Collagen Scaffolds Treated by Hydrogen Peroxide for Cell Cultivation
Abstract
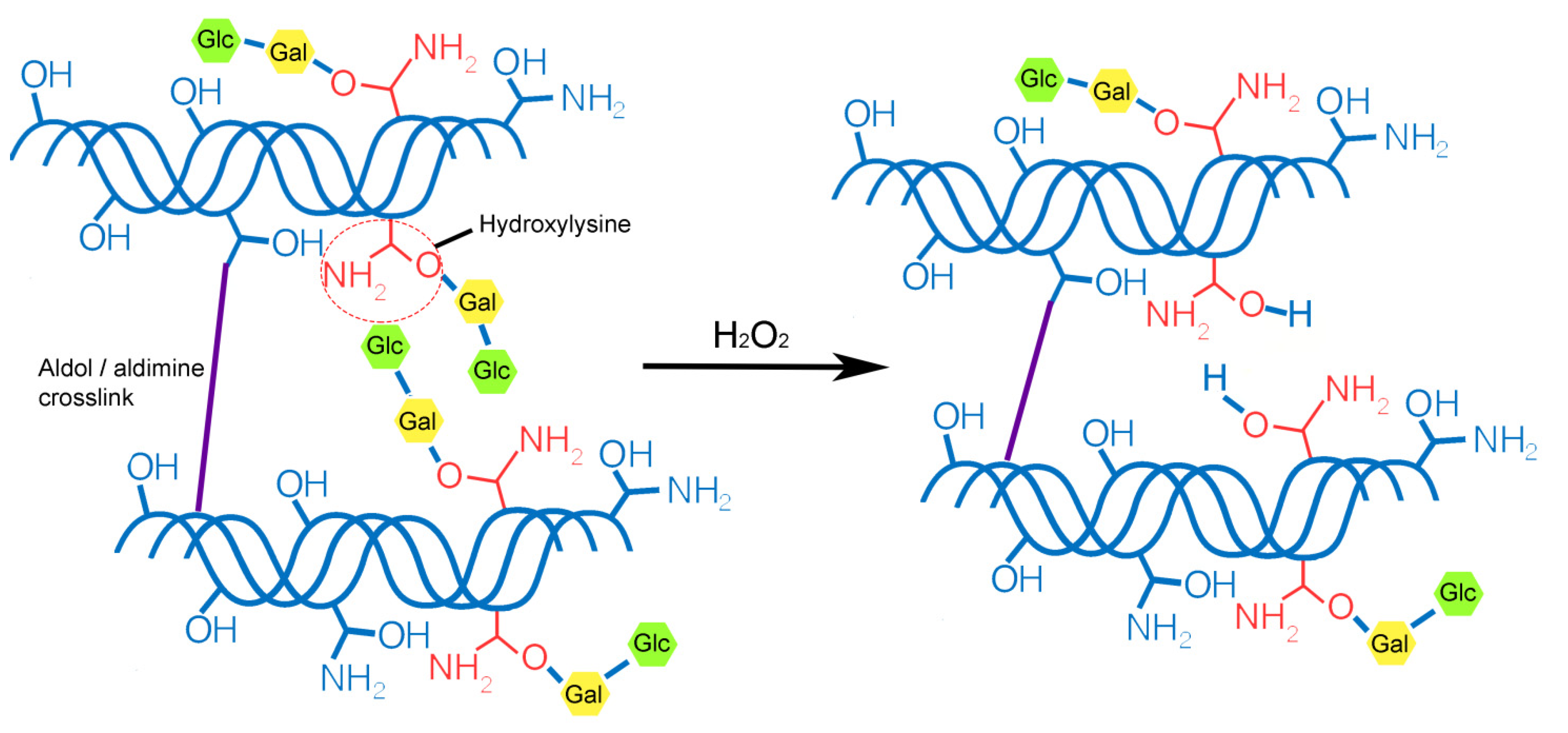
:1. Introduction
2. Materials and Methods
2.1. Collagen Fibril Formation and Modification
2.2. Scanning Electron Microscopy
2.3. Water Contact Angle
2.4. FTIR
2.5. Cell Cultivation
2.6. Fluorescence Staining of Cells
2.7. Cell Counts
2.8. Cell Viability
2.9. Statistical Analysis
3. Results and Discussion
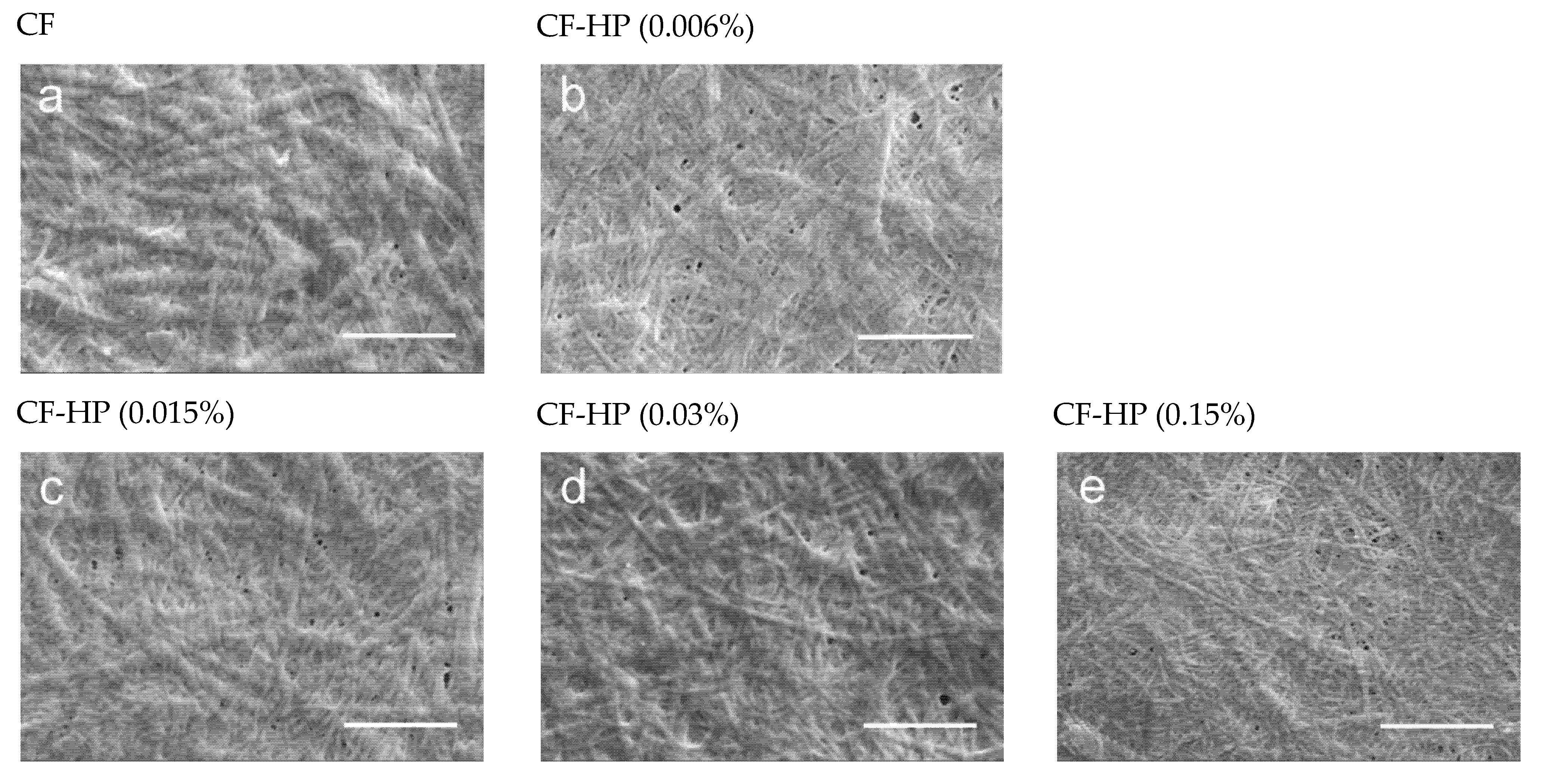
3.1. Scanning Electron Microscopy (SEM)
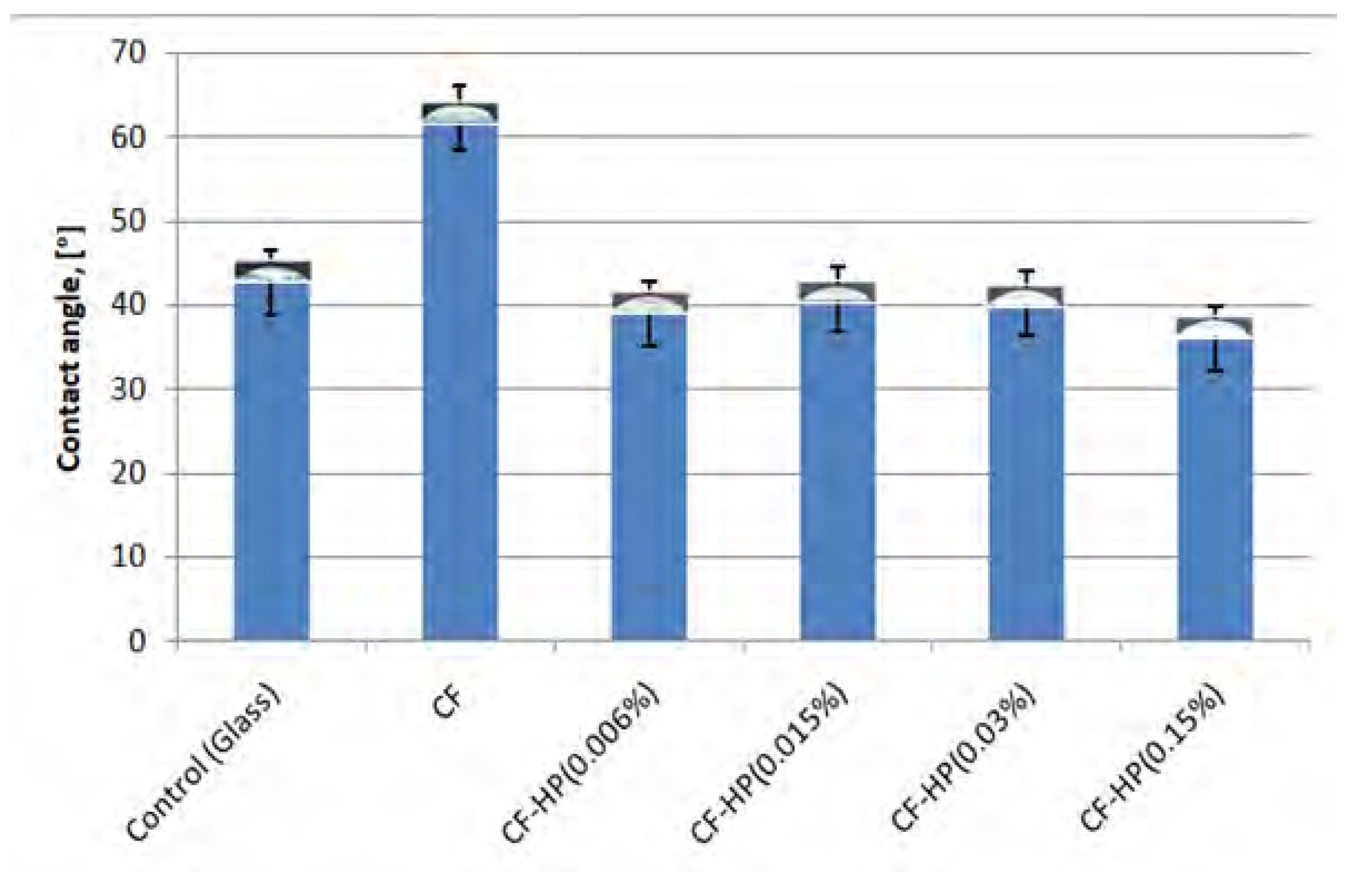
3.2. Water Contact Angle
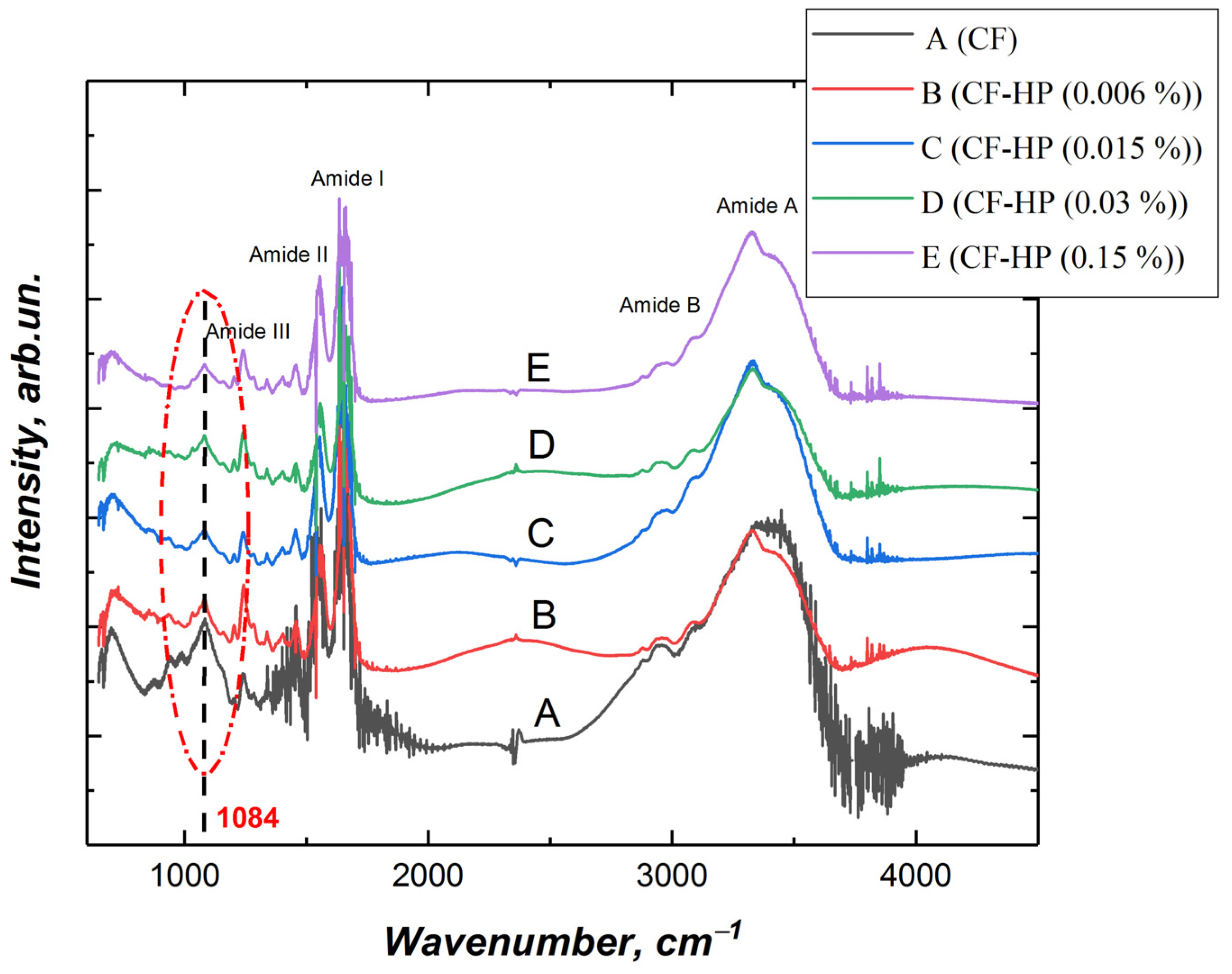
3.3. Fourier Transform Infrared (FTIR) Spectroscopy
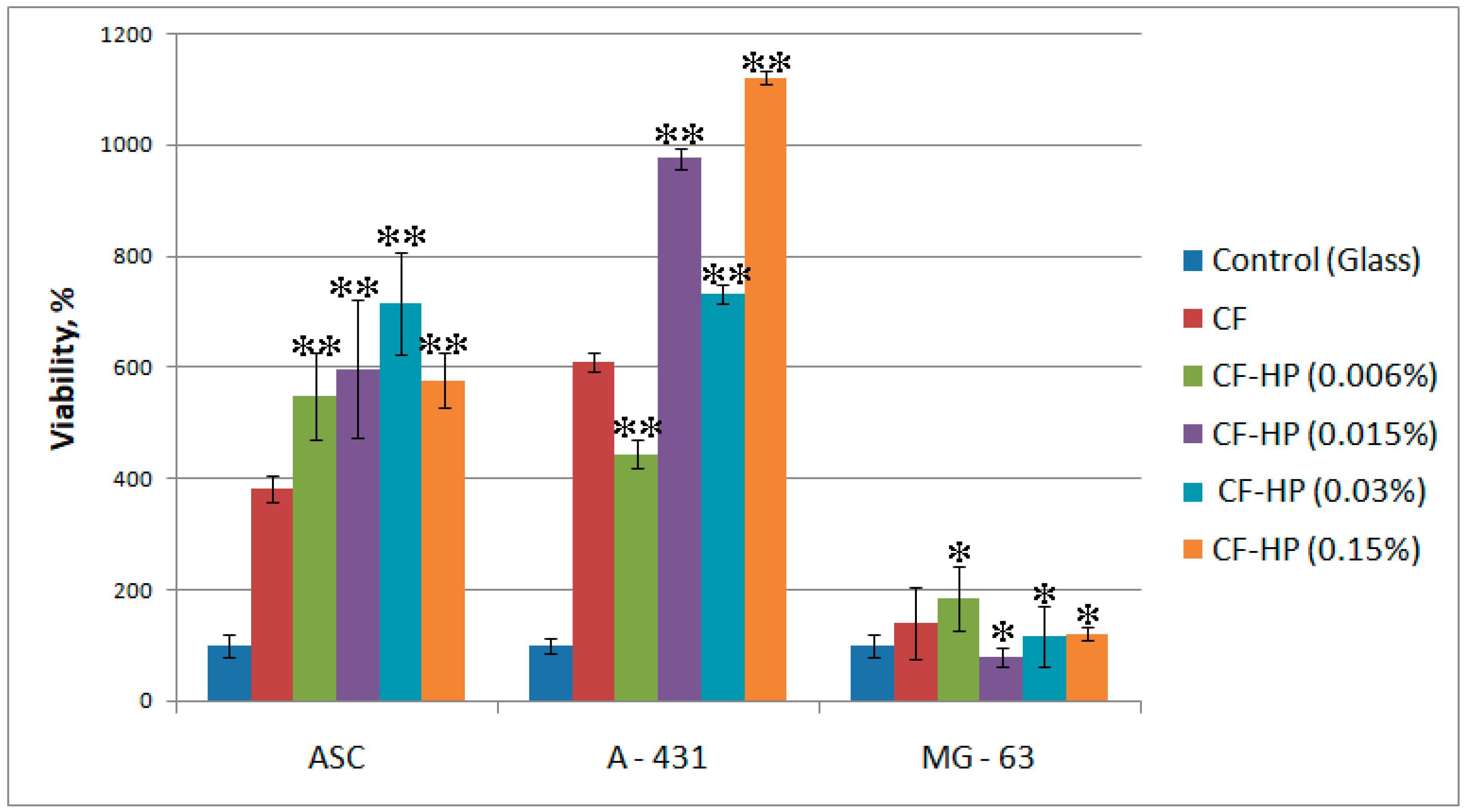
3.4. In Vitro Biological Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gelse, K. Collagens—Structure, function, and biosynthesis. Adv. Drug Deliv. Rev. 2003, 55, 1531–1546. [Google Scholar] [CrossRef] [Green Version]
- Revella, C.K.; Jensena, O.E.; Shearera, T.; Lu, Y.; Holmesban, D.F.; Kadler, K.E. Collagen fibril assembly: New approaches to unanswered questions. Matrix Biol. Plus. 2021, 12, 100079. [Google Scholar] [CrossRef]
- Shoulders, M.D.; Raines, R.T. Collagen structure and stability. Ann. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Svensson, R.B.; Mulder, H.; Kovanen, V.; Magnusson, S.P. Fracture mechanics of collagen fibrils: Influence of natural cross-links. Biophys. J. 2013, 104, 2476–2484. [Google Scholar] [CrossRef] [Green Version]
