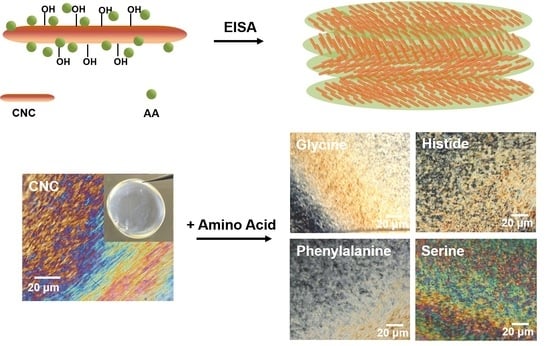
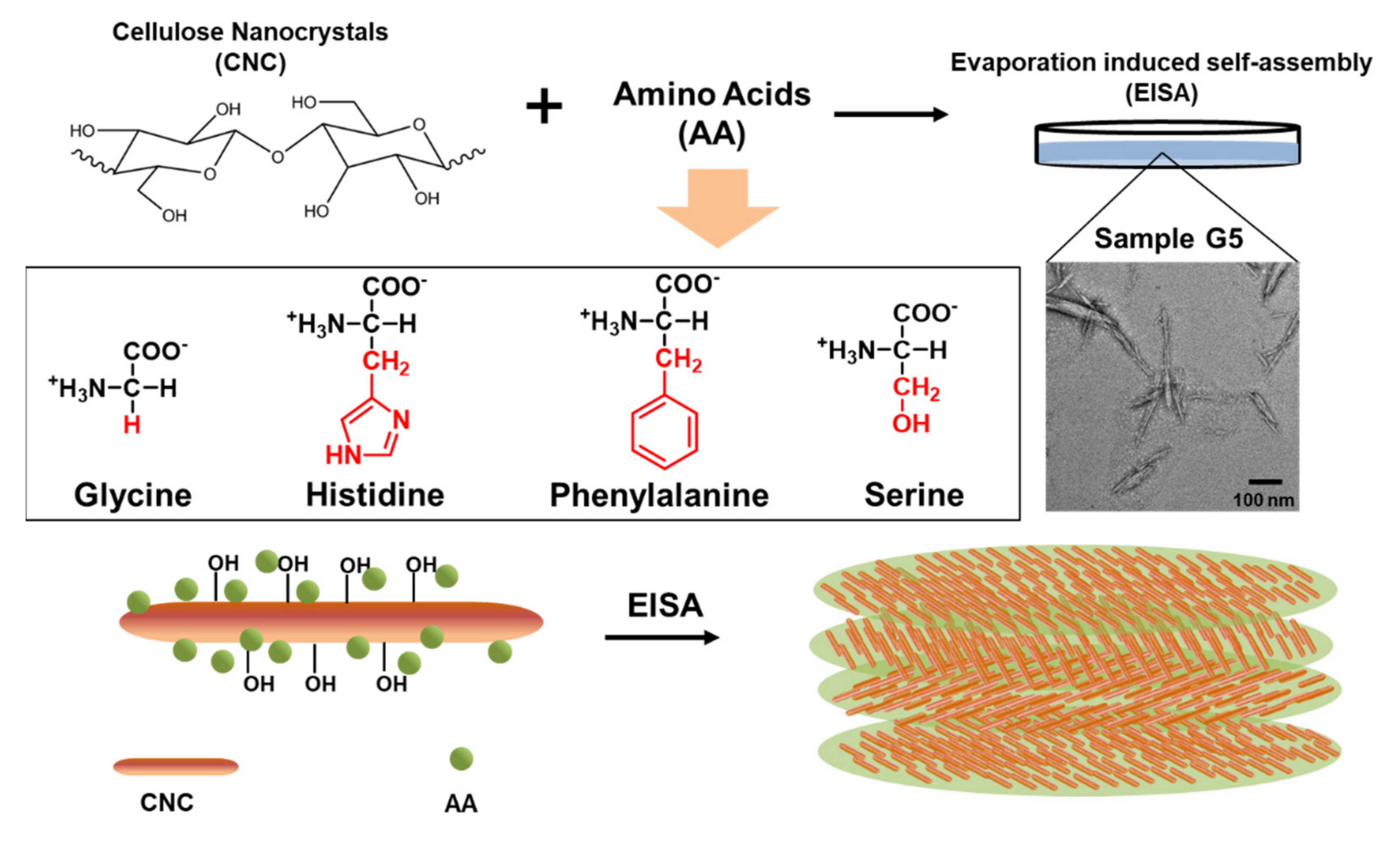
Chiral Nematic Cellulose Nanocrystal Films Cooperated with Amino Acids for Tunable Optical Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Modification and Preparation of CNCs Film
2.3. Characterizations
3. Results and Discussion
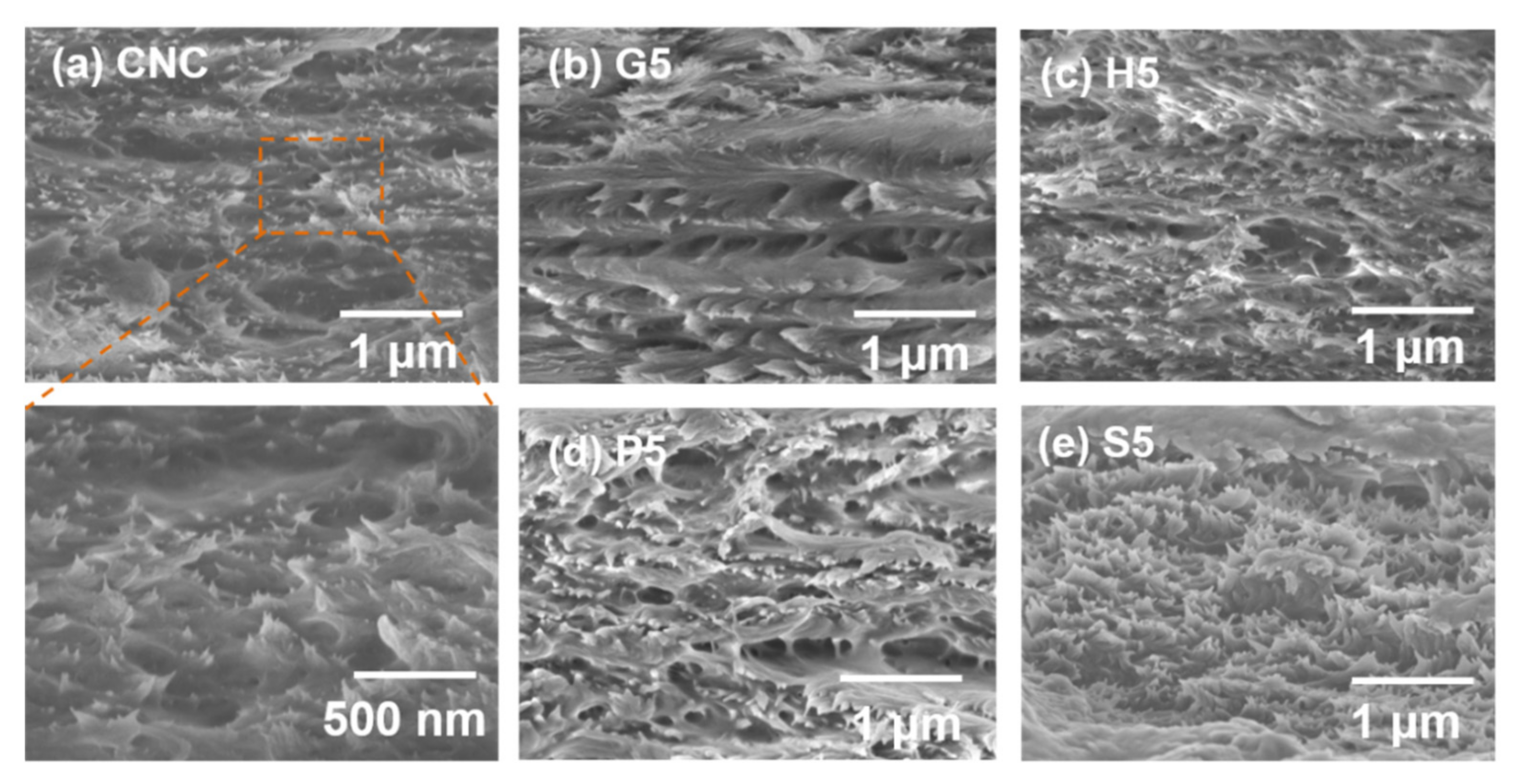
3.1. EISA of the Composite Films
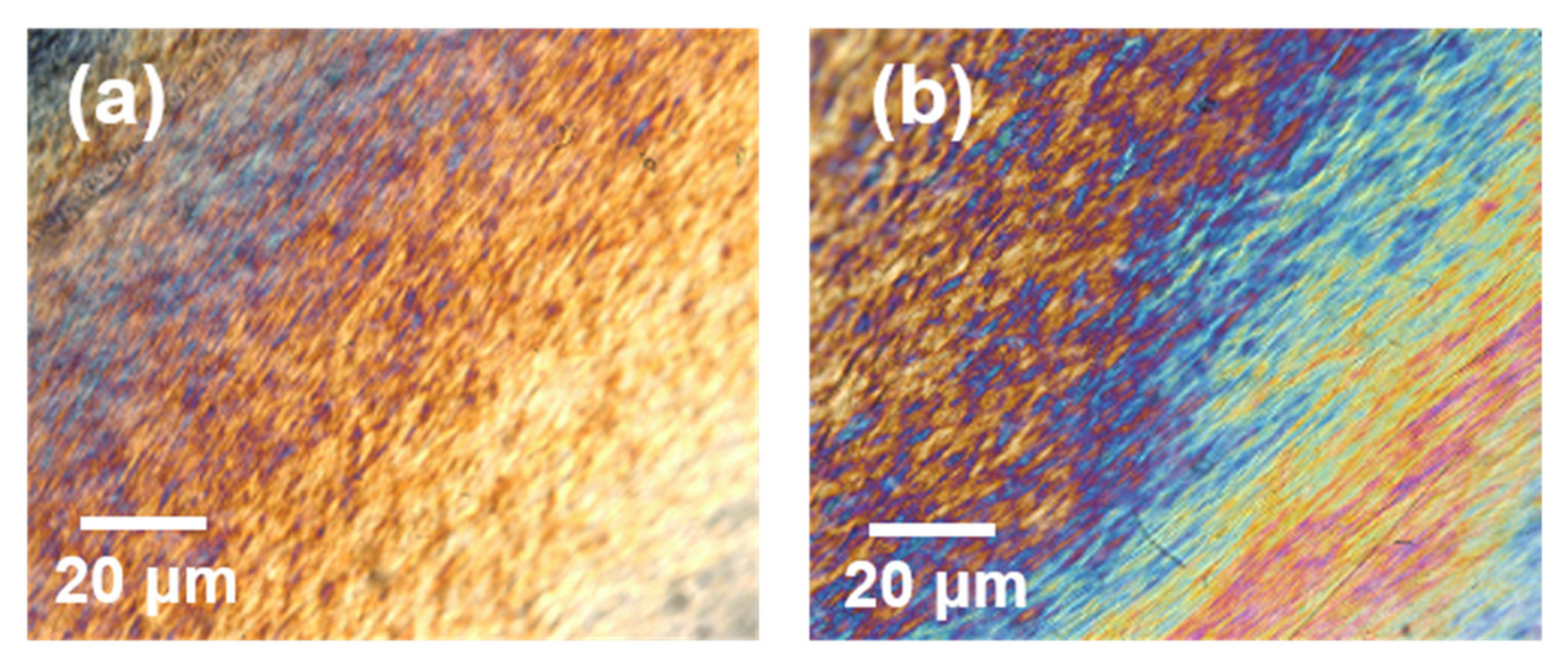
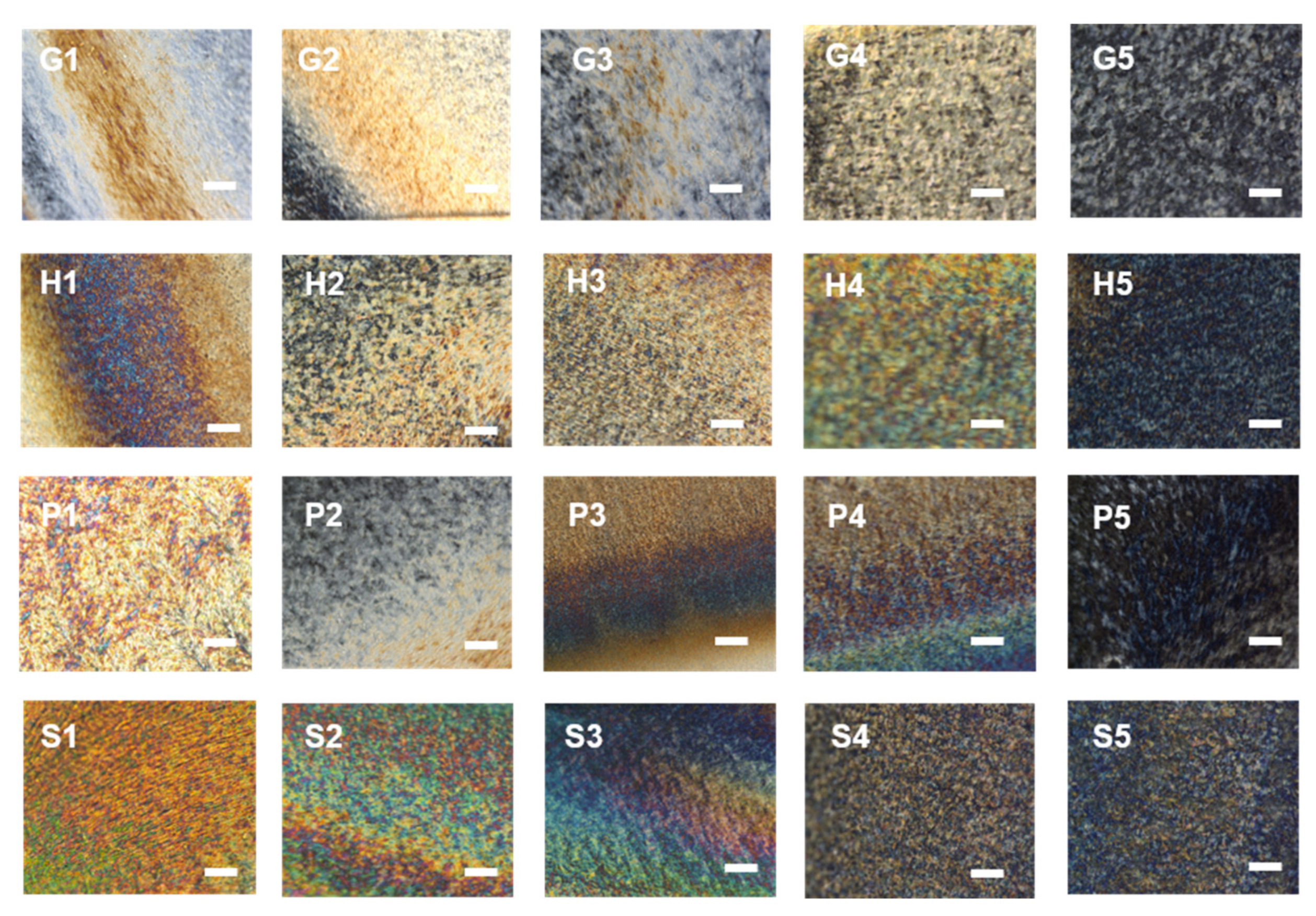
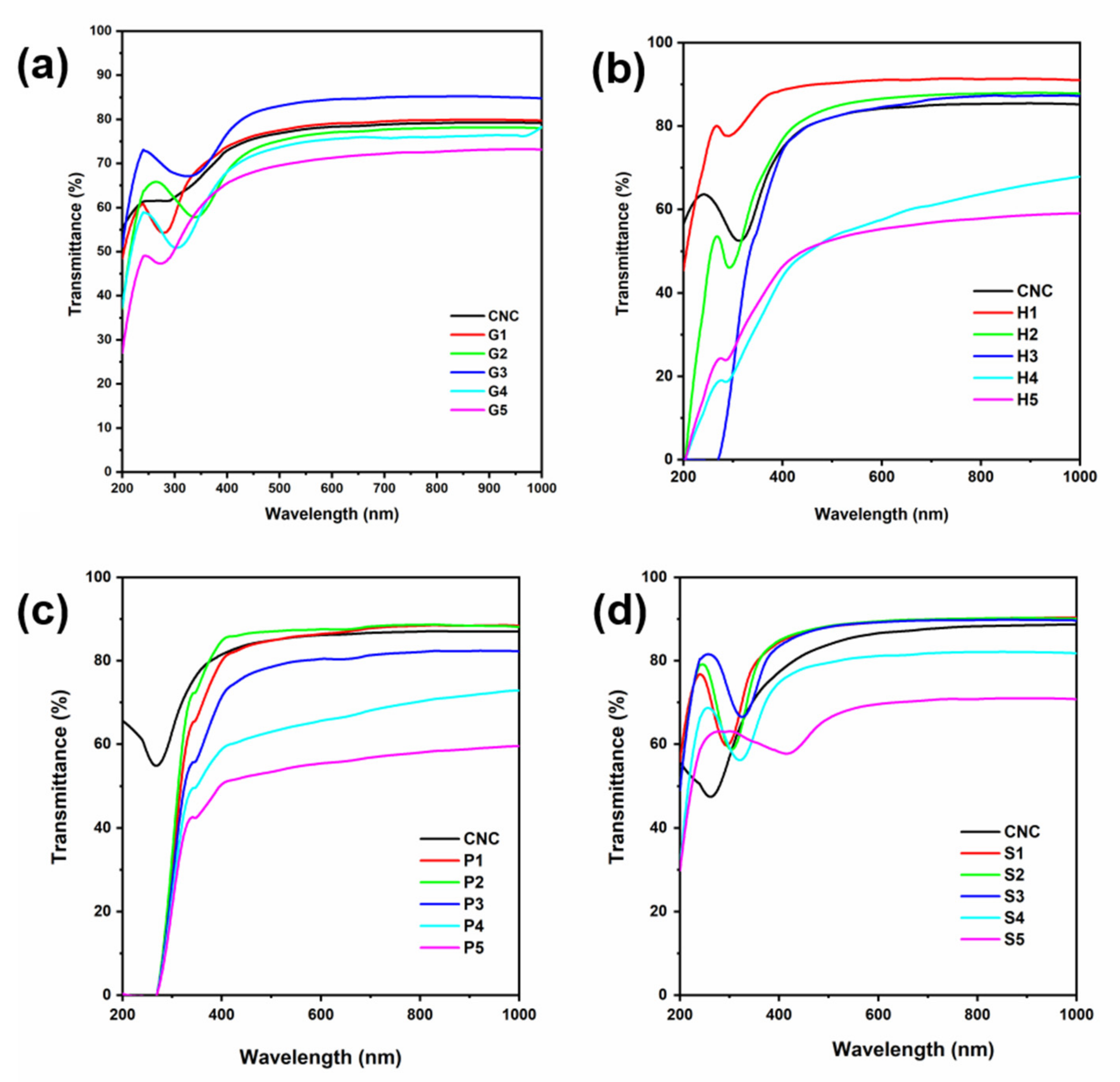
3.2. Optical Properties of the Composite Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Siqueira, G.; Bras, J.; Dufresne, A. Cellulosic bionanocomposites: A review of preparation, properties and applications. Polymers 2010, 2, 728–765. [Google Scholar] [CrossRef] [Green Version]
- Han, X.; Ding, L.; Tian, Z.; Wu, W.; Jiang, S. Extraction and characterization of novel ultrastrong and tough natural cellulosic fiber bundles from manau rattan (Calamus manan). Ind. Crops Prod. 2021, 173, 114103. [Google Scholar] [CrossRef]
- Yu, J.; Zhu, Y.; Ma, H.; Liu, L.; Hu, Y.; Xu, J.; Wang, Z.; Fan, Y. Contribution of hemicellulose to cellulose nanofiber-based nanocomposite films with enhanced strength, flexibility and UV-blocking properties. Cellulose 2019, 26, 6023–6034. [Google Scholar] [CrossRef]
