Abstract
In this study, a full organic and water-soluble material was synthesized by coupling low molecular weight polyethylenimine (PEI-800) with cyclotriveratrilene (CTV). The water-soluble cross-linked polymer contains hydrophobic holes with a high coordination capability towards different organic drug molecules. The coordinating capability towards hydrophilic drugs (doxorubicin, gatifloxacin and sinomenine) and hydrophobic drugs (camptothecin and celastrol) was analyzed in an aqueous medium by using NMR, UV-Vis and emission spectroscopies. The coordination of drug molecules with the armed CTV unit through hydrophobic interactions was observed. In particular, celastrol exhibited more ionic interactions with the PEI moiety of the hosting system. In the case of doxorubicin, the host–guest detachment was induced by the addition of ammonium chloride, suggesting that the intracellular environment can facilitate the release of the drug molecules.
1. Introduction
In the past 60 years, scientists have been inspired by biology concepts to prepare artificial systems by assembling different molecules with physical forces [1,2,3]. The process of replicating enzyme–substrate interactions, known as molecular recognition, entails the production of large molecules or multimolecular entities, as well as the examination of the hosting capability towards relatively tiny charged or neutral small molecules [4,5].
The requirement of limiting or rendering more discriminant drug toxicity interests researchers in preparing and investigating different drug-coordinating platforms [6,7,8]. Polymers have been found to have intensive applications for coordination with therapeutic agents due to their large molecular structures and functionalization [9].
Polyethyleneimine (PEI) consists of a mixture of primary, secondary and tertiary amines that can be protonated over a wide pH range and modified by exploiting the reactivity of the amino group [10,11]. Of note, the original or modified form of PEI mainly concerned the gene coordination due to the positive charges that the polymer exhibits in a water solution upon amino groups’ protonation [10,12,13]. Meanwhile, PEI is often conjugated to small aromatic molecules, which improves the cell transfection efficiency or facilitates the fluorescent polymeric nanoparticles preparation [14,15]. For instance, doxorubicin (DOX) can be linked to PEI through covalent connection or coordinated through supramolecular interactions for drug-delivery applications [16,17]. Our group previously also reported the coordination of doxorubicin with PEI-25k cross-linked cyclotriveratrylene (CTV) [18].
CTV is a cyclic trimer with a rigid conformation [19]. The most stable configuration of CTV exhibits a distance between the centers of the rings of ~4.77 Å, and the cavity dimension may vary depending on the length of appended side arm to the upper rim (Figure 1) [20]. The concave-shaped cavity is responsible for the vast variety of supramolecular interactions with ionic and neutral small molecules [21,22,23,24,25,26,27,28,29,30,31,32,33]. Moreover, armed CTV can even coordinate large structures such as fullerenes, showing high flexibility towards different sizes of molecules [32,33,34,35,36,37,38,39,40,41,42].
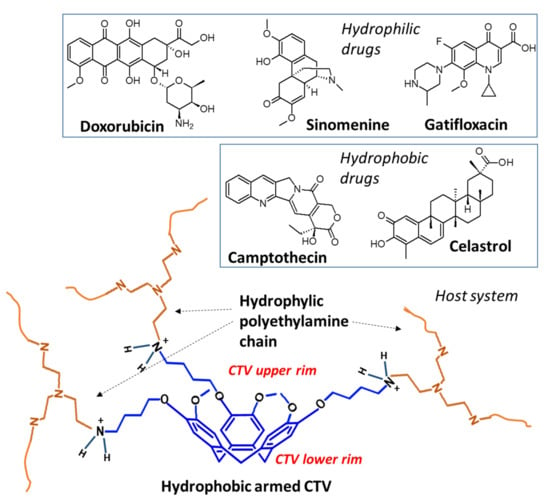
Figure 1.
Illustration of the synthesized coordinating system and molecular structures of drug molecules analyzed in this study.
Theoretically, the combination of CTV and PEI may provide a viable delivery platform for medicines with varied characteristics. The choice of the small molecular weighted polymer can induce drastic changes in the physical properties and coordinating capability, and therefore we intend to combine cyclotriveratrilene with PEI-800 for developing the platform.
The new cross-linked polymer (Cross-PEI-800) exhibited complete solubility in water, which facilitated the examination of the polymer itself in terms of its interactions with other compounds and cell viability. In addition, we also analyzed the coordination of Cross-PEI-800 with five different selected drug molecules to improve their water solubility: hydrophilic drugs—doxorubicin [43,44,45], gatifloxacin [46] and sinomenine [47]; hydrophobic drugs—camptothecin [48] and celastrol [49,50,51].
Formulations of the abovementioned drugs for selective targeting envisage two main strategies: chemical modifications and supramolecular encapsulation [52,53,54,55,56,57,58,59,60,61,62,63]. Supramolecular coordination is often preferable over other interactions due to its better external stimuli response, which modulates the coordination and releasing processes [63,64].
