nanoparticles anchored in carbon-ized bacterial cellulose with enhanced antibacterial properties. Colloids Surf. A Physicochem. Eng. Asp. 2020, 611, 125845. [Google Scholar] [CrossRef]
- Goi, Y.; Fujisawa, S.; Saito, T.; Yamane, K.; Kuroda, K.; Isogai, A. Dual Functions of TEMPO-Oxidized Cellulose Nanofibers in Oil-in-Water Emulsions: A Pickering Emulsifier and a Unique Dispersion Stabilizer. Langmuir 2019, 35, 10920–10926. [Google Scholar] [CrossRef]
- Khan, S.; Ul-Islam, M.; Khattak, W.A.; Ullah, M.W.; Park, J.K. Bacterial cellulose-titanium dioxide nanocomposites: Nanostructural characteristics, antibacterial mechanism, and biocompatibility. Cellulose 2015, 22, 565–579. [Google Scholar] [CrossRef]
- Zywicka, A.; Fijałkowski, K.; Junka, A.; Grzesiak, J.; El Fray, M. Modification of Bacterial Cellulose with Quater-nary Ammonium Compounds Based on Fatty Acids and Amino Acids and The Effect on Antimicrobial Activity. Biom-Acromolecules 2018, 19, 1528–1538. [Google Scholar] [CrossRef] [PubMed]
- Napavichayanun, S.; Ampawong, S.; Harnsilpong, T.; Angspatt, A.; Aramwit, P. Inflammatory reaction, clinical efficacy, and safety of bacterial cellulose wound dressing containing silk sericin and polyhexamethylene biguanide for wound treatment. Arch. Derm. Res. 2018, 310, 795–805. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Jiang, L.; Wu, J.; Su, C.; Huang, C.; Liu, X.; Shao, W. Flexible Amoxicillin-Grafted Bacterial Cellulose Sponges for Wound Dressing: In Vitro and in Vivo Evaluation. ACS Appl. Mater. Interfaces 2018, 10, 5862–5870. [Google Scholar] [CrossRef] [PubMed]
- Wijaya, C.J.; Saputra, S.N.; Soetaredjo, F.E.; Putro, J.N.; Lin, C.X.; Kurniawan, A.; Ju, Y.-H.; Ismadji, S. Cellulose nanocrystals from passion fruit peels waste as antibiotic drug carrier. Carbohydr. Polym. 2017, 175, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Yaşayan, G.; Karaca, G.; Akgüner, Z.P.; Öztürk, A.B. Chitosan/collagen composite films as wound dressings encapsulating allantoin and lidocaine hydrochloride. Int. J. Polym. Mater. 2020, 1–13, 1–13. [Google Scholar] [CrossRef]
- Beekmann, U.; Schmölz, L.; Lorkowski, S.; Werz, O.; Thamm, J.; Fischer, D.; Kralisch, D. Process control and scale-up of modified bacterial cellulose production for tailor-made anti-inflammatory drug delivery systems. Carbohydr. Polym. 2020, 236, 116062. [Google Scholar] [CrossRef]
- Wahid, F.; Hu, X.-H.; Chu, L.; Jia, S.-R.; Xie, Y.-Y.; Zhong, C. Development of bacterial cellulose/chitosan based semi-interpenetrating hydrogels with improved mechanical and antibacterial properties. Int. J. Biol. Macromol. 2019, 122, 380–387. [Google Scholar] [CrossRef]
- Müller, A.; Wesarg, F.; Hessler, N.; Müller, F.; Kralisch, D.; Fischer, D. Loading of Bacterial Nanocellulose Hy-drogels With Proteins Using A High-Speed Technique. Carbohydr. Polym. 2014, 106, 410–413. [Google Scholar] [CrossRef]
- Fürsatz, M.; Skog, M.; Sivlér, P.; Palm, E.; Aronsson, C.; Skallberg, A.; Greczynski, G.; Khalaf, H.; Bengtsson, T.; Aili, D. Functionalization of bacterial cellulose wound dressings with the antimicrobial peptide ε-poly-L-Lysine. Biomed. Mater. 2017, 13, 025014. [Google Scholar] [CrossRef]
- Yang, G.; Jiang, H.; Zheng, W.; Gong, N.; Chen, L.; Jiang, X.; Yanga, G. Correction: Bacterial cellulose–hyaluronan nanocomposite biomaterials as wound dressings for severe skin injury repair. J. Mater. Chem. B 2019, 7, 1962. [Google Scholar] [CrossRef]
- Fallacara, A.; Durini, E.; Vertuani, S.; Manfredini, S. Hyaluronic Acid Fillers in Soft Tissue Regeneration. Facial Plast. Surg. 2017, 33, 087–096. [Google Scholar] [CrossRef] [PubMed]
- 360researchreports.com. Global Microbial and Bacterial Cellulose Market—Industry Reports. 2020. Available online: https://www.360researchreports.com/global-microbial-and-bacterial-cellulose-market-15085121 (accessed on 16 December 2020).