- Kontturi, E.; Laaksonen, P.; Linder, M.B.; Gröschel, A.H.; Rojas, O.J.; Ikkala, O. Advanced Materials through Assembly of Nanocelluloses. Adv. Mater. 2018, 30, 1703779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, J.; Ye, W.; Yu, J.; Fan, Y.; Ono, Y.; Saito, T.; Isogai, A. Chitin nanocrystals prepared by oxidation of α-chitin using the O2/laccase/TEMPO system. Carbohydr. Polym. 2018, 189, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Bian, H.; Chen, L.; Dong, M.; Wang, L.; Wang, R.; Zhou, X.; Wu, C.; Wang, X.; Ji, X.; Dai, H. Natural lignocellulosic nanofibril film with excellent ultraviolet blocking performance and robust environment resistance. Int. J. Biol. Macromol. 2021, 166, 1578–1585. [Google Scholar] [CrossRef] [PubMed]
- Kedzior, S.A.; Marway, H.S.; Cranston, E.D. Tailoring Cellulose Nanocrystal and Surfactant Behavior in Miniemulsion Polymerization. Macromolecules 2017, 50, 2645–2655. [Google Scholar] [CrossRef]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose nanocrystals: Chemistry, self-assembly, and applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef]
- Bian, H.; Gao, Y.; Luo, J.; Jiao, L.; Wu, W.; Fang, G.; Dai, H. Lignocellulosic nanofibrils produced using wheat straw and their pulping solid residue: From agricultural waste to cellulose nanomaterials. Waste Manag. 2019, 91, 1–8. [Google Scholar] [CrossRef]
- Adstedt, K.; Popenov, E.A.; Pierce, K.J.; Xiong, R.; Geryak, R.; Cherpak, V.; Nepal, D.; Bunning, T.J.; Tsukruk, V.V. Chiral Cellulose Nanocrystals with Intercalated Amorphous Polysaccharides for Controlled Iridescence and Enhanced Mechanics. Adv. Funct. Mater. 2020, 30, 2003597. [Google Scholar] [CrossRef]