Although the selected compounds possess different physical properties, they exhibit similar structural features, including π-conjugated systems and heteroatoms. We herein developed a drug delivery platform, as shown in Figure 1, by exploiting these features for supramolecular coordination. The hydrophobic rigid CTV cup is suitable for interacting with the π-conjugated system of those molecules, and meanwhile, polyethyleneimine chains are more inclined to hydrogen bond coordination with the heteroatoms.
2. Materials and Methods
2.1. General
Human lung adenocarcinoma (A549), immortalized normal human hepatocytes (LO2), and human embryonic kidney cells (HEK293) were bought from ATCC (ATCC, Manassas, VA, USA). Cells were incubated in RPMI-1640 (A549 cells) and DMEM (LO2 and HEK293 cells) media, which contain 10% fetal bovine serum and antibiotics (penicillin, 50 U/Ml, and streptomycin, 50 mg/mL; Invitrogen, Paisley, Scotland, UK). All cells were cultured at 37 °C in a 5% humidified CO2 incubator. Stock solutions of all tested compounds were prepared by dissolving in DMSO and stored at −20 °C before use.
2.2. Cytotoxicity Assay
Cell viability was determined by the 3-[4,5-dimethylthiazole-2-yl]-2,5-diphenyltetrazolium bromide (MTT) assay as reported previously [65]. Cells with a density of 5 × 103 per well were seeded in 96-well plates before treatment of drugs. After overnight culture, cells were treated with different concentrations of each compound (0.191–100 μM) for 72 h. Cells without drug treatment were used as controls. After that, 10 μL of MTT solution (5 mg/mL) was added to the wells and incubated at 37 °C for another 4 h. Finally, 100 μL of solubilization buffer (12 mM of HCl in 10% SDS) was added to each well and incubated overnight. The absorbance of each well was then determined at 570 nm the next day. The percentage of cell viability was calculated, and the cytotoxicity was presented as % Viability = Atreated/Acontrol × 100.
2.3. Materials
Vanillyl alcohol, 1,4-dibromobutane, scandium triflate Sc(OTf)3 and branched polyethyleneimine (PEI-800) were purchased from Sigma-Aldrich (average Mw 800 by LS, average Mn 600 by GPC, branched) and used without further purification. Unless specified otherwise, acetone and acetonitrile (CH3CN) were used as acquired from commercial sources. The dialysis bag for the purification of polymer was Spectra/Por®6 Dialysis Membrane; MWCO: 1 kD. 1H and 13C NMR spectra were recorded on a Bruker Avance III 400 MHz BBFO Probe and AVANCE NEO 400 NMR in CDCl3, D2O and DMSO-d6, using the solvent residual proton signal as a reference. Flash column chromatography was performed using Silicycle Silica gel 60 (7–230 Mesh, pH 7). UV-Vis absorption spectra of all samples in H2O were obtained by Agilent Cary 60UV–Visible, Thermo Scientific Multiskan G0 microplate and cuvette spectrophotometer over a range of 200–1000 nm. Fluorescence spectra were acquired using Perkin Elmer, LS50B Luminescence Spectrometer equipped with an SC-05 standard cuvette holder.
2.4. Synthesis
[4-(2-bromobutoxy)-3-methoxyphenyl]methanol (2a): To a solution of 3-methoxy-4-hydroxybenzylalcohol 1a (2 g, 13 mmol, 1 equiv.) in acetone (24 mL), K2CO3 (8.97 g, 65 mmol, 5 equiv.) was added and stirred for 30 min at room temperature under argon. After addition of 1,4-dibromobutane (15.5 mL, 130 mmol, 10 equiv) in one portion, the resulting mixture was heated at 55 °C overnight. The reaction mixture was quenched with water (160 mL), extracted with ethyl acetate (3 × 80 mL) and concentrated in vacuo to obtain a yellow oil as the crude product. The crude product was purified on silica gel using ethyl acetate/hexane 4:6 as eluent to afford 2a (2.42 g, 8.40 mmol, 64%) as a white solid. 1H-NMR (CDCl3, 400 MHz, 25 °C): δ 6.92 (m, 1H), 6.86 (m, 1H), 4.62 (m, 2 H), 4.05 (t, J = 6.3 Hz, 2H), 3.88 (s, 3H), 3.50 (t, J = 6.35 Hz, 2H), 2.07 (m, 2H), 2.00 (m, 2H); 13C-NMR (CDCl3, 100 MHz, 25 °C): δ 149.5, 147.9, 134.0, 131.2, 119.9, 112.5, 110.5, 68.1, 65.2, 56.3, 33.5, 29.4, 27.8 ppm.
