Investigation of Crystallization and Relaxation Effects in Coarse-Grained Polyethylene Systems after Uniaxial Stretching
Abstract
:1. Introduction
2. Simulation Methodology
2.1. Force Field
2.2. Simulation Procedure
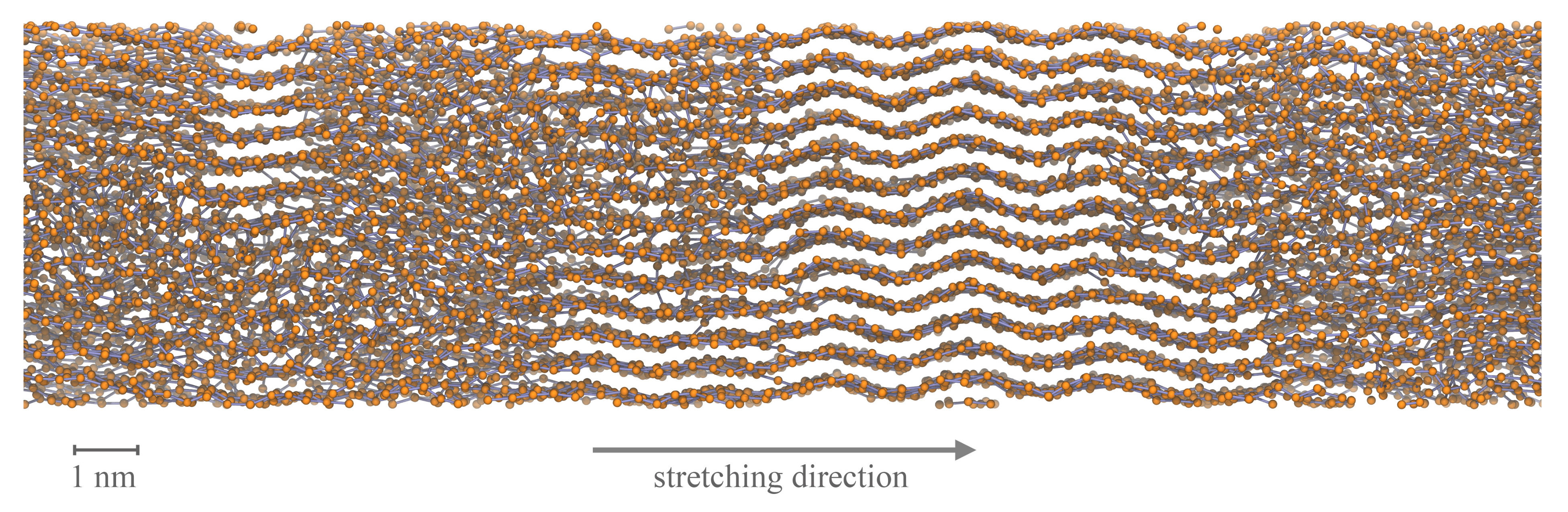
2.3. Evaluation of the Microscopic Structure
3. Results
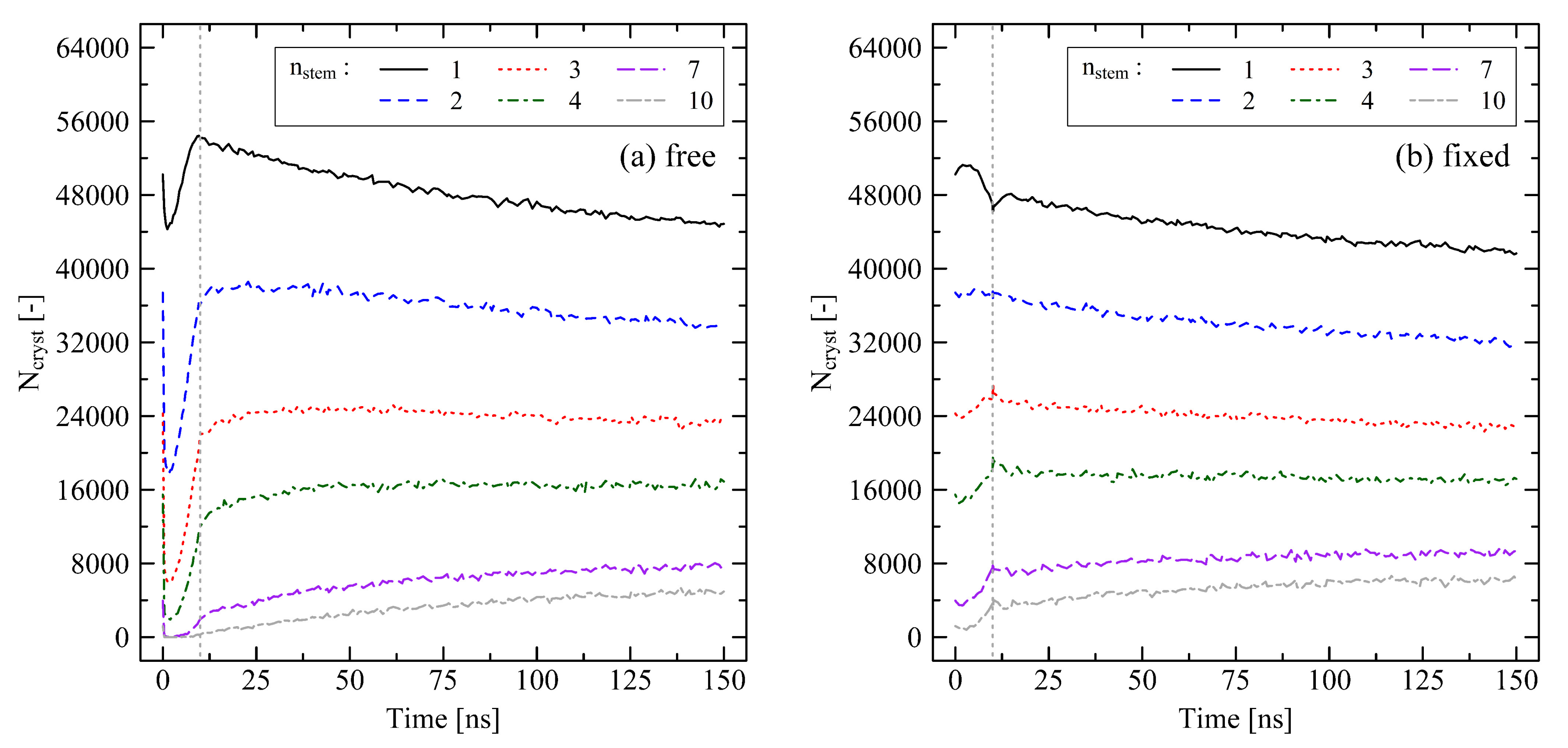
3.1. Relaxation in Short Simulation Runs (40 ns)
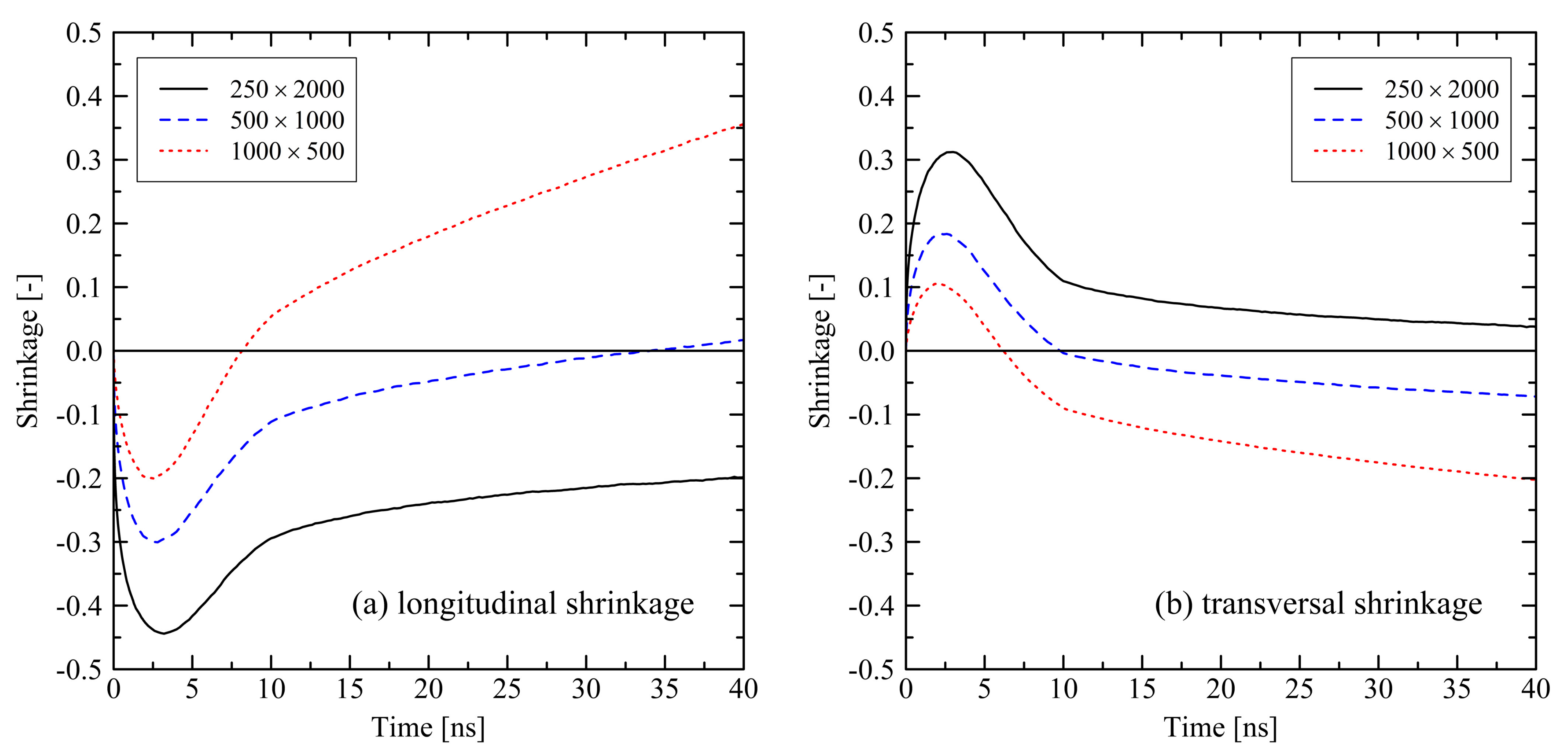
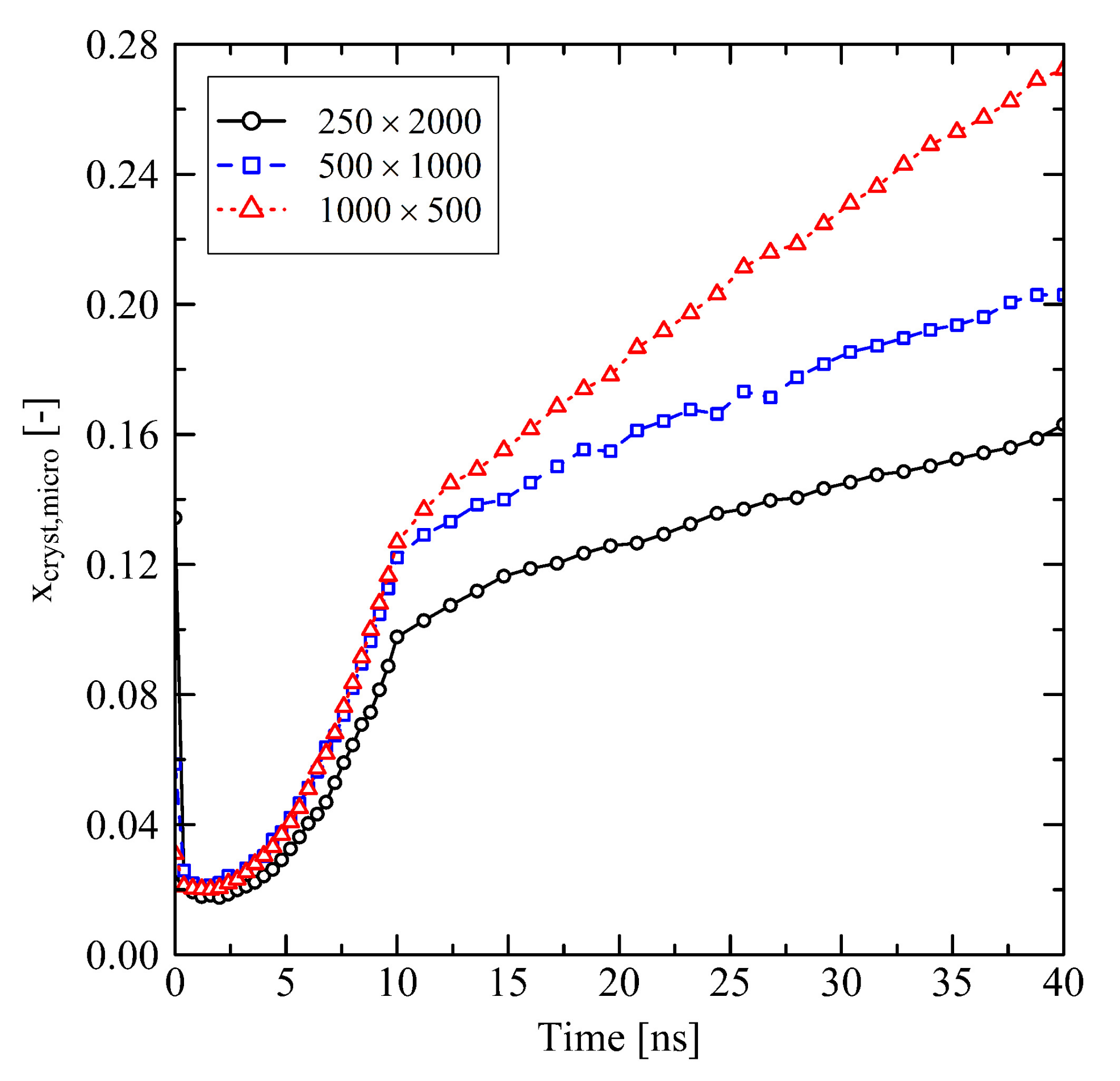
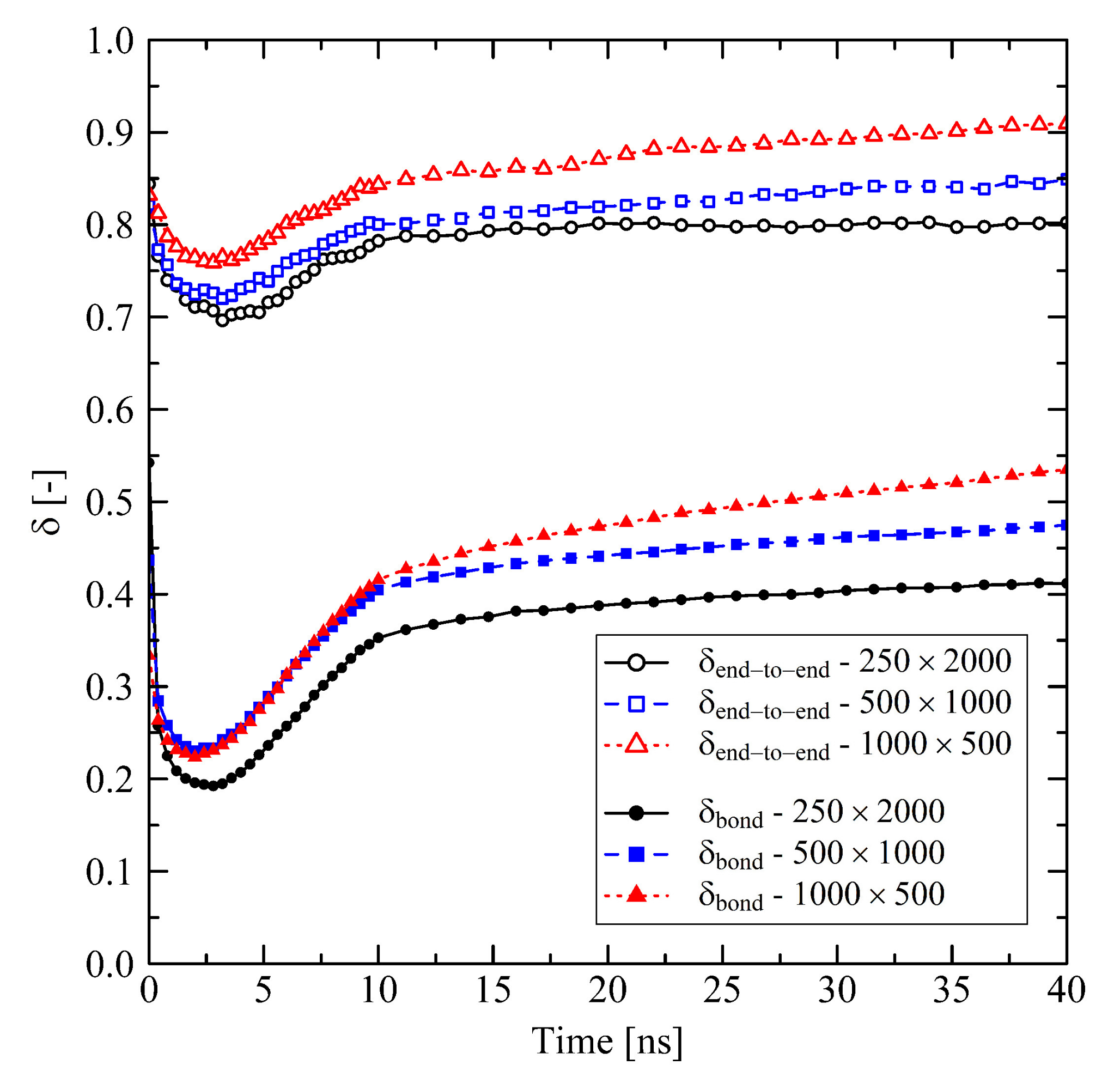
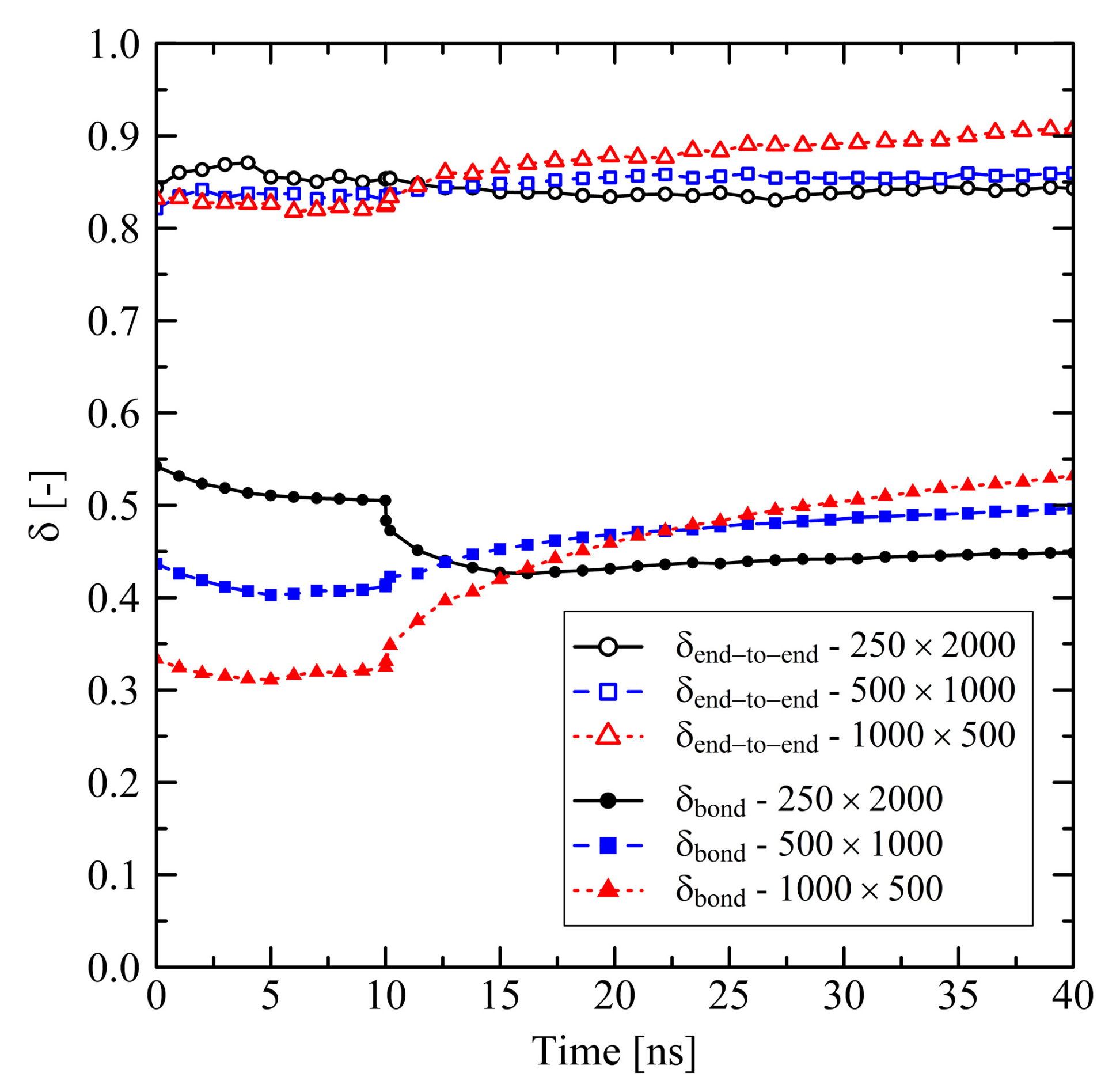
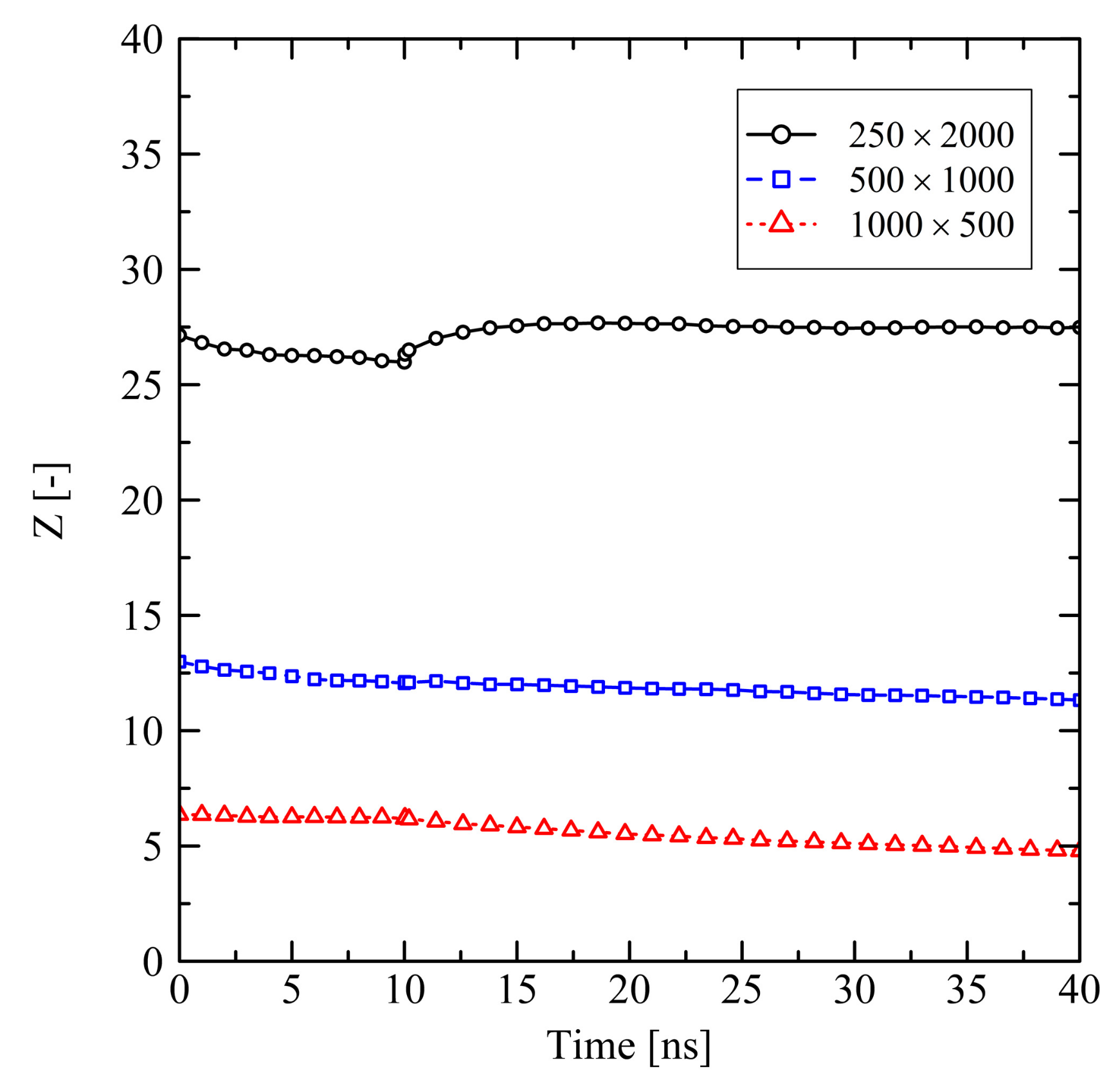
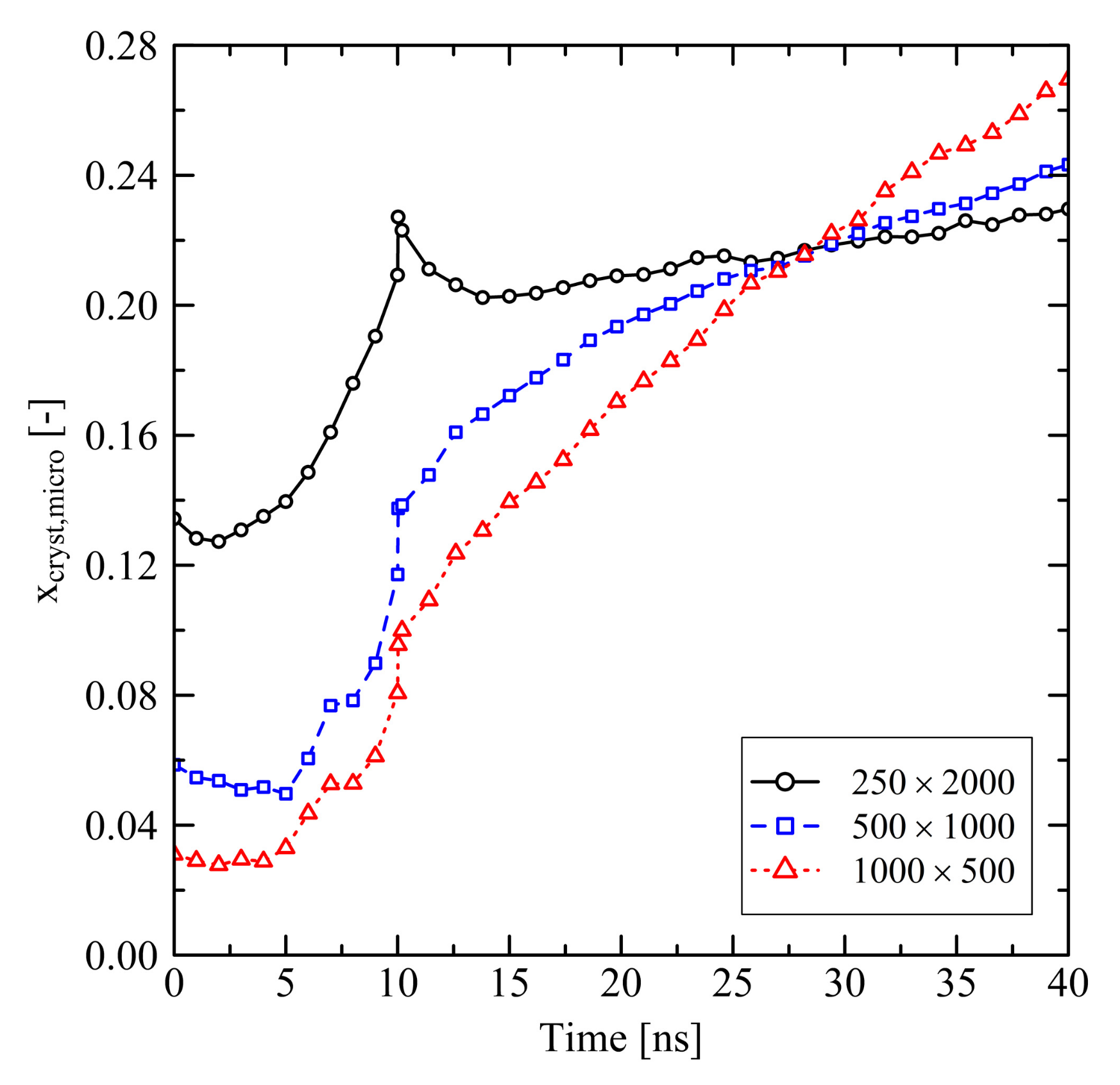
3.1.1. Chain Length Effects under Free Conditions
3.1.2. Chain Length Effects under Fixed Conditions
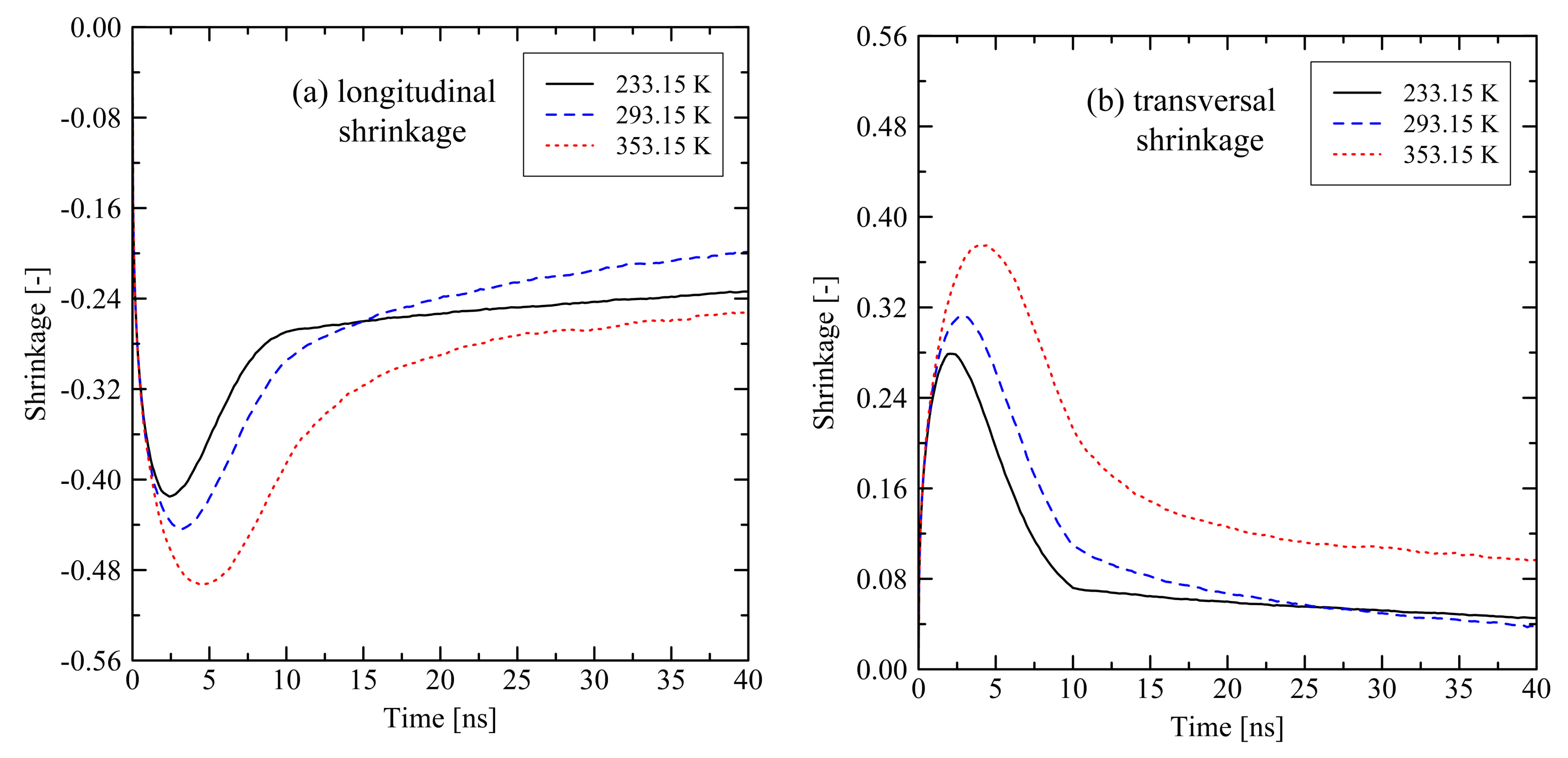
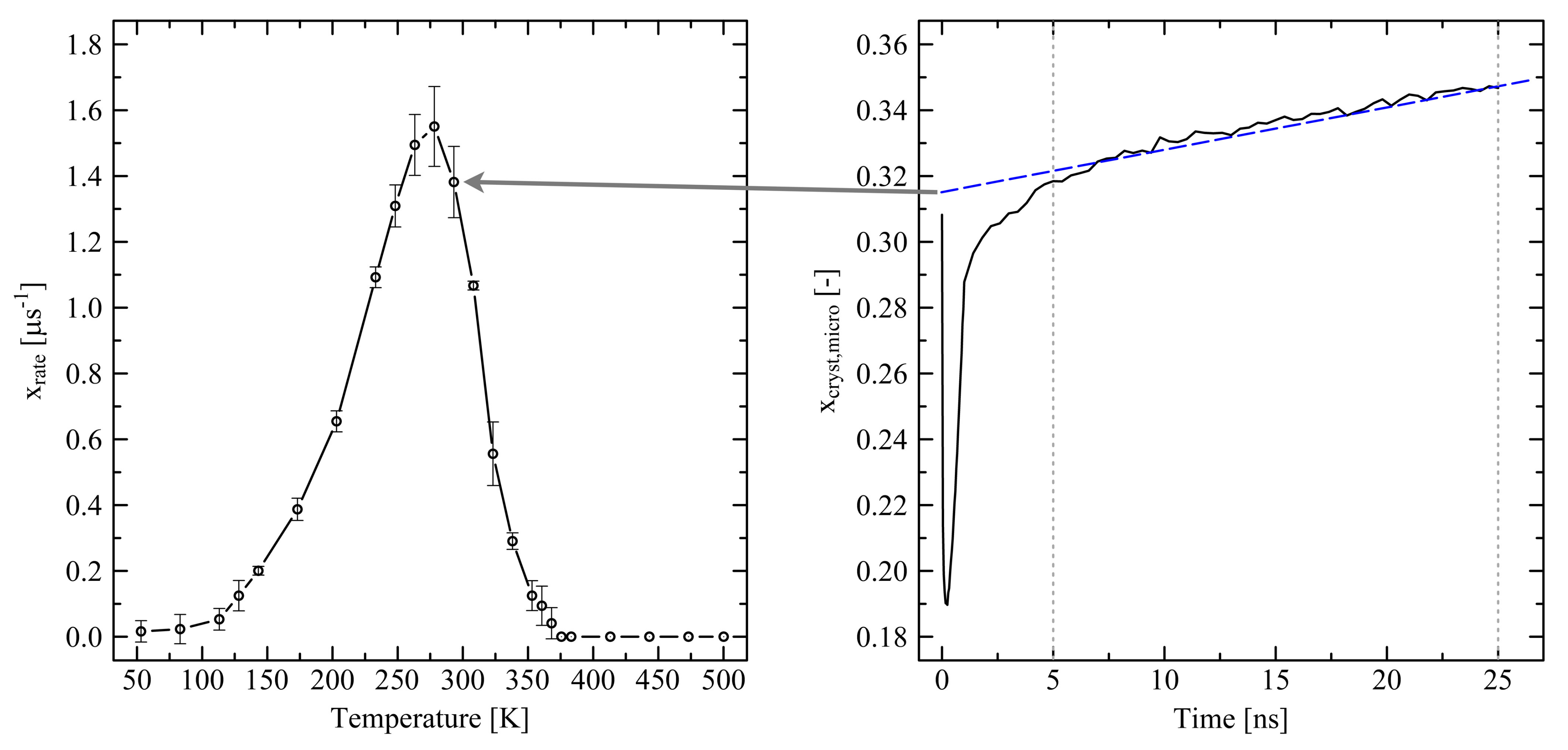
3.2. Temperature Dependent Effects and Crystal Growth
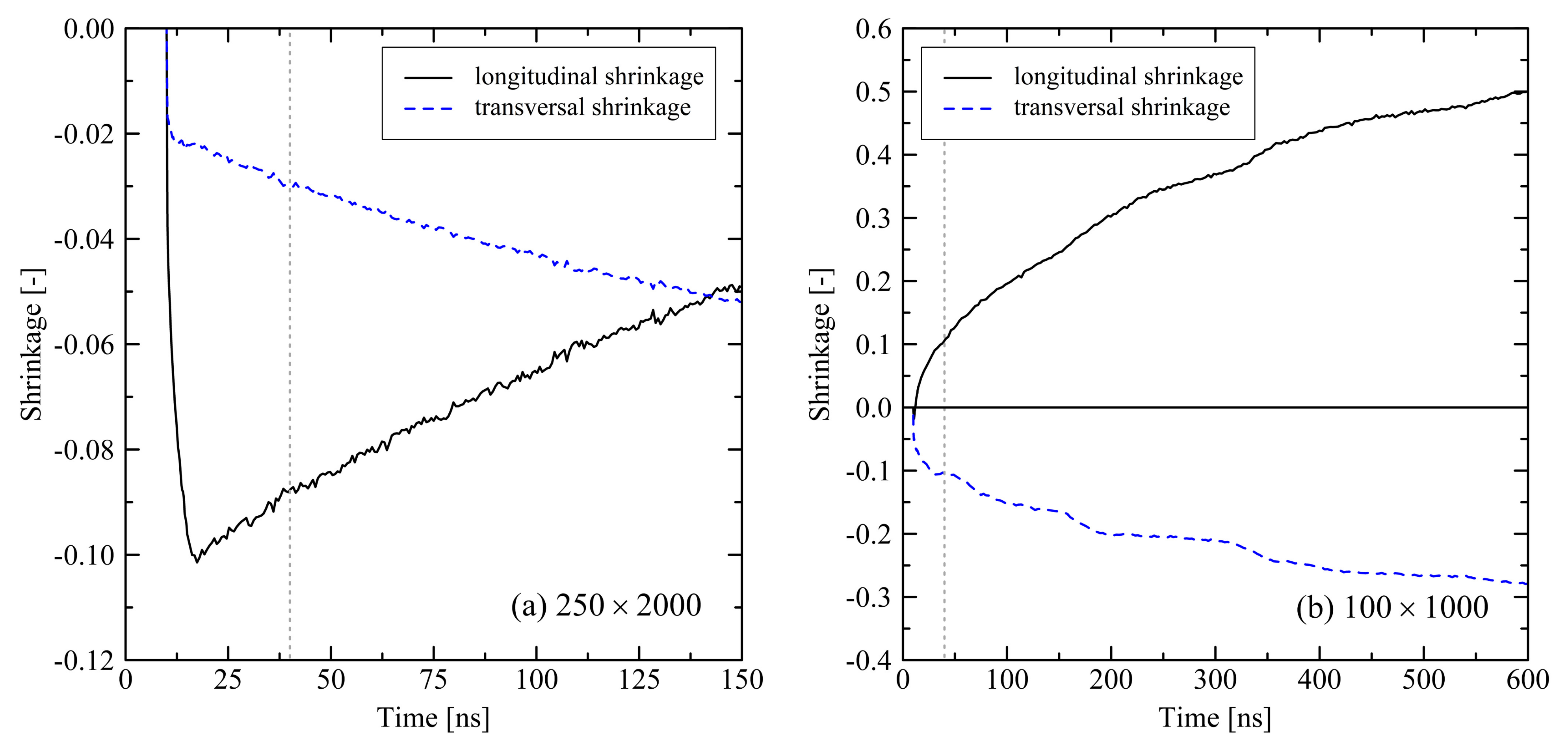
3.3. Relaxation in Long Simulation Runs (150 and 600 ns)
4. Discussion
5. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grommes, D.; Bruch, O.; Geilen, J. Investigation of the influencing factors on the process dependent elasticity modulus in extrusion blow molded plastic containers for material modelling in the finite element simulation. In Proceedings of the Regional Conference 2015 of the Polymer Processing Society PPS, Graz, Austria, 21–25 September 2015; AIP Publishing: Melville, NY, USA, 2016; Volume 1779, p. 050013. [Google Scholar]
