Determination of the Thermodynamic Parameters of the Pyrolysis Process of Post-Consumption Thermoplastics by Non-Isothermal Thermogravimetric Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Physicochemical Characterization of the Waste
2.3. Thermogravimetry of Thermoplastics
2.4. TGA Data Processing
2.4.1. Kinetic Models of Thermoplastics
2.4.2. Kinetic Model 1: Friedman Method (FR)
2.4.3. Kinetic Model 2: Kissinger–Akahira–Sunose (KAS) Method
2.4.4. Kinetic Model 3: Flynn–Wall–Ozawa (FWO) Method
2.4.5. Reaction Model
2.5. Validation and Tuning of the Models
Thermodynamic Parameters
3. Results
3.1. Characterization of Samples
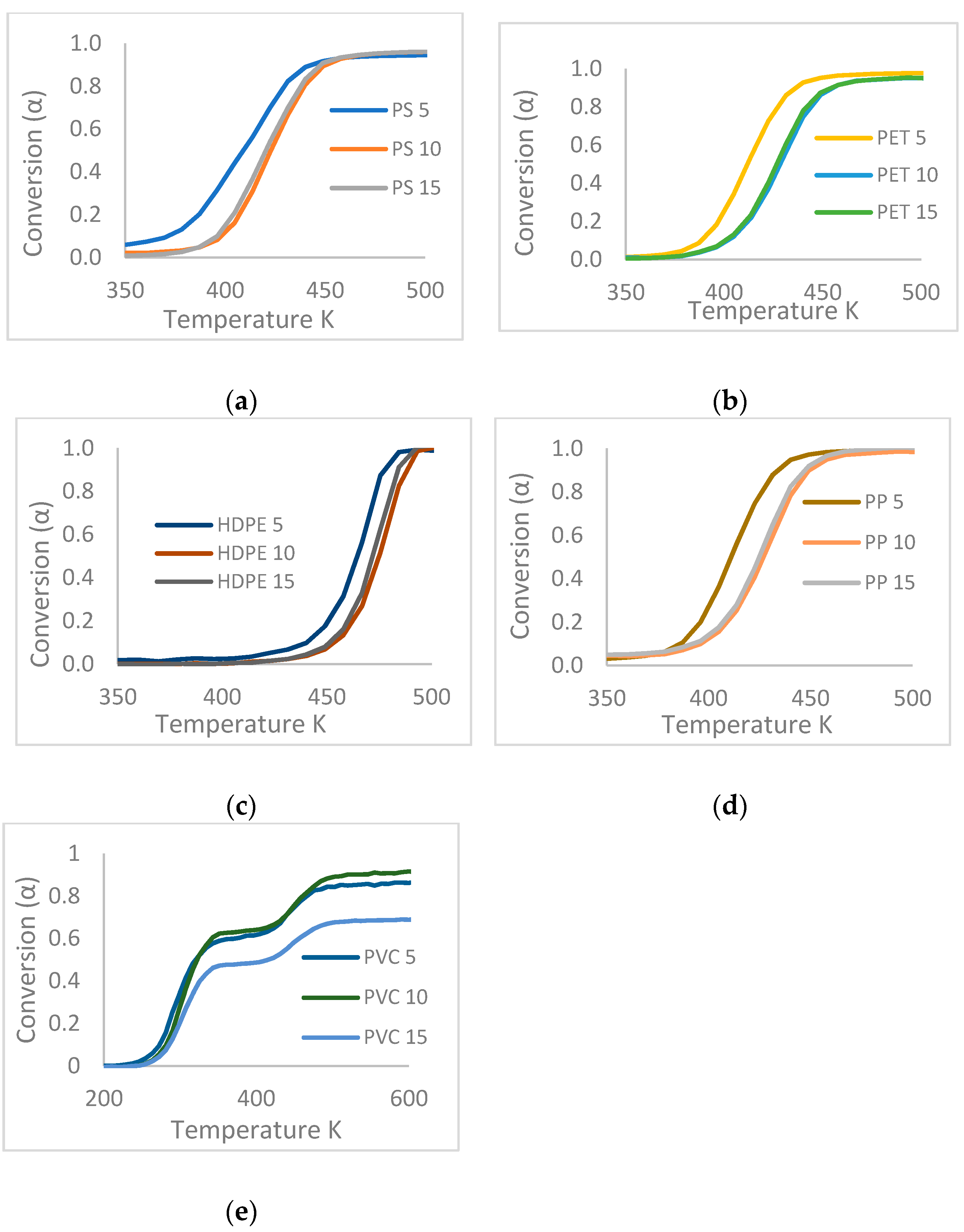
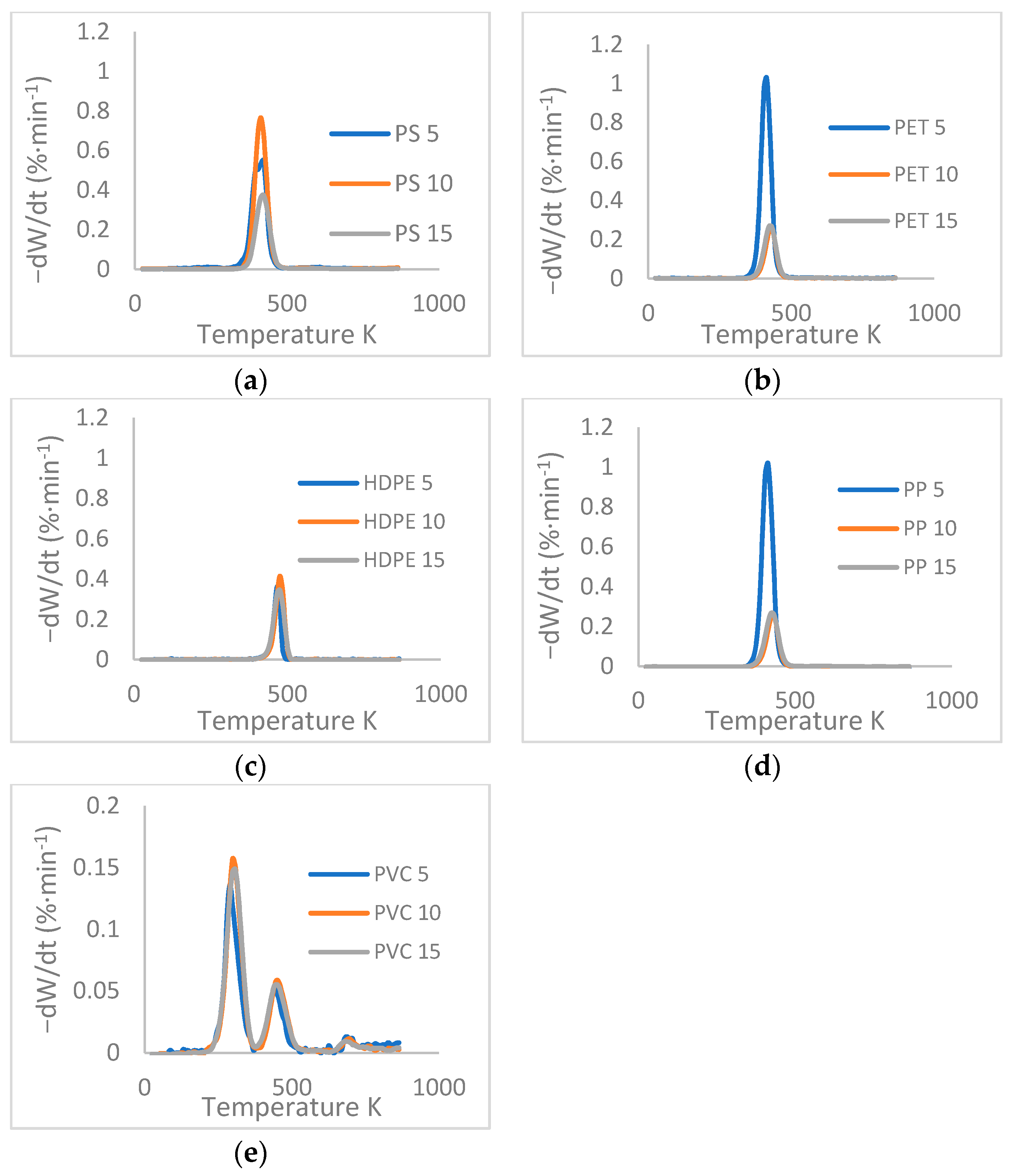
3.2. TGA Data Processing
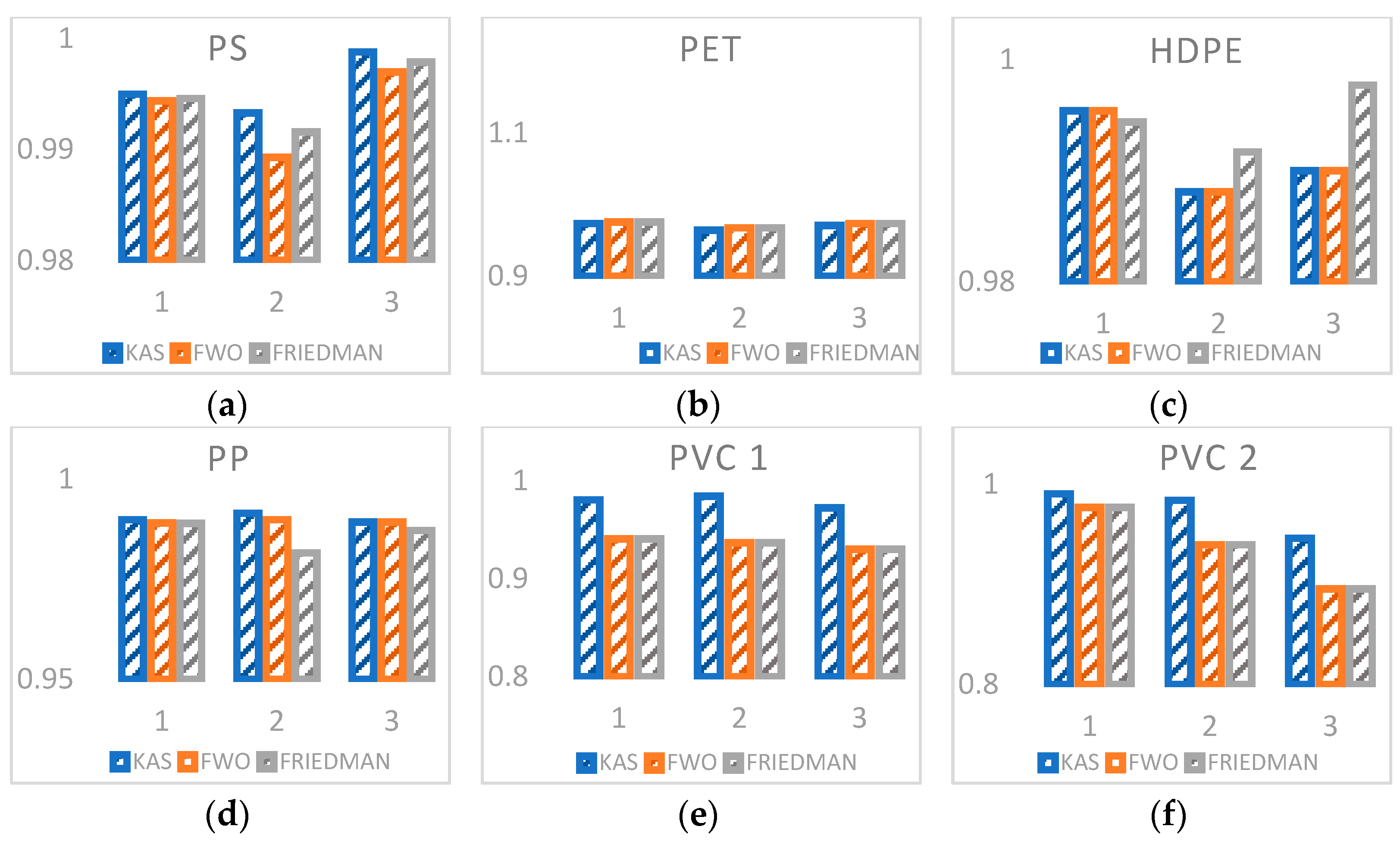
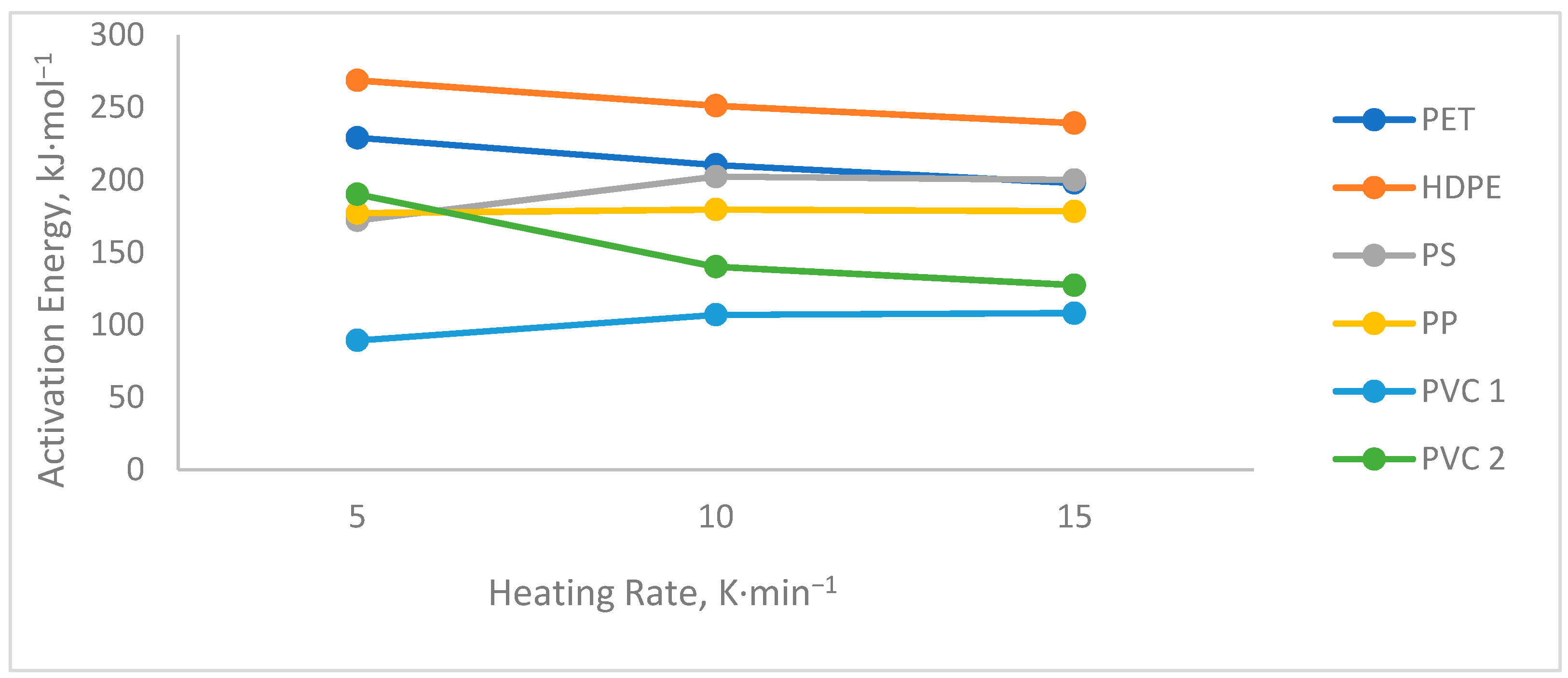
3.3. Kinetic Analysis
3.4. Thermodynamic Parameters
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De La Puente, G.; Klocker, C.; Sedran, U. Conversion of waste plastics into fuels: Recycling polyethylene in FCC. Appl. Catal. B Environ. 2002, 36, 279–285. [Google Scholar] [CrossRef]
- PNUD. Objetivo 12: Producción y Consumo Responsable. Available online: https://www1.undp.org/content/undp/es/home/sustainable-development-goals/goal-12-responsible-consumption-and-production.html (accessed on 5 August 2021).