2,7,12-Tris-(2-bromobutoxy)-3,8,13-trimethoxy-10,15-dihydro-2H-tribenzo[a,d,g]cyclononene (Armed CTV): Sc(OTf)3 (0.114 g, 0.231 mmol, 0.030 equiv) was added to a solution of 2a (2.22 g, 7.70 mmol, 1.0 equiv.) in dry CH3CN (50 mL) under nitrogen atmosphere and heated under reflux for 2 days. The solvent was removed in vacuo. The resulting mixture was redissolved in ethyl acetate (100 mL) and washed with water (2 × 200 mL). The combined organic layers were concentrated in vacuo to obtain a yellow oil as the crude product. The crude product was purified on silica gel using ethyl acetate/hexane 1:4 as eluent to afford the pure product as a white solid (983 mg, 1.21 mmol, 47%). 1H-NMR (CDCl3, 400 MHz, 25 °C): δ 6.85 (m, 6H), 4.76 (d, J = 14 Hz, 3H), 4.03 (m, 2H), 3.84 (s, 9H), 3.55 (d, J = 14 Hz, 3H), 3.49 (td, J1 = 6.24 Hz, J2 = 2.36 Hz, 6H), 2.07 (q, J = 5.84, 6H), 1.96 (q, J = 5.84, 6H); 13C-NMR (CDCl3, 100 MHz, 25 °C): δ 148.4, 147.1, 132.4, 131.9, 115.5, 113.9, 68.4, 56.3, 36.5, 33.5, 29.4, 27.9 ppm; MALDI-TOF mass [M]+ calc. 810.0766, exp. 810.0761, [M + Na]+ calc. 833.0658, exp. 833.0658.
Synthesis of cross-linked polymer (Cross-PEI-800). Branched PEI-800 (200 mg) was dissolved in 8 mL of dry DMSO and injected by a syringe in a dry round bottom flask containing Armed CTV (200 mg). The reaction solution was stirred at 75 °C for 5 days. After cooling to room temperature, the solution was transferred to dialysis bag 1000 Da Mw cut off, which was immersed in methanol for 24 h under magnetic stirring. During this time the methanol solution was changed three times. The solution in the bag was evaporated, and 228 mg of yellow gel was collected.
2.5. Spectroscopic Studies
UV spectra of doxorubicin, gatifloxacin, sinomenine, camptothecin, pure and mixed with Cross-PEI-800.
An amount of 1 mg of drug was dissolved in 10 mL of water, and the reported concentrations were obtained by sample dilution. The mixed solutions were prepared by the addition of Cross-PEI-800 to the solution of pure drug.
2.5.1. UV Spectra of Celastrol
Celastrol (1 mg, 2.2 × 10−3 mmol) was dissolved in water (1 mL) under argon. This solution (1 mL) was transferred into a UV cuvette under argon.
2.5.2. UV Spectra Celastrol with Addiction of Solution of Cross-PEI-800 (1%)
Celastrol (1 mg, 2.2 × 10−3 mmol) was dissolved in water (1 mL) under argon. This solution was added with 100 μL of solution to 10 mg/mL of Cross-PEI-800 (1% in weight of solution). An amount of 1 mL of this solution was transferred into a UV cuvette under argon.
3. Results
3.1. Synthesis and Characterization
The cross-linked polymer Cross-PEI-800 was prepared as shown in Scheme 1. Precursor 2a was obtained by reacting vanillyl alcohol with 1,4-dibromobutane, as reported in the literature [18,66,67,68]. The oligomerization of compound 2a with scandium triflate in CH3CN afforded Armed-CTV [18]. The PEI-800 was cross-linked with Armed-CTV in DMSO overnight to obtain the highly water-soluble product Cross-PEI-800.
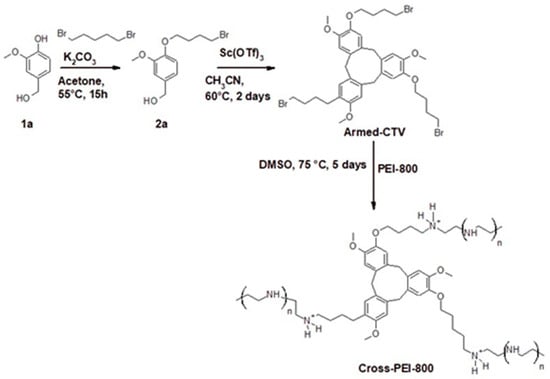
Scheme 1.
Synthesis of Cross-PEI-800 by cross-linking PEI-800 with Armed-CTV.
The 1H-NMR spectrum of Cross-PEI-800 in D2O exhibited three broad peaks around 8 ppm, 7 ppm and 1–4 ppm, which were assigned (based on the NMR signals of the precursors) to the –NH2+-, aromatic –CH- and non-aromatic protons (aliphatic, -NH-, -OCHn-), respectively (Figure 2).
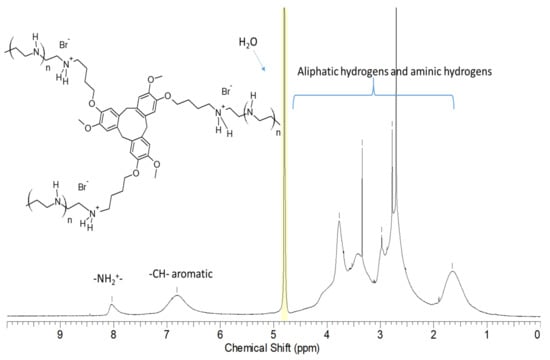
Figure 2.