- Leopold, T. Rechnergestützte Auslegung Streckblasgeformter Kunststoffhohlkörper. Ph.D. Thesis, RWTH Aachen, Aachen, Germany, 2011. [Google Scholar]
- Hopmann, C.; Michaeli, W.; Rasche, S. FE-Analysis of stretch-blow moulded bottles using an integrative process simulation. In Proceedings of the 14th International ESAFORM Conference on Material Forming, Belfast, UK, 27–29 April 2011; AIP Publishing: Melville, NY, USA, 2011; Volume 1353, pp. 868–873. [Google Scholar]
- Park, H.J.; Kim, J.; Yoon, I. Stretch blow molding of pet bottle: Simulation of blowing process and prediction of bottle properties. In Proceedings of the ANTEC 2003 Plastics: Annual Technical Conference (Volume 1: Processing), Nashville, TN, USA, 4–8 May 2003; pp. 859–865. [Google Scholar]
- Michels, P.; Grommes, D.; Oeckerath, A.; Reith, D.; Bruch, O. An integrative simulation concept for extrusion blow molded plastic bottles. Finite Elem. Anal. Des. 2019, 164, 69–78. [Google Scholar] [CrossRef]
- Allen, M.; Tildesley, D. Computer Simulation of Liquids, 2nd ed.; Oxford University Press: Oxford, UK, 2017. [Google Scholar]
- Kremer, K. Computer simulations for macromolecular science. Macromol. Chem. Phys. 2003, 204, 257–264. [Google Scholar] [CrossRef]
- Osswald, T.A.; Hernandez-Ortiz, J.P. Polymer Processing—Modelling and Simulation, 1st ed.; Carl Hanser Verlag: Munich, Germany, 2006. [Google Scholar]
- Kipping, A. Thermomechanische Analyse der Kühlphase beim Extusionsblasformen von Kunststoffen. Ph.D. Thesis, University of Siegen, Siegen, Germany, 2003. [Google Scholar]