- Ghisellini, P.; Cialani, C.; Ulgiati, S. A review on circular economy: The expected transition to a balanced interplay of environmental and economic systems. J. Clean. Prod. 2016, 114, 11–32. [Google Scholar] [CrossRef]
- Singh, R.K.; Ruj, B.; Sadhukhan, A.K.; Gupta, P.; Tigga, V.P. Waste plastic to pyrolytic oil and its utilization in CI engine: Performance analysis and combustion characteristics. Fuel 2020, 262, 116539. [Google Scholar] [CrossRef]
- López, A.; de Marco, I.; Caballero, B.M.; Laresgoiti, M.F.; Adrados, A. Influence of time and temperature on pyrolysis of plastic wastes in a semi-batch reactor. Chem. Eng. J. 2011, 173, 62–71. [Google Scholar] [CrossRef]
- Hartulistiyoso, E.; Sigiro, F.A.P.A.G.; Yulianto, M. Temperature Distribution of the Plastics Pyrolysis Process to Produce Fuel at 450 °C. Procedia Environ. Sci. 2015, 28, 234–241. [Google Scholar] [CrossRef] [Green Version]
- Kalargaris, I.; Tian, G.; Gu, S. Experimental characterisation of a diesel engine running on polypropylene oils produced at different pyrolysis temperatures. Fuel 2018, 211, 797–803. [Google Scholar] [CrossRef]
- Kalargaris, I.; Tian, G.; Gu, S. The utilisation of oils produced from plastic waste at different pyrolysis temperatures in a DI diesel engine. Energy 2017, 131, 179–185. [Google Scholar] [CrossRef]
- Park, S.S.; Seo, D.K.; Lee, S.H.; Yu, T.U.; Hwang, J. Study on pyrolysis characteristics of refuse plastic fuel using lab-scale tube furnace and thermogravimetric analysis reactor. J. Anal. Appl. Pyrolysis 2012, 97, 29–38. [Google Scholar] [CrossRef]
- Sharma, B.K.; Moser, B.R.; Vermillion, K.E.; Doll, K.M.; Rajagopalan, N. Production, characterization and fuel properties of alternative diesel fuel from pyrolysis of waste plastic grocery bags. Fuel Process. Technol. 2014, 122, 79–90. [Google Scholar] [CrossRef]
- Miandad, R.; Barakat, M.A.; Aburiazaiza, A.S.; Rehan, M.; Nizami, A.S. Catalytic pyrolysis of plastic waste: A review. Process Saf. Environ. Prot. 2016, 102, 822–838. [Google Scholar] [CrossRef]
- Wang, Z.; Wei, R.; Wang, X.; He, J.; Wang, J. Pyrolysis and Combustion of Polyvinyl Chloride (PVC) Sheath for New and Aged Cables via Thermogravimetric Analysis-Fourier Transform Infrared (TG-FTIR) and Calorimeter. Materials 2018, 11, 1997. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.K.; Ruj, B.; Sadhukhan, A.K.; Gupta, P. Impact of fast and slow pyrolysis on the degradation of mixed plastic waste: Product yield analysis and their characterization. J. Energy Inst. 2019, 92, 1647–1657. [Google Scholar] [CrossRef]
- Mani, M.; Subash, C.; Nagarajan, G. Performance, emission and combustion characteristics of a DI diesel engine using waste plastic oil. Appl. Therm. Eng. 2009, 29, 2738–2744. [Google Scholar] [CrossRef]
- Hujuri, U.; Ghoshal, A.K.; Gumma, S. Modeling pyrolysis kinetics of plastic mixtures. Polym. Degrad. Stab. 2008, 93, 1832–1837. [Google Scholar] [CrossRef]
