Physical Properties of Starch/Powdered Activated Carbon Composite Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
Film Preparation
2.2. X-ray Diffraction (XRD)
2.3. Mechanical Properties
2.3.1. Tensile Test
2.3.2. Nanoindentation Test
2.3.3. Nanoscratch Test
2.4. Surface Properties
2.4.1. Atomic Force Microscopy
2.4.2. Wettability
2.5. Barrier Properties
2.5.1. Water Vapor Permeability (WVP)
2.5.2. Water Solubility Index (WSI)
2.6. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Taggart, P.; Mitchell, J.R. Starch. In Handbook of Hydrocolloids, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2009; pp. 108–141. ISBN 9781845695873. [Google Scholar]
- Niranjana Prabhu, T.; Prashantha, K. A review on present status and future challenges of starch based polymer films and their composites in food packaging applications. Polym. Compos. 2018, 39, 2499–2522. [Google Scholar] [CrossRef]
- Majid, I.; Ahmad Nayik, G.; Mohammad Dar, S.; Nanda, V. Novel food packaging technologies: Innovations and future prospective. J. Saudi Soc. Agric. Sci. 2018, 17, 454–462. [Google Scholar] [CrossRef] [Green Version]
- Wilpiszewska, K.; Antosik, A.K.; Spychaj, T. Novel hydrophilic carboxymethyl starch/montmorillonite nanocomposite films. Carbohydr. Polym. 2015, 128, 82–89. [Google Scholar] [CrossRef]
- Ferreira, A.R.V.; Alves, V.D.; Coelhoso, I.M. Polysaccharide-based membranes in food packaging applications. Membranes 2016, 6, 22. [Google Scholar] [CrossRef] [Green Version]
- Averous, L.; Pollet, E. Enviromental Silicate Nano-Biocomposites; Averous, L., Pollet, E., Eds.; Springer: London, UK, 2012; ISBN 9781608054220. [Google Scholar]