- Fernandes, S.N.; Almeida, P.L.; Monge, N.; Aguirre, L.E.; Reis, D.; de Oliveira, C.L.P.; Neto, A.M.F.; Pieranski, P.; Godinho, M.H. Mind the Microgap in Iridescent Cellulose Nanocrystal Films. Adv. Mater. 2017, 29, 1603560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelly, J.A.; Giese, M.; Shopsowitz, K.E.; Hamad, W.Y.; MacLachlan, M.J. The development of chiral nematic mesoporous materials. Acc. Chem. Res. 2014, 47, 1088–1096. [Google Scholar] [CrossRef]
- Frka-Petesic, B.; Guidetti, G.; Kamita, G.; Vignolini, S. Controlling the Photonic Properties of Cholesteric Cellulose Nanocrystal Films with Magnets. Adv. Mater. 2017, 29, 1701469. [Google Scholar] [CrossRef] [PubMed]
- Vollick, B.; Kuo, P.Y.; Thérien-Aubin, H.; Yan, N.; Kumacheva, E. Composite Cholesteric Nanocellulose Films with Enhanced Mechanical Properties. Chem. Mater. 2017, 29, 789–795. [Google Scholar] [CrossRef]
- Droguet, B.E.; Liang, H.; Frka-Petesic, B.; Parker, R.M.; Volder, M.F.L.; Baumberg, J.J.; Vignolini, S. Large-scale fabrication of structurally coloured cellulose nanocrystal films and effect pigments. Nat. Mater. 2021. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhang, Z.; Xue, J.; Wang, X.; Song, F.; Wang, X.; Zhu, L.; Wang, Y. Biomimetic Optical Cellulose Nanocrystal Films with Controllable Iridescent Color and Environmental Stimuli-Responsive Chromism. ACS Appl. Mater. Interfaces 2018, 10, 5805–5811. [Google Scholar] [CrossRef]
