Application of Nanotechnology to Improve the Performance of Biodegradable Biopolymer-Based Packaging Materials
Abstract
:1. Introduction
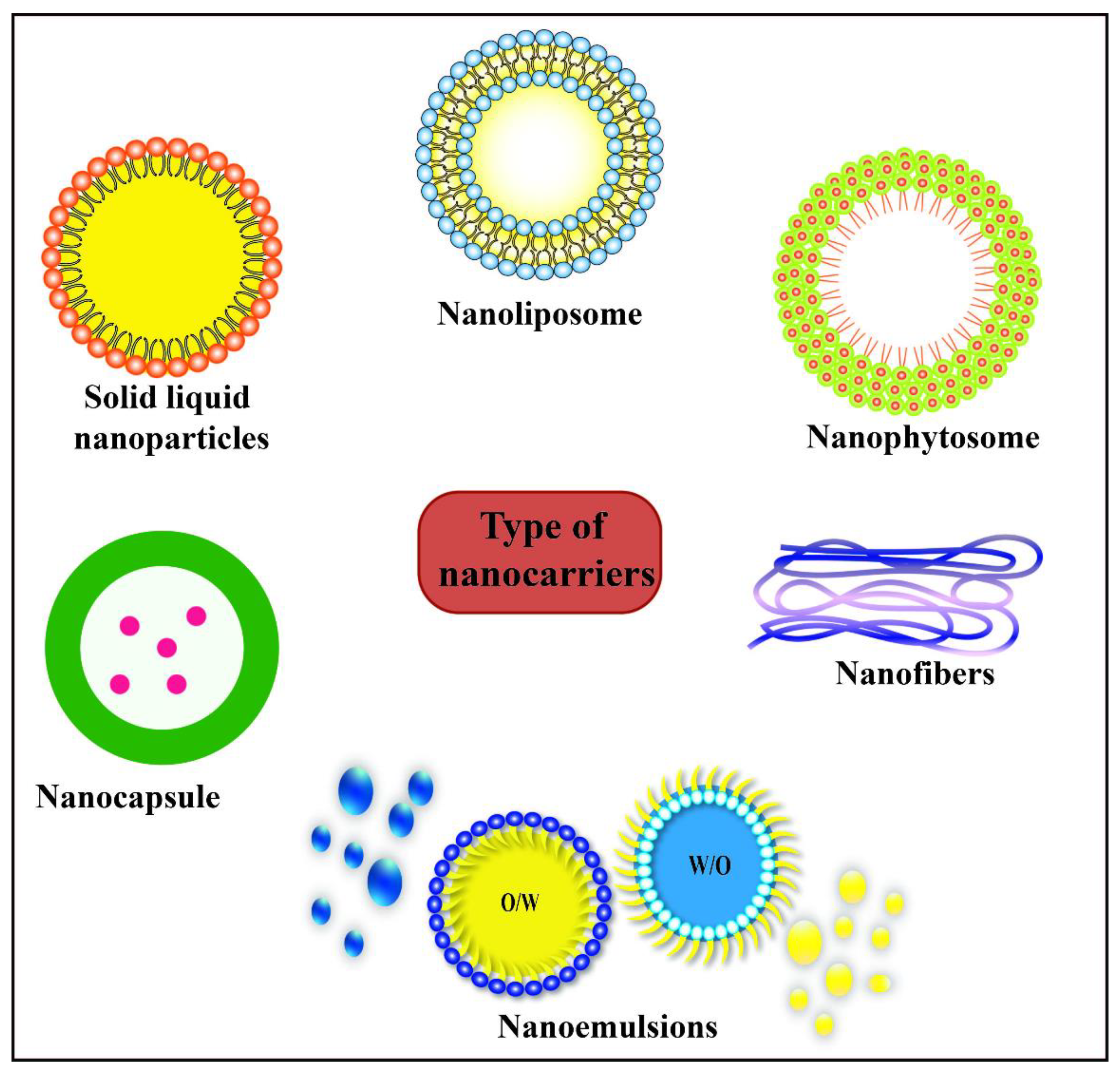
2. Overview of Nanocarriers for Active Compounds
3. Active Compounds for the Production of Smart/Active Packaging Materials
3.1. Antimicrobials
3.2. Antioxidants
3.3. Light Blockers
3.4. Plasticizers
3.5. Crosslinkers
3.6. Diffusion Blockers and Film Strengtheners
3.7. Sensors/Indicators
4. Impact of Bioactive Compounds on Packaging Properties
4.1. Physical Properties
4.2. Mechanical Properties
4.3. Microstructural Properties
4.4. Thermal Stability
4.5. Barrier Properties
4.6. Antioxidant Properties
4.7. Antimicrobial Properties
| Polymer | Bioactive Compound Type | Bioactive Compound (wt%) | Tensile Strength (MPa) | Elongation at Break (%) | Water Vapor Permeability | Antimicrobial | Antioxidant | Thermal | Microstructural | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| Cassava starch | Lycopene | 0% | 3.09 ± 0.10 | 134.59 ± 2.69 | 0.36 ± 0.05 | - | - | residual mass = 6% | Uniform and compact | [113] |
| 2% | 2.81 ± 0.06 | 233.13 ± 1.07 | 0.57 ± 0.02 | - | Porous and Non-uniform | |||||
| 5% | 2.92 ± 0.07 | 190.73 ± 0.96 | 0.55 ± 0.04 | residual mass = 7% | ||||||
| 8% | 2.66 ± 0.04 | 166.03 ± 0.93 | 0.55 ± 0.03 | - | ||||||
| Cassava starch | Bixin | 0% | 12.13 ± 0.95 | 6.05 ± 0.72 | 0.207 ± 0.014 | - | - | High thermal stability at least up to 270 °C | Compact and uniform structures | [114] |
| 2% | 14.40 ± 1.69 | 2.19 ± 0.35 | 0.202 ± 0.008 | |||||||
| 5% | 8.95 ± 1.32 | 15.55 ± 1.14 | 0.216 ± 0.007 | |||||||
| 8% | 2.06 ± 0.34 | 28.57 ± 3.44 | 0.243 ± 0.010 | Cracks surface | ||||||
| 10% | 1.94 ± 0.37 | 34.34 ± 3.40 | 0.273 ± 0.018 | |||||||
| Cassava starch | β-carotene | 0% | 3.09 ± 0.10 | 134.59 ± 2.69 | 0.36 ± 0.05 | - | - | No effect on thermal stability | Non-uniform structure | [115] |
| 2% | 2.74 ± 0.19 | 237.81 ± 7.49 | 0.45 ± 0.02 | - | ||||||
| 5% | 2.56 ± 0.15 | 311.82 ± 6.73 | 0.44 ± 0.05 | Smooth surface with pores | ||||||
| 8% | 2.63 ± 0.18 | 319.74 ± 3.35 | 0.44 ± 0.03 | Heterogeneous and cracks structure | ||||||
| HPMC | Nisin | 0% | 59.0 ± 6.8 | 6.0 ± 3.3 | 0.77 ± 0.03 | Antimicrobial activity against Listeria monocytogenes | - | - | Smooth surface | [116] |
| 100% | 37.0 ± 2.5 | 2.6 ± 0.7 | 0.95 ± 0.10 | Non-uniform surface with dome-shaped zones and holes | ||||||
| Pullulan | Lysozyme | 0% | 35.0 ± 4.4 | 6.63 ± 1.11 | - | Antimicrobial activity against Staphylococcus aureus | High antioxidant activity (77%) for 15% LNFs | High thermal stability at least up to 225 °C | Homogeneous, smooth, compact surface | [117] |
| 1% | 33.2 ± 3.7 | 2.57 ± 0.36 | ||||||||
| 3% | 35.6 ± 2.2 | 2.24 ± 0.27 | ||||||||
| 5% | 37.6 ± 2.2 | 1.84 ± 0.29 | ||||||||
| 10% | 34.1 ± 1.0 | 1.64 ± 0.61 | ||||||||
| 15% | 31.3 ± 2.3 | 1.34 ± 0.10 | ||||||||
| Chitosan | Epigallocatechin gallate | 0% | 6.44 ± 0.28 | 22.5 ± 4.3 | - | - | Higher DPPH scavenging activity | - | Smooth | [118] |
| 2.5%, | 10.4 ± 3.2 | 24.1 ± 4.6 | Rough and uneven surface | |||||||
| 4.5% | 19.2 ± 1.2 | 20.6 ± 3.5 | ||||||||
| 6.0% | 18.10 ± 4.10 | 3.9 ± 2.6 | ||||||||
| Chitosan | Cinnamaldehyde | 0% | 98.26 ± 5.69 | 4.16 ± 0.47 | 1.42 ± 0.29 | Better antifungal than antibacterial activity | - | - | Bubble-like surface | [81] |
| 0.1% | 62.29 ± 3.47 | 16.1 ± 2.6 | 1.31 ± 0.36 | Uniform and smooth | ||||||
| 0.2% | 51.78 ± 4.70 | 24.5 ± 0.6 | 1.15 ± 0.09 | |||||||
| 0.4% | 44.90 ± 4.11 | 12.2 ± 3.5 | 1.69 ± 0.05 | |||||||
| 0.6% | 37.42 ± 4.02 | 14.5 ± 2.9 | 1.74 ± 0.14 | |||||||
| 0.8% | 38.84 ± 4.74 | 14.6 ± 2.5 | 2.01 ± 0.27 | |||||||
| 1.0% | 29.57 ± 4.21 | 11.4 ± 2.6 | 2.24 ± 0.17 | |||||||
| 1.5% | 17.44 ± 3.48 | 14.9 ± 3.7 | 3.33 ± 0.47 | |||||||
| 2% | 7.57 ± 1.34 | 12.6 ± 2.2 | 3.91 ± 0.59 | |||||||
| Soy protein isolate | Cinnamaldehyde/ Carvacrol | 0 | 2.61 ± 0.54 | 172 ± 46 | 2.89 ± 0.24 | - | - | - | - | [119] |
| Carvacrol | 1.97 ± 0.11 | 418 ± 37 | 2.79 ± 0.28 | |||||||
| Cinnamaldehyde | 2.52 ± 0.21 | 374 ± 50 | 2.83 ± 0.09 | |||||||
| Low pectin | Cinnamaldehyde | 0 | 5.36 ± 0.42 | 246 ± 23 | 3.10 ± 0.10 | Good antimicrobial activity | - | - | - | [120] |
| 7 | 6.34 ± 0.71 | 174 ± 40 | 2.92 ± 0.09 | |||||||
| 12 | 5.99 ± 0.14 | 139 ± 29 | 2.15 ± 0.10 | |||||||
| 16 | 6.53 ± 0.68 | 146 ± 17 | 2.90 ± 0.10 | |||||||
| High pectin | Cinnamaldehyde | 0 | 4.84 ± 0.29 | 192 ± 39 | 3.26 ± 0.02 | - | - | - | ||
| 7 | 7.70 ± 1.17 | 155 ± 30 | 3.02 ± 0.02 | |||||||
| 12 | 7.62 ± 0.2 | 170 ± 36 | 2.70 ± 0.02 | |||||||
| 16 | 8.36 ± 0.15 | 180 ± 14 | 2.95 ± 0.02 | |||||||
| Quinoa protein/chitosan | Thymol | 0 | 4.4 ± 0.7 | 116 ± 22 | 0.35 ± 0.05 | - | - | - | Homogeneous | [121] |
| 10% | 2.9 ± 0.5 | 98 ± 11 | 0.40 ± 0.04 | Porous and heterogeneous surface |
5. Application of Active-Loaded Packaging Materials in Foods
5.1. Seafood
5.2. Meat
5.3. Cheese
5.4. Bread
5.5. Fruit and Vegetables
| Active Agent | Matrix | Natural Compounds | Nanocarrier | Food Model | Condition Storage | Ref. |
|---|---|---|---|---|---|---|
| Phenolic compounds | Aloe vera | Eugenol essential oil (EO) | Nanoemulsion | Shrimp | - | [122] |
| Aloe vera | Eugenol EO | Nanoemulsion | Shrimp | - | [140] | |
| Gelatin-Carrageenan | Curcumin Gallic acid Quercetin | Nanoemulsion | Raw broiler meat | 20 days at 4 °C | [141] | |
| Alginate-CMC | Vanillin Ascorbic acid | Nanoemulsion | Fresh cut kiwi slices | 7 days at 5 ± 1 °C | [142] | |
| Pectin | Resveratrol | Nanoemulsion | Pork | 15 days at 4 °C | [130] | |
| Chitosan | Thymol or thyme EO | Nanoemulsion | Pork | 12 days at 4 °C | [143] | |