- Kwaśniewska, A.; Chocyk, D.; Gładyszewski, G.; Borc, J.; Świetlicki, M.; Gładyszewska, B. The influence of kaolin clay on the mechanical properties and structure of thermoplastic starch films. Polymers 2020, 12, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López, O.V.; Castillo, L.A.; García, M.A.; Villar, M.A.; Barbosa, S.E. Food packaging bags based on thermoplastic corn starch reinforced with talc nanoparticles. Food Hydrocoll. 2015, 43, 18–24. [Google Scholar] [CrossRef]
- Oleyaei, S.A.; Zahedi, Y.; Ghanbarzadeh, B.; Moayedi, A.A. Modification of physicochemical and thermal properties of starch films by incorporation of TiO2 nanoparticles. Int. J. Biol. Macromol. 2016, 89, 256–264. [Google Scholar] [CrossRef]
- Cheviron, P.; Gouanvé, F.; Espuche, E. Preparation, characterization and barrier properties of silver/montmorillonite/starch nanocomposite films. J. Memb. Sci. 2016, 497, 162–171. [Google Scholar] [CrossRef]
- Ng, C.; Losso, J.N.; Marshall, W.E.; Rao, R.M. Physical and chemical properties of selected agricultural byproduct-based activated carbons and their ability to adsorb geosmin. Bioresour. Technol. 2002, 84, 177–185. [Google Scholar] [CrossRef]
- Manocha, S. Porous Carbon. In Carbon Alloys: Novel Concepts to Develop Carbon Science and Technology; Elsevier Science: Amsterdam, The Netherlands, 2003; Volume 28, pp. 109–127. [Google Scholar] [CrossRef]
- Baker, F.S.; Miller, C.E.; Repik, A.J.; Tolles, E.D. Activated Carbon. In Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2000; pp. 1–34. [Google Scholar]
- Sharififard, H.; Nabavinia, M.; Soleimani, M. Evaluation of adsorption efficiency of activated carbon/chitosan composite for removal of Cr (VI) and Cd (II) from single and bi-solute dilute solution. Adv. Environ. Technol. 2016, 2, 215–227. [Google Scholar] [CrossRef]
