Molecular Weight Distribution and Dissolution Behavior of Lignin in Alkaline Solutions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Feedstock and Chemicals
2.2. Preparation of Alkali Lignin
2.3. Products Separation
2.4. Product Analysis
2.4.1. Composition Analysis of Raw Materials
2.4.2. Analysis of FT-IR
2.4.3. Analysis of GPC
2.4.4. Analysis of SEM
2.4.5. Analysis of NMR
3. Results
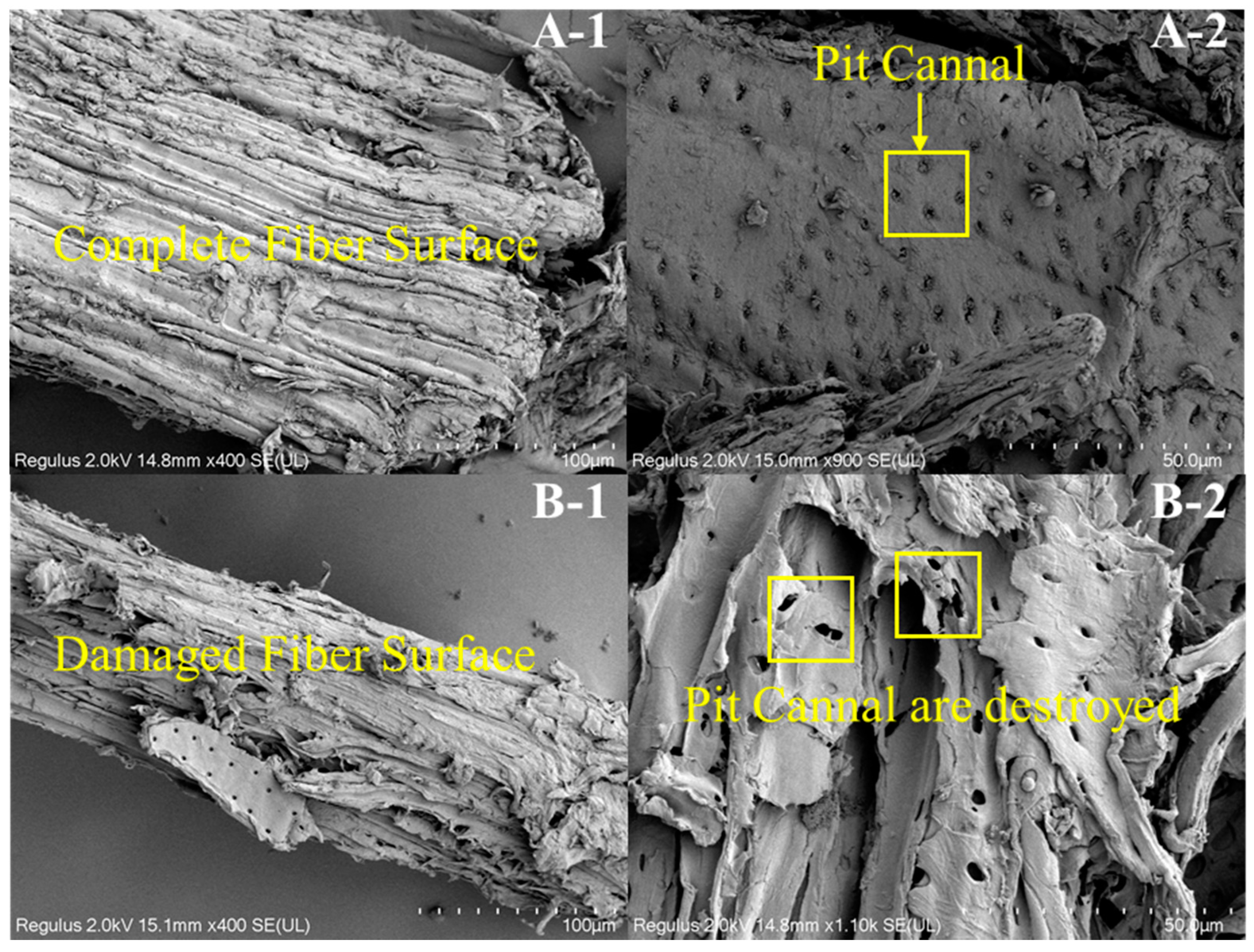
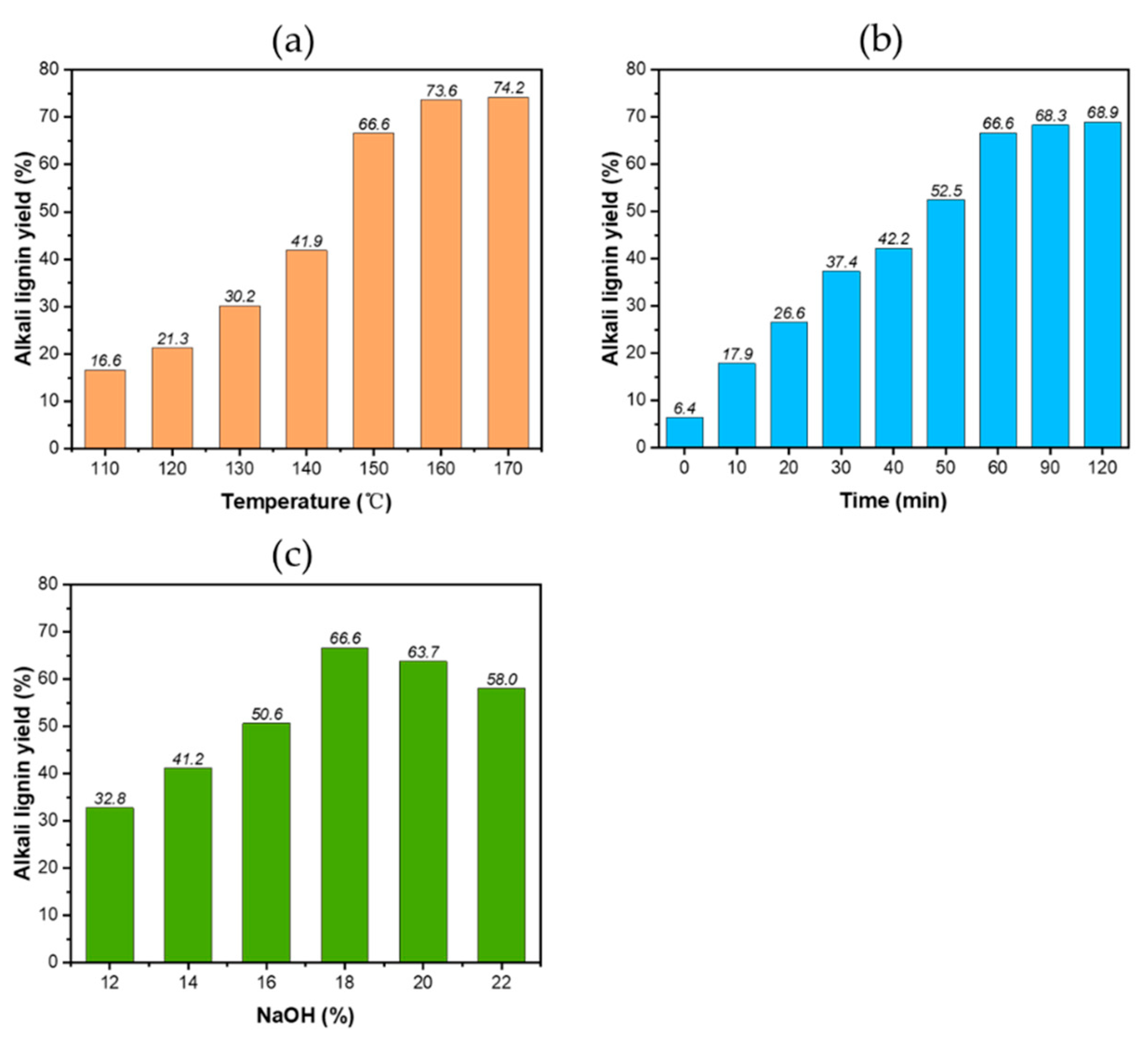
3.1. The Morphologies and Yields of the Residual Lignin and Pulp
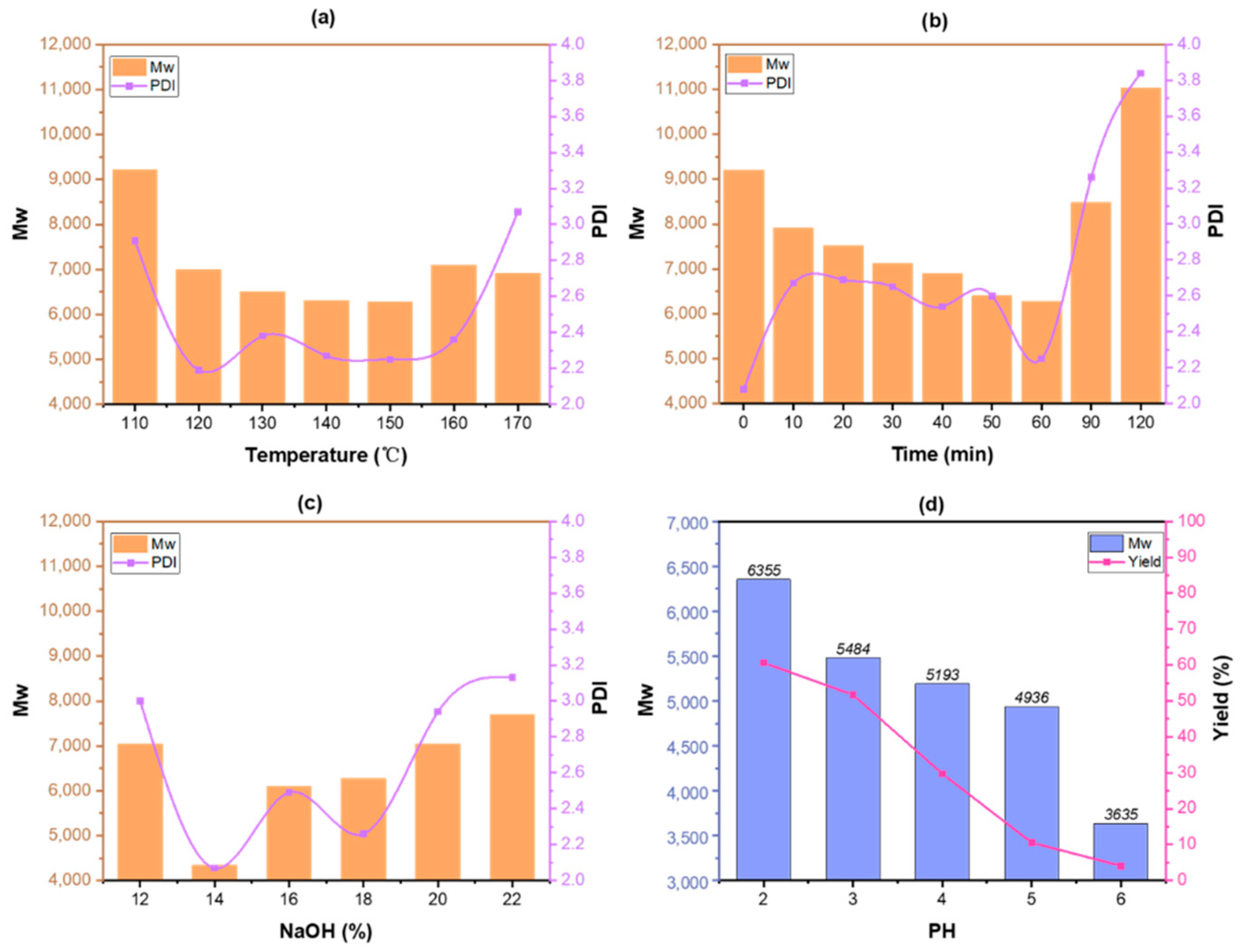
3.2. The Molecular Weight Distribution of Lignin
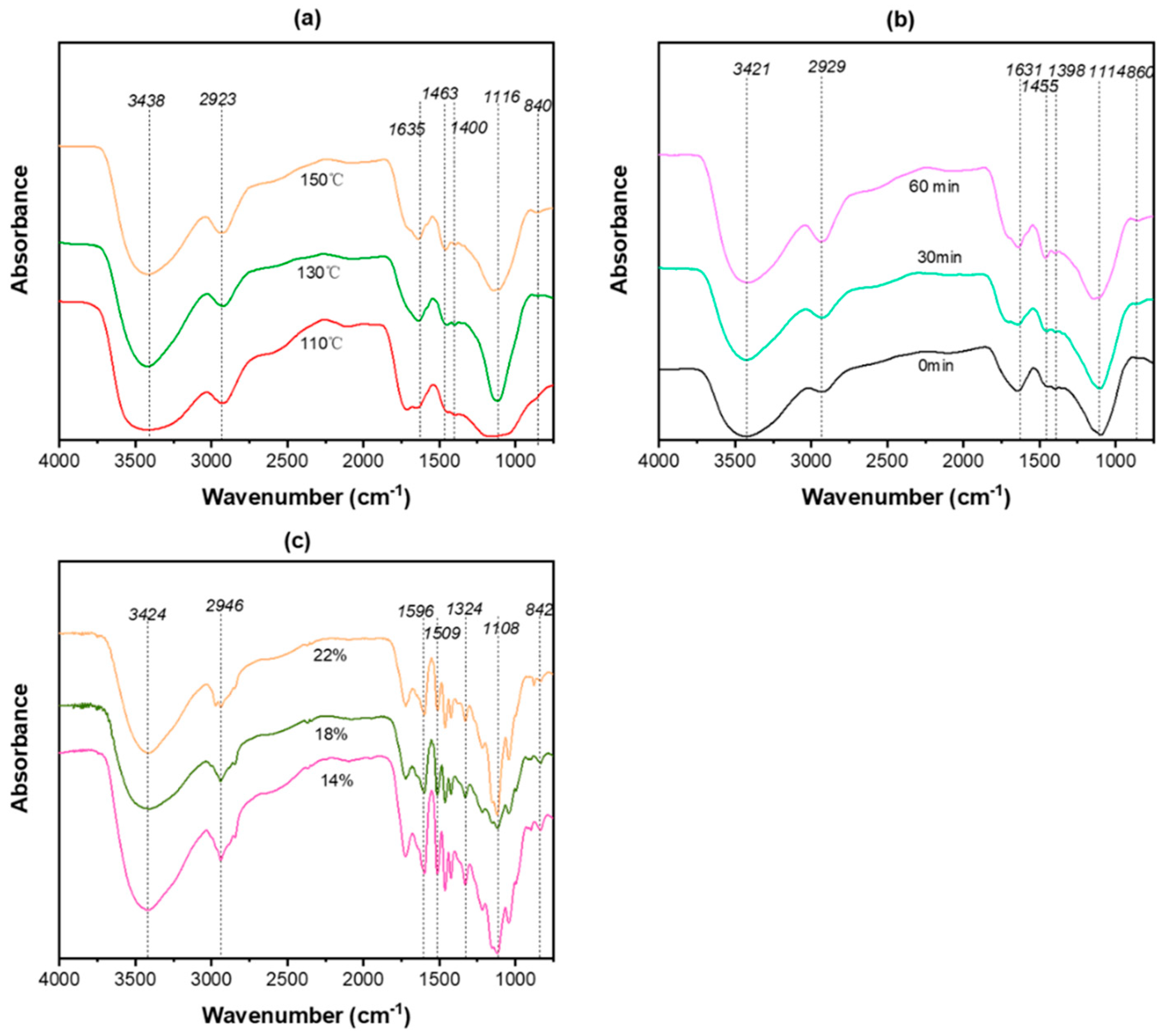
3.3. The Structural Change of Lignin
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kamm, B.; Gruber, P.R.; Kamm, M. Chapter 1. Biorefinery Systems—An Overview. In Biorefineries-Industrial Processes and Products: Status Quo and Future Directions; Wiley Online Library: Hoboken, NJ, USA, 2008. [Google Scholar]
- Vanholme, R.; Demedts, B.B.; Morreel, K.; Ralph, J.; Boerjan, W. Lignin Biosynthesis and Structure. Plant Physiol. 2010, 153, 895–905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Z.; Fridrich, B.; Santi, A.D.; Elangovan, S.; Barta, K. Bright Side of Lignin Depolymerization: Toward New Platform Chemicals. Chem. Rev. 2018, 118, 614–678. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Wang, Y.; Wang, J.; Zhou, J.; Zhang, S. Decomposition of benzyl phenyl ether over char-supported Ni: The effect of char structures. Fuel Process. Technol. 2021, 221, 106941. [Google Scholar] [CrossRef]
- Li, N.; Bian, H.; Zhu, J.Y.; Ciesielski, P.N.; Pan, X. Tailorable cellulose II nanocrystals (CNC II) prepared in mildly acidic lithium bromide trihydrate (MALBTH). Green Chem. 2021, 23, 2778–2791. [Google Scholar] [CrossRef]
- Norgren, M.; Edlund, H. Lignin: Recent advances and emerging applications. Curr. Opin. Colloid Interface Sci. 2014, 19, 409–416. [Google Scholar] [CrossRef]
- Olsén, P.; Jawerth, M.E.; Lawoko, M.; Johansson, M.; Berglund, L.A. Transforming technical lignins to structurally defined star-copolymers under ambient conditions. Green Chem. 2019, 21, 2478–2486. [Google Scholar] [CrossRef]
- Rossi, L.M.; Costa, N.; Silva, F.P.; Wojcieszak, R. Magnetic nanomaterials in catalysis: Advanced catalysts for magnetic separation and beyond. Green Chem. 2014, 16, 2906–2933. [Google Scholar] [CrossRef]
- Xu, T.T.; Gu, X.H.; Li, L.Z.; Pan, J.M.; Liu, Q.C.; Zhang, H.H.; Dai, H.Q. Study on the Biocompatibility and Antibacterial Properties of Lignin Sulfonate/Polyacrylamide/Carboxymethyl Cellulose Sodium Hydrogel. Basic Clin. Pharmacol. Toxicol. 2020, 127, 16. [Google Scholar]