| Essential oil | Chitosan | Cinnamodendron dinisii Schwanke | Nanoparticle | Ground beef | 12 days at 6 ± 2 °C | [128] |
| Chitosan | Paulownia Tomentosa | Nanoparticle | Pork chop | 16 days at 4 °C | [144] | |
| Chitosan-gelatin | Tarragon | Nanoparticle | Pork | 16 days at 4 °C | [131] | |
| Corn starch | Zataria multiflora EO | Nanoemulsion | Chicken meat | |||
| PVA | Cinnamon | Nanophytosome | Shrimp | 7 days at 4 °C | [123] | |
| Pullulan | Cinnamon | Nanoemulsion | Strawberries | 6 days at 20 ± 2 °C | [145] | |
| Nanochitosan | Cuminum cyminum EO | Nanoliposome | Sardine | 16 days at 4 °C | [124] | |
| Chitosan | Ferulago angulata EO | Nanoemulsion | Rainbow trout fillets | 16 days at 4 °C | ||
| Gelatin/Hydroxypropyl | Mustard | Nanoemulsion | Turkey | 20 days at 4 ± 1 °C | [146] | |
| Chitosan | Garlic | Nanoliposome | Chicken breast fillets | at 4 °C | [9] | |
| Alginate | Oregano EO | Nanoemulsion | Tomatoes | 14 days at 14 days at 24 ± 1 °C | [147] | |
| Alginate | Trachyspermum ammi | Nanoemulsion | Turkey fillets | 12 days at 4 ± 1 °C | [126] | |
| Carotenoid | Xanthan gum | β-carotene | Nanocapsule | Fresh cut melon | - | [138] |
| Peptide | Soy Protein Isolate | Star anise essential oil Polylysine Nisin | Nanoemulsion | Yao meat | 20 days at 4 °C | [147] |
| Poly (ethylene oxide) | Nisin | Nanoparticle | Cheese | - | [134] | |
| Others | Gelatin | Betanin | Nanoliposome | Beef | 16 days at 4 °C | [129] |
6. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khezerlou, A.; Zolfaghari, H.; Banihashemi, S.A.; Forghani, S.; Ehsani, A. Plant gums as the functional compounds for edible films and coatings in the food industry: A review. Polym. Adv. Technol. 2021, 32, 2306–2326. [Google Scholar] [CrossRef]
- Alizadeh-Sani, M.; Ehsani, A.; Kia, E.M.; Khezerlou, A. Microbial gums: Introducing a novel functional component of edible coatings and packaging. Appl. Microbiol. Biotechnol. 2019, 103, 6853–6866. [Google Scholar] [CrossRef]
- Cazón, P.; Velazquez, G.; Ramírez, J.A.; Vázquez, M. Polysaccharide-based films and coatings for food packaging: A review. Food Hydrocoll. 2017, 68, 136–148. [Google Scholar] [CrossRef]
- Magazù, S.; Migliardo, F.; Telling, M. Structural and dynamical properties of water in sugar mixtures. Food Chem. 2008, 106, 1460–1466. [Google Scholar] [CrossRef]
- Dehghani, S.; Hosseini, S.V.; Regenstein, J.M. Edible films and coatings in seafood preservation: A review. Food Chem. 2018, 240, 505–513. [Google Scholar] [CrossRef]
- Dammak, I.; de Carvalho, R.A.; Trindade, C.S.F.; Lourenço, R.V.; do Amaral Sobral, P.J. Properties of active gelatin films incorporated with rutin-loaded nanoemulsions. Int. J. Biol. Macromol. 2017, 98, 39–49. [Google Scholar] [CrossRef]
- Alizadeh-Sani, M.; Kia, E.M.; Ghasempour, Z.; Ehsani, A. Preparation of active nanocomposite film consisting of sodium caseinate, ZnO nanoparticles and rosemary essential oil for food packaging applications. J. Polym. Environ. 2021, 29, 588–598. [Google Scholar] [CrossRef]
- Azizi-Lalabadi, M.; Alizadeh-Sani, M.; Divband, B.; Ehsani, A.; McClements, D.J. Nanocomposite films consisting of functional nanoparticles (TiO2 and ZnO) embedded in 4A-Zeolite and mixed polymer matrices (gelatin and polyvinyl alcohol). Food Res. Int. 2020, 137, 109716. [Google Scholar] [CrossRef] [PubMed]
- Kamkar, A.; Molaee-aghaee, E.; Khanjari, A.; Akhondzadeh-basti, A.; Noudoost, B.; Shariatifar, N.; Alizadeh Sani, M.; Soleimani, M. Nanocomposite active packaging based on chitosan biopolymer loaded with nano-liposomal essential oil: Its characterizations and effects on microbial, and chemical properties of refrigerated chicken breast fillet. Int. J. Food Microbiol. 2021, 342, 109071. [Google Scholar] [CrossRef] [PubMed]
- Tavassoli, M.; Sani, M.A.; Khezerlou, A.; Ehsani, A.; McClements, D.J. Multifunctional nanocomposite active packaging materials: Immobilization of quercetin, lactoferrin, and chitosan nanofiber particles in gelatin films. Food Hydrocoll. 2021, 118, 106747. [Google Scholar] [CrossRef]
- Ahmadi, A.; Ahmadi, P.; Sani, M.A.; Ehsani, A.; Ghanbarzadeh, B. Functional biocompatible nanocomposite films consisting of selenium and zinc oxide nanoparticles embedded in gelatin/cellulose nanofiber matrices. Int. J. Biol. Macromol. 2021, 175, 87–97. [Google Scholar] [CrossRef]
- Alizadeh-Sani, M.; Tavassoli, M.; Mohammadian, E.; Ehsani, A.; Khaniki, G.J.; Priyadarshi, R.; Rhim, J.-W. pH-responsive color indicator films based on methylcellulose/chitosan nanofiber and barberry anthocyanins for real-time monitoring of meat freshness. Int. J. Biol. Macromol. 2021, 166, 741–750. [Google Scholar] [CrossRef]
- Alizadeh-Sani, M.; Tavassoli, M.; McClements, D.J.; Hamishehkar, H. Multifunctional halochromic packaging materials: Saffron petal anthocyanin loaded-chitosan nanofiber/methyl cellulose matrices. Food Hydrocoll. 2021, 111, 106237. [Google Scholar] [CrossRef]
- Ranjbaryan, S.; Pourfathi, B.; Almasi, H. Reinforcing and release controlling effect of cellulose nanofiber in sodium caseinate films activated by nanoemulsified cinnamon essential oil. Food Packag. Shelf Life 2019, 21, 100341. [Google Scholar] [CrossRef]
- Esfanjani, A.F.; Assadpour, E.; Jafari, S.M. Improving the bioavailability of phenolic compounds by loading them within lipid-based nanocarriers. Trends Food Sci. Technol. 2018, 76, 56–66. [Google Scholar] [CrossRef]
- Nejatian, M.; Saberian, H.; Jafari, S.M. Encapsulation of food ingredients by double nanoemulsions. In Lipid-Based Nanostructures for Food Encapsulation Purposes, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 89–128. [Google Scholar]