- Benrabah, Z.; Mir, H. Thermo-viscoelastic model for shrinkage and warpage predicition during cooling and solidification of automotive blow molded parts. SAE Int. J. Mater. Manuf. 2013, 6, 349–364. [Google Scholar] [CrossRef]
- Xu, A.; Kazmer, D.O. Thermoforming shrinkage prediction. Polym. Eng. Sci. 2003, 41, 1553–1563. [Google Scholar] [CrossRef]
- Hsu, H.P.; Kremer, K. Primitive Path analysis and stress distribution in highly strained macromolecules. ACS Macro Lett. 2017, 7, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.P.; Kremer, K. Clustering of entanglement points in highly strained polymer melts. Macromolecules 2019, 52, 6756–6772. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.S.; Carrillo, J.M.Y.; Lam, C.N.; Sumpter, B.G.; Wang, Y. Molecular dynamics investigation of the relaxation mechanism of entangled polymers after a large step deformation. ACS Macro Lett. 2018, 17, 190–195. [Google Scholar] [CrossRef]
- O’Connor, T.C.; Hopkins, A.; Robbins, M.O. Stress relaxation in highly oriented melts of entangled polymers. Macromolecules 2019, 52, 8540–8550. [Google Scholar] [CrossRef]
- Jeong, S.; Baig, C. Molecular process of stress relaxation for sheared polymer melts. Polymer 2020, 202, 122683. [Google Scholar] [CrossRef]
- Lavine, M.S.; Waheed, N.; Rutledge, G.C. Molecular dynamics simulation of orientation and crystallization of polyethylene during uniaxial extension. Polymer 2003, 44, 1771–1779. [Google Scholar] [CrossRef]
