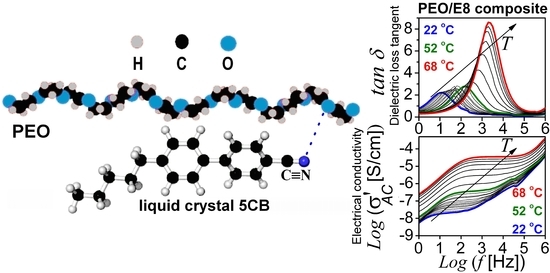
Ion-Conducting Flexible Thin Films of Composites from Poly(ethylene oxide) and Nematic Liquid Crystals E8—Characterization by Impedance and Dielectric Relaxation Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
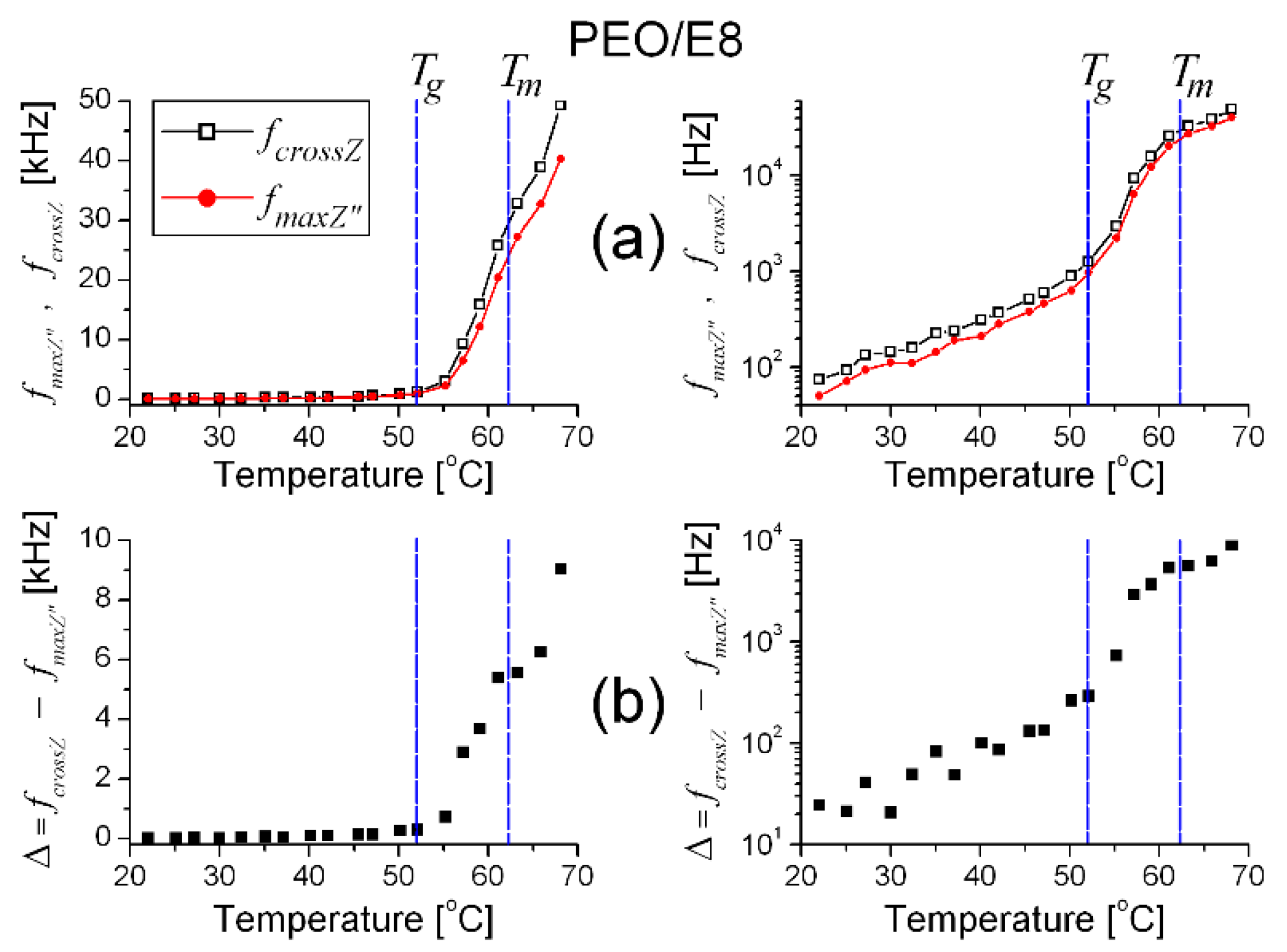
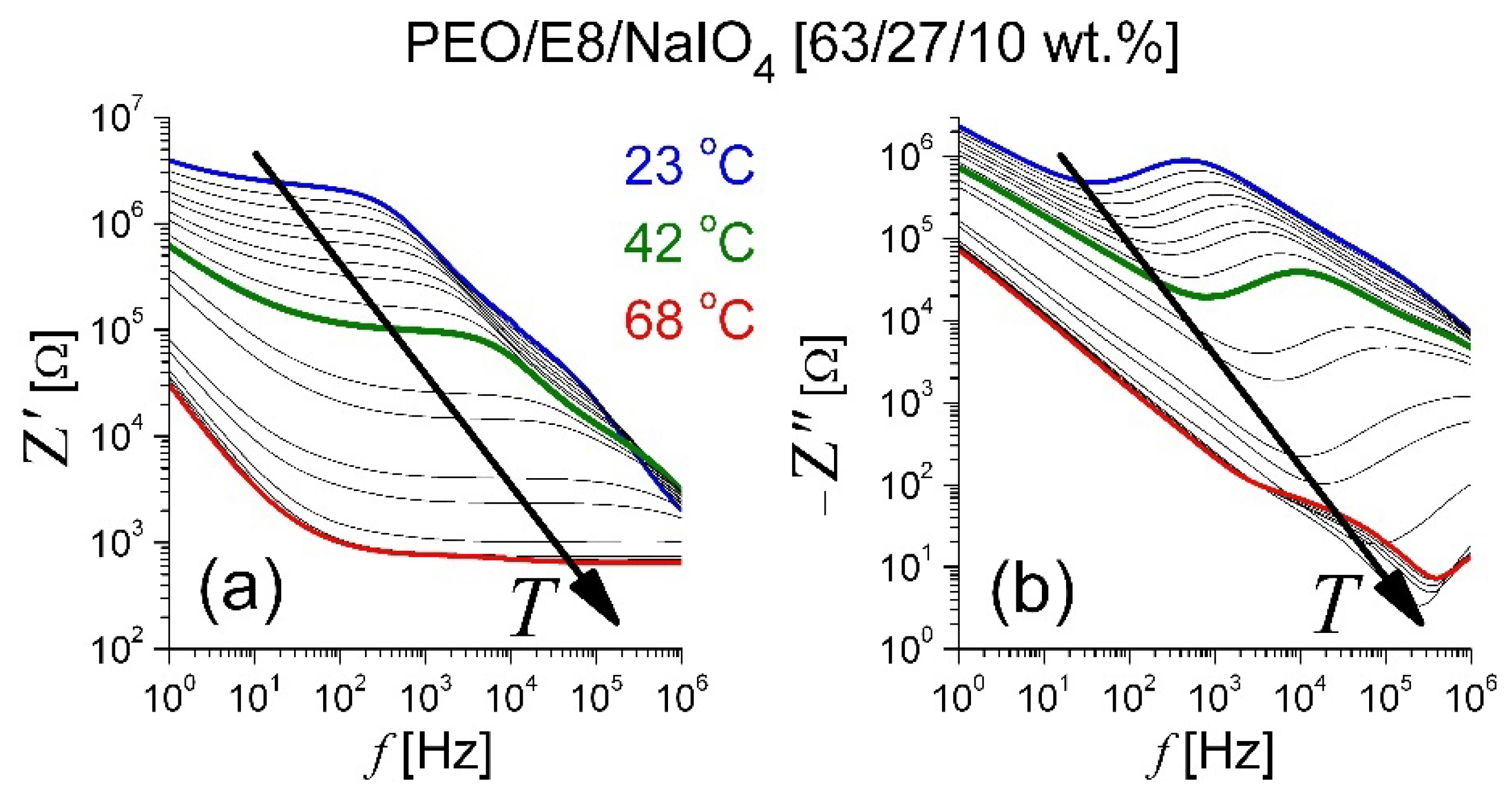
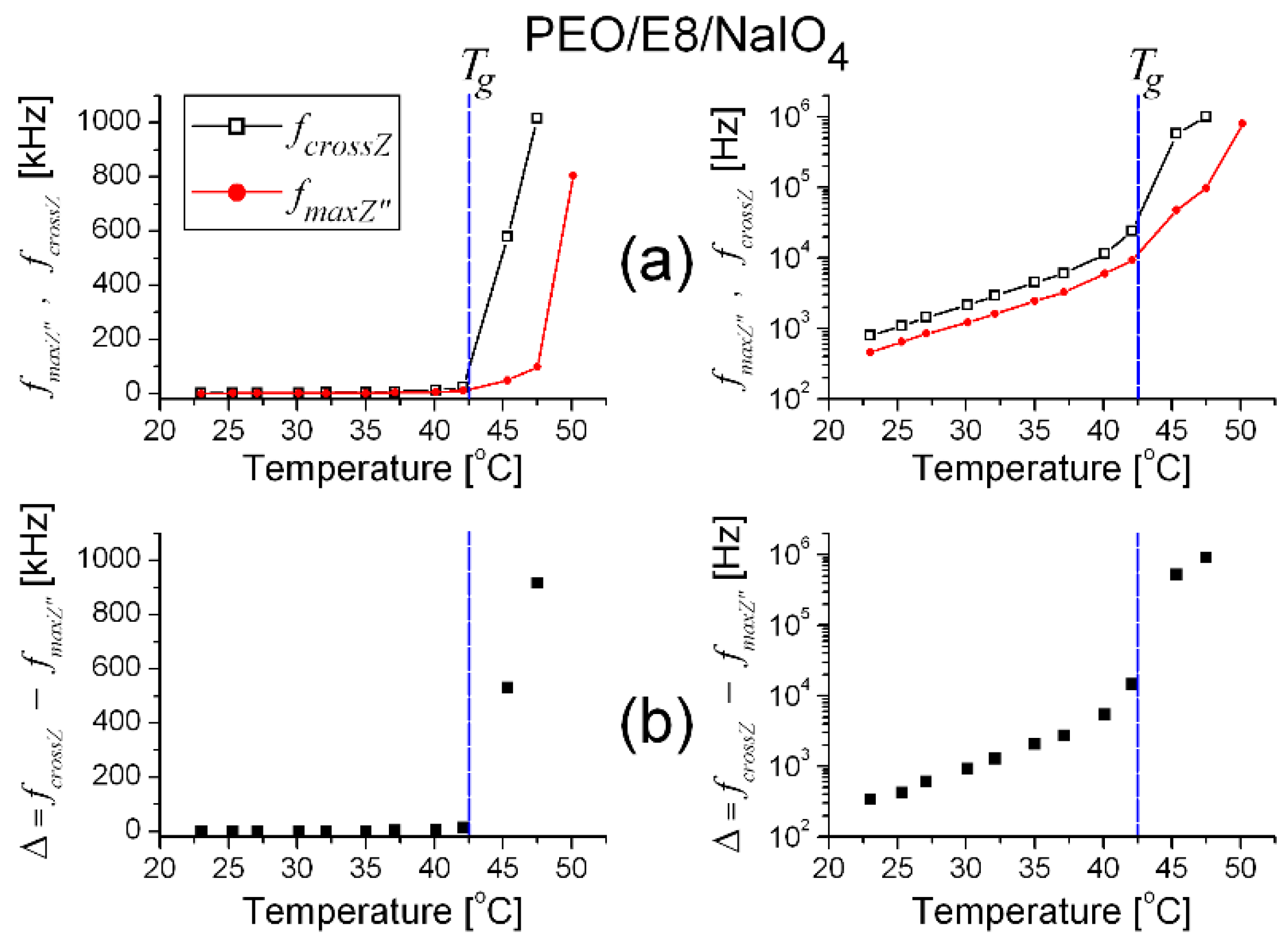
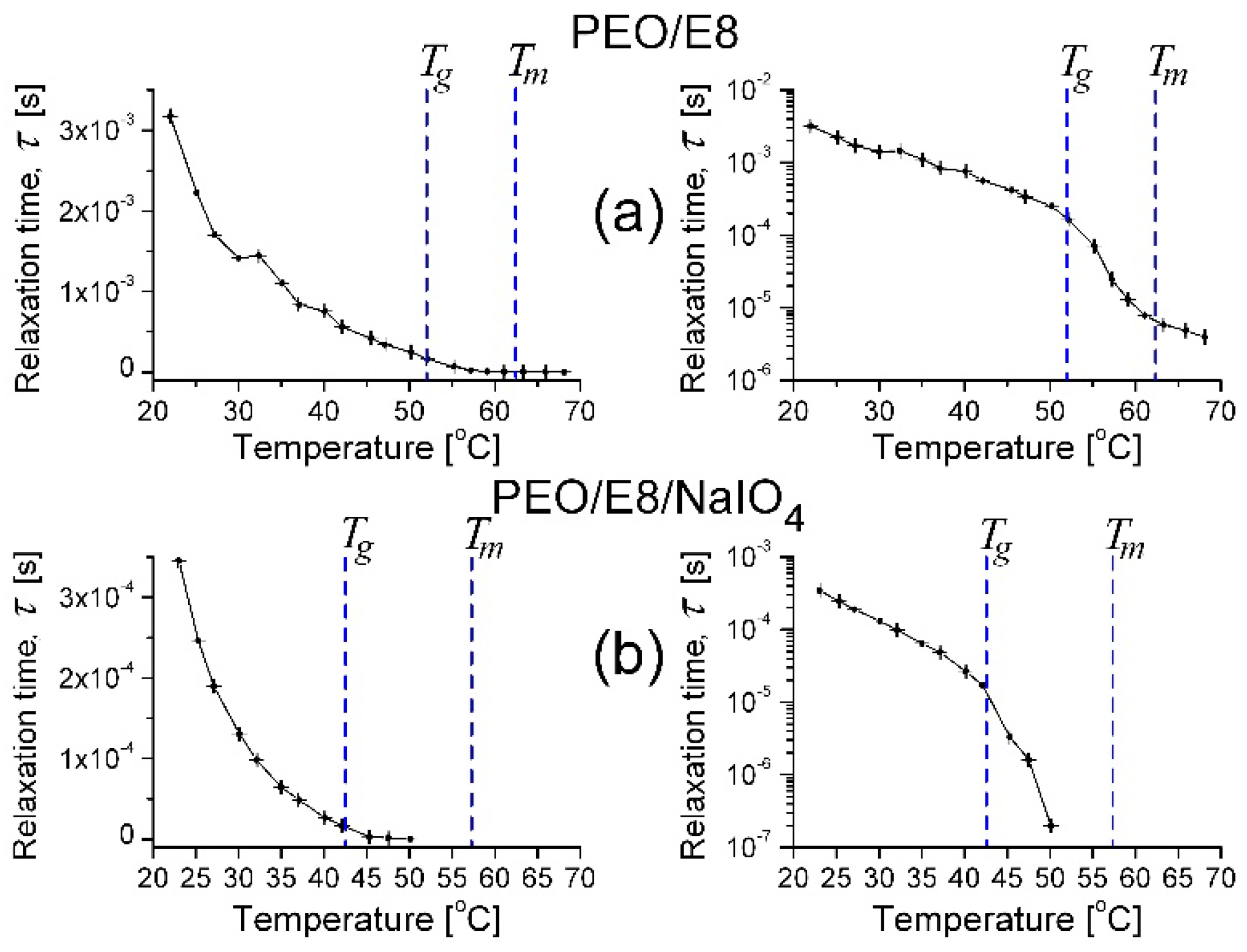