- Zhang, Z.; Song, J.; Han, B. Catalytic Transformation of Lignocellulose into Chemicals and Fuel Products in Ionic Liquids. Chem. Rev. 2016, 117, 6834–6880. [Google Scholar] [CrossRef]
- Zakzeski, J.; Bruijnincx, P.; Jongerius, A.L.; Weckhuysen, B.M. The catalytic valorization of lignin for the production of renewable chemicals. Chem. Rev. 2013, 110, 3552–3599. [Google Scholar] [CrossRef]
- Vanholme, R.; Meester, B.D.; Ralph, J.; Boerjan, W. Lignin biosynthesis and its integration into metabolism. Curr. Opin. Biotechnol. 2019, 56, 230–239. [Google Scholar] [CrossRef]
- Ma, Y.; Du, Z.; Liu, J.; Xia, F.; Xu, J. Selective oxidative C–C bond cleavage of a lignin model compound in the presence of acetic acid with a vanadium catalyst. Green Chem. 2015, 17, 4968–4973. [Google Scholar] [CrossRef]
- Li, J.; Liu, Z.; Feng, C.; Liu, X.; Qin, F.; Liang, C.; Bian, H.; Qin, C.; Yao, S. Green, efficient extraction of bamboo hemicellulose using freeze-thaw assisted alkali treatment. Bioresour. Technol. 2021, 333, 125107. [Google Scholar] [CrossRef]
- Hanson, S.K.; Wu, R.; Silks, L.A.P. C-C or C-O Bond Cleavage in a Phenolic Lignin Model Compound: Selectivity Depends on Vanadium Catalyst. Angew. Chem. Int. Ed. 2012, 51, 3410–3413. [Google Scholar] [CrossRef]