1H-NMR spectrum and peak assignments of Cross-PEI-800 in D2O.
Cross-PEI-800 displayed UV-Vis spectra with absorption maxima at 286 and 328 nm, while PEI-800 exhibited no absorption maxima (Figure 3a,b). Notably, Cross-PEI-800 exhibited a better absorption band at 328 nm in a more concentrated solution.
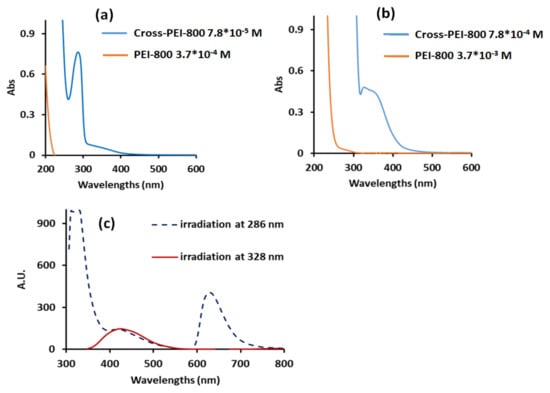
Figure 3.
UV-Vis spectra in water of Cross-PEI-800 at concentrations 7.8 × 10−5 M (a), 7.8 × 10−4 M (b) and emission spectrum at concentration 1.5 × 10−4 M (c). The concentration refers to the moles of monomers, whose molecular weight calculations are shown in the Supplementary Materials in the appendix of Figure S5.
The irradiation of Cross-PEI-800 solution at 286 nm produced a sharp emission at 330 nm and a broad emission at 450 and 630 nm, while the irradiation at 328 nm produced a broad emission at 450 nm (Figure 3c). Of note, the precursor Armed-CTV did not show any signals because of its insolubility in water.
The cytotoxicity of Cross-PEI-800 was evaluated against the human normal cells (LO2 and HEK293) and human lung cancer cells (A549) using an MTT assay. Cross-PEI-800 showed higher IC50 values against A549 and HEK293 cells than against LO2 cells [65].
The optical properties and the cell viability values of Cross-PEI-800 are summarized in Table 1.

Table 1.
The spectroscopic and biological properties of Cross-PEI-800.
3.2. Drug Coordination Analysis
3.2.1. Doxorubicin (Hydrophilic Drug)
The 1H NMR spectra of DOX and the coordinating system (Cross-PEI-800) were obtained in D2O, and their corresponding NMR signals were assigned as reported in the literature (Figure 4a,b) [69]. After mixing Cross-PEI-800 with DOX in D2O, the aromatic signals of the drug were broadened, and the aromatic signal of the polymer was shifted upfield by ~0.2 ppm (Figure 4c), showing a weak π−π stacking interaction between the cross-linked polymer and DOX phenyl rings. The broadening and shifting of the 1′ proton signal of DOX from 5.44 to 5.38 ppm were also observed (Figure 4c). After seven days, the broadened proton signal at 5.38 ppm was further split into two peaks at 5.38 and 5.41 ppm (Figure 4d). According to the NOESY spectrum, the peak at 5.41 ppm interacted with the aliphatic protons of DOX, while the signal at 5.38 ppm did not show intramolecular interactions except for the interaction with residual H2O (Figure 4e). Thus, the signal at 5.41 ppm corresponds to the 1′ proton of DOX, and the signal at 5.38 ppm corresponds to the –OH linked to the aliphatic carbons of the drug molecule (Figure 4d). In the solution of pure DOX, the –OH signal was not visible because of the proton exchange with D2O. Once the polymer was added, the hydroxyl proton was shielded from the interactions with solvent molecules and finally became detectable in 1H NMR spectrum. The 1H-NMR analysis (full 1HNMR spectra, Figures S6–S8 in the Supplementary Materials) unequivocally showed the hydrophobic interactions between Cross-PEI-800 and DOX. In addition, the NOESY spectrum also revealed that the aromatic signals of the cross-linked polymer and drug molecules interacted with the signals at 3.8–4.1 ppm that correspond to –OMe and -OCH2- signals (full NOESY spectrum, Figure S9, Supplementary Materials). This demonstrates that the DOX located inside the hydrophobic environment of armed CTV is oriented with the –OMe group pointing towards the aromatic rings of the CTV unit.
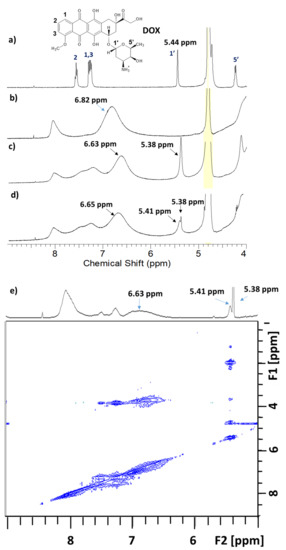
Figure 4.