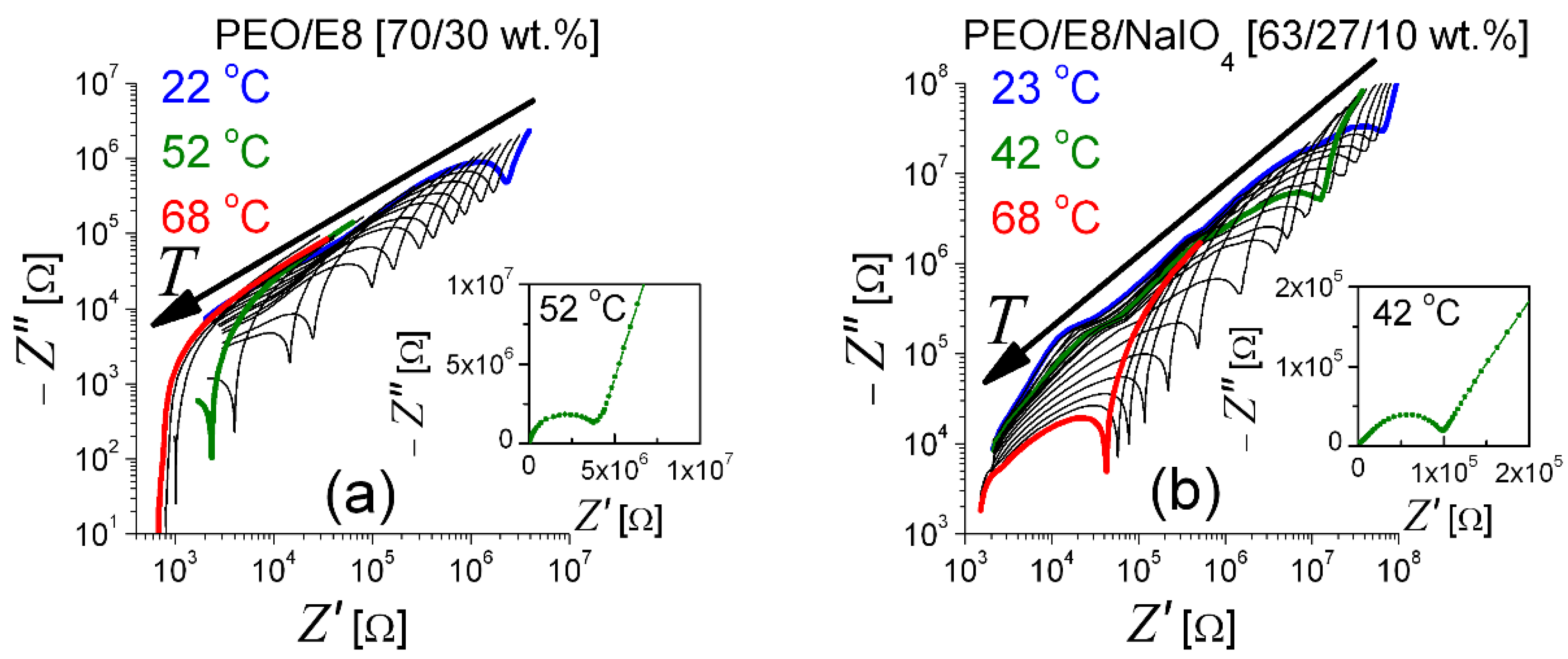
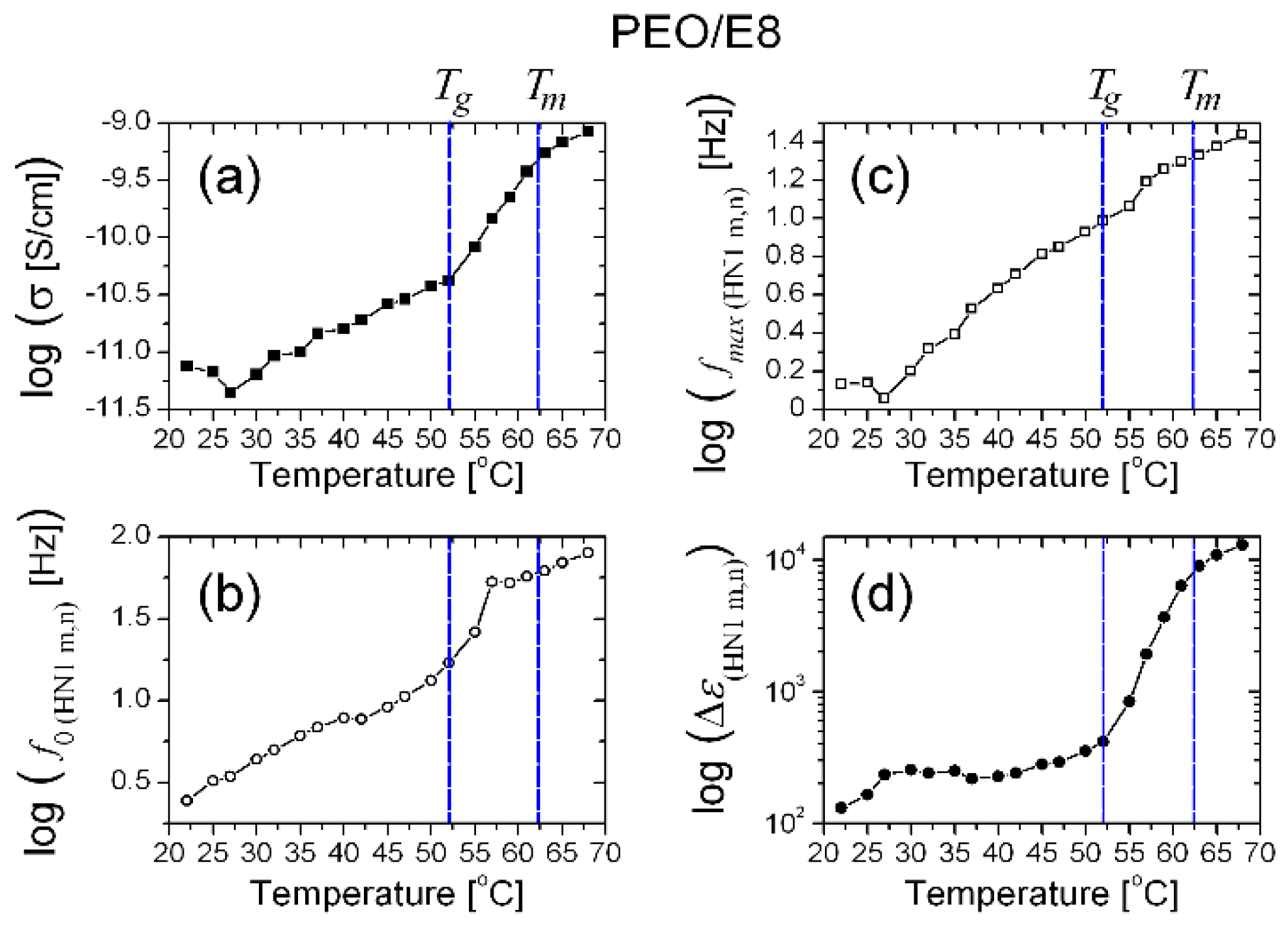
3.1. Impedance Spectra Analysis
- (i)
- fcrossZ > fmaxZ″ in the measured range of temperature (T), from Troom to the temperature slightly above the melting point Tm (Figure 2a) when an utterly amorphous phase of PEO is present (amorphous rubbery state);
- (ii)
- (iii)
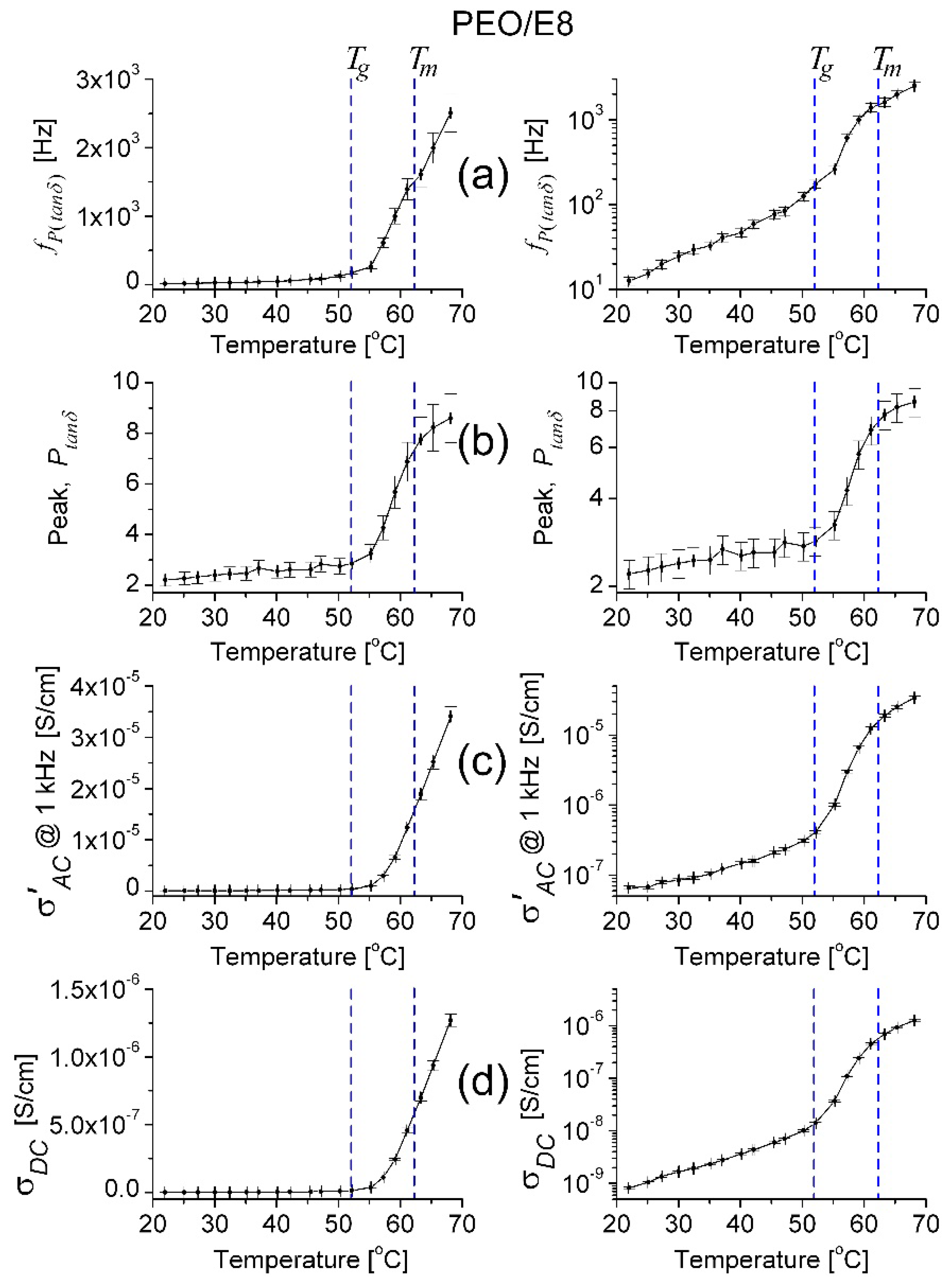
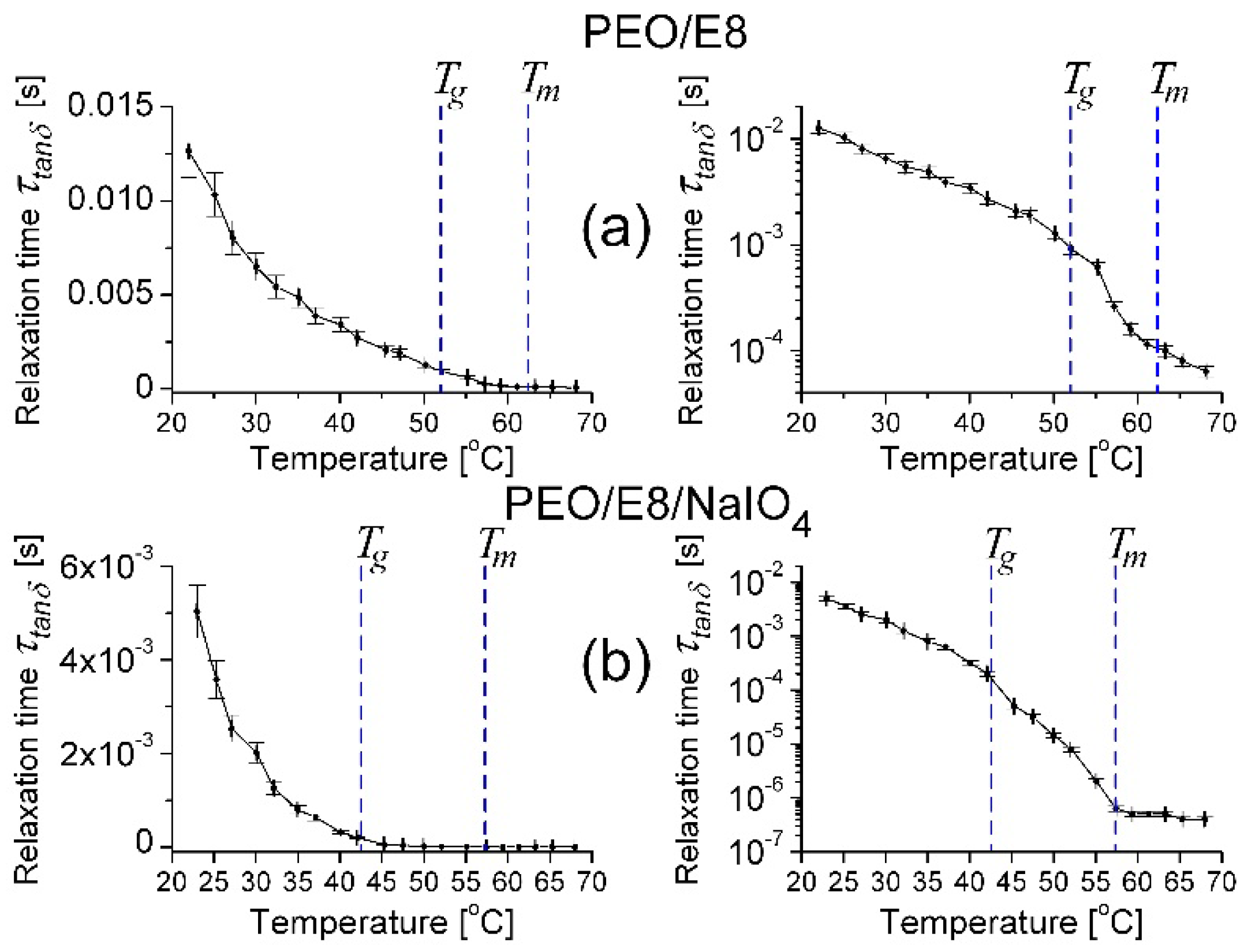
- In the temperature dependencies of both fcrossZ and fmaxZ″, there was a distinct threshold (Tth)—above the temperature Tth up to Tm, these dependencies were enhanced (at T > Tm the increment was lowered); the threshold Tth was equal for both fcrossZ and fmaxZ″, and Tth ≡ Tg (Figure 2a);
- (iv)
- The difference Δ = fcrossZ − fmaxZ″ was also an increasing function of T; Δ was steeply enlarged above the same temperature threshold Tth ≡ Tg (Figure 2b).