- Chen, L.Y.; Remondetto, G.E.; Subirade, M. Food protein-based materials as nutraceutical delivery systems. Trends Food Sci. Technol. 2006, 17, 272–283. [Google Scholar] [CrossRef]
- Fathi, M.; Martin, A.; McClements, D.J. Nanoencapsulation of food ingredients using carbohydrate based delivery systems. Trends Food Sci. Technol. 2014, 39, 18–39. [Google Scholar] [CrossRef]
- Fathi, M.; Mozafari, M.R.; Mohebbi, M. Nanoencapsulation of food ingredients using lipid based delivery systems. Trends Food Sci. Technol. 2012, 23, 13–27. [Google Scholar] [CrossRef]
- Esfanjani, A.F.; Jafari, S.M. Biopolymer nano-particles and natural nano-carriers for nano-encapsulation of phenolic compounds. Colloids Surf. B Biointerfaces 2016, 146, 532–543. [Google Scholar] [CrossRef]
- Rezaei, A.; Fathi, M.; Jafari, S.M. Nanoencapsulation of hydrophobic and low-soluble food bioactive compounds within different nanocarriers. Food Hydrocoll. 2019, 88, 146–162. [Google Scholar] [CrossRef]
- Maqsoudlou, A.; Assadpour, E.; Mohebodini, H.; Jafari, S.M. Improving the efficiency of natural antioxidant compounds via different nanocarriers. Adv. Colloid Interface Sci. 2020, 278, 102122. [Google Scholar] [CrossRef]
- Shewan, H.M.; Stokes, J.R. Review of techniques to manufacture micro-hydrogel particles for the food industry and their applications. J. Food Eng. 2013, 119, 781–792. [Google Scholar] [CrossRef]
- Tamjidi, F.; Shahedi, M.; Varshosaz, J.; Nasirpour, A. Nanostructured lipid carriers (NLC): A potential delivery system for bioactive food molecules. Innov. Food Sci. Emerg. Technol. 2013, 19, 29–43. [Google Scholar] [CrossRef]
- Weiss, J.; Decker, E.A.; McClements, D.J.; Kristbergsson, K.; Helgason, T.; Awad, T. Solid lipid nanoparticles as delivery systems for bioactive food components. Food Biophys. 2008, 3, 146–154. [Google Scholar] [CrossRef]
- Brandelli, A.; Brum, L.F.W.; dos Santos, J.H.Z. Nanostructured bioactive compounds for ecological food packaging. Environ. Chem. Lett. 2017, 15, 193–204. [Google Scholar] [CrossRef]
- Jafarzadeh, S.; Jafari, S.M.; Salehabadi, A.; Nafchi, A.M.; Uthaya Kumar, U.S.; Khalil, H.P.S.A. Biodegradable green packaging with antimicrobial functions based on the bioactive compounds from tropical plants and their by-products. Trends Food Sci. Technol. 2020, 100, 262–277. [Google Scholar] [CrossRef]
- Li, C.; Qiu, X.-L.; Lu, L.-X.; Tang, Y.-L.; Long, Q.; Dang, J.-G. Preparation of low-density polyethylene film with quercetin and α-tocopherol loaded with mesoporous silica for synergetic-release antioxidant active packaging. J. Food Process. Eng. 2019, 42, e13088. [Google Scholar] [CrossRef]
- Yadav, S.; Mehrotra, G.K.; Bhartiya, P.; Singh, A.; Dutta, P.K. Preparation, physicochemical and biological evaluation of quercetin based chitosan-gelatin film for food packaging. Carbohydr. Polym. 2020, 227, 115348. [Google Scholar] [CrossRef]
- Hassoun, A.; Carpena, M.; Prieto, M.A.; Simal-Gandara, J.; Özogul, F.; Özogul, Y.; Çoban, Ö.E.; Guðjónsdóttir, M.; Barba, F.J.; Marti-Quijal, F.J.; et al. Use of Spectroscopic Techniques to Monitor Changes in Food Quality during Application of Natural Preservatives: A Review. Antioxidants 2020, 9, 882. [Google Scholar] [CrossRef] [PubMed]
- Tonyali, B.; McDaniel, A.; Amamcharla, J.; Trinetta, V.; Yucel, U. Release kinetics of cinnamaldehyde, eugenol, and thymol from sustainable and biodegradable active packaging films. Food Packag. Shelf Life 2020, 24, 100484. [Google Scholar] [CrossRef]
- Papadimitriou, A.; Ketikidis, I.; Stathopoulou, M.E.K.; Banti, C.N.; Papachristodoulou, C.; Zoumpoulakis, L.; Agathopoulos, S.; Vagenas, G.V.; Hadjikakou, S.K. Innovative material containing the natural product curcumin, with enhanced antimicrobial properties for active packaging. Mater. Sci. Eng. C 2018, 84, 118–122. [Google Scholar] [CrossRef]
- Gutierrez-del-Rio, I.; Fernandez, J.; Lombo, F. Plant nutraceuticals as antimicrobial agents in food preservation: Terpenoids, polyphenols and thiols. Int. J. Antimicrob. Agents 2018, 52, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Seow, Y.X.; Yeo, C.R.; Chung, H.L.; Yuk, H.G. Plant Essential Oils as Active Antimicrobial Agents. Crit. Rev. Food Sci. Nutr. 2014, 54, 625–644. [Google Scholar] [CrossRef]
- Calo, J.R.; Crandall, P.G.; O’Bryan, C.A.; Ricke, S.C. Essential oils as antimicrobials in food systems—A review. Food Control. 2015, 54, 111–119. [Google Scholar] [CrossRef]
- Donsi, F.; Ferrari, G. Essential oil nanoemulsions as antimicrobial agents in food. J. Biotechnol. 2016, 233, 106–120. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.D.; Holley, R.A. Antimicrobial and Antioxidative Strategies to Reduce Pathogens and Extend the Shelf Life of Fresh Red Meats. Compr. Rev. Food Sci. Food Saf. 2012, 11, 340–354. [Google Scholar] [CrossRef]
- Valdés, A.; Mellinas, A.C.; Ramos, M.; Burgos, N.; Jiménez, A.; Garrigós, M.C. Use of herbs, spices and their bioactive compounds in active food packaging. RSC Adv. 2015, 5, 40324–40335. [Google Scholar] [CrossRef] [Green Version]
- Mohammadi, K.; Alizadeh Sani, M.; Nattagh-Eshtivani, E.; Yaribash, S.; Rahmani, J.; Shokrollahi Yancheshmeh, B.; Julian McClements, D. A systematic review and meta-analysis of the impact of cornelian cherry consumption on blood lipid profiles. Food Sci. Nutr. 2021, 9, 4629–4638. [Google Scholar] [CrossRef]
- Al-Bandak, G.; Oreopoulou, V. Antioxidant properties and composition of Majorana syriaca extracts. Eur. J. Lipid Sci. Technol. 2007, 109, 247–255. [Google Scholar] [CrossRef]