- Chapman, J.A. The regulation of size and form in the assembly of collagen fibrils in vivo. Biopolymers 1989, 28, 1367–1382. [Google Scholar] [CrossRef] [PubMed]
- Snowden, J.M.; Swann, D.A. Vitreous structure. V. The morphology and thermal stability of vitreous collagen fibers and comparison to articular cartilage (type II) collagen. Investig. Ophthalmol. Vis. Sci. 1980, 19, 19. [Google Scholar]
- Bos, K.J.; Holmes, D.F.; Kadler, K.; McLeod, D.; Morris, N.P.; Bishop, P. Axial structure of the heterotypic collagen fibrils of vitreous humour and cartilage. J. Mol. Biol. 2001, 306, 1011–1022. [Google Scholar] [CrossRef] [PubMed]
- Young, R.D.; Knupp, C.; Pinali, C.; Png, K.M.Y.; Ralphs, J.R.; Bushby, A.J.; Starborg, T.; Kadler, K.E.; Quantock, A.J. Three-dimensional aspects of matrix assembly by cells in the developing cornea. Proc. Natl. Acad. Sci. USA 2014, 111, 687–692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orgel, J.P.; San Antonio, J.D.; Antipova, O. Molecular and structural mapping of collagen fibril interactions. Connect. Tissue Res. 2011, 52, 2–17. [Google Scholar] [CrossRef]
- Gistelinck, C.; Witten, P.E.; Huysseune, A.; Symoens, S.; Malfait, F.; Larionova, D.; Simoens, P.; Dierick, M.; Van Hoorebeke, L.; De Paepe, A.; et al. Loss of Type I Collagen Telopeptide Lysyl Hydroxylation Causes Musculoskeletal Abnormalities in a Zebrafish Model of Bruck Syndrome. J. Bone Miner. Res. 2016, 31, 1930–1942. [Google Scholar] [CrossRef] [PubMed]