- Yao, K.; Meng, Q.; Bulone, V.; Zhou, Q. Flexible and responsive chiral nematic cellulose nanocrystal/poly (ethylene glycol) composite films with uniform and tunable structural color. Adv. Mater. 2017, 29, 1701323. [Google Scholar] [CrossRef]
- Wang, B.; Walther, A. Self-Assembled, Iridescent, Crustacean-Mimetic Nanocomposites with Tailored Periodicity and Layered Cuticular Structure. ACS Nano 2015, 9, 10637–10646. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, D.; Qin, H.; Feng, L.; Liang, X.; Qing, G. Chemoselectivity of Pristine Cellulose Nanocrystal Films Driven by Carbohydrate–Carbohydrate Interactions. ACS Appl. Mater. Interfaces 2019, 11, 13114–13122. [Google Scholar] [CrossRef]
- Meng, Y.; Long, Z.; He, Z.; Fu, X.; Dong, C. Chiral Cellulose Nanocrystal Humidity-Responsive Iridescent Films with Glucan for Tuned Iridescence and Reinforced Mechanics. Biomacromolecules 2021, 22, 4479–4488. [Google Scholar] [CrossRef]
- Moreau, C.; Villares, A.; Capron, I.; Cathala, B. Tuning supramolecular interactions of cellulose nanocrystals to design innovative functional materials. Ind. Crops Prod. 2015, 93, 96–107. [Google Scholar] [CrossRef]