- Portela, R.; Leal, C.; Almeida, P.; Sobral, R. Bacterial Cellulose: A Versatile Biopolymer for Wound Dressing Ap-plications. Microb. Biotechnol. 2019, 12, 586–610. [Google Scholar] [CrossRef] [PubMed]
- Leppiniemi, J.; Lahtinen, P.; Paajanen, A.; Mahlberg, R.; Metsä-Kortelainen, S.; Pinormaa, T.; Pajari, H.; Vikholm-Lundin, I.; Pursula, P.; Blazevic, V. 3D-Printable Bioactivated Nanocellulose–Alginate Hydrogels. ACS Appl. Mater. Interfaces 2017, 9, 21959–21970. [Google Scholar] [CrossRef] [PubMed]
- Núñez-Carmona, E.; Bertuna, A.; Abbatangelo, M.; Sberveglieri, V.; Comini, E.; Sberveglieri, G. BC-MOS: The novel bacterial cellulose based MOS gas sensors. Mater. Lett. 2019, 237, 69–71. [Google Scholar] [CrossRef]
- Vuorinen, T.; Laurila, M.-M.; Mangayil, R.; Karp, M.; Mäntysalo, M. High Resolution E-Jet Printed Temperature Sensor on Artificial Skin. In XXVI Brazilian Congress on Biomedical Engineering; Springer Nature: London, UK, 2018; pp. 839–842. [Google Scholar]
- Golubnitschaja, O.; Veeser, L.S.; Avishai, E.; Costigliola, V. Wound Healing: Proof-of-Principle Model for the Modern Hospital: Patient Stratification, Prediction, Prevention and Personalisation of Treatment. In The Modern Hospital; Springer Nature: London, UK, 2019; pp. 357–366. [Google Scholar]
- Badshah, M.; Ullah, H.; Wahid, F.; Khan, T. Bacterial Cellulose Based Metallic Green Nanocomposites for Bio-medical and Pharmaceutical Applications. Curr. Pharm. Des. 2020, 26, 5866–5880. [Google Scholar] [CrossRef]
- Eslahi, N.; Mahmoodi, A.; Mahmoudi, N.; Zandi, N.; Simchi, A. Processing and Properties of Nanofibrous Bacte-rial Cellulose-Containing Polymer Composites: A Review of Recent Advances for Biomedical Applications. Polym. Rev. 2019, 60, 144–170. [Google Scholar] [CrossRef]
- Gorgieva, S.; Trcek, J. Bacterial Cellulose: Production, Modification and Perspectives in Biomedical Applications. Nanomaterials 2019, 9, 1352. [Google Scholar] [CrossRef]
- De Oliveira Barud, H.G.; da Silva, R.R.; da Silva Barud, H.; Tercjak, A.; Gutierrez, J.; Lustri, W.R.; de Oliveira, O.B.; Ri-beiro, S.J.L. A multipurpose natural and renewable polymer in medical applications: Bacterial cellulose. Carbohydr. Polym. 2016, 153, 406–420. [Google Scholar] [CrossRef]
- Rjwade, J.M.; Paknikar, K.M.; Kumbhar, J.V. Applications of bacterial cellulose and its composites in biomedi-cine. Appl. Microbiol. Biotechnol. 2015, 99, 2491–2511. [Google Scholar] [CrossRef]
- Hasan, N.; Rahman, L.; Kim, S.-H.; Cao, J.; Arjuna, A.; Lallo, S.; Jhun, B.H.; Yoo, J.-W. Recent advances of nanocellulose in drug delivery systems. J. Pharm. Investig. 2020, 50, 553–572. [Google Scholar] [CrossRef]
- Mohite, B.V.; Patil, S.V. A novel biomaterial: Bacterial cellulose and its new era applications. Biotechnol. Appl. Biochem. 2014, 61, 101–110. [Google Scholar] [CrossRef] [PubMed]
| Application | Description | Reference |
|---|---|---|
| Wound dressing | Low cytotoxicity, swelling ratio, physicochemical and mechanical properties of BC make it an ideal biomaterial for wound dressings | [38,39,43,44,45,46,48,50,51,53,54] |
| Antimicrobial activity | Capable of being biofunctionalized with various antimicrobial agents | [33,37] |
| Tissue regeneration | Intracellular matrix of BC allows it to be used a scaffold for tissue growth | [4,5,31,32,60,63,66,67,68,69,70] |
| Drug delivery | Water holding capabilities allow for pharmaceuticals to be loaded | [10,48,56,57] |
| Vascular grafts | Elasticity and tensile strength allow it to be used as artificial blood vessels | [63,72] |