- Sobhan, A.; Muthukumarappan, K.; Cen, Z.; Wei, L. Characterization of nanocellulose and activated carbon nanocomposite films’ biosensing properties for smart packaging. Carbohydr. Polym. 2019, 225, 115189. [Google Scholar] [CrossRef] [PubMed]
- de Freitas, F.P.; Carvalho, A.M.M.L.; Carneiro, A.D.C.O.; de Magalhães, M.A.; Xisto, M.F.; Canal, W.D. Adsorption of neutral red dye by chitosan and activated carbon composite films. Heliyon 2021, 7, e07629. [Google Scholar] [CrossRef]
- Somsesta, N.; Sricharoenchaikul, V.; Aht-Ong, D. Adsorption removal of methylene blue onto activated carbon/cellulose biocomposite films: Equilibrium and kinetic studies. Mater. Chem. Phys. 2020, 240, 122221. [Google Scholar] [CrossRef]
- Akhmetova, A.; Saliev, T.; Allan, I.U.; Illsley, M.J.; Nurgozhin, T.; Mikhalovsky, S. A Comprehensive Review of Topical Odor-Controlling Treatment Options for Chronic Wounds. J. Wound Ostomy Cont. Nurs. 2016, 43, 598–609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Illsley, M.J.; Akhmetova, A.; Bowyer, C.; Nurgozhin, T.; Mikhalovsky, S.V.; Farrer, J.; Dubruel, P.; Allan, I.U. Activated carbon-plasticised agarose composite films for the adsorption of thiol as a model of wound malodour. J. Mater. Sci. Mater. Med. 2017, 28, 154. [Google Scholar] [CrossRef] [Green Version]
- Kwasniewska, A.; Muszynski, S.; Tatarczak, J.; Gładyszewski, G.; Gładyszewska, B. Wollastonite-filled and Arabic gum-modified starch films. Part 1. Mechanical and structural properties. Przem. Chem. 2016, 95, 109–111. [Google Scholar] [CrossRef]