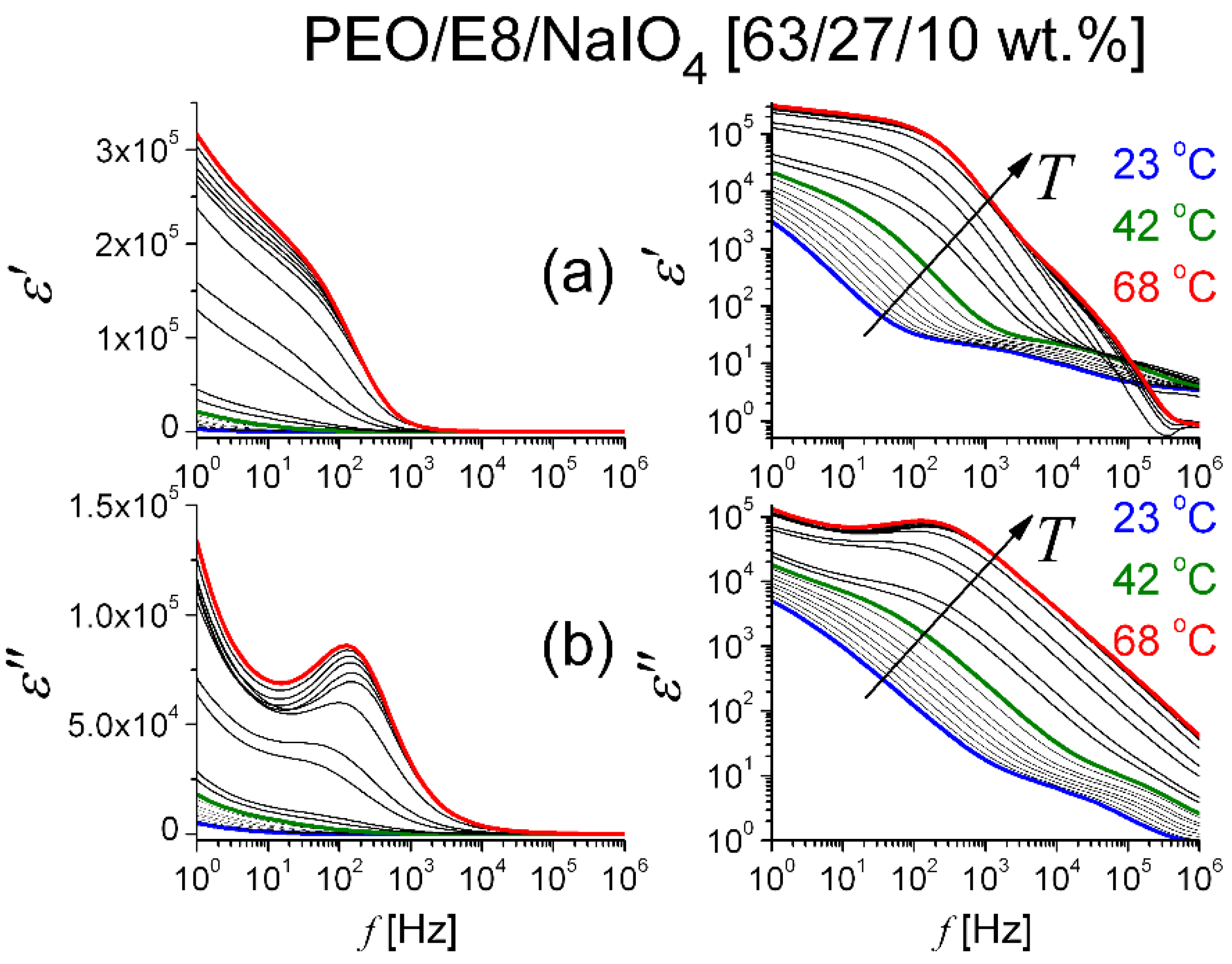
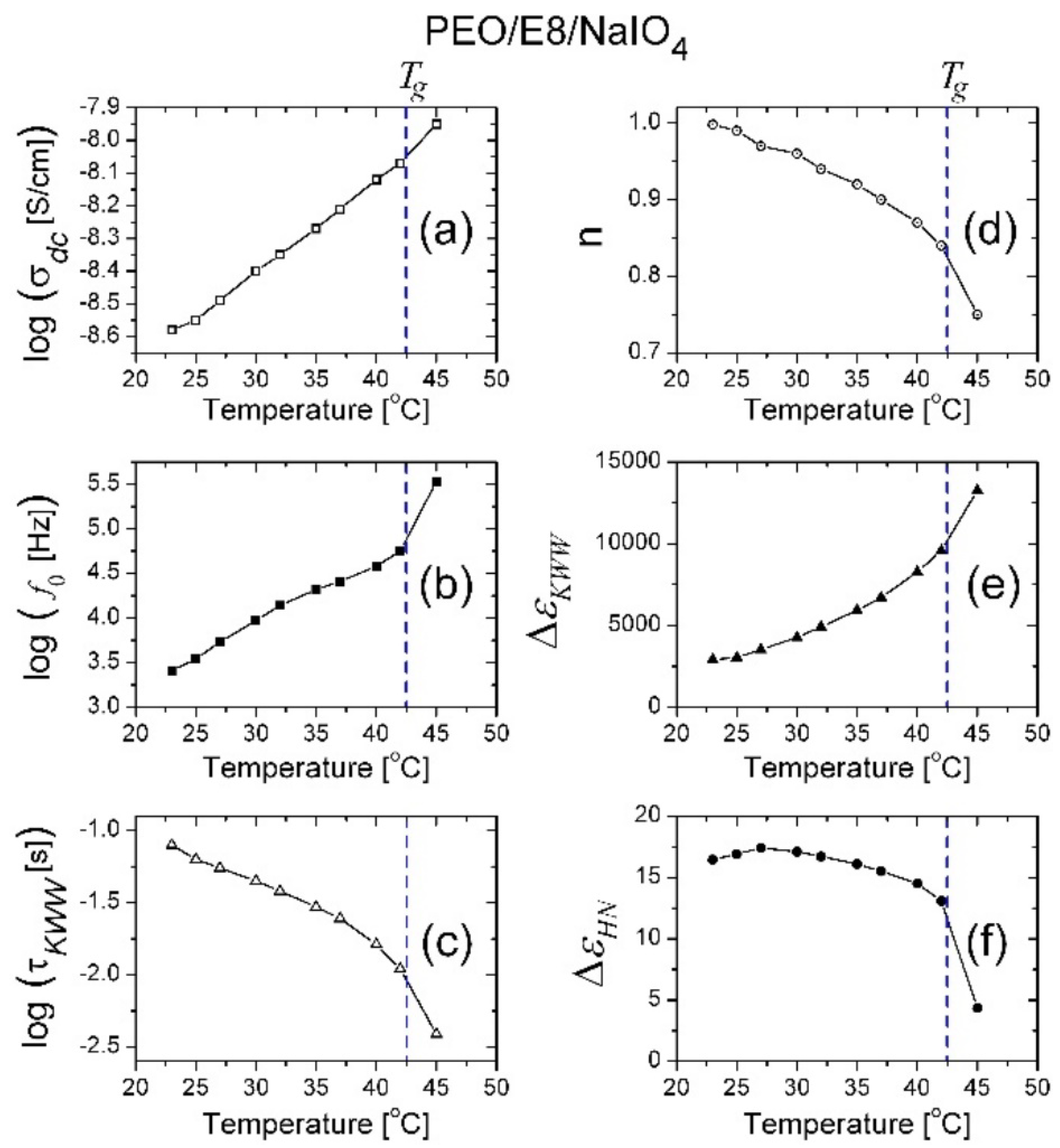
3.2. Dielectric Spectroscopic Analysis
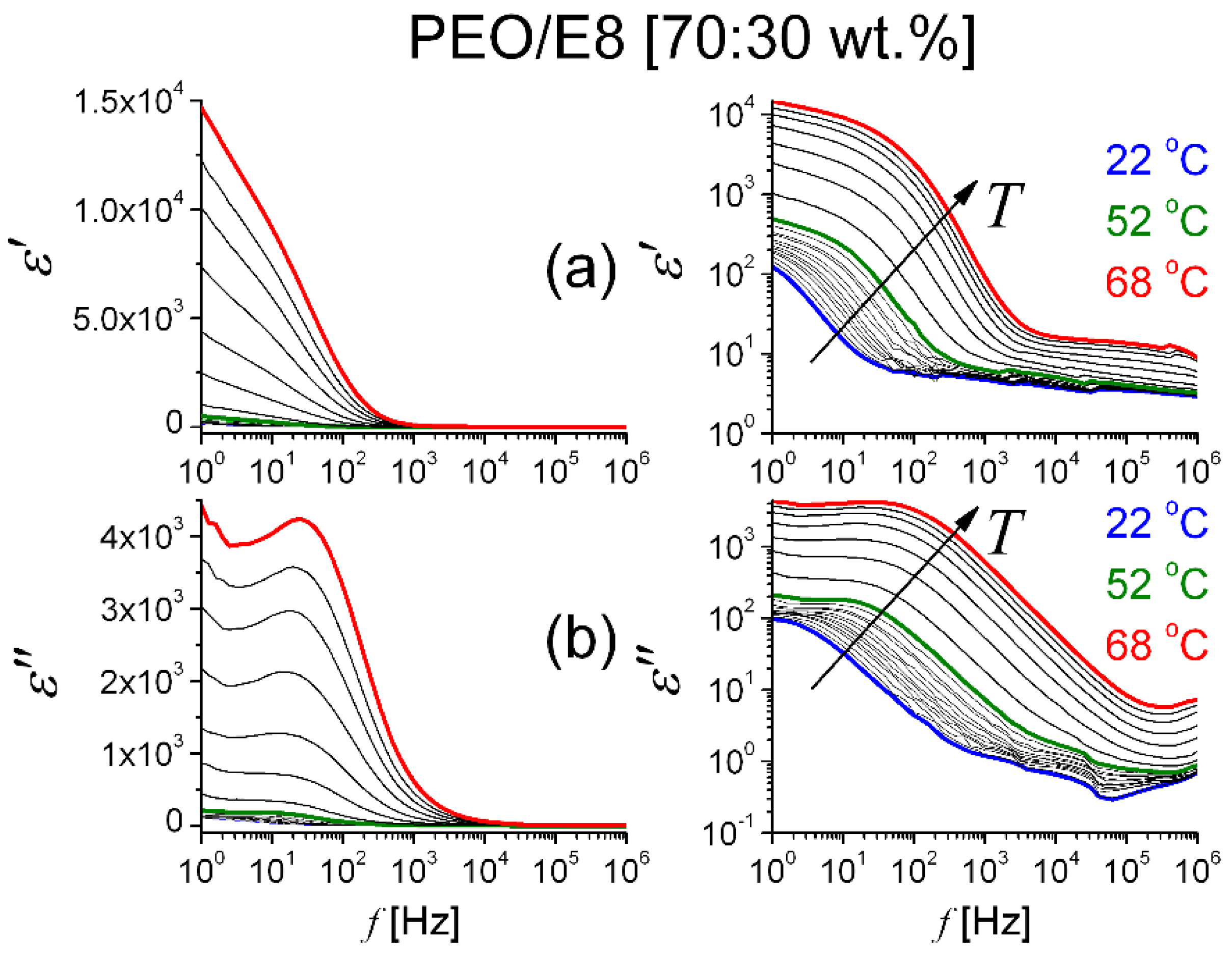
3.2.1. Dielectric Permittivity
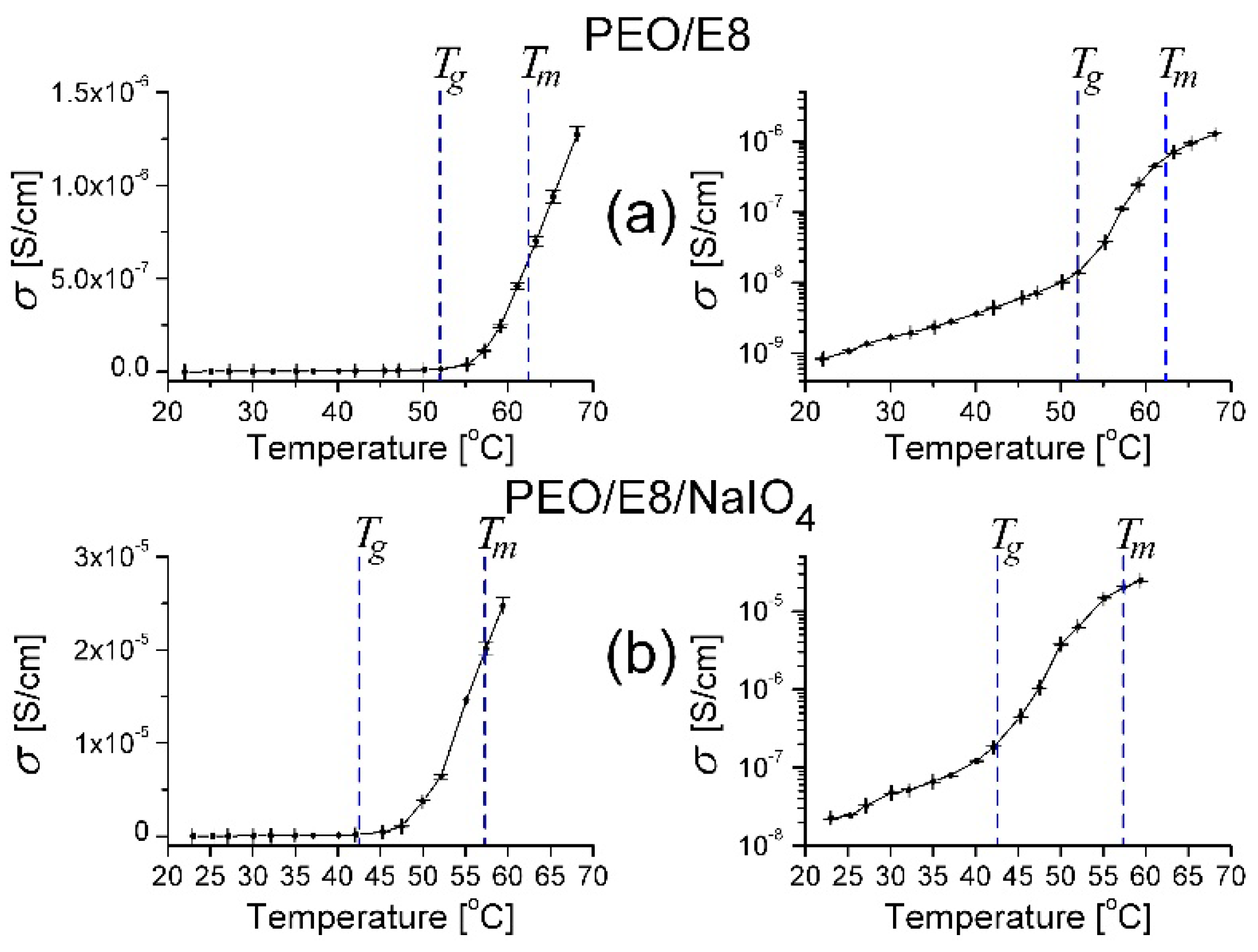
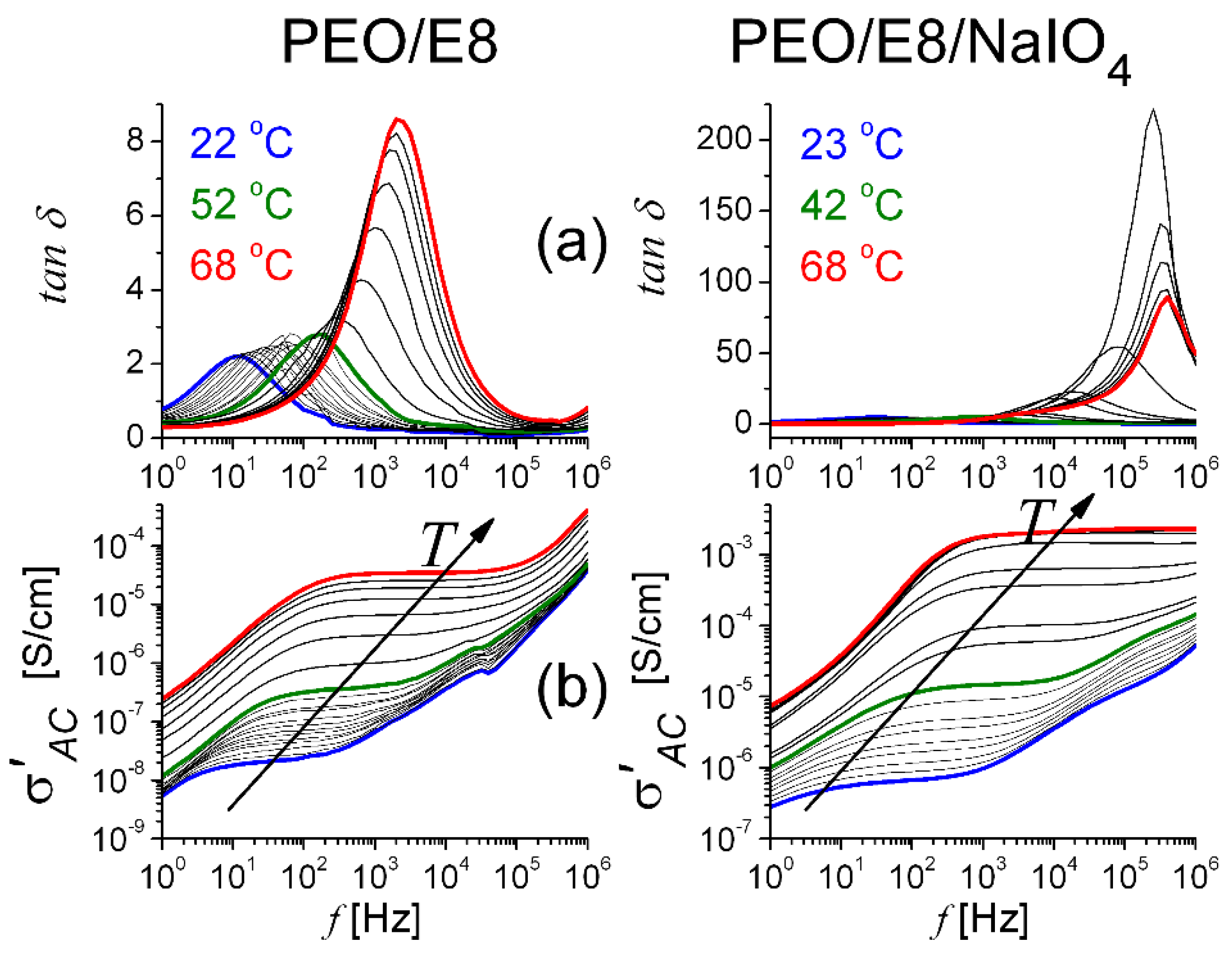
3.2.2. Dielectric Loss Tangent and AC Conductivity
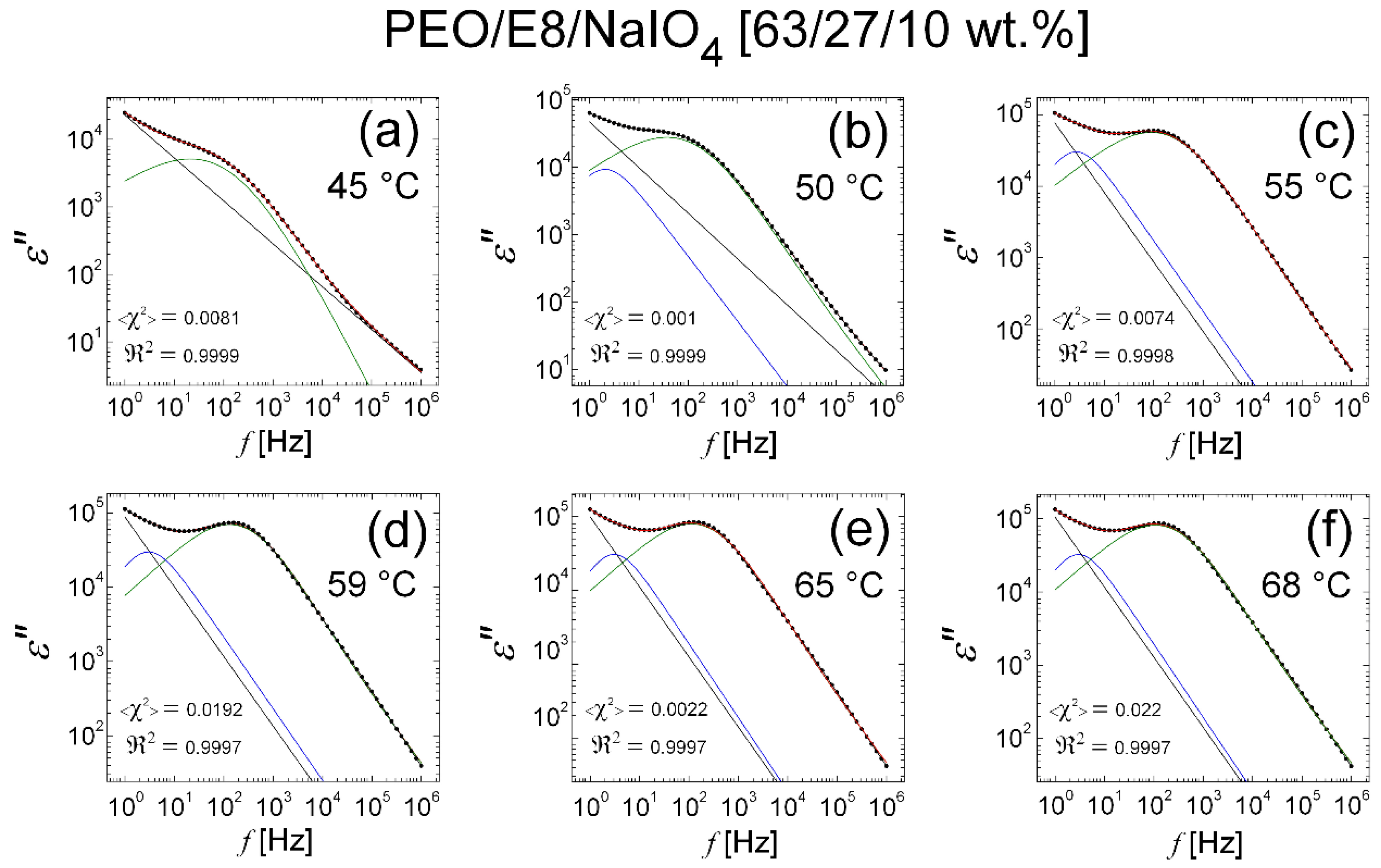
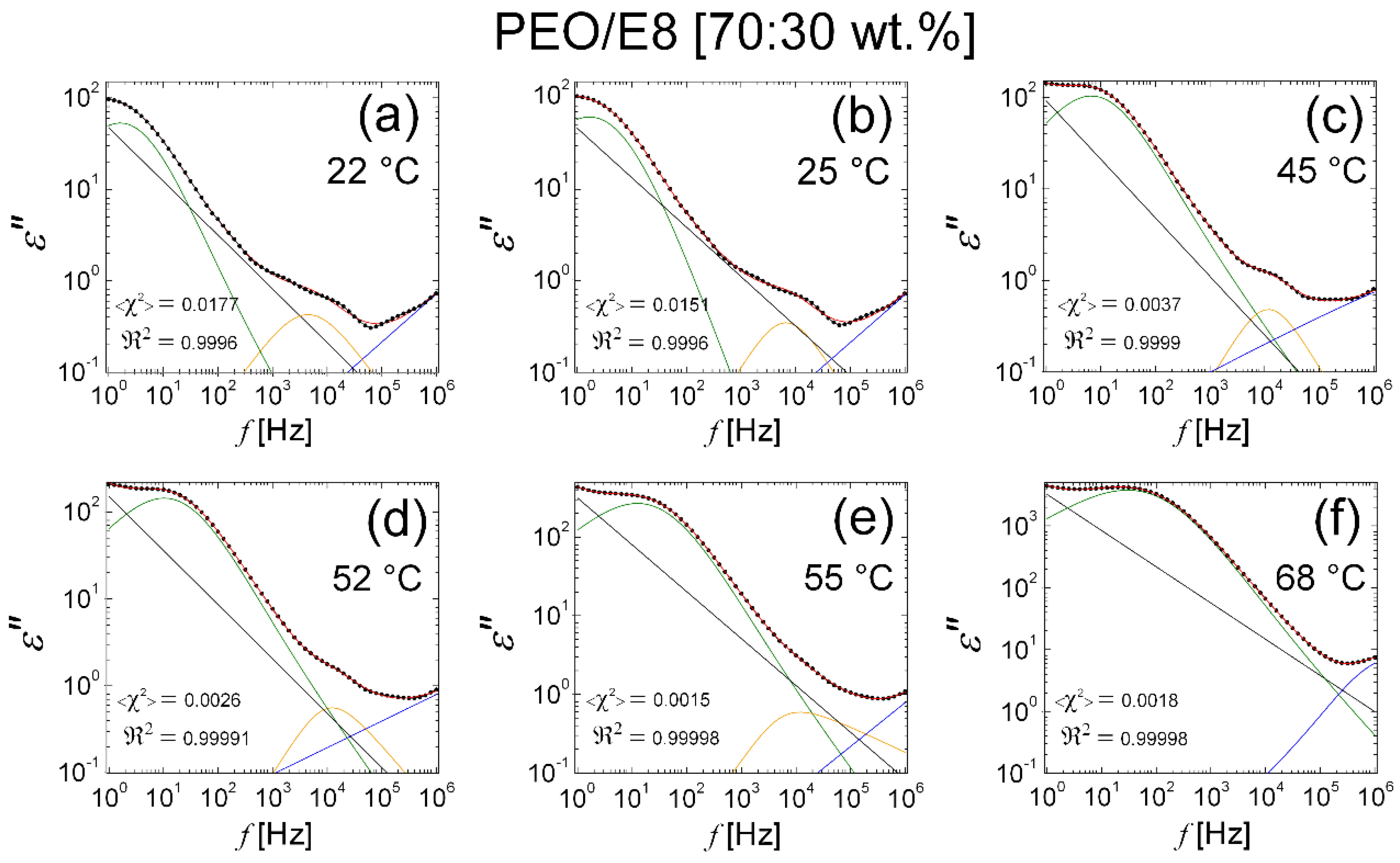
3.2.3. Modelling of Dielectric Behaviors
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bloisi, F.; Vicari, L.R.M.; Nasti, L. Liquid Crystal Polymer Composite Materials for LCDs. In Handbook of Visual Display Technology; Chen, J., Cranton, W., Fihn, M., Eds.; Springer: Cham, Switzerland, 2016; pp. 2223–2250. [Google Scholar]
- Vicari, L. Optical Applications of Liquid Crystals; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Kim, T.H.; Kim, M.; Manda, R.; Lim, Y.J.; Cho, K.J.; Hee, H.; Kang, J.W.; Lee, G.D.; Lee, S.H. Flexible liquid crystal displays using liquid crystal-polymer composite film and colorless polyimide substrate. Curr. Opt. Photonics 2019, 3, 66–71. [Google Scholar]
- Jain, A.K.; Deshmukh, R.R. An Overview of Polymer-Dispersed Liquid Crystals Composite Films and Their Applications. In Liquid Crystals and Display Technology; Ghamsari, M.S., Carlescu, I., Eds.; IntechOpen: London, UK, 2020; pp. 11–78. [Google Scholar]
- Lin, J.D.; Zhang, Y.S.; Lee, J.Y.; Mo, T.S.; Yeh, H.C.; Lee, C.R. Electrically tunable liquid-crystal-polymer composite laser with symmetric sandwich structure. Macromolecules 2020, 53, 913–921. [Google Scholar] [CrossRef]
- Saeed, M.H.; Zhang, S.; Cao, Y.; Zhou, L.; Hu, J.; Muhammad, I.; Xiao, J.; Zhang, L.; Yang, H. Recent advances in the polymer dispersed liquid crystal composite and its applications. Molecules 2020, 25, 5510. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.C.; Song, M.; Ryu, T.I.; Lee, M.J.; Jin, S.H.; Gal, Y.S.; Kim, H.K.; Lee, G.D.; Kang, Y.S. Liquid crystals embedded in polymeric electrolytes for quasi-solid state dye-sensitized solar cell applications. Macromol. Chem. Phys. 2009, 210, 1844–1850. [Google Scholar] [CrossRef]
- Karim, M.A.; Song, M.; Park, J.S.; Kim, Y.H.; Lee, M.J.; Lee, J.W.; Lee, C.W.; Cho, Y.R.; Gal, Y.S.; Lee, J.H.; et al. Development of liquid crystal embedded in polymer electrolytes composed of click polymers for dye-sensitized solar cell applications. Dyes Pigm. 2010, 86, 259–265. [Google Scholar] [CrossRef]
- Yang, D.K.; Wu, S.T. Fundamentals of Liquid Crystal Devices, 2nd ed.; John Wiley & Sons: Chichester, UK, 2015; pp. 363–413. [Google Scholar]
- Said, S.M.; Zulkifli, A.Z.S.; Kamarudin, M.A.; Mainal, A.; Subramanian, B.; Mohamed, N.S. Polymer electrolyte liquid crystal system for improved optical and electrical properties. Europ. Polym. J. 2015, 66, 266–272. [Google Scholar] [CrossRef]
- Said, S.M.; Sahamir, S.R.; Sabri, M.F.M.; Kamarudin, M.A.; Hayashi, K.; Zulkifli, A.Z.S.; Nakajo, T.; Kubouchi, M.; Miyazaki, Y. Polymer electrolyte liquid crystal mixtures as phase-dependent thermoelectric materials. Mol. Cryst. Liq. Cryst. 2017, 642, 9–17. [Google Scholar] [CrossRef]
- Wang, S.; Liu, X.; Wang, A.; Wang, Z.; Chen, J.; Zeng, Q.; Wang, X.; Zhang, L. An ionic liquid crystal-based solid polymer electrolyte with desirable ion-conducting channels for superior performance ambient-temperature lithium batteries. Polym. Chem. 2018, 9, 4674–4682. [Google Scholar] [CrossRef]
- Kamarudin, M.A.; Khan, A.A.; Said, S.M.; Qasim, M.M.; Wilkinson, T.D. Composite liquid crystal-polymer electrolytes in dye-sensitised solar cells: Effects of mesophase alkyl chain length. Liq. Cryst. 2018, 45, 112–121. [Google Scholar] [CrossRef]
- Nasir, N.; Hong, H.; Rehman, M.A.; Kumar, S.; Seo, Y. Polymer-dispersed liquid-crystal-based switchable glazing fabricated via vacuum glass coupling. RSC Adv. 2020, 10, 32225–32231. [Google Scholar] [CrossRef]
- Labeeb, A.M.; Ibrahim, S.A.; Ward, A.A.; Abd-El-Messieh, S.L. Polymer/liquid crystal nanocomposites for energy storage applications. Polym. Eng. Sci. 2020, 60, 2529–2540. [Google Scholar] [CrossRef]
- Armand, M.B.; Chabagno, J.M.; Duclot, M. Polyether as Solid Electrolytes. In Fast Ion Transport in Solids; Vashishta, P., Mundy, J.N., Shenoy, G.K., Eds.; North Holland: Amsterdam, The Netherlands, 1979; pp. 131–136. [Google Scholar]
- Armand, M. The history of polymer electrolytes. Solid State Ionics 1994, 69, 309–319. [Google Scholar] [CrossRef]