- Xu, K.; Zhang, Y.; Ye, Q.; Wu, J.; Li, Q.; Su, G.; Harper, D.P. Natural cuticle-inspired chitin/silk fibroin/cellulose nanocrystal biocomposite films: Fabrication and characterization. Mater. Res. Express 2021, 8, 036402. [Google Scholar] [CrossRef]
- Gu, Z.; Lu, M.; Feng, K.; Jin, Z. The different composites of cellulose nanocrystals with d- or l-histidine. Nanoscale 2021, 13, 8174–8180. [Google Scholar] [CrossRef] [PubMed]
- Bast, L.K.; Klockars, K.W.; Greca, L.G.; Rojas, O.J.; Tardy, B.L.; Bruns, N. Infiltration of proteins in cholesteric cellulose structures. Biomacromolecules 2021, 22, 2067–2080. [Google Scholar] [CrossRef]
- Jiang, F.; Hsieh, Y. Lo Chemically and mechanically isolated nanocellulose and their self-assembled structures. Carbohydr. Polym. 2013, 95, 32–40. [Google Scholar] [CrossRef]
- Litowski, J.R.; Semchuk, P.D.; Mant, C.T.; Hodges, R.S. Hydrophilic interaction/cation-exchange chromatography for the purification of synthetic peptides from closely related impurities: Serine side-chain acetylated peptides. J. Pept. Res. 1999, 54, 1–11. [Google Scholar] [CrossRef]
- Gençer, A.; Schütz, C.; Thielemans, W. Influence of the particle concentration and marangoni flow on the formation of cellulose nanocrystal films. Langmuir 2017, 33, 228–234. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.; Li, Y.; Li, B.; Yang, Y. Control of the Handedness of Self-assemblies of Dipeptides by the Chirality of Phenylalanine and Steric Hindrance of Phenylglycine. Langmuir 2016, 32, 7420–7426. [Google Scholar] [CrossRef]