- Egea, G.; Jiménez-Altayó, F.; Campuzano, V. Reactive Oxygen Species and Oxidative Stress in the Pathogenesis and Progression of Genetic Diseases of the Connective Tissue. Antioxidants 2020, 9, 1013. [Google Scholar] [CrossRef]
- Magder, S. Reactive oxygen species: Toxic molecules or spark of life? Crit. Care 2006, 10, 208. [Google Scholar] [CrossRef] [Green Version]
- Dröge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef] [PubMed]
- Lambeth, J.D. Nox enzymes, ROS, and chronic disease: An example of antagonistic pleiotropy. Free Radic. Biol. Med. 2007, 43, 332–347. [Google Scholar] [CrossRef] [Green Version]
- Giorgio, M.; Trinei, M.; Migliaccio, E.; Pelicc, P.G. Hydrogen peroxide: A metabolic by-product or a common mediator of ageing signals? Nat. Rev. Mol. Cell Biol. 2007, 8, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Lennicke, C.; Rahn, J.; Lichtenfels, R.; Wessjohann, L.A.; Seliger, B. Hydrogen peroxide–production, fate and role in redox signaling of tumor cells. Cell Commun. Signal. 2015, 13, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Branco, A.C.; Ribeiro, N.; Figueiredo-Pina, C.G.; Colaço, R.; Serro, A.P. Characterization of the Nanostructure of Collagen Fibers Following the Application of Dilute Hydrogen Peroxide used in Dental Whitening Treatments. Anal. Lett. 2020, 53, 705–713. [Google Scholar] [CrossRef]