1H-NMR spectra, range 4–9 ppm of (a) Doxorubicin, (b) Cross-PEI-800, (c) Cross-PEI-800 + DOX, (d) Cross-PEI-800 + DOX after 7 days, and (e) NOESY spectrum of Cross-PEI-800 + DOX after 7 days. The ppm full-range spectra shown in the Supplementary Materials (Figures S6–S9).
The UV-Vis spectrum of DOX after adding Cross-PEI-800 displayed absorption broadening with a lowered absorbance (abs) maximum, while after adding PEI-800, it showed a red shift of absorption (Figure S10 in the Supplementary Materials). The UV titration analysis revealed that the coordination between Cross-PEI-800 and DOX followed stoichiometry 1:1, and the equilibrium constant of the complex was 107 (Figure S11 in the Supplementary Materials). As shown in Figure 5 (red line), the irradiation of the DOX solution at 490 nm exhibited a fluorescence maximum at 550 nm, whereas there was no absorption at 490 nm or emission at 550 nm for Cross-PEI-800 (Figure 3). The addition of Cross-PEI-800 and the irradiation at the same frequency (490 nm) showed two fluorescence maxima at 550 and 630 nm (blue dashed line, Figure 5). The irradiation at the absorption maxima of the cross-linked polymer (286 and 328 nm) produced only the fluorescence maxima of the polymer (Figure S12 in the Supplementary Materials).
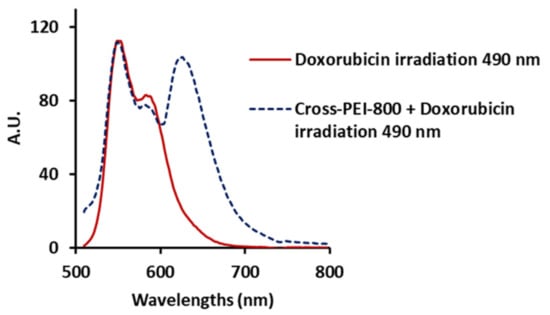
Figure 5.
Fluorescence spectra upon irradiation at 490 nm of DOX 5.7 × 10−6 M (red line) and DOX 5.7 × 10−6 M mixed with Cross-PEI-800 1.5 × 10−4 M (blue dashed line).
Based on the fluorescence profile of Cross-PEI-800 incorporated with DOX, the excited electrons of DOX are proposed to migrate to the LUMO orbital of Cross-PEI-800 and subsequently generate the emission maximum of the cross-linked polymer at 630 nm. Accordingly, this provides further evidence of the molecular interactions between DOX and Cross-PEI-800.
3.2.2. Gatifloxacin (Hydrophilic Drug)
The NMR assignment of gatifloxacin in D2O was carried out as reported in the literature [70]. The proton signal of –CH- linked to the secondary amine of gatifloxacine was shifted from 4.12 ppm to 4.07 ppm after the addition of Cross-PEI-800 (Figure 6a,b, full spectra shown in Figures S13–S15 in the Supplementary Materials). At the same time, the proton signals of Cross-PEI-800 were overlapped with the –CH2- signals of the piperazine moiety of gatifloxacine. In the NOESY spectrum, the signal at 4.07 ppm was found to interact with the aromatic signals of Cross-PEI-800 (Figure 6c, full spectrum shown in Figure S16 in the Supplementary Materials). NMR data clearly indicated the interaction of the gatifloxacin’s piperazine ring with the CTV moiety of Cross-PEI-800.
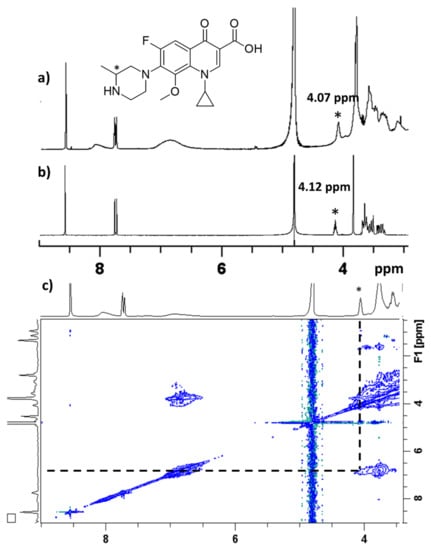
Figure 6.
(a) 1H NMR spectrum, D2O, 3–9 ppm, of gatifloxacine mixed with Cross-PEI-800, (b) 1H NMR spectrum D2O, 3–9 ppm, of gatifloxacin, (c) NOESY spectrum in D2O, 2–9 ppm of gatifloxacine mixed with Cross-PEI-800. The 1HNMR signal marked with * refers to the hydrogen linked to the tertiary carbon of gatifloxacine piperazine group.