- Tran, A.; Hamad, W.Y.; MacLachlan, M.J. Tactoid Annealing Improves Order in Self-Assembled Cellulose Nanocrystal Films with Chiral Nematic Structures. Langmuir 2018, 34, 646–652. [Google Scholar] [CrossRef]
- Honorato-Rios, C.; Kuhnhold, A.; Bruckner, J.R.; Dannert, R.; Schilling, T.; Lagerwall, J.P.F. Equilibrium Liquid Crystal Phase Diagrams and Detection of Kinetic Arrest in Cellulose Nanocrystal Suspensions. Front. Mater. 2016, 3, 21. [Google Scholar] [CrossRef] [Green Version]
- Ling, Z.; Wang, K.; Liu, W.; Tang, W.; Yong, Q. Tuning cellulose nanocrystals alignments for supramolecular assembly of chiral nematic films with highly efficient UVB shielding capability. J. Mater. Chem. C 2020, 8, 8493–8501. [Google Scholar] [CrossRef]
- De France, K.J.; Kummer, N.; Ren, Q.; Campioni, S.; Nyström, G. Assembly of Cellulose Nanocrystal–Lysozyme Composite Films with Varied Lysozyme Morphology. Biomacromolecules 2020, 21, 5139–5147. [Google Scholar] [CrossRef] [PubMed]
- Parker, R.M.; Guidetti, G.; Williams, C.A.; Zhao, T.; Narkevicius, A.; Vignolini, S.; Frka-Petesic, B. The Self-Assembly of Cellulose Nanocrystals: Hierarchical Design of Visual Appearance. Adv. Mater. 2018, 30, 1704477. [Google Scholar] [CrossRef]
- Lou, Z.; Wang, Q.; Kara, U.; Mamtani, R.; Zhou, X.; Bian, H.; Yang, Z.H.; Li, Y.J.; Lv, H.L.; Adera, S.; et al. Biomass-derived carbon heterostructures enable environmentally adaptive wideband electromagnetic wave absorbers. Nano-Macro Lett. 2021. [Google Scholar] [CrossRef]