- Heikkinen, J.M.; Hordijk, J.C.; de Jong, W.; Spliethoff, H. Thermogravimetry as a tool to classify waste components to be used for energy generation. J. Anal. Appl. Pyrolysis 2004, 71, 883–900. [Google Scholar] [CrossRef]
- Al-Salem, S.M.; Antelava, A.; Constantinou, A.; Manos, G.; Dutta, A. A review on thermal and catalytic pyrolysis of plastic solid waste (PSW). J. Environ. Manag. 2017, 197, 177–198. [Google Scholar] [CrossRef]
- Saha, B.; Ghoshal, A.K. Model-free kinetics analysis of decomposition of polypropylene over Al-MCM-41. Thermochim. Acta 2007, 460, 77–84. [Google Scholar] [CrossRef]
- Khedri, S.; Elyasi, S. Kinetic analysis for thermal cracking of HDPE: A new isoconversional approach. Polym. Degrad. Stab. 2016, 129, 306–318. [Google Scholar] [CrossRef]
- Sharuddin, S.D.A.; Abnisa, F.; Daud, W.M.A.W.; Aroua, M.K. A review on pyrolysis of plastic wastes. Energy Convers. Manag. 2016, 115, 308–326. [Google Scholar] [CrossRef]
- Wang, Z.; Xie, T.; Ning, X.; Liu, Y.; Wang, J. Thermal degradation kinetics study of polyvinyl chloride (PVC) sheath for new and aged cable. Waste Manag. 2019, 99, 146–153. [Google Scholar] [CrossRef]
- Peterson, J.D.; Vyazovkin, S. Wight, Kinetics of the Thermal and Thermo-Oxidative Degradation of Polystyrene, Polyethylene and Poly(propylene). Macromol. Chem. Phys. 2001, 202, 775–784. [Google Scholar] [CrossRef]
- Das, P.; Tiwari, P. Thermal degradation kinetics of plastics and model selection. Thermochim. Acta 2017, 654, 191–202. [Google Scholar] [CrossRef]
- Aboulkas, A.; El Harfi, K.; El Bouadili, A. Thermal degradation behaviors of polyethylene and polypropylene. Part I: Pyrolysis kinetics and mechanisms. Energy Convers. Manag. 2010, 51, 1363–1369. [Google Scholar] [CrossRef]
- Li, D.; Lei, S.; Wang, P.; Zhong, L.; Ma, W.; Chen, G. Study on the pyrolysis behaviors of mixed waste plastics. Renew. Energy 2021, 173, 662–674. [Google Scholar] [CrossRef]
- Nisar, J.; Ali, G.; Shah, A.; Shah, M.R.; Iqbal, M.; Ashiq, M.N.; Bhatti, H.N. Pyrolysis of Expanded Waste Polystyrene: Influence of Nickel-Doped Copper Oxide on Kinetics, Thermodynamics, and Product Distribution. Energy Fuels 2019, 33, 12666–12678. [Google Scholar] [CrossRef]
- Al-Salem, S.M.; Karam, H.J.; Al-Wadi, M.H.; Alsamaq, S.; Jiang, G.; Wang, J.; Leeke, G.A. Thermal degradation kinetics of real-life reclaimed plastic solid waste (PSW) from an active landfill site: The mining of an unsanitary arid landfill. Ain Shams Eng. J. 2021, 12, 983–993. [Google Scholar] [CrossRef]
- Xu, F.; Wang, B.; Yang, D.; Hao, J.; Qiao, Y.; Tian, Y. Thermal degradation of typical plastics under high heating rate conditions by TG-FTIR: Pyrolysis behaviors and kinetic analysis. Energy Convers. Manag. 2018, 171, 1106–1115. [Google Scholar] [CrossRef]