- Mark, H.B., Jr.; Rubinson, J.F. Challenges and Opportunities: Where Do We Go from Here? In Conducting Polymers and Polymer Electrolytes: From Biology to Photovoltaics; Mark, H.B., Jr., Rubinson, J.F., Eds.; Oxford University Press: Washington, DC, USA, 2003; pp. 203–204. [Google Scholar]
- Bailey, F.E., Jr.; Koleske, J.V. Poly(Ethylene Oxide); Academic Press: New York, NY, USA, 1976. [Google Scholar]
- Porcarelli, L.; Gerbaldi, C.; Bella, F.; Nair, J.R. Super soft all-ethylene oxide polymer electrolyte for safe all-solid lithium batteries. Sci. Rep. 2016, 6, 19892. [Google Scholar] [CrossRef] [Green Version]
- Koduru, H.K.; Marino, L.; Scarpelli, F.; Petrov, A.G.; Marinov, Y.G.; Hadjichristov, G.B.; Iliev, M.T.; Scaramuzza, N. Structural and dielectric properties of NaIO4-complexed PEO/PVP blended solid polymer electrolytes. Curr. Appl. Phys. 2017, 17, 1518–1531. [Google Scholar] [CrossRef]
- Arya, A.; Sharma, A.L. Insights into the use of oxide in energy storage/conversion devices: A critical review. J. Phys. D Appl. Phys. 2017, 50, 443002. [Google Scholar] [CrossRef]
- Dave, G.; Kanchan, D.K. Dielectric relaxation and modulus studies of PEO-PAM blend based sodium salt electrolyte system. Indian J. Pure Appl. Phys. 2018, 56, 978–988. [Google Scholar]
- Sundaramahalingam, K.; Nallamuthu, N.; Manikandan, A.; Vanitha, D.; Muthuvinayagam, M. Studies on sodium nitrate based polyethylene oxide/polyvinyl pyrrolidone polymer blend electrolytes. Phys. B Cond. Matter 2018, 547, 55–63. [Google Scholar] [CrossRef]
- Kulova, T.L.; Skundin, A.M. Polymer electrolytes for sodium-ion batteries. Electrochem. Energetics 2018, 18, 26–47. (In Russian) [Google Scholar] [CrossRef]
- Arya, A.; Sharma, A.L. Tailoring the structural, morphological, electrochemical, and dielectric properties of solid polymer electrolyte. Ionics 2019, 25, 1617–1632. [Google Scholar] [CrossRef]
- Pritam, S.; Arya, A.; Sharma, A.L. Dielectric relaxations and transport properties parameter analysis of novel blended solid polymer electrolyte for sodium-ion rechargeable batteries. J. Mater. Sci. 2019, 54, 7131–7155. [Google Scholar] [CrossRef]
- Pritam, S.; Arya, A.; Sharma, A.L. Selection of best composition of Na+ ion conducting PEO-PEI blend solid polymer electrolyte based on structural, electrical, and dielectric spectroscopic analysis. Ionics 2020, 26, 745–766. [Google Scholar] [CrossRef]
- Ganta, K.K.; Jeedi, V.R.; Kumar, K.V.; Narsaiah, E.L. Effect of NaClO4 concentration on the ionic conductivity and dielectric properties of sodium ion-conducting PEO/PVDF solid polymer electrolytes for energy storage applications. J. Green Eng. 2020, 10, 5589–5606. [Google Scholar]
- Ganta, K.K.; Jeedi, V.R.; Kumar, K.V.; Narsaiah, E.L. Preparation, characterization and impedance spectroscopic studies of Na+ ion conducting PEO+ PVDF-blended polymer electrolytes. Int. J. Polym. Anal. Charact. 2021, 26, 130–144. [Google Scholar] [CrossRef]
- Li, J.; Kamata, K.; Komura, M.; Yamada, T.; Yoshida, H.; Iyoda, T. Anisotropic ion conductivity in liquid crystalline diblock copolymer membranes with perpendicularly oriented PEO cylindrical domains. Macromolecules 2007, 40, 8125–8128. [Google Scholar] [CrossRef]
- Stoeva, Z.; Lu, Z.; Ingram, M.D.; Imrie, C.T. A new polymer electrolyte based on a discotic liquid crystal triblock copolymer. Electrochim. Acta 2013, 93, 279–286. [Google Scholar] [CrossRef] [Green Version]
- Tong, Y.; Chen, L.; He, X.; Chen, Y. Free mesogen assisted assembly of the star-shaped liquid-crystalline copolymer/Polyethylene Oxide solid electrolytes for Lithium ion batteries. Electrochim. Acta 2014, 118, 33–40. [Google Scholar] [CrossRef]
- Zhang, A.; Cao, F.; Na, G.; Wang, S.; Li, S.; Liu, J. A novel PEO-based blends solid polymer electrolytes doping liquid crystalline ionomers. Ionics 2016, 22, 2103–2112. [Google Scholar] [CrossRef]
- Hosseini, M.; Makhlouf, A.S.H. Industrial Applications for Intelligent Polymers and Coatings; Springer International Publ.: Cham, Switzerland, 2016. [Google Scholar]
- Koduru, H.K.; Marinov, Y.G.; Scarpelli, F.; Hadjichristov, G.B.; Petrov, A.G.; Godbert, N.; Scaramuzza, N. Polyethylene oxide (PEO)-Liquid crystal (E8) composite electrolyte membranes: Microstructural, electrical conductivity and dielectric studies. J. Non-Cryst. Solids 2018, 499, 107–116. [Google Scholar] [CrossRef]