- Tardy, B.L.; Mattos, B.D.; Greca, L.G.; Kämäräinen, T.; Klockars, K.W.; Rojas, O.J. Tessellation of Chiral-Nematic Cellulose Nanocrystal Films by Microtemplating. Adv. Funct. Mater. 2019, 29, 1808518. [Google Scholar] [CrossRef]
- Bardet, R.; Belgacem, N.; Bras, J. Flexibility and Color Monitoring of Cellulose Nanocrystal Iridescent Solid Films Using Anionic or Neutral Polymers. ACS Appl. Mater. Interfaces 2015, 7, 4010–4018. [Google Scholar] [CrossRef]
- Niu, X.; Liu, Y.; Fang, G.; Huang, C.; Rojas, O.J.; Pan, H. Highly Transparent, Strong, and Flexible Films with Modified Cellulose Nanofiber Bearing UV Shielding Property. Biomacromolecules 2018, 19, 4565–4575. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, T.; Tao, Y.; Ling, Z.; Huang, C.; Lai, C.; Yong, Q. Fabrication of anti-bacterial, hydrophobic and UV resistant galactomannan-zinc oxide nanocomposite films. Polymer 2021, 215, 123412. [Google Scholar] [CrossRef]
- Ling, Z.; Liu, W.; Ren, Y.; Chen, H.; Huang, C.; Lai, C.; Yong, Q. Bioinspired manufacturing of oriented polysaccharides scaffolds for strong, optical haze and anti-UV/bacterial membranes. Carbohydr. Polym. 2021, 270, 118328. [Google Scholar] [CrossRef] [PubMed]
- Fleming, S.; Frederix, P.W.J.M.; Ramos Sasselli, I.; Hunt, N.T.; Ulijn, R.V.; Tuttle, T. Assessing the Utility of Infrared Spectroscopy as a Structural Diagnostic Tool for β-Sheets in Self-Assembling Aromatic Peptide Amphiphiles. Langmuir 2013, 29, 9510–9515. [Google Scholar] [CrossRef]