- Shved, Y.A.; Kukhareva, L.B.; Zorin, I.M.; Bilibin, A.Y.; Blinova, M.I.; Pinaev, G.P. Interaction of cultured skin cells with the polylactide matrix coved with different collagen structural isoforms. Cell Tiss. Biol. 2007, 1, 89–95. [Google Scholar] [CrossRef]
- Jung, J.-S.; Volk, C.; Marga, C.; Santos, A.N.; Jung, M.; Rujescu, D.; Santos, A.N. Adipose-Derived Stem/Stromal Cells Recapitulate Aging Biomarkers and Show Reduced Stem Cell Plasticity Affecting Their Adipogenic Differentiation Capacity. Cell. Reprogram. 2019, 21, 187–199. [Google Scholar] [CrossRef]
- Nashchekina, Y.; Chabina, A.; Nashchekin, A.; Mikhailova, N. 2020 Polycaprolactone Films Modified by L-Arginine for Mesenchymal Stem Cell Cultivation. Polymers 2020, 12, 1042. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, L.; Gallo, N.; Natali, M.L.; Terzi, A.; Sannino, A.; Madaghiele, M. Mimicking the Hierarchical Organization of Natural Collagen: Toward the Development of Ideal Scaffolding Material for Tissue Regeneration. Front. Bioeng. Biotechnol. 2021, 9, 1–32. [Google Scholar] [CrossRef]
- Collins, C.J.; Andriotis, O.G.; Nedelkovski, V.; Frank, M. Bone micro- and nanomechanics. In Encyclopedia of Biomedical Engineering; Roger, N., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 22–44. [Google Scholar]
- Hodge, A.J.; Petruska, J.A. Recent studies with the electron microscope on ordered aggregates of the tropocollagen macromolecules. In Aspects of Protein Structure; Ramachandran, G.N., Ed.; London Academic: London, UK, 1963; pp. 289–300. [Google Scholar]