- Schuh, C.A. Nanoindentation studies of materials. Mater. Today 2006, 9, 32–40. [Google Scholar] [CrossRef]
- Hay, J. Introduction to instrumented indentation testing. Exp. Tech. 2009, 33, 66–72. [Google Scholar] [CrossRef]
- Kumar, N.; Kataria, S.; Dash, S.; Tyagi, A.K. Deformation of SS 304 LN during scratch test and influence on evolution of coefficient of friction. Adv. Tribol. 2009, 2009, 648075. [Google Scholar] [CrossRef]
- Kwaśniewska, A.; Świetlicki, M.; Prószyński, A.; Gładyszewski, G. The quantitative nanomechanical mapping of starch/kaolin film surfaces by Peak Force AFM. Polymers 2021, 13, 244. [Google Scholar] [CrossRef]
- Derjaguin, B.V.; Muller, V.M.; Toporov, Y.P. Effect of contact deformations on the adhesion of particles. J. Colloid Interface Sci. 1975, 53, 314–326. [Google Scholar] [CrossRef]
- Kokoszka, S.; Debeaufort, F.; Lenart, A.; Voilley, A. Water vapour permeability, thermal and wetting properties of whey protein isolate based edible films. Int. Dairy J. 2010, 20, 53–60. [Google Scholar] [CrossRef]
- Ait Laziz, N.; Abou-Rjeily, J.; Darwiche, A.; Toufaily, J.; Outzourhit, A.; Ghamouss, F.; Tahar Sougrati, M. Li- and Na-ion storage performance of natural graphite via simple flotation process. J. Electrochem. Sci. Technol. 2018, 9, 320–329. [Google Scholar] [CrossRef] [Green Version]
- Chocyk, D.; Gładyszewska, B.; Ciupak, A.; Oniszczuk, T.; Leszek, M.; Rejak, A. Influence of water addition on mechanical properties of thermoplastic starch foils. Int. Agrophysics 2015, 29, 267–273. [Google Scholar] [CrossRef] [Green Version]