- French, A.D. Idealized powder diffraction patterns for cellulose polymorphs. Cellulose 2014, 21, 885–896. [Google Scholar] [CrossRef]
- French, A.D.; Cintrón, M.S. Cellulose polymorphy, crystallite size, and the Segal Crystallinity Index. Cellulose 2013, 20, 583–588. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, X.; Chen, J.; Ling, Z.; Guo, J.; Huang, J.; Ma, J.; Jin, Z. Chiral Nematic Cellulose Nanocrystal Films Cooperated with Amino Acids for Tunable Optical Properties. Polymers 2021, 13, 4389. https://doi.org/10.3390/polym13244389
Xiao X, Chen J, Ling Z, Guo J, Huang J, Ma J, Jin Z. Chiral Nematic Cellulose Nanocrystal Films Cooperated with Amino Acids for Tunable Optical Properties. Polymers. 2021; 13(24):4389. https://doi.org/10.3390/polym13244389
Chicago/Turabian StyleXiao, Xiao, Jie Chen, Zhe Ling, Jiaqi Guo, Jianbin Huang, Jianfeng Ma, and Zhi Jin. 2021. "Chiral Nematic Cellulose Nanocrystal Films Cooperated with Amino Acids for Tunable Optical Properties" Polymers 13, no. 24: 4389. https://doi.org/10.3390/polym13244389
APA StyleXiao, X., Chen, J., Ling, Z., Guo, J., Huang, J., Ma, J., & Jin, Z. (2021). Chiral Nematic Cellulose Nanocrystal Films Cooperated with Amino Acids for Tunable Optical Properties. Polymers, 13(24), 4389. https://doi.org/10.3390/polym13244389