- Ko, M.J.; Waheed, N.; Lavine, M.S.; Rutledge, G.C. Characterization of polyethylene crystallization from an oriented melt by molecular dynamics simulation. J. Chem. Phys. 2004, 121, 2823–2832. [Google Scholar] [CrossRef]
- Sliozberg, Y.R.; Yeh, I.C.; Kroeger, M.; Masser, K.A.; Lenhart, J.L.; Andzelm, J.W. Ordering and crystallization of entangled polyethylene melt under uniaxial tension: A molecular dynamics study. Macromolecules 2018, 51, 9635–9648. [Google Scholar] [CrossRef]
- Yamamoto, T. Molecular dynamics simulation of stretch-induced crystallization in polyethylene: Emergence of fiber structure and molecular network. Macromolecules 2019, 52, 1695–1706. [Google Scholar] [CrossRef]
- Moyassari, A.; Gkourmpis, T.; Hedenqvist, M.S.; Gedde, U.W. Molecular dynamics simulation of linear polyethylene blends: Effect of molar masss bimodality on topological characteristics and mechanical behavior. Polymer 2019, 161, 139–150. [Google Scholar] [CrossRef]
- Moyassari, A.; Gkourmpis, T.; Hedenqvist, M.S.; Gedde, U.W. Molecular dynamics simulations of short-chain branched bimodal polyethylene: Topological characteristics and mechanical behavior. Macromolecules 2019, 52, 807–818. [Google Scholar] [CrossRef] [Green Version]
- Verho, T.; Paajanen, A.; Vaari, J.; Laukkannen, A. Crystal growth in polyethylene by molecular dynamics: The crystal edge and lamellar thickness. Macromolecules 2018, 51, 4865–4873. [Google Scholar] [CrossRef]
- Hall, K.W.; Sirk, T.W.; Klein, M.L.; Shinoda, W. A coarse-grain model for entangled polyethylene melts and polyethylene crystallization. J. Chem. Phys. 2019, 150, 244901. [Google Scholar] [CrossRef] [PubMed]