- Brown, M.E.; Walker, M.C.; Nakashige, T.G.; Iavarone, A.T.; Chang, M. Discovery and Characterization of Heme Enzymes from Unsequenced Bacteria: Application to Microbial Lignin Degradation. J. Am. Chem. Soc. 2011, 133, 18006–18009. [Google Scholar] [CrossRef]
- Kumar, M.; Morya, R.; Gupta, A.; Kumar, V.; Thakur, I.S. Bacterial-Mediated Depolymerization and Degradation of Lignin. In Environmental Microbiology and Biotechnology; Springer: Singapore, 2021. [Google Scholar]
- Ramos-Corona, A.; Rangel, R.; Espino, J.; Nuez, R.; Alvarado-Gil, J.J. High-yield of Lignin degradation under N-ZnO/Graphene Oxide compounds. Catal. Today 2021. [Google Scholar] [CrossRef]
- Feghali, E.; Jacquet, O.; Thuéry, P.; Cantat, T. Catalytic hydrosilylation of oxalic acid: Chemoselective formation of functionalizedC2-products. Catal. Sci. Technol. 2014, 4, 2230–2234. [Google Scholar] [CrossRef]
- Li, C.; Zheng, M.; Wang, A.; Zhang, T. One-pot catalytic hydrocracking of raw woody biomass into chemicals over supported carbide catalysts: Simultaneous conversion of cellulose, hemicellulose and lignin. Energy Environ. Sci. 2012, 5, 6383–6390. [Google Scholar] [CrossRef]