- García, N.L.; Famá, L.; Dufresne, A.; Aranguren, M.; Goyanes, S. A comparison between the physico-chemical properties of tuber and cereal starches. Food Res. Int. 2009, 42, 976–982. [Google Scholar] [CrossRef]
- Gironès, J.; López, J.P.; Mutjé, P.; Carvalho, A.J.F.; Curvelo, A.A.S.; Vilaseca, F. Natural fiber-reinforced thermoplastic starch composites obtained by melt processing. Compos. Sci. Technol. 2012, 72, 858–863. [Google Scholar] [CrossRef]
- Cyras, V.P.; Manfredi, L.B.; Ton-That, M.T.; Vázquez, A. Physical and mechanical properties of thermoplastic starch/montmorillonite nanocomposite films. Carbohydr. Polym. 2008, 73, 55–63. [Google Scholar] [CrossRef]
- Zhao, L.; Luo, F.; Zhai, M.; Mitomo, H.; Yoshii, F. Study on CM-chitosan/activated carbon hybrid gel films formed with EB irradiation. Radiat. Phys. Chem. 2008, 77, 622–629. [Google Scholar] [CrossRef]
- Om Prakash, M.; Gujjala, R.; Panchal, M.; Ojha, S. Mechanical characterization of arhar biomass based porous nano activated carbon polymer composites. Polym. Compos. 2020, 41, 3113–3123. [Google Scholar] [CrossRef]
- Jullien, R. Aggregation phenomena and fractal aggregates. Contemp. Phys. 1987, 28, 477–493. [Google Scholar] [CrossRef]
- McKee, C.T.; Last, J.A.; Russell, P.; Murphy, C.J. Indentation versus tensile measurements of Young’s modulus for soft biological tissues. Tissue Eng. Part B Rev. 2011, 17, 155–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhushan, B.; Jung, Y.C. Natural and biomimetic artificial surfaces for superhydrophobicity, self-cleaning, low adhesion, and drag reduction. Prog. Mater. Sci. 2011, 56, 1–108. [Google Scholar] [CrossRef] [Green Version]