- Hagita, K.; Fujiwara, S.; Iwaoka, N. Structure formation of a quenched single polyethylene chain with different force fields in united atom molecular dynamics simulations. AIP Adv. 2018, 8, 115108. [Google Scholar] [CrossRef] [Green Version]
- Eichenberger, A.P. Molecular Dynamics Simulation of Alkanes and Proteins: Methodology, Prediction of Properties and Comparison to Experimental Data. Ph.D. Thesis, ETH Zürich, Zürich, Switzerland, 2013. [Google Scholar]
- Grommes, D.; Reith, D. Determination of Relevant Mechanical Properties for the Production Process of Polyethylene by Using Mesoscale Molecular Simulation Techniques. Soft Mater. 2020, 18, 242–261. [Google Scholar] [CrossRef]
- Moreira, L.; Zhang, G.; Müller, F.; Stuehn, T.; Kremer, K. Direct equilibration and characterization of polymer melts for computer simulations. Macromol. Theory Simul. 2015, 24, 419–431. [Google Scholar] [CrossRef]
- Auhl, R.; Everaers, R.; Grest, G.S.; Kremer, K.; Plimpton, S.J. Equilibration of long chain polymer melts in computer simulations. J. Chem. Phys. 2003, 119, 12718–12728. [Google Scholar] [CrossRef] [Green Version]
- Hedesiu, C.E. Structure–Property Relationships in Polyolefins. Ph.D. Thesis, RWTH Aachen, Aachen, Germany, 2007. [Google Scholar]
- Sato, Y.; Hashiguchi, H.; Inohara, K.; Takishima, S.; Masuoka, H. PVT properties of polyethylene copolymer melts. Fluid Phase Equilibria 2007, 257, 124–130. [Google Scholar] [CrossRef]