- Molinari, V.; Giordano, C.; Antonietti, M.; Esposito, D. Titanium nitride-nickel nanocomposite as heterogeneous catalyst for the hydrogenolysis of aryl ethers. J. Am. Chem. Soc. 2014, 136, 1758–1761. [Google Scholar] [CrossRef]
- Yw, A.; Zl, A.; Xun, Z.B.; Xun, H.C.; Mg, D.; Hs, E.; Yong, H.A.; Shu, Z.A.; Hong, Z.A. Hydrogenolysis of lignin to phenolic monomers over Ru based catalysts with different metal-support interactions: Effect of partial hydrogenation of C(sp 2 )-O/C. Fuel 2021, 302, 121184. [Google Scholar]
- Sl, A.; Gang, W.A.; Sh, B.; Bl, C.; Xun, H.D.; Jz, A.; Yong, H.A.; Shu, Z.A.; Hong, Z.A. Catalytic pyrolysis of pine wood over char-supported Fe: Bio-oil upgrading and catalyst regeneration by CO2 /H2O. Fuel 2021, 307, 121778. [Google Scholar]
- Qi, S.C.; Zhang, L.; Kudo, S.; Hayashi, J.I. Correction: Catalytic hydrogenolysis of kraft lignin to monomers at high yield in alkaline water. Green Chem. 2017, 19, 2636–2645. [Google Scholar] [CrossRef]
- Ding, N.; Liu, H.; Sun, Y.; Tang, X.; Lin, L. Lignin Degradation in Cooking with Active Oxygen and Solid Alkali process: A mechanism study. J. Clean. Prod. 2020, 278, 123984. [Google Scholar] [CrossRef]
- Cc, A.; Ms, B.; Wq, A. Lignin utilization: A review of lignin depolymerization from various aspects—ScienceDirect. Renew. Sustain. Energy Rev. 2019, 107, 232–249. [Google Scholar]