- Embuscado, M.; Huber, K. Edible Films and Coatings for Food Applications; Springer Science & Business Media: New York, NY, USA, 2009; ISBN 9780387928234. [Google Scholar]
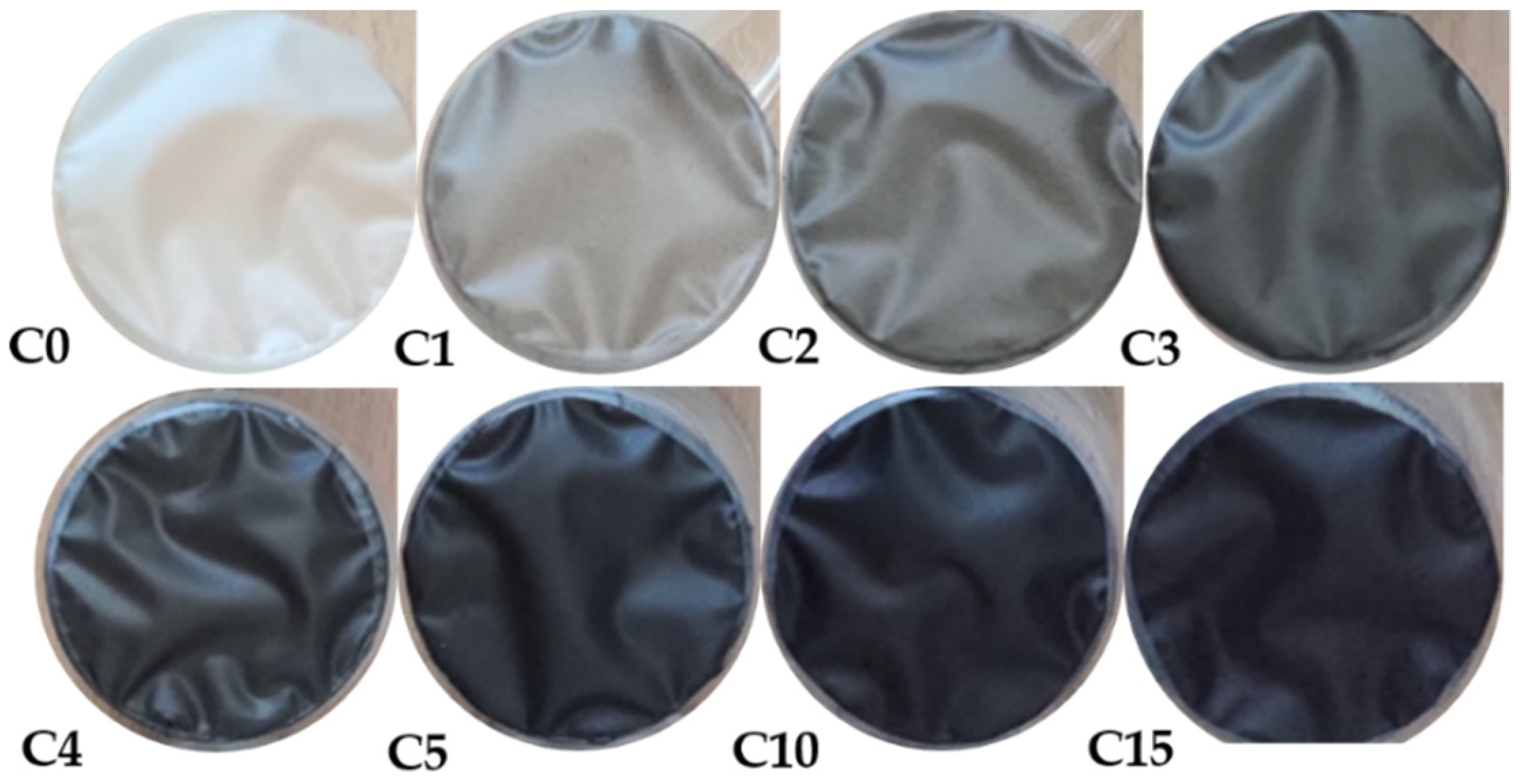


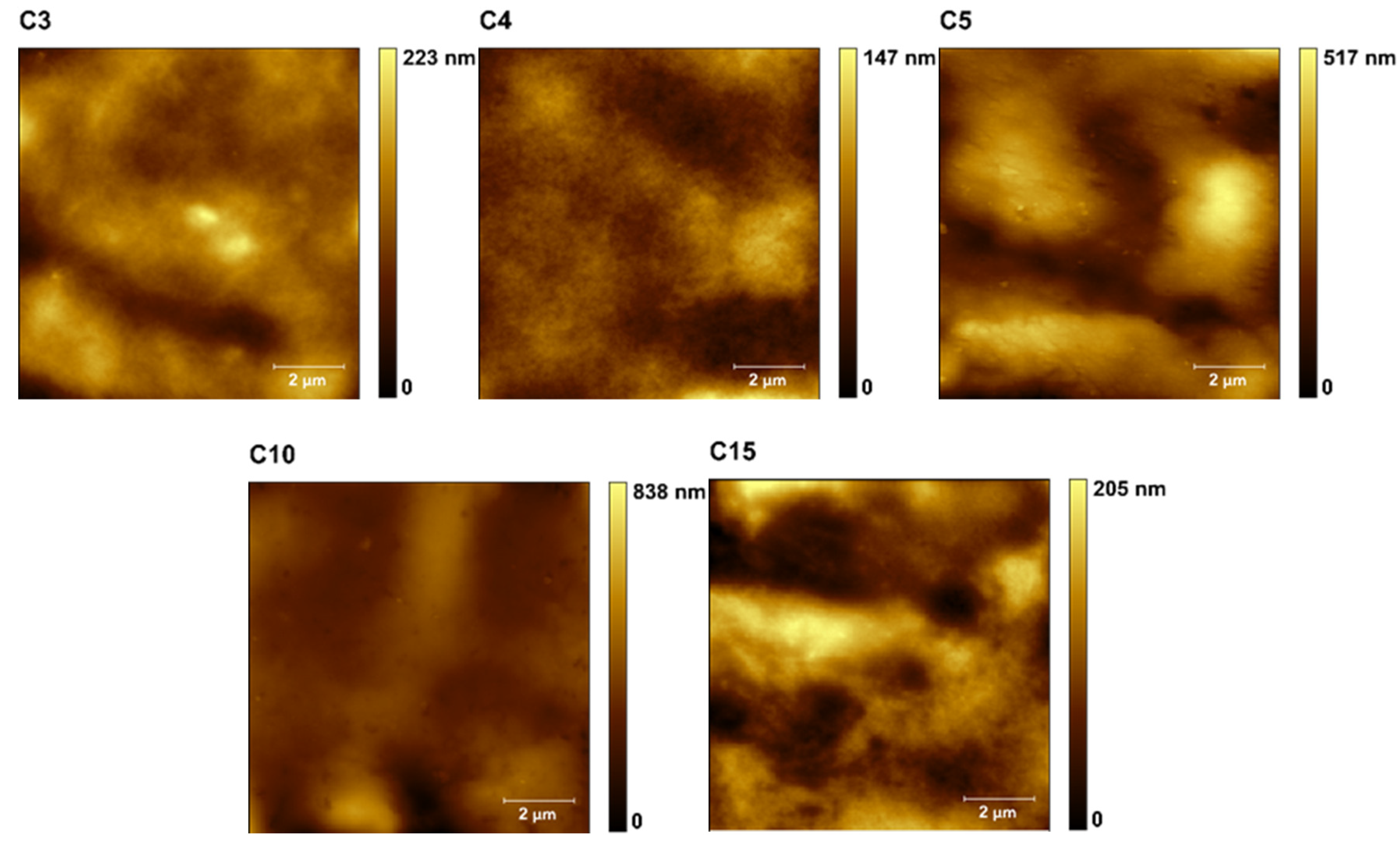
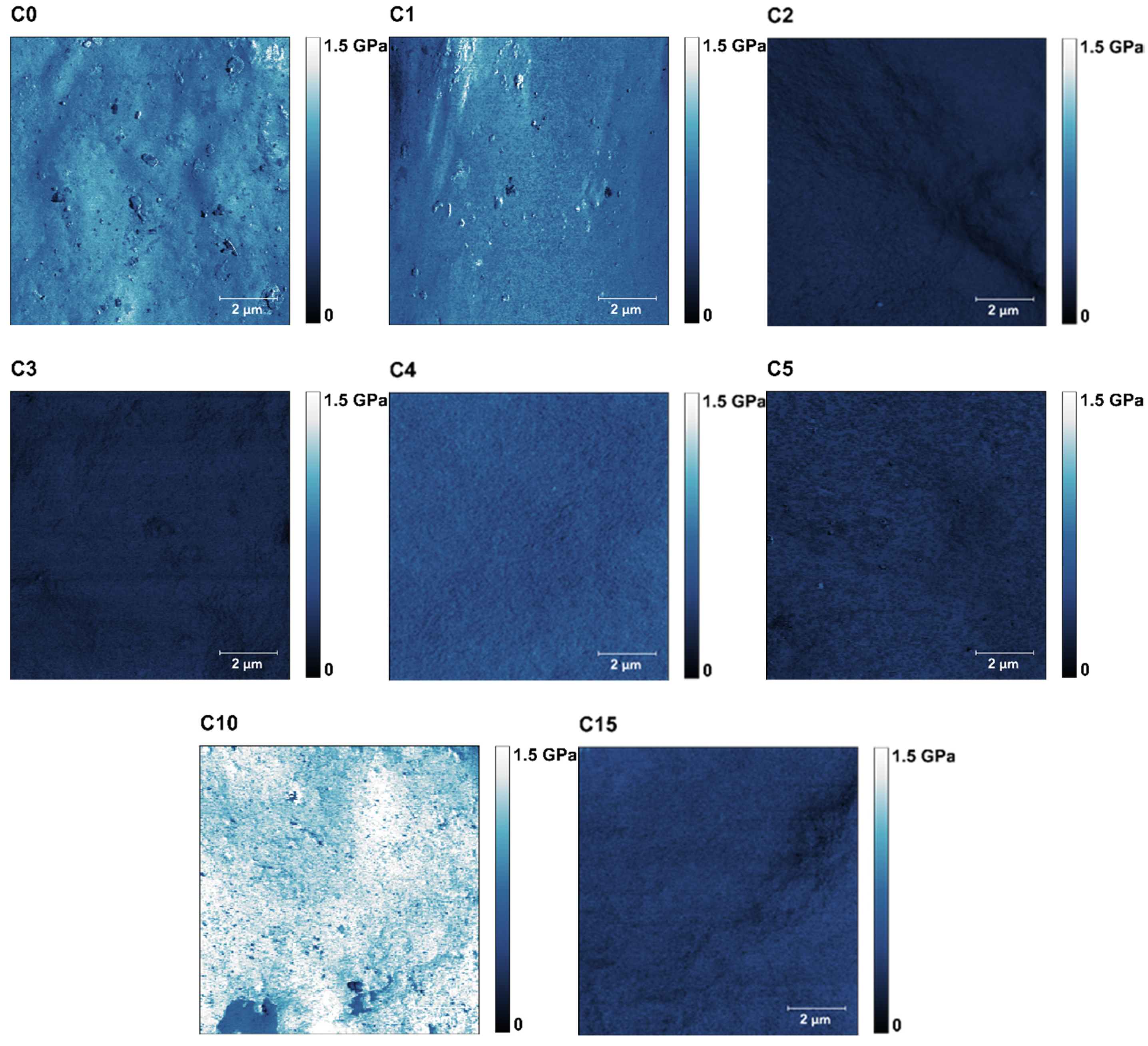

| Sample | Thickness [mm] | Maximum Strain εmax [–] | Tensile Strength σmax [MPa] | Young’s Modulus E [MPa] | EUNHT [MPa] | HUNHT [MPa] | ERNST [–] |
|---|---|---|---|---|---|---|---|
| C0 | 0.070 ± 0.001 a | 0.53 ± 0.08 a | 3.40 ± 0.36 a | 35.01 ± 3.05 abc | 85.25 ± 19.99 | 5.57 ± 0.70 | 0.89 a |
| C1 | 0.076 ± 0.002 b | 0.39 ± 0.04 b | 3.09 ± 0.36 ad | 37.97 ± 8.68 abc | 19.50 ± 4.51 | 1.64 ± 0.29 | 0.88 a |
| C2 | 0.080 ± 0.002 c | 0.31 ± 0.02 c | 2.55 ± 0.18 bd | 17.95 ± 7.44 a | 32.25 ± 5.68 | 3.08 ± 0.32 | 0.76 a |
| C3 | 0.082 ± 0.001 cd | 0.26 ± 0.04 ce | 2.43 ± 0.32 bc | 24.26 ± 11.64 ab | 30.25 ± 5.32 | 3.73 ± 1.29 | 0.83 a |
| C4 | 0.084 ± 0.001 d | 0.28 ± 0.03 ce | 3.09 ± 0.12 ad | 45.72 ± 3.02 bc | 145.00 ± 18.55 | 8.14 ± 0.18 | 0.80 a |
| C5 | 0.094 ± 0.001 e | 0.22 ± 0.02 de | 1.86 ± 0.31 c | 20.66 ± 8.68 a | 90.75 ± 34.53 | 7.03 ± 1.34 | 0.81 a |
| C10 | 0.095 ± 0.002 e | 0.21 ± 0.01 de | 3.42 ± 0.27 a | 79.65 ± 27.11 d | 96.00 ± 17.93 | 8.49 ± 0.56 | 0.55 b |
| C15 | 0.111 ± 0.003 f | 0.16 ± 0.01 d | 2.52 ± 0.47 bc | 55.08 ± 12.77 c | 232.00 ± 71.34 | 16.51 ± 7.22 | 0.81 a |
| Sample | RMSUNHT [µm] | RaUNHT [µm] | DMT Modulus [MPa] | Adhesion Force [nN] | Water Contact Angle [°] | WSI [%] | WVP [10−10 g/(m·s·Pa)] |
|---|---|---|---|---|---|---|---|
| C0 | 2.02 ± 0.15 | 1.64 ± 0.18 | 685.68 ± 31,40 ab | 4.05 ± 0.33 ab | 70.11 ± 3.84 a | 31.20 ± 0.16 ab | 2.60 |