| Surgical reconstruction | Can be used as a biodegradable alternative to synthetic surgical consumables | [60,61,62,63,64,65,66] |
| Emulsion stabiliser | Fractionated BC can be used as an emulsifier in oil-water mixtures | [86] |
| Dietary supplement | BC is pure fiber and can be eaten to aide digestion | [85,87] |
| Enzyme immobilisation | Surface modifications allow for enzymes to be immobilised | [72] |
| Filtration system | Nanofibrillar matrix and carbon coatings allow BC to be used an effective filter | [79] |
| Conductive material | Modification with metal nanoparticles make the BC conductive for use in nanowires | [76,78,81,82,83,84,85,86] |
| Biosensors | Biofunctionalised BC can be made into patches that are sensitive to biochemical tests | [76,77,78] |
| Active Compound in Bacterial Cellulose Composite | Property | Reference |
|---|---|---|
| Polyhexanide | Antimicrobial activity | [126] |
| Octenidine dihydrochloride | Antimicrobial activity | [48,52] |
| Benzalkonium chloride | Antimicrobial activity | [126] |
| Tetracycline | Antimicrobial activity | [47,55,62] |
| Amoxicillin | Antimicrobial activity | [127] |
| Povidone iodine | Antimicrobial activity | [48] |
| Antimicrobial peptides | Antimicrobial activity | [133] |
| Lysozyme | Antimicrobial activity | [37] |
| Dehydrogenative polymers | Antimicrobial activity | [5] |
| Silver Nanoparticles | Antimicrobial activity | [54,115,116,117,118,119,120,121,122] |
| Chitosan | Antimicrobial activity | [38,40,41,51,107,108,129,131] |
| Zinc Oxide | Antimicrobial activity | [80] |
| Antibiotics | Antimicrobial activity | [101,120] |
| Gold nanoparticles | Antimicrobial activity | [114] |
| Diclofenac/Ibuprofen | Anti-inflammatory | [56,57] |
| Lidocaine/Benzocaine | Analgesia | [129] |
| Silk sericin | Angiogenesis and re-epithelisation | [126] |
| Mesenchymal stem cells | Angiogenesis and re-epithelisation | [4] |
| Epidermal keratinocytes | Angiogenesis and re-epithelisation | [44] |
| Dermal fibroblasts | Angiogenesis and re-epithelisation | [44,67,71] |
| Polyvinyl alcohol | Mechanical performance | [41] |
| Plasticisers | Mechanical performance | [109] |
| Sodium alginate | Mechanical performance | [98,108] |
| Polylactic acids | Physicochemical properties | [112] |
| Hyaluronic acid | Physicochemical properties | [134,135] |
| Hydrolysed gelatine | Physicochemical properties | [43,98,107] |
| Acrylic acid | Faster wound healing | [44] |
| Magnetite | Faster wound healing | [68,81] |
| Radioactive isotopes | Cancer wound therapy | [95] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Swingler, S.; Gupta, A.; Gibson, H.; Kowalczuk, M.; Heaselgrave, W.; Radecka, I. Recent Advances and Applications of Bacterial Cellulose in Biomedicine. Polymers 2021, 13, 412. https://doi.org/10.3390/polym13030412
Swingler S, Gupta A, Gibson H, Kowalczuk M, Heaselgrave W, Radecka I. Recent Advances and Applications of Bacterial Cellulose in Biomedicine. Polymers. 2021; 13(3):412. https://doi.org/10.3390/polym13030412
Chicago/Turabian StyleSwingler, Sam, Abhishek Gupta, Hazel Gibson, Marek Kowalczuk, Wayne Heaselgrave, and Iza Radecka. 2021. "Recent Advances and Applications of Bacterial Cellulose in Biomedicine" Polymers 13, no. 3: 412. https://doi.org/10.3390/polym13030412
APA StyleSwingler, S., Gupta, A., Gibson, H., Kowalczuk, M., Heaselgrave, W., & Radecka, I. (2021). Recent Advances and Applications of Bacterial Cellulose in Biomedicine. Polymers, 13(3), 412. https://doi.org/10.3390/polym13030412