- Ramakers-van Dorp, E.; Möginger, B.; Hausnerova, B. Thermal expansion of semi-crystalline polymers: Anisotropic thermal strain and crystallite orientation. Polymer 2020, 191, 122249. [Google Scholar] [CrossRef]
- Boyer, R.F.; Snyder, R.G. The glass temperature of amorphous polyethylene. J. Polym. Sci. Polym. Lett. Ed. 1977, 15, 315–320. [Google Scholar] [CrossRef]
- Halverson, J.D.; Brandes, T.; Lenz, O.; Arnold, A.; Bevc, S.; Starchenko, V.; Kremer, K.; Stuehn, T.; Reith, D. ESPResSo++: A modern multiscale simulation package for soft matter systems. Comput. Phys. Commun. 2013, 184, 1129–1149. [Google Scholar] [CrossRef]
- Guzman, H.V.; Tretyakov, N.; Kobayashi, H.; Fogarty, A.C.; Kreis, K.; Krajniak, J.; Junghans, C.; Kremer, K.; Stuehn, T. ESPResSo++ 2.0: Advanced methods for multiscale molecular simulation. Comput. Phys. Commun. 2019, 238, 66–76. [Google Scholar] [CrossRef] [Green Version]
- Everaers, R.; Sukumaran, S.K.; Grest, G.S.; Svaneborg, C.; Sivasubramanian, A.; Kremer, K. Rheology and microscopic topology of entangled polymeric liquids. Science 2004, 303, 823–826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edwards, S.F. The statistical mechanics of polymerized material. Proc. Phys. Soc. 1967, 92, 9–16. [Google Scholar] [CrossRef]
- Edwards, S.F. The theory of rubber elasticity. Br. Polym. J. 1977, 9, 140–143. [Google Scholar] [CrossRef]
- De Gennes, P.G. Reptation of a polymer chain in the presence of fixed obstacles. J. Chem. Phys. 1971, 55, 572–579. [Google Scholar] [CrossRef]