- Amorati, R.; Valgimigli, L. Methods To Measure the Antioxidant Activity of Phytochemicals and Plant Extracts. J. Agric. Food Chem. 2018, 66, 3324–3329. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-del-Rio, I.; Lopez-Ibanez, S.; Magadan-Corpas, P.; Fernandez-Calleja, L.; Perez-Valero, A.; Tunon-Granda, M.; Miguelez, E.M.; Villar, C.J.; Lombo, F. Terpenoids and Polyphenols as Natural Antioxidant Agents in Food Preservation. Antioxidants 2021, 10, 1264. [Google Scholar] [CrossRef] [PubMed]
- Basavegowda, N.; Baek, K.H. Synergistic Antioxidant and Antibacterial Advantages of Essential Oils for Food Packaging Applications. Biomolecules 2021, 11, 1267. [Google Scholar] [CrossRef]
- Cardoso-Ugarte, G.A.; Sosa-Morales, M.E. Essential Oils from Herbs and Spices as Natural Antioxidants: Diversity of Promising Food Applications in the past Decade. Food Rev. Int. 2021, 1–31. [Google Scholar] [CrossRef]
- Ferrentino, G.; Morozova, K.; Horn, C.; Scampicchio, M. Extraction of Essential Oils from Medicinal Plants and their Utilization as Food Antioxidants. Curr. Pharm. Des. 2020, 26, 519–541. [Google Scholar] [CrossRef] [PubMed]
- Sani, M.A.; Azizi-Lalabadi, M.; Tavassoli, M.; Mohammadi, K.; McClements, D.J. Recent Advances in the Development of Smart and Active Biodegradable Packaging Materials. Nanomaterials 2021, 11, 1331. [Google Scholar] [CrossRef] [PubMed]
- Stoll, L.; Domenek, S.; Hickmann Flôres, S.; Nachtigall, S.M.B.; de Oliveira Rios, A. Polylactide films produced with bixin and acetyl tributyl citrate: Functional properties for active packaging. J. Appl. Polym. Sci. 2021, 138, 50302. [Google Scholar] [CrossRef]
- Pristouri, G.; Badeka, A.; Kontominas, M.G. Effect of packaging material headspace, oxygen and light transmission, temperature and storage time on quality characteristics of extra virgin olive oil. Food Control 2010, 21, 412–418. [Google Scholar] [CrossRef]
- Scotter, M. The chemistry and analysis of annatto food colouring: A review. Food Addit. Contam. Part A 2009, 26, 1123–1145. [Google Scholar] [CrossRef]
- Nur Hanani, Z.A.; Roos, Y.H.; Kerry, J.P. Use and application of gelatin as potential biodegradable packaging materials for food products. Int. J. Biol. Macromol. 2014, 71, 94–102. [Google Scholar] [CrossRef]
- Sobral, P.J.A.; Menegalli, F.C.; Hubinger, M.D.; Roques, M.A. Mechanical, water vapor barrier and thermal properties of gelatin based edible films. Food Hydrocoll. 2001, 15, 423–432. [Google Scholar] [CrossRef]
- Ghasemlou, M.; Khodaiyan, F.; Oromiehie, A. Physical, mechanical, barrier, and thermal properties of polyol-plasticized biodegradable edible film made from kefiran. Carbohydr. Polym. 2011, 84, 477–483. [Google Scholar] [CrossRef]
- Peña, C.; de la Caba, K.; Eceiza, A.; Ruseckaite, R.; Mondragon, I. Enhancing water repellence and mechanical properties of gelatin films by tannin addition. Bioresour. Technol. 2010, 101, 6836–6842. [Google Scholar] [CrossRef]
- Azeredo, H.M.C.; Waldron, K.W. Crosslinking in polysaccharide and protein films and coatings for food contact—A review. Trends Food Sci. Technol. 2016, 52, 109–122. [Google Scholar] [CrossRef]
- Tillet, G.; Boutevin, B.; Ameduri, B. Chemical reactions of polymer crosslinking and post-crosslinking at room and medium temperature. Prog. Polym. Sci. 2011, 36, 191–217. [Google Scholar] [CrossRef]
- Nogueira, G.F.; Oliveira, R.A.; Velasco, J.I.; Fakhouri, F.M. Methods of Incorporating Plant-Derived Bioactive Compounds into Films Made with Agro-Based Polymers for Application as Food Packaging: A Brief Review. Polymers 2020, 12, 2518. [Google Scholar] [CrossRef]
- Yu, S.-H.; Tsai, M.-L.; Lin, B.-X.; Lin, C.-W.; Mi, F.-L. Tea catechins-cross-linked methylcellulose active films for inhibition of light irradiation and lipid peroxidation induced β-carotene degradation. Food Hydrocoll. 2015, 44, 491–505. [Google Scholar] [CrossRef]
- Rouhi, M.; Razavi, S.H.; Mousavi, S.M. Optimization of crosslinked poly(vinyl alcohol) nanocomposite films for mechanical properties. Mater. Sci. Eng. C 2017, 71, 1052–1063. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Shen, J.; Chen, S.; Cooper, M.A.; Fu, H.; Wu, D.; Yang, Z. Nanofiller Reinforced Biodegradable PLA/PHA Composites: Current Status and Future Trends. Polymers 2018, 10, 505. [Google Scholar] [CrossRef] [Green Version]
- Lin, D.; Yang, Y.; Wang, J.; Yan, W.; Wu, Z.; Chen, H.; Zhang, Q.; Wu, D.; Qin, W.; Tu, Z. Preparation and characterization of TiO2-Ag loaded fish gelatin-chitosan antibacterial composite film for food packaging. Int. J. Biol. Macromol. 2020, 154, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Sani, M.A.; Tavassoli, M.; Hamishehkar, H.; McClements, D.J. Carbohydrate-based films containing pH-sensitive red barberry anthocyanins: Application as biodegradable smart food packaging materials. Carbohydr. Polym. 2021, 255, 117488. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.F.; Rezaei, M.; Zandi, M.; Farahmandghavi, F. Development of bioactive fish gelatin/chitosan nanoparticles composite films with antimicrobial properties. Food Chem. 2016, 194, 1266–1274. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, F.M.; Kaffashi, B.; Shokrollahi, P.; Seyedjafari, E.; Ardeshirylajimi, A. PCL/chitosan/Zn-doped nHA electrospun nanocomposite scaffold promotes adipose derived stem cells adhesion and proliferation. Carbohydr. Polym. 2015, 118, 133–142. [Google Scholar] [CrossRef]
- Ramos, M.; Fortunati, E.; Peltzer, M.; Dominici, F.; Jiménez, A.; Garrigós, M.d.C.; Kenny, J.M. Influence of thymol and silver nanoparticles on the degradation of poly(lactic acid) based nanocomposites: Thermal and morphological properties. Polym. Degrad. Stab. 2014, 108, 158–165. [Google Scholar] [CrossRef] [Green Version]