After mixing gatifloxacin with the cross-linked polymer, the UV-Vis spectrum of gatifloxacin remained unchanged (Figure S17 in the Supplementary Materials). Upon irradiation at the absorption maxima (232, 286 and 330 nm), gatifloxacin produced a blue emission at 430 nm. In the presence of Cross-PEI-800, this emission was also observed upon irradiation at 286 and 330 nm. However, irradiation at 232 nm exhibited the emission of the cross-linked polymer (Figure S18 in the Supplementary Materials). According to the previous studies, the absorption of quinolone derivatives at 230 nm is attributed to the fluorophenyl piperazine unit [71]. The irradiation at 230 nm in the presence of the polymer illustrated the disappearance of the drug emission, showing the interaction of fluorophenyl piperazine moiety with Cross-PEI-800 (Figure S18 in the Supplementary Materials). Accordingly, NMR and UV analyses conclude that Cross-PEI-800, with gatifloxacin, exhibits hydrophobic interaction between the piperazine substituent and the proximity of the armed CTV.
3.2.3. Sinomenine (Hydrophilic Drug)
After the addition of Cross-PEI-800 to sinomenine in the D2O solution, the aromatic proton signals of sinomenine were shifted upfield, and a new signal at 5.43 ppm was observed. In accordance with the 1H NMR assignment of sinomenine in DMSO-d6 [72], the new peak corresponds to the proton at position a (Figure 7, full spectra shown in Figures S19–S21 in the Supplementary Materials) that was absent in the pure drug due to deuterium exchange. Therefore, NMR data demonstrated that the cross-linked polymer partially shielded sinomenine from the interactions with the solvent. Moreover, the upfield shift of the aromatic proton signals proved the existence of π−π interactions between sinomenine and Cross-PEI-800. The shifting of b signal and its NOE correlations with the –OMe signal of polymer indicated the hydrophobic and p-p interaction between sinomenine and armed CTV unit.
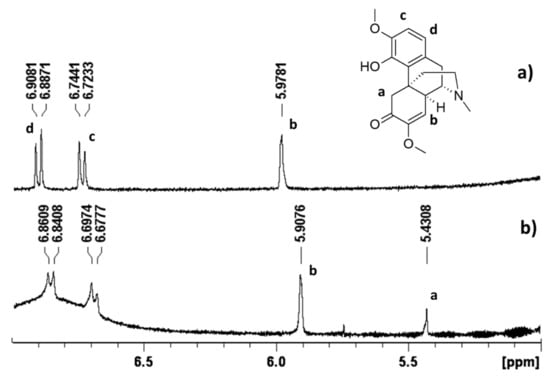
Figure 7.
1H-NMR spectrum in D2O, 5–7 ppm of (a) pure sinomenine and (b) sinomenine mixed with Cross-PEI-800. The full-range 1H-NMR and NOESY spectra were provided in the Supplementary Materials.
Besides, the UV-Vis spectrum of sinomenine showed an absorption band at 264 nm, and its intensity was enhanced after treatment with Cross-PEI-800 (Figure S22a). Similar to other drug molecules, the emission spectra can also identify the associated intermolecular interactions. The irradiation of sinomenine at 264 nm (abs maximum) showed no emission. Upon treatment with Cross-PEI-800, the emission maxima of the cross-linked polymer (320 and 630 nm upon irradiation at 286 nm) along with an additional maximum at 430 nm were found (Figure S22b). Irradiations of the mixture at 286 and 324 nm generated the same absorption maxima as pure Cross-PEI-800 (Figure S22c,d).
3.2.4. Camptothecin (Hydrophobic Drug)
Camptothecin in D2O alone did not exhibit any NMR signals. In the presence of a large amount of Cross-PEI-800, some proton peaks of camptothecin were found and assigned as a, b and c (Figure 8b) based on the reported NMR assignments of camptothecin in DMSO-d6, Trifluoroacetic acid-d and modified camptothecin inD2O solutions [73,74,75]. By reducing the amount of Cross-PEI-800, the aromatic proton signals of camptothecin became overlapped with those of the cross-linked polymer, and they were assigned as d in Figure 8c (enlarged spectra shown in Figures S23–S25).
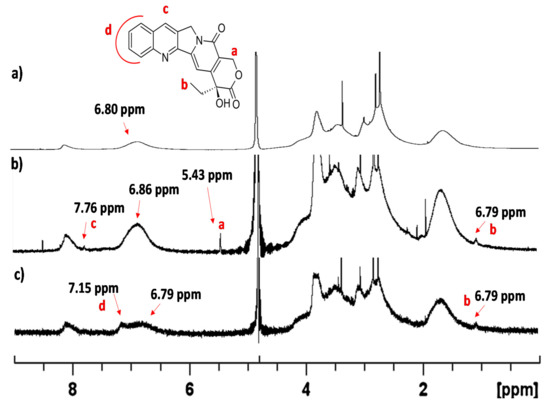
Figure 8.