- Aranzazu, L.M.; Cárdenas, P.V.; Cárdenas, J.M.; Gaviria, G.H.; Rojas, A.F.; Carrero, J.I. Kinetic Models of Polymer Thermal Decomposition: A Review. Rev. Ing. Univ. Medellín 2013, 12, 113–129. [Google Scholar]
- Jiang, G.; Wei, L. Analysis of Pyrolysis Kinetic Model for Processing of Thermogravimetric Analysis Data. Phase Chang. Mater. Their Appl. 2018. [Google Scholar] [CrossRef] [Green Version]
- Kassargy, C.; Awad, S.; Burnens, G.; Kahine, K.; Tazerout, M. Experimental study of catalytic pyrolysis of polyethylene and polypropylene over USY zeolite and separation to gasoline and diesel-like fuels. J. Anal. Appl. Pyrolysis 2017, 127, 31–37. [Google Scholar] [CrossRef]
- Xiang, S.; Feng, L.; Bian, X.; Li, G.; Chen, X. Evaluation of PLA content in PLA/PBAT blends using TGA. Polym. Test 2020, 81, 106211. [Google Scholar] [CrossRef]
- Patel, S.R.; Kundu, S.K.; Halder, P.K.; Setiawan, A.; Paz-Ferreiro, J.; Surapaneni, A.; Shah, K.V. A Hybrid Kinetic Analysis of the Biosolids Pyrolysis using Thermogravimetric Analyser. Chem. Select. 2018, 3, 13400–13407. [Google Scholar] [CrossRef]
- Singh, R.K.; Ruj, B.; Sadhukhan, A.K.; Gupta, P. A TG-FTIR investigation on the co-pyrolysis of the waste HDPE, PP, PS and PET under high heating conditions. J. Energy Inst. 2020, 93, 1020–1035. [Google Scholar] [CrossRef]
- Yu, J.; Sun, L.; Ma, C.; Qiao, Y.; Yao, H. Thermal degradation of PVC: A review. Waste Manag. 2016, 48, 300–314. [Google Scholar] [CrossRef] [PubMed]
- Klaimy, S.; Lamonier, J.F.; Casetta, M.; Heymans, S.; Duquesne, S. Recycling of plastic waste using flash pyrolysis—Effect of mixture composition. Polym. Degrad. Stab. 2021, 187, 109540. [Google Scholar] [CrossRef]
- Moldoveanu, S.C. General Information about Pyrolysis. Pyrolysis Org. Mol. 2019, 1–33. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Pérez-Maqueda, L.A.; Popescu, C.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Nisar, J.; Ali, G.; Shah, A.; Iqbal, M.; Khan, R.A.; Sirajuddin; Anwar, F.; Ullah, R.; Akhter, M.S. Fuel production from waste polystyrene via pyrolysis: Kinetics and products distribution. Waste Manag 2019, 88, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Artetxe, M.; Lopez, G.; Amutio, M.; Barbarias, I.; Arregi, A.; Aguado, R.; Bilbao, J.; Olazar, M. Styrene recovery from polystyrene by flash pyrolysis in a conical spouted bed reactor. Waste Manag. 2015, 45, 126–133. [Google Scholar] [CrossRef]
- Zhou, C.; Yang, Y.; Li, W.; Shi, Y.; Jin, L.; Zhang, Z.; Wang, G. Free radical reaction model for n-pentane pyrolysis. Chin. J. Chem. Eng. 2018, 26, 514–520. [Google Scholar] [CrossRef]
- Wen, Y.; Zaini, I.N.; Wang, S.; Mu, W.; Jönsson, P.G.; Yang, W. Synergistic effect of the co-pyrolysis of cardboard and polyethylene: A kinetic and thermodynamic study. Energy 2021, 229, 120693. [Google Scholar] [CrossRef]
- Xue, Y.; Johnston, P.; Bai, X. Effect of catalyst contact mode and gas atmosphere during catalytic pyrolysis of waste plastics. Energy Convers. Manag. 2017, 142, 441–451. [Google Scholar] [CrossRef]