- Mohammadian, E.; Alizadeh-Sani, M.; Jafari, S.M. Smart monitoring of gas/temperature changes within food packaging based on natural colorants. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2885–2931. [Google Scholar] [CrossRef]
- Alizadeh-Sani, M.; Mohammadian, E.; Rhim, J.-W.; Jafari, S.M. pH-sensitive (halochromic) smart packaging films based on natural food colorants for the monitoring of food quality. Trends Food Sci Technol. 2020, 105, 93–144. [Google Scholar] [CrossRef]
- Mustafa, F.; Andreescu, S. Chemical and Biological Sensors for Food-Quality Monitoring and Smart Packaging. Foods 2018, 7, 168. [Google Scholar] [CrossRef] [Green Version]
- Alizadeh Sani, M.; Tavassoli, M.; Salim, S.A.; Azizi-lalabadi, M.; McClements, D.J. Development of green halochromic smart and active packaging materials: TiO2 nanoparticle- and anthocyanin-loaded gelatin/κ-carrageenan films. Food Hydrocoll. 2022, 124, 107324. [Google Scholar] [CrossRef]
- Kuswandi, B.; Nurfawaidi, A. On-package dual sensors label based on pH indicators for real-time monitoring of beef freshness. Food Control 2017, 82, 91–100. [Google Scholar] [CrossRef]
- Lee, J.Y.; Garcia, C.V.; Shin, G.H.; Kim, J.T. Antibacterial and antioxidant properties of hydroxypropyl methylcellulose-based active composite films incorporating oregano essential oil nanoemulsions. LWT 2019, 106, 164–171. [Google Scholar] [CrossRef]
- Ma, Q.; Zhang, Y.; Zhong, Q. Physical and antimicrobial properties of chitosan films incorporated with lauric arginate, cinnamon oil, and ethylenediaminetetraacetate. LWT-Food Sci. Technol. 2016, 65, 173–179. [Google Scholar] [CrossRef]
- Fathi, N.; Almasi, H.; Pirouzifard, M.K. Effect of ultraviolet radiation on morphological and physicochemical properties of sesame protein isolate based edible films. Food Hydrocoll. 2018, 85, 136–143. [Google Scholar] [CrossRef]
- Pirouzifard, M.; Yorghanlu, R.A.; Pirsa, S. Production of active film based on potato starch containing Zedo gum and essential oil of Salvia officinalis and study of physical, mechanical, and antioxidant properties. J. Thermoplast. Compos. Mater. 2020, 33, 915–937. [Google Scholar] [CrossRef]
- Pérez-Córdoba, L.J.; Norton, I.T.; Batchelor, H.K.; Gkatzionis, K.; Spyropoulos, F.; Sobral, P.J. Physico-chemical, antimicrobial and antioxidant properties of gelatin-chitosan based films loaded with nanoemulsions encapsulating active compounds. Food Hydrocoll. 2018, 79, 544–559. [Google Scholar] [CrossRef]
- Khezerlou, A.; Ehsani, A.; Tabibiazar, M.; Moghaddas Kia, E. Development and characterization of a Persian gum–sodium caseinate biocomposite film accompanied by Zingiber officinale extract. J. Appl. Polym. Sci. 2019, 136, 47215. [Google Scholar] [CrossRef]
- Li, X.; Yang, X.; Deng, H.; Guo, Y.; Xue, J. Gelatin films incorporated with thymol nanoemulsions: Physical properties and antimicrobial activities. Int. J. Biol. Macromol. 2020, 150, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Cheng, W.; Feng, X.; Gao, C.; Wu, D.; Meng, L.; Zhang, Y.; Tang, X. Fabrication, structure and properties of pullulan-based active films incorporated with ultrasound-assisted cinnamon essential oil nanoemulsions. Food Packag. Shelf Life 2020, 25, 100547. [Google Scholar] [CrossRef]
- Behjati, J.; Yazdanpanah, S. Nanoemulsion and emulsion vitamin D3 fortified edible film based on quince seed gum. Carbohydr. Polym. 2021, 262, 117948. [Google Scholar] [CrossRef]
- Alizadeh-Sani, M.; Khezerlou, A.; Ehsani, A. Fabrication and characterization of the bionanocomposite film based on whey protein biopolymer loaded with TiO2 nanoparticles, cellulose nanofibers and rosemary essential oil. Ind. Crop. Prod. 2018, 124, 300–315. [Google Scholar] [CrossRef]
- Sanchez-Gonzalez, L.; Cháfer, M.; Hernández, M.; Chiralt, A.; González-Martínez, C. Antimicrobial activity of polysaccharide films containing essential oils. Food Control 2011, 22, 1302–1310. [Google Scholar] [CrossRef]
- Chen, H.; Hu, X.; Chen, E.; Wu, S.; McClements, D.J.; Liu, S.; Li, B.; Li, Y. Preparation, characterization, and properties of chitosan films with cinnamaldehyde nanoemulsions. Food Hydrocoll. 2016, 61, 662–671. [Google Scholar] [CrossRef]
- Agudelo-Cuartas, C.; Granda-Restrepo, D.; Sobral, P.J.A.; Hernandez, H.; Castro, W. Characterization of whey protein-based films incorporated with natamycin and nanoemulsion of α-tocopherol. Heliyon 2020, 6, e03809. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei-Mohkam, A.; Garavand, F.; Dehnad, D.; Keramat, J.; Nasirpour, A. Physical, mechanical, thermal and structural characteristics of nanoencapsulated vitamin E loaded carboxymethyl cellulose films. Prog. Org. Coat. 2020, 138, 105383. [Google Scholar] [CrossRef]
- Fattahi, R.; Ghanbarzadeh, B.; Dehghannya, J.; Hosseini, M.; Falcone, P.M. The effect of Macro and Nano-emulsions of cinnamon essential oil on the properties of edible active films. Food Sci. Nutr. 2020, 8, 6568–6579. [Google Scholar] [CrossRef]
- Aziz, S.G.-G.; Almasi, H. Physical Characteristics, Release Properties, and Antioxidant and Antimicrobial Activities of Whey Protein Isolate Films Incorporated with Thyme (Thymus vulgaris L.) Extract-Loaded Nanoliposomes. Food Bioprocess Technol. 2018, 11, 1552–1565. [Google Scholar] [CrossRef]
- Han, J.H. Edible films and coatings: A review. In Innovations in Food Packaging, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 213–255. [Google Scholar] [CrossRef]