- Everaers, R. Topological versus rheological entanglement length in primitive-path analysis protocols, tube models, and slip-link models. Phys. Rev. E 2012, 86, 022801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kröger, M. Shortest multiple disconnected path for the analysis of entanglements in two- and three-dimensional polymeric systems. Comput. Phys. Commun. 2005, 168, 209–232. [Google Scholar] [CrossRef]
- Shanbhag, S.; Kröger, M. Primitive path networks generated by annealing and geometrical methods: Insights into differences. Marcromolecules 2007, 40, 2897–2903. [Google Scholar] [CrossRef]
- Teare, P.W.; Holmes, D.R. Extra reflections in the x-ray diffraction pattern of polyethylenes and polymethylenes. J. Polym. Sci. 1957, 24, 496–499. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, J. Non-isothermal crystallization kinetics of high density polyethylene/titanium dioxide composites via melt blending. J. Therm. Anal. Calorim. 2014, 115, 63–71. [Google Scholar] [CrossRef]
- Toda, A.; Taguchi, K.; Nozaki, K.; Konishi, M. Melting behaviors of polyethylene crystals: An application of fast-scan DSC. Polymer 2014, 55, 3186–3194. [Google Scholar] [CrossRef]
- Patki, R.P.; Phillips, P.J. Crystallization kinetics of linear polyethylene: The maximum in crystal growth rate-temperature dependence. Eur. Polym. J. 2008, 44, 534–541. [Google Scholar] [CrossRef]
- Yamamoto, T. Molecular dynamics simulations of steady-state crystal growth and homogeneous nucleation in polyethylene-like polymer. J. Chem. Phys. 2008, 129, 184903. [Google Scholar] [CrossRef]




| Bead Type | Bond Length | Bond Angle | Dihedral Angle | |||