- Osman, A.I.; Farrell, C.; Al-Muhtaseb, A.H.; Al-Fatesh, A.S.; Harrison, J.; Rooney, D.W. Pyrolysis kinetic modelling of abundant plastic waste (PET) and in-situ emission monitoring. Environ. Sci. Eur. 2020, 32, 1–12. [Google Scholar] [CrossRef]
- Fakhrhoseini, S.M.; Dastanian, M. Predicting pyrolysis products of PE, PP, and PET using NRTL activity coefficient model. J. Chem. 2013, 2013. [Google Scholar] [CrossRef]

| Plastic Waste | Reaction Model | Model Code | f(α) | g(α) |
|---|---|---|---|---|
| HDPE | Contracting cylinder: two-dimensional phase boundary reaction | R2 | 2(1 − α)1/2 | 1 − (1 − α)1/2 |
| PP | Contracting cylinder: three-dimensional phase boundary reaction | R3 | 3(1 − α)2/3 | 1 − (1 − α)1/3 |
| PS | Avrami–Erofeev: two-dimensional nucleation | A2 | 2(1 − α) × [−ln(1−α)]1/2 | [−ln(1 − α)]1/2 |
| PET | Power law | P2 | 2(α)1/2 | α1/2 |
| PVC | Three-dimensional diffusion | D3 | 3/2(1 − α)2/3[1 − (1 − α)1/3]−1 | [1 − (1 − α)1/3]2 |
| Assigned Wave Number/cm−1 | Group | Vibrating Mode |
|---|---|---|
| 800–600 | -C-Cl | Stretching |
| 909–670 | Mononuclear aromatic hydrocarbons | C-H bending force of the plane |
| 1000–650 | AR-H=C-H | Deformation vibration |
| 1000–675 | -C=C- | Bending |
| 1000–800 | =C-H | Bending |
| 1000–960; 940–900 | R-CH=CH2 | Bending |
| 1300–1000 | Mononuclear aromatic hydrocarbons | Bending in plane |
| 1300–1000 | C-O | Stretching |
| 1380–1460 | -CH3 | C-H bending |
| 1460 | -CH2- | Scissor |
| 1470–1350 | -CH3 | Bending |
| 1500–1400 | Mononuclear aromatic hydrocarbons | Skeletal vibrations |
| 1680–1600 | -C=C- | Stretching |
| 1750–1715 | C=O | Stretching |
| 2400–2100 | -C≡C- | Stretching |
| 3000–2700 | -C-H | C-H stretch |
| 3100–2600 | H-Cl | Asymmetric stretch |
| 3100–3000 | =C-H | Stretching |
| 3300–3000 | Mononuclear aromatic hydrocarbons | C-H stretch |
| 3300–3270 | C≡C | Stretching |
| Plastic Waste | Model | β (°C × min−1) | Ea (kJ × mol−1) | A (K−1) |
|---|---|---|---|---|
| PS | KAS | 5 | 172.02 | 8.41 × 1011 |
| 10 | 202.22 | 2.41 × 1014 | ||
| 15 | 199.93 | 1.37 × 1014 | ||
| FWO | 5 | 170.38 | 1.42 × 1013 | |
| 10 | 167.68 | 4.65 × 1013 | ||
| 15 | 205.61 | 4.27 × 1012 | ||
| FRIEDMAN | 5 | 174.64 | 1.34 × 1012 | |
| 10 | 168.26 | 4.78 × 1013 | ||
| 15 | 210.19 | 4.31 × 1012 | ||
| PET | KAS | 5 | 229.04 | 3.08 × 1016 |
| 10 | 210.33 | 2.52 × 1014 | ||
| 15 | 197.87 | 3.51 × 1013 | ||
| FWO | 5 | 229.07 | 3.10 × 1016 | |
| 10 | 210.36 | 2.53 × 1014 | ||
| 15 | 197.44 | 3.26 × 1013 | ||
| FRIEDMAN | 5 | 229.05 | 3.08 × 1016 | |
| 10 | 210.35 | 2.52 × 1014 | ||
| 15 | 197.87 | 3.51 × 1013 | ||
| HDPE | KAS | 5 | 268.62 | 1.09 × 1017 |
| 10 | 251.12 | 9.00 × 1015 | ||
| 15 | 239.12 | 1.32 × 1015 | ||
| FWO | 5 | 266.78 | 8.11 × 1016 | |
| 10 | 250.33 | 7.93 × 1015 | ||
| 15 | 224.39 | 1.27 × 1014 | ||
| FRIEDMAN | 5 | 281.24 | 8.99 × 1017 | |