(a) 1H-NMR in D2O spectrum of Cross-PEI-800 range 0–9 ppm, (b) 1H-NMR spectrum in D2O of camptothecin (5 mg) in the presence of Cross-PEI-800 (5 mg), range 0–9 ppm, (c) 1H-NMR spectrum in D2O of camptothecin (7 mg) in the presence of Cross-PEI-800 (2 mg), range 0–9 ppm.
Regarding the NOESY spectrum of camptothecin in the presence of the cross-linked polymer, the aromatic signal at 7.15 ppm interacted with the –OMe signals at 3.82 ppm of Cross-PEI-800 (Figure S26 in the Supplementary Materials). On the other hand, camptothecin in H2O exhibited weak UV absorption, which increased the intensity after the addition of Cross-PEI-800 (Figure S27 in the Supplementary Materials). When switching to PEI-800, the intensity also increased with a red shift. These results further prove the drug’s tendency to coordinate the hydrophobic armed CTV unit of Cross-PEI-800. Pure camptothecin upon irradiation generated emission at 430 nm that was also visible in the presence of Cross-PEI-800 (Figure S28 in the Supplementary Materials). The titration experiments of the drug with the polymer revealed that two units of armed CTV were involved in the coordination with camptothecin (Figure S29 in the Supplementary Materials).
3.2.5. Celastrol (Hydrophobic Drug)
In the D2O solution, no proton signal of pure celastrol was observed, whereas only the polymer’s signals were found after adding Cross-PEI-800, owing to the overlapping by intense proton signals of the polymer (Figures S30–32 in the Supplementary Materials). In the solution with fewer polymers, there was a very weak additional signal at 7.15 ppm, similar to the case of camptothecin. This was due to the poor water solubility of the drug. This signal was more distinguishable in the NOESY spectrum (Figure S33 in the Supplementary Materials).
Other studies have reported that the UV-Visible spectroscopy method was employed for the reversibility studies of celastrol [76]. The spectra of celastrol in phosphate-buffered saline (pH 7.4) were reported to exhibit a peak at 440 nm [77]. Further studies revealed that quinone methide rapidly reacted with thiol groups under physiological conditions and subsequently caused a decrease in the UV-Visible absorption, indicating the formation of covalent adducts.
In our experiments, the UV-Vis absorption spectrum of celastrol in water only displayed a weak absorption at 280 nm (Figure 9a). The addition of 1% (in weight) of Cross-PEI-800 formed a new absorption band at 435 nm and resulted in a color change in the solution (Figure 9a,b, as detailed by the UV-Vis analysis shown in Figure S34 in the Supplementary Materials).
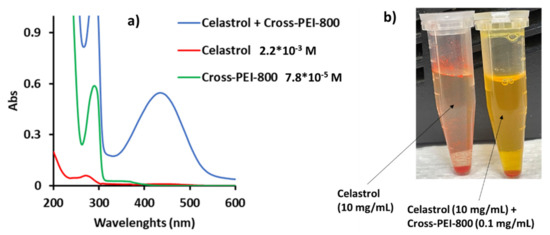
Figure 9.
(a) UV-Vis spectrum of pure celastrol, pure Cross-PEI-800, celastrol mixed with Cross-PEI-800 (1% of cross-linked polymer), (b) Chromatic solution variation after addition of polymer to celastrol.
In addition, the irradiation of the mixture at 435 nm displayed the emission peaks of Cross-PEI-800. In other words, the new absorption at 435 nm cannot be ascribed to a modification of celastrol but to a complex formation between the drug and the cross-linked polymer (Figure S35 in the Supplementary Materials).
Subsequently, titration experiments found that the coordination between celastrol and Cross-PEI-800 was stoichiometric, in a ratio of 1:1 (Figure S36 in the Supplementary Materials). The drug solubility in water increased 100 times after adding 1% of Cross-PEI-800; more importantly, the solubility further increased after 10 days (Figure S37 in the Supplementary Materials). Furthermore, the absorption band of the mixture was shifted from 435 to 360 nm after 10 days, as shown in the UV-Vis spectra (Figure S38 in the Supplementary Materials). This absorption change was postulated to involve a change of interactions between celastrol and Cross-PEI-800. The absorption of the drug-polymer mixture at 435 nm (blue line, Figure 9) was similar to that of celastrol in phosphate buffer at pH 7. This suggested a similar interaction due to the buffering power of the polyethylenimine group. Meanwhile, the blue shift of the absorption band after 10 days is attributed to a hydrophobic or π−π stacking interaction.
3.3. Guest Molecule Releasing Preliminary Analysis
To understand the capability of releasing the guest molecule from the hosting system, DOX (hydrophilic) and celastrol (hydrophobic), which interacted with Cross-PEI-800, were subjected to a pH change using NH4Cl. With the addition of NH4Cl to the solutions, the pH of the solution was maintained at 7, and the recovery of DOX absorption was found except for celastrol (Figure S39 in the Supplementary Materials). The results suggested that the DOX can be easily released from Cross-PEI-800 under an acidic intracellular environment. In the case of celastrol, the releasing process requires different conditions, for example, the addition of a competitive guest compound.