- Chen, W.; Ma, S.; Wang, Q.; McClements, D.J.; Liu, X.; Ngai, T.; Liu, F. Fortification of edible films with bioactive agents: A review of their formation, properties, and application in food preservation. Crit Rev Food Sci Nutr. 2021, 8, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Ghasempour, Z.; Khodaivandi, S.; Ahangari, H.; Hamishehkar, H.; Amjadi, S.; Kia, E.M.; Ehsani, A. Characterization and Optimization of Persian Gum/Whey Protein Bionanocomposite Films Containing Betanin Nanoliposomes for Food Packaging Utilization. 2021. Available online: https://assets.researchsquare.com/files/rs-420388/v1_covered.pdf?c=1631863559 (accessed on 27 April 2021).
- Najafi, Z.; Kahn, C.J.F.; Bildik, F.; Arab-Tehrany, E.; Şahin-Yeşilçubuk, N. Pullulan films loading saffron extract encapsulated in nanoliposomes; preparation and characterization. Int. J. Biol. Macromol. 2021, 188, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Kong, R.; Wang, J.; Cheng, M.; Lu, W.; Chen, M.; Zhang, R.; Wang, X. Development and characterization of corn starch/PVA active films incorporated with carvacrol nanoemulsions. Int. J. Biol. Macromol. 2020, 164, 1631–1639. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, Y.; Kang, S.; Cui, M.; Xu, H. Development of pH-responsive antioxidant soy protein isolate films incorporated with cellulose nanocrystals and curcumin nanocapsules to monitor shrimp freshness. Food Hydrocoll. 2021, 120, 106893. [Google Scholar] [CrossRef]
- de Carvalho, S.M.; Noronha, C.M.; da Rosa, C.G.; Sganzerla, W.G.; Bellettini, I.C.; Nunes, M.R.; Bertoldi, F.C.; Manique Barreto, P.L. PVA antioxidant nanocomposite films functionalized with alpha-tocopherol loaded solid lipid nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2019, 581, 123793. [Google Scholar] [CrossRef]
- Sahraee, S.; Milani, J.M.; Regenstein, J.M.; Kafil, H.S. Protection of foods against oxidative deterioration using edible films and coatings: A review. Food Biosci. 2019, 32, 100451. [Google Scholar] [CrossRef]
- Acosta, S.; Chiralt, A.; Santamarina, P.; Rosello, J.; González-Martínez, C.; Cháfer, M. Antifungal films based on starch-gelatin blend, containing essential oils. Food Hydrocoll. 2016, 61, 233–240. [Google Scholar] [CrossRef]
- Acevedo-Fani, A.; Salvia-Trujillo, L.; Rojas-Graü, M.A.; Martín-Belloso, O. Edible films from essential-oil-loaded nanoemulsions: Physicochemical characterization and antimicrobial properties. Food Hydrocoll. 2015, 47, 168–177. [Google Scholar] [CrossRef] [Green Version]
- Homayounpour, P.; Shariatifar, N.; Alizadeh-Sani, M. Development of nanochitosan-based active packaging films containing free and nanoliposome caraway (Carum carvi. L) seed extract. Food Sci. Nutr. 2021, 9, 553–563. [Google Scholar] [CrossRef]
- Ji, M.; Wu, J.; Sun, X.; Guo, X.; Zhu, W.; Li, Q.; Shi, X.; Tian, Y.; Wang, S. Physical properties and bioactivities of fish gelatin films incorporated with cinnamaldehyde-loaded nanoemulsions and vitamin C. LWT 2021, 135, 110103. [Google Scholar] [CrossRef]
- Sun, X.; Wang, J.; Zhang, H.; Dong, M.; Li, L.; Jia, P.; Bu, T.; Wang, X.; Wang, L. Development of functional gelatin-based composite films incorporating oil-in-water lavender essential oil nano-emulsions: Effects on physicochemical properties and cherry tomatoes preservation. LWT 2021, 142, 110987. [Google Scholar] [CrossRef]
- Liu, D.; Dang, S.; Zhang, L.; Munsop, K.; Li, X. Corn starch/polyvinyl alcohol based films incorporated with curcumin-loaded Pickering emulsion for application in intelligent packaging. Int. J. Biol. Macromol. 2021, 188, 974–982. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Li, S.; Chen, S.; Wang, C.; Liu, D.; Li, X. Antibacterial and antioxidant activities of sodium starch octenylsuccinate-based Pickering emulsion films incorporated with cinnamon essential oil. Int. J. Biol. Macromol. 2020, 159, 696–703. [Google Scholar] [CrossRef]
- Bi, F.; Qin, Y.; Chen, D.; Kan, J.; Liu, J. Development of active packaging films based on chitosan and nano-encapsulated luteolin. Int. J. Biol. Macromol. 2021, 182, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Wang, Y. Physicochemical and antioxidant properties based on fish sarcoplasmic protein/chitosan composite films containing ginger essential oil nanoemulsion. Food Bioprocess Technol. 2021, 14, 151–163. [Google Scholar] [CrossRef]
- Mirzaei-Mohkam, A.; Garavand, F.; Dehnad, D.; Keramat, J.; Nasirpour, A. Optimisation, antioxidant attributes, stability and release behaviour of carboxymethyl cellulose films incorporated with nanoencapsulated vitamin E. Prog. Org. Coat. 2019, 134, 333–341. [Google Scholar] [CrossRef]
- Shen, Y.; Ni, Z.-J.; Thakur, K.; Zhang, J.-G.; Hu, F.; Wei, Z.-J. Preparation and characterization of clove essential oil loaded nanoemulsion and pickering emulsion activated pullulan-gelatin based edible film. Int. J. Biol. Macromol. 2021, 181, 528–539. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Gelatin/agar-based functional film integrated with Pickering emulsion of clove essential oil stabilized with nanocellulose for active packaging applications. Colloids Surf. A Physicochem. Eng. Asp. 2021, 627, 127220. [Google Scholar] [CrossRef]
- Darbasi, M.; Askari, G.; Kiani, H.; Khodaiyan, F. Development of chitosan based extended-release antioxidant films by control of fabrication variables. Int. J. Biol. Macromol. 2017, 104, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Calderón, J.; Calderón-Jaimes, L.; Guerra-Hernández, E.; García-Villanova, B. Antioxidant capacity, phenolic content and vitamin C in pulp, peel and seed from 24 exotic fruits from Colombia. Food Res. Int. 2011, 44, 2047–2053. [Google Scholar] [CrossRef]
- Khezerlou, A.; Azizi-Lalabadi, M.; Mousavi, M.-M.; Ehsani, A. Incorporation of essential oils with antibiotic properties in edible packaging films. J. Food Bioprocess Eng. 2019, 2, 77–84. [Google Scholar]