- Quan, B.D.; Sone, E.D. Chapter Nine-Cryo-TEM Analysis of Collagen Fibrillar Structure. Methods Enzymol. 2013, 532, 189–205. [Google Scholar]
- Orgel, J.P.R.O.; Irving, T.C.; Miller, A.; Wess, T.J. Microfibrillar structure of type I collagen in situ. Proc. Natl. Acad. Sci. USA 2006, 103, 9001–9005. [Google Scholar] [CrossRef] [Green Version]
- Sorushanova, A.; Delgado, L.M.; Wu, Z.; Shologu, N.; Kshirsagar, A.; Raghunath, R.; Mullen, A.M.; Bayon, Y.; Pandit, A.; Raghunath, M.; et al. The Collagen Suprafamily: From Biosynthesis to Advanced Biomaterial Development. Adv. Mater. 2019, 31, e1801651. [Google Scholar] [CrossRef] [Green Version]
- Terzi, A.; Gallo, N.; Bettini, S.; Sibillano, T.; Altamura, D.; Madaghiele, M.; De Caro, L.; Valli, L.; Salvatore, L.; Sannino, A.; et al. Sub- and Supramolecular X-Ray Characterization of Engineered Tissues from Equine Tendon, Bovine Dermis, and Fish Skin Type-I Collagen. Macromol. Biosci. 2020, 20, 2000017. [Google Scholar] [CrossRef] [PubMed]
- Hennet, T. Collagen glycosylation Curr. Opin. Struct. Biol. 2019, 56, 131–138. [Google Scholar] [CrossRef]
- Birk, D.E.; Bruckner, P. Collagens, suprastructures and collagen fibril assembly. In The Extracellular Matrix: An Overview, Biology of Extracellular Matrix; Mecham, R.P., Ed.; Springer: Berlin, Germany, 2011; pp. 77–115. [Google Scholar]