| 10 | 247.75 | 5.07 × 1015 | ||
| 15 | 231.84 | 4.15 × 1014 | ||
| PP | KAS | 5 | 177.03 | 1.22 × 1011 |
| 10 | 179.54 | 1.50 × 1011 | ||
| 15 | 178.29 | 1.44 × 1011 | ||
| FWO | 5 | 178.25 | 2.84 × 1012 | |
| 10 | 183.11 | 1.55 × 1012 | ||
| 15 | 180.27 | 2.07 × 1011 | ||
| FRIEDMAN | 5 | 188.51 | 9.99 × 1011 | |
| 10 | 190.37 | 1.22 × 1012 | ||
| 15 | 189.64 | 9.45 × 1011 | ||
| PVC 1 | KAS | 5 | 89.24 | 2.23 × 107 |
| 10 | 106.95 | 1.62 × 109 | ||
| 15 | 107.99 | 2.19 × 109 | ||
| FWO | 5 | 89.31 | 2.27 × 107 | |
| 10 | 107.03 | 1.65 × 109 | ||
| 15 | 107.99 | 2.20 × 109 | ||
| FRIEDMAN | 5 | 89.30 | 2.26 × 107 | |
| 10 | 107.02 | 1.64 × 109 | ||
| 15 | 107.99 | 2.20 × 109 | ||
| PVC 2 | KAS | 5 | 190.08 | 5.07 × 1012 |
| 10 | 140.07 | 1.44 × 109 | ||
| 15 | 127.29 | 2.06 × 108 | ||
| FWO | 5 | 158.12 | 1.83 × 1010 | |
| 10 | 140.08 | 1.44 × 109 | ||
| 15 | 127.41 | 2.10 × 108 | ||
| FRIEDMAN | 5 | 168.51 | 1.06 × 1011 | |
| 10 | 136.74 | 8.49 × 108 | ||
| 15 | 127.41 | 2.10 × 108 |
| Thermodynamic Parameters | Heating Rates K × min−1 | |||
|---|---|---|---|---|
| (kJ × mol−1) | 5 | 10 | 15 | |
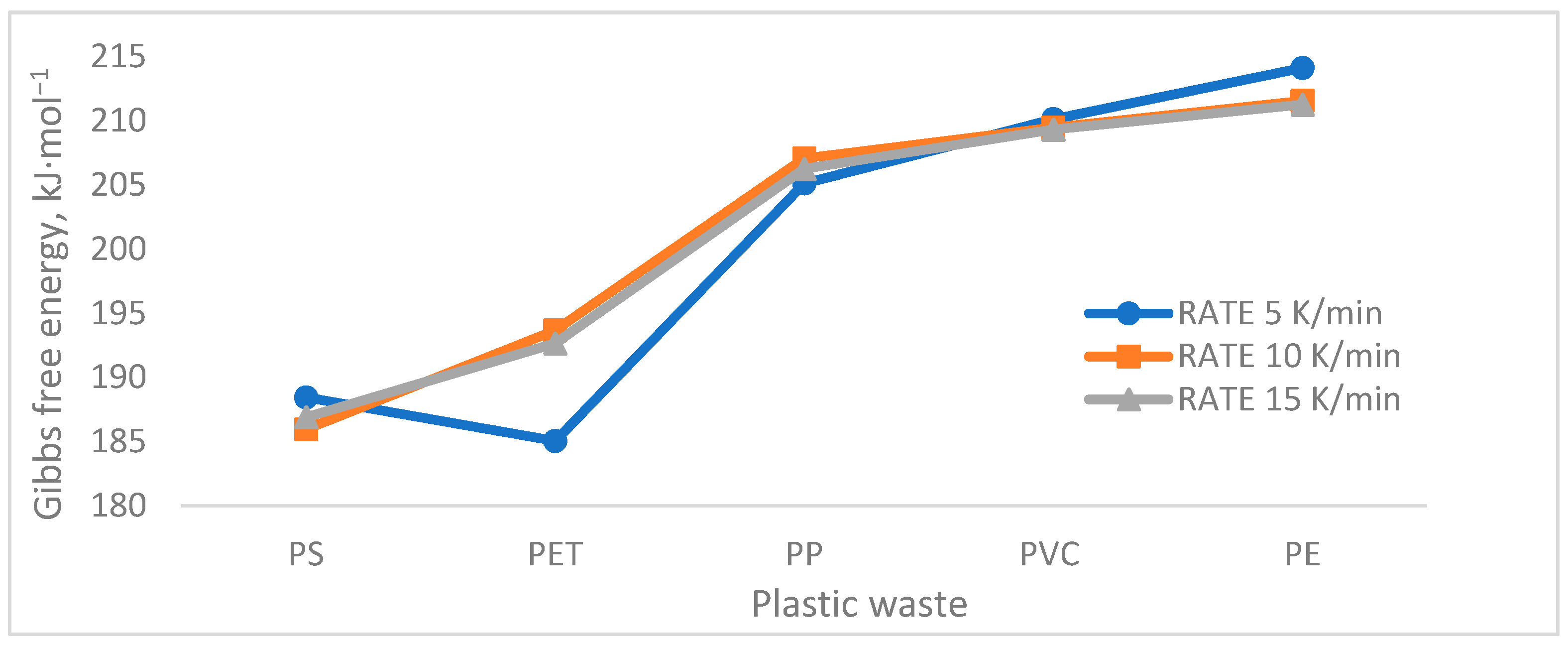
| PS | H | 166.252 | 196.439 | 194.105 |
| G | 188.435 | 185.955 | 186.897 | |
| S | −0.032 | 0.015 | 0.010 | |
| PET | H | 223.307 | 224.409 | 204.847 |
| G | 185.041 | 193.657 | 192.655 | |
| S | 0.055 | 0.044 | 0.017 | |
| HDPE | H | 262.472 | 244.897 | 232.824 |
| G | 214.105 | 211.552 | 211.255 | |
| S | 0.065 | 0.045 | 0.028 | |
| PP | H | 171.169 | 173.562 | 172.279 |
| G | 205.111 | 207.055 | 206.243 | |
| S | −0.048 | −0.217 | −0.047 | |
| PVC | H | 184.148 | 134.063 | 121.218 |
| G | 210.114 | 209.467 | 209.313 | |
| S | −0.036 | −0.104 | −0.121 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palmay, P.; Puente, C.; Barzallo, D.; Bruno, J.C. Determination of the Thermodynamic Parameters of the Pyrolysis Process of Post-Consumption Thermoplastics by Non-Isothermal Thermogravimetric Analysis. Polymers 2021, 13, 4379. https://doi.org/10.3390/polym13244379
Palmay P, Puente C, Barzallo D, Bruno JC. Determination of the Thermodynamic Parameters of the Pyrolysis Process of Post-Consumption Thermoplastics by Non-Isothermal Thermogravimetric Analysis. Polymers. 2021; 13(24):4379. https://doi.org/10.3390/polym13244379
Chicago/Turabian StylePalmay, Paul, Cesar Puente, Diego Barzallo, and Joan Carles Bruno. 2021. "Determination of the Thermodynamic Parameters of the Pyrolysis Process of Post-Consumption Thermoplastics by Non-Isothermal Thermogravimetric Analysis" Polymers 13, no. 24: 4379. https://doi.org/10.3390/polym13244379
APA StylePalmay, P., Puente, C., Barzallo, D., & Bruno, J. C. (2021). Determination of the Thermodynamic Parameters of the Pyrolysis Process of Post-Consumption Thermoplastics by Non-Isothermal Thermogravimetric Analysis. Polymers, 13(24), 4379. https://doi.org/10.3390/polym13244379