- Almasi, H.; Jahanbakhsh Oskouie, M.; Saleh, A. A review on techniques utilized for design of controlled release food active packaging. Crit. Rev. Food Sci. Nutr. 2020, 61, 2601–2621. [Google Scholar] [CrossRef] [PubMed]
- Khezerlou, A.; Jafari, S.M. Nanoencapsulated bioactive components for active food packaging. In Handbook of Food Nanotechnology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 493–532. [Google Scholar]
- Beikzadeh, S.; Akbarinejad, A.; Swift, S.; Perera, J.; Kilmartin, P.A.; Travas-Sejdic, J. Cellulose acetate electrospun nanofibers encapsulating Lemon Myrtle essential oil as active agent with potent and sustainable antimicrobial activity. React. Funct. Polym. 2020, 157, 104769. [Google Scholar] [CrossRef]
- Elshamy, S.; Khadizatul, K.; Uemura, K.; Nakajima, M.; Neves, M.A. Chitosan-based film incorporated with essential oil nanoemulsion foreseeing enhanced antimicrobial effect. J. Food Sci. Technol. 2021, 58, 3314–3327. [Google Scholar] [CrossRef]
- Assis, R.Q.; Lopes, S.M.; Costa, T.M.H.; Flôres, S.H.; de Oliveira Rios, A. Active biodegradable cassava starch films incorporated lycopene nanocapsules. Ind. Crop. Prod. 2017, 109, 818–827. [Google Scholar] [CrossRef]
- Pagno, C.H.; de Farias, Y.B.; Costa, T.M.H.; de Oliveira Rios, A.; Flôres, S.H. Synthesis of biodegradable films with antioxidant properties based on cassava starch containing bixin nanocapsules. J. Food Sci. Technol. 2016, 53, 3197–3205. [Google Scholar] [CrossRef] [Green Version]
- Assis, R.Q.; Pagno, C.H.; Costa, T.M.H.; Flôres, S.H.; Rios, A.d.O. Synthesis of biodegradable films based on cassava starch containing free and nanoencapsulated β-carotene. Packag. Technol. Sci. 2018, 31, 157–166. [Google Scholar] [CrossRef]
- Imran, M.; Revol-Junelles, A.-M.; René, N.; Jamshidian, M.; Akhtar, M.J.; Arab-Tehrany, E.; Jacquot, M.; Desobry, S. Microstructure and physico-chemical evaluation of nano-emulsion-based antimicrobial peptides embedded in bioactive packaging films. Food Hydrocoll. 2012, 29, 407–419. [Google Scholar] [CrossRef]
- Silva, N.H.; Vilela, C.; Almeida, A.; Marrucho, I.M.; Freire, C.S. Pullulan-based nanocomposite films for functional food packaging: Exploiting lysozyme nanofibers as antibacterial and antioxidant reinforcing additives. Food Hydrocoll. 2018, 77, 921–930. [Google Scholar] [CrossRef]
- Liang, J.; Yan, H.; Zhang, J.; Dai, W.; Gao, X.; Zhou, Y.; Wan, X.; Puligundla, P. Preparation and characterization of antioxidant edible chitosan films incorporated with epigallocatechin gallate nanocapsules. Carbohydr. Polym. 2017, 171, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Otoni, C.G.; Avena-Bustillos, R.J.; Olsen, C.W.; Bilbao-Sáinz, C.; McHugh, T.H. Mechanical and water barrier properties of isolated soy protein composite edible films as affected by carvacrol and cinnamaldehyde micro and nanoemulsions. Food Hydrocoll. 2016, 57, 72–79. [Google Scholar] [CrossRef]
- Otoni, C.G.; Pontes, S.F.; Medeiros, E.A.; Soares, N.d.F. Edible films from methylcellulose and nanoemulsions of clove bud (Syzygium aromaticum) and oregano (Origanum vulgare) essential oils as shelf life extenders for sliced bread. J. Agric. Food Chem. 2014, 62, 5214–5219. [Google Scholar] [CrossRef] [PubMed]
- Robledo, N.; Vera, P.; López, L.; Yazdani-Pedram, M.; Tapia, C.; Abugoch, L. Thymol nanoemulsions incorporated in quinoa protein/chitosan edible films; antifungal effect in cherry tomatoes. Food Chem. 2018, 246, 211–219. [Google Scholar] [CrossRef]
- Sharifimehr, S.; Soltanizadeh, N.; Hossein Goli, S.A. Effects of edible coating containing nano-emulsion of Aloe vera and eugenol on the physicochemical properties of shrimp during cold storage. J. Sci. Food Agric. 2019, 99, 3604–3615. [Google Scholar] [CrossRef] [PubMed]
- Nazari, M.; Majdi, H.; Milani, M.; Abbaspour-Ravasjani, S.; Hamishehkar, H.; Lim, L.-T. Cinnamon nanophytosomes embedded electrospun nanofiber: Its effects on microbial quality and shelf-life of shrimp as a novel packaging. Food Packag. Shelf Life 2019, 21, 100349. [Google Scholar] [CrossRef]
- Homayonpour, P.; Jalali, H.; Shariatifar, N.; Amanlou, M. Effects of nano-chitosan coatings incorporating with free /nano-encapsulated cumin (Cuminum cyminum L.) essential oil on quality characteristics of sardine fillet. Int. J. Food Microbiol. 2021, 341, 109047. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, E.U.; Morey, A. Intelligent Packaging for Poultry Industry. J. Appl. Poult. Res. 2019, 28, 791–800. [Google Scholar] [CrossRef]
- Kazemeini, H.; Azizian, A.; Adib, H. Inhibition of Listeria monocytogenes growth in turkey fillets by alginate edible coating with Trachyspermum ammi essential oil nano-emulsion. Int. J. Food Microbiol. 2021, 344, 109104. [Google Scholar] [CrossRef] [PubMed]
- Lambert, A.D.; Smith, J.P.; Dodds, K.L. Shelf life extension and microbiological safety of fresh meat—A review. Food Microbiol. 1991, 8, 267–297. [Google Scholar] [CrossRef]
- Xavier, L.O.; Sganzerla, W.G.; Rosa, G.B.; da Rosa, C.G.; Agostinetto, L.; Veeck, A.P.d.L.; Bretanha, L.C.; Micke, G.A.; Dalla Costa, M.; Bertoldi, F.C.; et al. Chitosan packaging functionalized with Cinnamodendron dinisii essential oil loaded zein: A proposal for meat conservation. Int. J. Biol. Macromol. 2021, 169, 183–193. [Google Scholar] [CrossRef]
- Amjadi, S.; Nazari, M.; Alizadeh, S.A.; Hamishehkar, H. Multifunctional betanin nanoliposomes-incorporated gelatin/chitosan nanofiber/ZnO nanoparticles nanocomposite film for fresh beef preservation. Meat Sci. 2020, 167, 108161. [Google Scholar] [CrossRef]