- Ayala, R.; Zhang, C.; Yang, D.; Hwang, Y.; Aung, A.; Shroff, S.S.; Arce, F.T.; Lal, R.; Arya, G.; Varghese, S. Engineering the cell-material interface for controlling stem cell adhesion, migration, and differentiation. Biomaterials 2011, 32, 3700–3711. [Google Scholar] [CrossRef] [PubMed]
- Sionkowska, A. Effects of solar radiation on collagen and chitosan films. J. Photochem. Photobiol. B Biol. 2006, 82, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Kaminska, A.; Sionkowska, A. The effect of UV radiation on the thermal parameters of collagen degradation. Polym. Degrad. Stab. 1996, 51, 15–18. [Google Scholar] [CrossRef]
- Rabotyagova, O.S.; Cebe, P.; Kaplan, D.L. Collagen structural hierarchy and susceptibility to degradation by ultraviolet radiation. Mater. Sci. Eng. C 2008, 28, 1420–1429. [Google Scholar] [CrossRef] [Green Version]
- Torikai, A.; Shibata, H. Effect of ultraviolet radiation on photodegradation of collagen. J. Appl. Polym. Sci. 1999, 73, 1259–1265. [Google Scholar] [CrossRef]
- Riaz, T.; Zeeshan, R.; Zarif, F.; Ilyas, K.; Muhammad, N.; Safi, S.Z.; Rahim, A.; Rizvi, S.A.A.; Rehman, I.U. FTIR analysis of natural and synthetic collagen. Appl. Spectrosc. Rev. 2018, 53, 703–746. [Google Scholar] [CrossRef]
- Nashchekina, Y.А.; Starostina, А.А.; Trusova, N.А.; Sirotkina, М.Y.; Lihachev, А.I.; Nashchekin, А.V. Molecular and fibrillar structure collagen analysis by FTIR spectroscopy. J. Phys. Conf. Ser. 2020, 1697, 012053. [Google Scholar] [CrossRef]