- Yan, R.; Shen, F.; Jiang, W.; Zhang, Y.; Li, Z.; Zhang, X.; Deng, S.; Lin, L. Enhanced Enzymatic Hydrolysis of Palm Pressed Fiber Based on the Three Main Components: Cellulose, Hemicellulose, and Lignin. Appl. Biochem. Biotechnol. Part A Enzym. Eng. Biotechnol. 2014, 173, 409–420. [Google Scholar]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templaton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass; Technical Report NREL/TP-510-42618; National Renewable Energy Laboratory: Golden, CO, USA, 2010. [Google Scholar]
- Wen, J.L.; Sun, S.L.; Xue, B.L.; Sun, R.C. Recent Advances in Characterization of Lignin Polymer by Solution-State Nuclear Magnetic Resonance (NMR) Methodology. Materials 2013, 6, 359–391. [Google Scholar] [CrossRef] [Green Version]
- Zeller, W. Estimation of procyanidin/prodelphinidin and cis/trans flavanol ratios of condensed tannin fractions by 1H-13C HSQC NMR spectroscopy: Correlation with thiolysis. Zhonghua Wai Ke Za Zhi Chin. J. Surg. 2009, 47, 857. [Google Scholar]
- Rencoret, J.; Marques, G.; Gutierrez, A.; Nieto, L.; Santos, J.I.; Jimenez-Barbero, J.; Martinez, A.T.; Rio, J.C.D. HSQC-NMR analysis of lignin in woody (Eucalyptus globulus and Picea abies) and non-woody (Agave sisalana) ball-milled plant materials at the gel state 10th EWLP, Stockholm, Sweden, August 25–28, 2008. Holzforschung 2009, 63, 691–698. [Google Scholar] [CrossRef]