|---|---|---|---|---|---|---|
| [nm] | [kJ mol nm] | [] | [kJ mol] | [-] | [kJ mol] | |
| CG3 | 0.353 | 19730 | 146.4 | 56.6 | 1 | 0.74 |
| Bead Type | Position | |||||
| [nm] | [kJ mol] | [nm] | [kJ mol] | |||
| CG3mid | middle | 0.457 | 2.214 | 0.401 | 2.213 | |
| CG3end | end | 0.468 | 2.415 | 0.421 | 2.415 | |
| CTE | Tg | |||
|---|---|---|---|---|
| [g cm] | [g cm] | [ K] | [K] | |
| sim. | 1 [27] | [27] | ||
| exp. | [30] | [31] | 2 [32] | [33] |
| 3 [32] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grommes, D.; Schenk, M.R.; Bruch, O.; Reith, D. Investigation of Crystallization and Relaxation Effects in Coarse-Grained Polyethylene Systems after Uniaxial Stretching. Polymers 2021, 13, 4466. https://doi.org/10.3390/polym13244466
Grommes D, Schenk MR, Bruch O, Reith D. Investigation of Crystallization and Relaxation Effects in Coarse-Grained Polyethylene Systems after Uniaxial Stretching. Polymers. 2021; 13(24):4466. https://doi.org/10.3390/polym13244466
Chicago/Turabian StyleGrommes, Dirk, Martin R. Schenk, Olaf Bruch, and Dirk Reith. 2021. "Investigation of Crystallization and Relaxation Effects in Coarse-Grained Polyethylene Systems after Uniaxial Stretching" Polymers 13, no. 24: 4466. https://doi.org/10.3390/polym13244466
APA StyleGrommes, D., Schenk, M. R., Bruch, O., & Reith, D. (2021). Investigation of Crystallization and Relaxation Effects in Coarse-Grained Polyethylene Systems after Uniaxial Stretching. Polymers, 13(24), 4466. https://doi.org/10.3390/polym13244466