- Belbachir, K.; Noreen, R.; Gouspillou, G.; Petibois, C. Collagen types analysis and differentiation by FTIR spectroscopy. Anal. Bioanal. Chem. 2009, 395, 829–837. [Google Scholar] [CrossRef]
- Fisher, G.J.; Quan, T.; Purohit, T.; Shao, Y.; Cho, M.K.; He, T.; Varani, J.; Kang, S.; Voorhees, J.J. Collagen Fragmentation Promotes Oxidative Stress and Elevates Matrix Metalloproteinase-1 in Fibroblasts in Aged Human Skin. Am. J. Pathol. 2009, 174, 101–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrianarivo, A.G.; Robinson, J.A.; Mann, K.G.; Tracy, R.P. Growth on type I collagen promotes expression of the osteoblastic phenotype in human osteosarcoma MG-63 cells. J. Cell Physiol. 1992, 153, 256–265. [Google Scholar] [CrossRef]
- Somaiah, C.; Kumar, A.; Mawrie, D.; Sharma, A.; Patil, S.D.; Bhattacharyya, J.; Swaminathan, R.; Jaganathan, B.G. Collagen Promotes Higher Adhesion, Survival and Proliferation of Mesenchymal Stem Cells. PLoS ONE 2015, 10, e0145068. [Google Scholar]
- Clark, A.G.; Maitra, A.; Jacques, C.; Simon, A.; Pérez-González, C.; Trepat, X.; Voituriez, R.; Vignjevic, D.M. Viscoelastic relaxation of collagen networks provides a self-generated directional cue during collective migration. bioRxiv 2020. [Google Scholar] [CrossRef]
- Giraud-Guille, M.-M. Twisted liquid crystalline supramolecular arrangements in morphogenesis. Int. Rev. Cytol. 1996, 166, 59–101. [Google Scholar]
- Ottania, V.; Raspantib, M.; Ruggeria, A. Collagen structure and functional implications. Micron 2001, 32, 251–260. [Google Scholar] [CrossRef]