| C1 | 2.12 ± 0.23 | 1.71 ± 0.18 | 637.15 ± 40.24 ab | 3,30 ± 1.71 a | 86.68 ± 6.67 b | 31.45 ± 0.05 ab | 3.14 |
| C2 | 2.19 ± 0.59 | 1.76 ± 0.53 | 270.56 ± 143.49 a | 10.75 ± 1.02 e | 100.64 ± 4.9 c | 31.57 ± 0.38 ab | 3.27 |
| C3 | 2.48 ± 0.57 | 1.86 ± 0.38 | 514.44 ± 9.16 ab | 13.70 ± 1.54 e | 101.70 ± 4,24 c | 31.44 ± 0.12 ab | 3.34 |
| C4 | 2.20 ± 0.24 | 1.77 ± 0.18 | 1052.41 ± 52.29 bc | 7.51 ± 0.32 bcd | 100.12 ± 2.20 c | 32.14 ± 0.26 b | 3.34 |
| C5 | 2.58 ± 0.50 | 2.07 ± 0.43 | 337.53 ± 18,77 a | 10.72 ± 0.87 ce | 102.04 ± 2.87 c | 31.95 ± 0.52 b | 3.57 |
| C10 | 3.64 ± 0.98 | 2.75 ± 0.51 | 1399,73 ± 491.40 c | 8.79 ± 1.92 cd | 87.06 ± 5.37 b | 30.54 ± 0.55 a | 3.96 |
| C15 | 5.44 ± 01.51 | 4.47 ± 1.25 | 772.43 ± 190.1 ab | 6.79 ± 1.83 abd | 64.82 ± 11.87 a | 30.56 ± 0.62 a | 4.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwaśniewska, A.; Świetlicki, M.; Prószyński, A.; Gładyszewski, G. Physical Properties of Starch/Powdered Activated Carbon Composite Films. Polymers 2021, 13, 4406. https://doi.org/10.3390/polym13244406
Kwaśniewska A, Świetlicki M, Prószyński A, Gładyszewski G. Physical Properties of Starch/Powdered Activated Carbon Composite Films. Polymers. 2021; 13(24):4406. https://doi.org/10.3390/polym13244406
Chicago/Turabian StyleKwaśniewska, Anita, Michał Świetlicki, Adam Prószyński, and Grzegorz Gładyszewski. 2021. "Physical Properties of Starch/Powdered Activated Carbon Composite Films" Polymers 13, no. 24: 4406. https://doi.org/10.3390/polym13244406
APA StyleKwaśniewska, A., Świetlicki, M., Prószyński, A., & Gładyszewski, G. (2021). Physical Properties of Starch/Powdered Activated Carbon Composite Films. Polymers, 13(24), 4406. https://doi.org/10.3390/polym13244406