- Bahadur, B.; Sarna, R.K.; Bhide, V.G. Dielectric studies of three liquid crystals HBT, E8 and PCH-1132. Mol. Cryst. Liq. Cryst. 1982, 88, 151–165. [Google Scholar] [CrossRef]
- Koduru, H.K.; Marinov, Y.G.; Hadjichristov, G.B.; Scaramuzza, N. Characterization of polymer/liquid crystal composite based electrolyte membranes for sodium ion battery applications. Solid State Ionics 2019, 335, 86–96. [Google Scholar] [CrossRef]
- Hadjichristov, G.B.; Marinov, Y.G.; Ivanov, T.E.; Koduru, H.K.; Scaramuzza, N. PEO/E8 Polymer-Liquid Crystal Flexible Complex Blend Electrolyte System for Na Ions. In Liquid and Single Crystals: Properties, Manufacturing and Uses; Goosen, J., Ed.; Nova Science Publ.: New York, NY, USA, 2020; pp. 1–64. [Google Scholar]
- Kandpal, M.C.; Bhide, V.G. A mössbauer study of anisotropic molecular diffusion in the supercooled nematic liquid crystalline phase. Phys. B+C 1982, 112, 57–66. [Google Scholar] [CrossRef]
- Bahadur, B.; Sarna, R.K.; Bhide, V.G. Refractive indices, density and order parameter of some technologically important liquid crystalline mixtures. Mol. Cryst. Liq. Cryst. 1982, 72, 139–145. [Google Scholar] [CrossRef]
- Debye, P.J.W. Polar Molecules; The Chemical Catalog Company: New York, NY, USA, 1929. [Google Scholar]
- Jonscher, A.K. Dielectric Relaxation in Solids; Chelsea Dielectric Press: London, UK, 1983. [Google Scholar]
- Gaiduk, V.I. Dielectric Relaxation and Dynamics of Polar Molecules; World Scientific: Singapore, 1999. [Google Scholar]
- Kremer, F.; Schönhals, A. Broadband Dielectric Spectroscopy; Springer: Berlin, Germany, 2003. [Google Scholar]
- Barsoukov, E.; Macdonald, J.R. Impedance Spectroscopy: Theory, Experiment, and Applications, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Lvovich, V.F. Impedance Spectroscopy: Applications to Electrochemical and Dielectric Phenomena; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Deshmukh, K.; Sankaran, S.; Ahamed, B.; Sadasivuni, K.K.; Pasha, K.S.K.; Ponnamma, D.; Sreekanth, P.S.R.; Chidambaram, K. Dielectric Spectroscopy. In Spectroscopic Methods for Nanomaterials Characterization; Thomas, S., Thomas, R., Zachariah, A.K., Kumar, R.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 238–299. [Google Scholar]
- Lust, E. Zero Charge Potentials and Electrical Double Layer at Solid Electrodes. In Encyclopedia of Interfacial Chemistry: Surface Science and Electrochemistry; Wandelt, K., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 5.1, pp. 316–344. [Google Scholar]
- Delgado, A.V.; Ahualli, S.; Fernandez, M.M.; Iglesias, G.R.; Jimenez, M.L. Charge and potential distribution in the electrical double layer of porous materials: Models. In Charge and Energy Storage in Electrical Double Layers; Ahualli, S., Delgado, A.V., Eds.; Interface Science and Technology; Academic Press: London, UK, 2018; Volume 24, pp. 3–18. [Google Scholar]
- Aliev, F.M.; Breganov, M.N. Electric polarization and dynamics of molecular motion of polar liquid crystals in micropores and macropores. Sov. Phys. JETP 1989, 68, 70–79. [Google Scholar]
- Hadjichristov, G.B.; Ivanov, T.E.; Marinov, Y.G.; Koduru, H.K.; Scaramuzza, N. PEO-PVP-NaIO4 Ion-conducting polymer electrolyte: Inspection for ionic space charge polarization and charge trapping. Phys. Status Solidi (A) Appl. Mater. Sci. 2019, 216, 1800739. [Google Scholar] [CrossRef]
- Chan, C.H.; Kammer, H.W. Characterization of polymer electrolytes by dielectric response using electrochemical impedance spectroscopy. Pure Appl. Chem. 2018, 90, 939–953. [Google Scholar] [CrossRef] [Green Version]
- Chan, C.H.; Kammer, H.W. Low frequency dielectric relaxation and conductance of solid polymer electrolytes with PEO and blends of PEO and PMMA. Polymers 2020, 12, 1009. [Google Scholar] [CrossRef]
- Boyd, R.H.; Porter, C.H. Effects of melting on dielectric relaxation in poly-(hexamethylene Sebacamide) (Nylon 610). J. Polym. Sci. Part A-2 Polym. Phys 1972, 10, 647–656. [Google Scholar] [CrossRef]
- Karim, S.R.B.A.; Chan, C.H.; Sim, L.H. Impedance Spectroscopy: A practical Guide to Evaluate Parameters of a Nyquist Plot for Solid Polymer Electrolyte Applications. In Functional Polymeric Composites: Macro to Nanoscales; Chia, C.H., Chan, C.H., Thomas, S., Eds.; Apple Academic Press: Oakville, ON, Canada, 2018; pp. 97–128. [Google Scholar]
- Binks, A.E.; Sharples, A. Electrical conduction in olefin oxide polymers. J. Polym. Sci. Part A-2 Polym. Phys. 1968, 6, 407–420. [Google Scholar] [CrossRef]