- Varani, J.; Dame, M.K.; Rittie, L.; Fligiel, S.E.; Kang, S.; Fisher, G.J.; Voorhees, J.J. Decreased collagen production in chronologically aged skin: Roles of age-dependent alteration in fibroblast function and defective mechanical stimulation. Am. J. Pathol. 2006, 168, 1861–1868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lavker, R.M.; Gilchrest, B.A. (Eds.) Cutaneous agingchronologic versus photoaging. In Photoaging; Blackwell Science: Cambridge, MA, USA, 1995; pp. 123–135. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nashchekina, Y.; Nikonov, P.; Mikhailova, N.; Nashchekin, A. Collagen Scaffolds Treated by Hydrogen Peroxide for Cell Cultivation. Polymers 2021, 13, 4134. https://doi.org/10.3390/polym13234134
Nashchekina Y, Nikonov P, Mikhailova N, Nashchekin A. Collagen Scaffolds Treated by Hydrogen Peroxide for Cell Cultivation. Polymers. 2021; 13(23):4134. https://doi.org/10.3390/polym13234134
Chicago/Turabian StyleNashchekina, Yuliya, Pavel Nikonov, Nataliya Mikhailova, and Alexey Nashchekin. 2021. "Collagen Scaffolds Treated by Hydrogen Peroxide for Cell Cultivation" Polymers 13, no. 23: 4134. https://doi.org/10.3390/polym13234134
APA StyleNashchekina, Y., Nikonov, P., Mikhailova, N., & Nashchekin, A. (2021). Collagen Scaffolds Treated by Hydrogen Peroxide for Cell Cultivation. Polymers, 13(23), 4134. https://doi.org/10.3390/polym13234134