- Xiong, Y.; Li, S.; Warner, R.D.; Fang, Z. Effect of oregano essential oil and resveratrol nanoemulsion loaded pectin edible coating on the preservation of pork loin in modified atmosphere packaging. Food Control 2020, 114, 107226. [Google Scholar] [CrossRef]
- Zhang, H.; Liang, Y.; Li, X.; Kang, H. Effect of chitosan-gelatin coating containing nano-encapsulated tarragon essential oil on the preservation of pork slices. Meat Sci. 2020, 166, 108137. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.J.; Maciel, L.C.; Teixeira, J.A.; Vicente, A.A.; Cerqueira, M.A. Use of edible films and coatings in cheese preservation: Opportunities and challenges. Food Res. Int. 2018, 107, 84–92. [Google Scholar] [CrossRef] [Green Version]
- Al-Moghazy, M.; El-sayed, H.S.; Salama, H.H.; Nada, A.A. Edible packaging coating of encapsulated thyme essential oil in liposomal chitosan emulsions to improve the shelf life of Karish cheese. Food Biosci. 2021, 43, 101230. [Google Scholar] [CrossRef]
- Cui, H.; Wu, J.; Li, C.; Lin, L. Improving anti-listeria activity of cheese packaging via nanofiber containing nisin-loaded nanoparticles. LWT-Food Sci. Technol. 2017, 81, 233–242. [Google Scholar] [CrossRef]
- Lei, K.; Wang, X.; Li, X.; Wang, L. The innovative fabrication and applications of carvacrol nanoemulsions, carboxymethyl chitosan microgels and their composite films. Colloids Surf. B Biointerfaces 2019, 175, 688–696. [Google Scholar] [CrossRef]
- Duan, C.; Meng, X.; Meng, J.; Khan, M.I.H.; Dai, L.; Khan, A.; An, X.; Zhang, J.; Huq, T.; Ni, Y. Chitosan as A Preservative for Fruits and Vegetables: A Review on Chemistry and Antimicrobial Properties. J. Bioresour. Bioprod. 2019, 4, 11–21. [Google Scholar] [CrossRef]
- Zambrano-Zaragoza, M.L.; Quintanar-Guerrero, D.; Del Real, A.; Piñon-Segundo, E.; Zambrano-Zaragoza, J.F. The release kinetics of β-carotene nanocapsules/xanthan gum coating and quality changes in fresh-cut melon (cantaloupe). Carbohydr. Polym. 2017, 157, 1874–1882. [Google Scholar] [CrossRef] [PubMed]
- Ansarifar, E.; Moradinezhad, F. Preservation of strawberry fruit quality via the use of active packaging with encapsulated thyme essential oil in zein nanofiber film. Int. J. Food Sci. Technol. 2021, 56, 4239–4247. [Google Scholar] [CrossRef]
- Sharifimehr, S.; Soltanizadeh, N.; Goli, S.A.H. Physicochemical properties of fried shrimp coated with bio-nano-coating containing eugenol and Aloe vera. LWT 2019, 109, 33–39. [Google Scholar] [CrossRef]
- Khan, M.R.; Sadiq, M.B.; Mehmood, Z. Development of edible gelatin composite films enriched with polyphenol loaded nanoemulsions as chicken meat packaging material. CyTA-J. Food 2020, 18, 137–146. [Google Scholar] [CrossRef] [Green Version]
- Manzoor, S.; Gull, A.; Wani, S.M.; Ganaie, T.A.; Masoodi, F.A.; Bashir, K.; Malik, A.R.; Dar, B.N. Improving the shelf life of fresh cut kiwi using nanoemulsion coatings with antioxidant and antimicrobial agents. Food Biosci. 2021, 41, 101015. [Google Scholar] [CrossRef]
- Liu, T.; Liu, L. Fabrication and characterization of chitosan nanoemulsions loading thymol or thyme essential oil for the preservation of refrigerated pork. Int. J. Biol. Macromol. 2020, 162, 1509–1515. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, X.; Kang, H. Chitosan coatings incorporated with free or nano-encapsulated Paulownia Tomentosa essential oil to improve shelf-life of ready-to-cook pork chops. LWT 2019, 116, 108580. [Google Scholar] [CrossRef]
- Chu, Y.; Gao, C.; Liu, X.; Zhang, N.; Xu, T.; Feng, X.; Yang, Y.; Shen, X.; Tang, X. Improvement of storage quality of strawberries by pullulan coatings incorporated with cinnamon essential oil nanoemulsion. LWT 2020, 122, 109054. [Google Scholar] [CrossRef]
- Milani, M.A.; Dana, M.G.; Ghanbarzadeh, B.; Alizadeh, A.; Afshar, P.G. Effect of novel bioactive coating enriched with nanoemulsion of mustard essential oil on the quality of turkey meat. J. Food Nutr. Res. 2020, 59, 71–80. [Google Scholar]
- Pirozzi, A.; Del Grosso, V.; Ferrari, G.; Donsì, F. Edible Coatings Containing Oregano Essential Oil Nanoemulsion for Improving Postharvest Quality and Shelf Life of Tomatoes. Foods 2020, 9, 1605. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhang, M.; Bhandari, B.; Xu, J.; Yang, C. Effects of nanoemulsion-based active coatings with composite mixture of star anise essential oil, polylysine, and nisin on the quality and shelf life of ready-to-eat Yao meat products. Food Control 2020, 107, 106771. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khezerlou, A.; Tavassoli, M.; Alizadeh Sani, M.; Mohammadi, K.; Ehsani, A.; McClements, D.J. Application of Nanotechnology to Improve the Performance of Biodegradable Biopolymer-Based Packaging Materials. Polymers 2021, 13, 4399. https://doi.org/10.3390/polym13244399
Khezerlou A, Tavassoli M, Alizadeh Sani M, Mohammadi K, Ehsani A, McClements DJ. Application of Nanotechnology to Improve the Performance of Biodegradable Biopolymer-Based Packaging Materials. Polymers. 2021; 13(24):4399. https://doi.org/10.3390/polym13244399
Chicago/Turabian StyleKhezerlou, Arezou, Milad Tavassoli, Mahmood Alizadeh Sani, Keyhan Mohammadi, Ali Ehsani, and David Julian McClements. 2021. "Application of Nanotechnology to Improve the Performance of Biodegradable Biopolymer-Based Packaging Materials" Polymers 13, no. 24: 4399. https://doi.org/10.3390/polym13244399
APA StyleKhezerlou, A., Tavassoli, M., Alizadeh Sani, M., Mohammadi, K., Ehsani, A., & McClements, D. J. (2021). Application of Nanotechnology to Improve the Performance of Biodegradable Biopolymer-Based Packaging Materials. Polymers, 13(24), 4399. https://doi.org/10.3390/polym13244399