- Bruce, P.G.; Vincent, C.A. Polymer electrolytes. J. Chem. Soc. Faraday Trans. 1993, 89, 3187–3203. [Google Scholar] [CrossRef]
- Druger, S.D.; Nitzan, A.; Ratner, M.A. Dynamic bond percolation theory: A microscopic model for diffusion in dynamically disordered systems. I. Definition and one-dimensional case. J. Chem. Phys. 1983, 79, 3133–3142. [Google Scholar] [CrossRef]
- MacCallum, J.R.; Vincent, C.A. (Eds.) Polymer Electrolyte Reviews; Elsevier: London, UK, 1987. [Google Scholar]
- Garca-Coln, L.S.; del Castillo, L.F.; Goldstein, P. Theoretical basis for the Vogel-Fulcher-Tammann equation. Phys. Rev. B Cond. Matter Mater. Phys. 1989, 40, 7040–7044. [Google Scholar] [CrossRef]
- Diederichsen, K.M.; Buss, H.G.; McCloskey, B.D. The compensation effect in the Vogel-Tammann-Fulcher (VTF) equation for polymer-based electrolytes. Macromolecules 2017, 50, 3831–3840. [Google Scholar] [CrossRef]
- Porter, C.H.; Boyd, R.H. A dielectric study of the effects of melting on molecular relaxation in poly (ethylene oxide) and polyoxymethylene. Macromolecules 1971, 4, 589–594. [Google Scholar] [CrossRef]
- Porter, C.H.; Boyd, R.H. The Measurement of Dielectric Constant and Loss of Polymeric Solids and Melts Over a Wide Frequency Range. In Dielectric Properties of Polymers; Karasz, F.E., Ed.; Plenum Press: New York, NY, USA, 1972; pp. 147–215. [Google Scholar]
- Jonscher, A.K. Dielectric relaxation in solids. J. Phys. D Appl. Phys. 1999, 32, R57–R70. [Google Scholar] [CrossRef]
- Polizos, G.; Tuncer, E.; Tomer, V.; Sauers, I.; Randall, C.A.; Manias, E. Dielectric Spectroscopy of Polymer-Based Nanocomposite Dielectrics with Tailored Interfaces and Structured Spatial Distribution of Fillers. In Nanoscale Spectroscopy with Applications; Musa, S.M., Ed.; CRC Press: Boca Raton, FL, USA, 2014; pp. 93–130. [Google Scholar]
- Stevels, J.M. The Electrical Properties of Glass. In Encyclopedia of Physics; Flügge, S., Ed.; Springer: Berlin, Germany, 1957; pp. 350–391. [Google Scholar]
- Smyth, C.P. Dielectric Behavior and Structure: Dielectric Constant and Loss, Dipole Moment and Molecular Structure; McGraw-Hill: New York, NY, USA, 1955. [Google Scholar]
- Grant, F.A. Use of complex conductivity in the representation of dielectric phenomena. J. Appl. Phys. 1958, 29, 76. [Google Scholar] [CrossRef]
- Havriliak, S.; Negami, S. A complex plane analysis of α-dispersions in some polymer systems. J. Polym. Sci. C Polym. Lett. 1966, 16, 99–117. [Google Scholar] [CrossRef]
- Havriliak, S.; Negami, S. A complex plane representation of dielectric and mechanical relaxation processes in some polymers. Polymer 1967, 8, 161–210. [Google Scholar] [CrossRef]
- Kohlrausch, R. Theorie des elektrischen Rückstandes in der Leidener Flasche. Ann. Phys. Chem. 1854, 91, 179–214. [Google Scholar] [CrossRef] [Green Version]
- Williams, G.; Watts, D.C. Non-symmetrical dielectric relaxation behaviour arising from a simple empirical decay function. Trans. Faraday Soc. 1970, 66, 80–85. [Google Scholar] [CrossRef]
- Carbone, F.; Mazzulla, A.; Ciuchi, F.; Scaramuzza, N. Dielectric relaxation in non-polar nematic liquid crystal devices. Eur. Phys. J. Plus 2015, 130, 151. [Google Scholar] [CrossRef]
- Williams, G. Comprehensive Polymer Science; Allen, G., Bevington, J.C., Eds.; Pergamon Press: Oxford, UK, 1989; Volume 2, pp. 601–632. [Google Scholar]
- Williams, G. Dielectric relaxation spectroscopy of polymers revealing dynamics in isotropic and anisotropic stationary systems and changes in molecular mobility in non-stationary systems. Polymer 1994, 35, 1915–1922. [Google Scholar] [CrossRef]
- Karmakar, A.; Ghosh, A. Dielectric permittivity and electric modulus of polyethylene oxide (PEO)–LiClO4 composite electrolytes. Curr. Appl. Phys. 2012, 12, 539–543. [Google Scholar] [CrossRef]
- Kumar, M.; Tiwari, T.; Chauhan, J.K.; Srivastava, N. Understanding the ion dynamics and relaxation behavior from impedance spectroscopy of NaI doped zwitterionic polymer system. Mater. Res. Express 2014, 1, 045003. [Google Scholar] [CrossRef]
- Kumar, M.; Srivastava, N. Conductivity and dielectric investigation of NH4I-doped synthesized polymer electrolyte system. Ionics 2015, 21, 1301–1310. [Google Scholar] [CrossRef]
- Das, S.; Ghosh, A. Ion conduction and relaxation in PEO-LiTFSI-Al2O3 polymer nanocomposite electrolytes. J. Appl. Phys. 2015, 117, 174103. [Google Scholar] [CrossRef]