- Kishimoto, T.; Uraki, Y.; Ubukata, M. Synthesis of beta-O-4-type artificial lignin polymers and their analysis by NMR spectroscopy. Org. Biomol. Chem. 2008, 6, 2982–2987. [Google Scholar] [CrossRef]




| Material | Holocellulose (%) | Acid-Insoluble Lignin (%) | Acid Soluble Lignin (%) | Ash Content (%) |
|---|---|---|---|---|
| Eucalyptus | 71.54 | 19.60 | 4.71 | 0.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Sun, M.; Jiao, L.; Dai, H. Molecular Weight Distribution and Dissolution Behavior of Lignin in Alkaline Solutions. Polymers 2021, 13, 4166. https://doi.org/10.3390/polym13234166
Yang J, Sun M, Jiao L, Dai H. Molecular Weight Distribution and Dissolution Behavior of Lignin in Alkaline Solutions. Polymers. 2021; 13(23):4166. https://doi.org/10.3390/polym13234166
Chicago/Turabian StyleYang, Jie, Mengya Sun, Liang Jiao, and Hongqi Dai. 2021. "Molecular Weight Distribution and Dissolution Behavior of Lignin in Alkaline Solutions" Polymers 13, no. 23: 4166. https://doi.org/10.3390/polym13234166
APA StyleYang, J., Sun, M., Jiao, L., & Dai, H. (2021). Molecular Weight Distribution and Dissolution Behavior of Lignin in Alkaline Solutions. Polymers, 13(23), 4166. https://doi.org/10.3390/polym13234166