- Alzari, V.; Nuvoli, D.; Sanna, V.; Caruso, T.; Marino, S.; Scaramuzza, N. Study of polymeric nanocomposites prepared by inserting graphene and/or Ag, Au and ZnO nanoparticles in a TEGDA polymer matrix, by means of the use of dielectric spectroscopy. AIP Adv. 2016, 6, 035005. [Google Scholar] [CrossRef] [Green Version]
- Pal, P.; Ghosh, A. Investigation of ionic conductivity and relaxation in plasticized PMMA-LiClO4 solid polymer electrolytes. Solid State Ionics 2018, 319, 117–124. [Google Scholar] [CrossRef]
- Williams, G. Molecular motion in glass-forming systems. J. Non-Cryst. Solids 1991, 131, 1–12. [Google Scholar] [CrossRef]
- Alvarez, F.; Alegria, A.; Colmenero, J. Relationship between the time-domain Kohlrausch-Williams-Watts and frequency-domain Havriliak-Negami relaxation functions. Phys. Rev. B Cond. Matter Mater. Phys. 1991, 44, 7306. [Google Scholar] [CrossRef]
- Williams, G.; Shinton, S.E.; Aldridge, G.A. Dielectric relaxation spectroscopy and alignment behavior of a polymer-dispersed liquid crystal and its component materials. J. Polym. Sci. B Polym. Phys. 2001, 39, 1173–1194. [Google Scholar] [CrossRef]
- Brás, A.R.E.; Viciosa, M.T.; Rodrigues, C.M.; Dias, C.J.; Dionísio, M. Changes in molecular dynamics upon formation of a polymer dispersed liquid crystal. Phys. Rev. E Stat. Nonliner Soft Matter Phys. 2006, 73, 061709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganesan, L.M.; Frübing, P.; Mellinger, A.; Gerhard, R. Dielectric relaxation behaviour of nematic liquid crystals dispersed in poly(vinylidene fluoride-trifluoroethylene). J. Phys. D Appl. Phys. 2009, 42, 092006. [Google Scholar] [CrossRef]
- Deshmukh, R.R. Electro-Optic and DielectricResponses in PDLC Composite Systems. In Liquid Crystalline Polymers; Thakur, V.K., Kessler, M.R., Eds.; Springer: Cham, Switzerland, 2015; Volume 2, pp. 169–195. [Google Scholar]
- Williams, G. Dielectric Relaxation Behaviour of Liquid Crystals. In Molecular Dynamics of Liquid Crystals; NATO ASI Series, Vol. 431; Luckhurst, G.R., Veracini, C.A., Eds.; Kluwer Academic: Dordrecht, The Netherlands, 1994; pp. 431–450. [Google Scholar]
- McCrum, N.G.; Read, B.E.; Williams, G. Anelastic and Dielectric Effects in Polymeric Solids; Dover: New York, NY, USA, 1991. [Google Scholar]
- Williams, G. Dielectric Properties of Polymers. In Structure and Properties of Polymers; Materials Science and Technology: A Compressive Treatment, Vol. 12; Thomas, E.L., Ed.; Wiley-VCH: Weinheim, Germany, 1993; pp. 471–528. [Google Scholar]
- Wübbenhorst, M.; van Turnhout, J. Analysis of complex dielectric spectra. I. One-dimensional derivative techniques and three-dimensional modeling. J. Non-Cryst. Solids 2002, 305, 40–49. [Google Scholar] [CrossRef]
- Kammer, H.W. Dielectric relaxation in PEO-based polymer electrolytes. Ionics 2018, 24, 1415–1428. [Google Scholar] [CrossRef]
- Das, S.; Ghosh, A. Ionic conductivity and dielectric permittivity of PEO-LiClO4 solid polymer electrolyte plasticized with propylene carbonate. AIP Adv. 2015, 5, 027125. [Google Scholar] [CrossRef] [Green Version]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hadjichristov, G.B.; Vlakhov, T.E.; Marinov, Y.G.; Scaramuzza, N. Ion-Conducting Flexible Thin Films of Composites from Poly(ethylene oxide) and Nematic Liquid Crystals E8—Characterization by Impedance and Dielectric Relaxation Spectroscopy. Polymers 2021, 13, 4465. https://doi.org/10.3390/polym13244465
Hadjichristov GB, Vlakhov TE, Marinov YG, Scaramuzza N. Ion-Conducting Flexible Thin Films of Composites from Poly(ethylene oxide) and Nematic Liquid Crystals E8—Characterization by Impedance and Dielectric Relaxation Spectroscopy. Polymers. 2021; 13(24):4465. https://doi.org/10.3390/polym13244465
Chicago/Turabian StyleHadjichristov, Georgi B., Todor E. Vlakhov, Yordan G. Marinov, and Nicola Scaramuzza. 2021. "Ion-Conducting Flexible Thin Films of Composites from Poly(ethylene oxide) and Nematic Liquid Crystals E8—Characterization by Impedance and Dielectric Relaxation Spectroscopy" Polymers 13, no. 24: 4465. https://doi.org/10.3390/polym13244465
APA StyleHadjichristov, G. B., Vlakhov, T. E., Marinov, Y. G., & Scaramuzza, N. (2021). Ion-Conducting Flexible Thin Films of Composites from Poly(ethylene oxide) and Nematic Liquid Crystals E8—Characterization by Impedance and Dielectric Relaxation Spectroscopy. Polymers, 13(24), 4465. https://doi.org/10.3390/polym13244465