4. Discussion
The 1H-NMR spectrum of Cross-PEI-800 in D2O revealed that the PEI moiety assisted in the water dissolution of the hydrophobic armed CTV functional group. In the 1H-NMR spectrum of Cross-PEI-800 (Figure 2), the assignment of all proton signals of armed CTV unit can be achieved by comparison with the spectrum of Armed-CTV in CDCl3 (Figure S3 in the Supplementary Materials). The same information was obtained by comparing the UV spectra of Armed-CTV (spectrum in MeOH, Figure S40 in the Supplementary Materials) with Cross-PEI-800 (Figure 3). The two compounds displayed the same absorption band at ~290 nm with similar coefficients. The irradiation at this frequency exhibited emission (dashed line, Figure 4c), and this indicated that the CTV unit generated the emission.
The 1H-NMR and NOESY spectra showed that the aromatic groups of DOX, sinomenine and camptothecin, interacted with the armed CTV unit, respectively. On the contrary, the piperazine group of gatifloxacin was involved in the major interaction with the cross-linked polymer. Furthermore, the interaction of celastrol was detectable from UV-Vis spectra, where the coordination involves the amino and CTV groups of Cross-PEI-800 and the heteroatoms of the drug.
The emission spectra analysis further proves the drug-CTV unit interaction. Irradiation of the solution mixtures at the drugs’ absorption maxima, the photoluminescence (PL) spectra generated the emission of the cross-linked polymer upon irradiation at 290 nm.
Finally, the addition of NH4Cl to the DOX-Cross-PEI-800 conjugate induced the recovery of the drug’s UV-absorption, owing to the detachment of the drug from the cross-linked polymer by acidic proton. This shows that Cross-PEI-800 is a viable and potential platform for drug delivery.
5. Conclusions
A full-organic, water-soluble material, Cross-PEI-800, was prepared by combining two molecular units (CTV and PEI-800) often used in supramolecular chemistry that exhibit different coordinating features. CTV is a concave macrocycle that interacts with guest molecules through Van der Waals, π-π stacking and hydrophobic interactions. PEI-800 is a polyethylenimine that is highly soluble in water and coordinates other molecules through hydrogen bonding or electrostatic interactions. Cross-PEI-800 consists of a water-soluble gel that contains hydrophobic holes with a high coordinating power towards organic molecules. This material has been demonstrated to encapsulate molecules with different characteristics via various interaction modalities.
Owing to the water solubility and optical properties of the prepared coordinating systems, various spectroscopic methods were employed to examine the host–guest complexes in water under normal conditions. Of note, other research groups also reported coordinating materials, including synthetic organic macrocycles that coordinate drugs in organic solvents and subcritical water [78,79], and the assembly of an organic macrocycle and inorganic nanoparticle that coordinates drugs in water [80].
The synthesized system was found to coordinate both hydrophilic and hydrophobic molecules. Both CTV and PEI-800 moieties were involved in all drugs’ coordination. The results show that the armed CTV unit works as a hole trap for small aromatic organic molecules. Only celastrol exhibited different coordination induced by the buffering power of the polyethyleneimine unit. The main feature of Cross-PEI-800 consists of its complexity or multifunctionality, which contributes to various physical properties and coordinating modalities.
In this work, the molecular structure and the coordinating capability of Cross-PEI-800 were examined. Cross-PEI-800 not only showed its capability to coordinate the drugs in water but also exhibited relatively low cytotoxicity. Two drug molecules, DOX and celastrol, were selected for the preliminary analysis of the drug-releasing capability of Cross-PEI-800 using ammonium chloride. The material has shown its capability to slowly release DOX under a simulated intracellular acidic environment, possibly due to the contribution of H+ ions to the separation of the guest–host complex.
The presented data demonstrate that the system, along with some modifications, merits further studies about the drug-delivery capability. An in-depth study on the delivery selectivity of Cross-PEI-800 would be needed. The presented study could be of interest to medical applications. Due to the capability of PEI-800 in coordinating nucleic acids, the prepared material Cross-PEI-800 could find application as a gene-drug co-delivery system.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/polym13234133/s1, Figure S1: 1H-NMR spectrum compound 1a. Figure S2: 13C-NMR spectrum compound 1a. Figure S3: 1H-NMR second reaction. Figure S4: 13C-NMR second reaction. Figure S5: 1H-NMR spectrum CossPEI-800, Evaluation of monomer weight from the integration of the NMR signals. Figure S6: 1H-NMR spectrum in D2O of Cross-PEI-800 mixed with doxorubicin. Figure S7: 1H-NMR spectrum in D2O of Cross-PEI-800 mixed with doxorubicin after 1 week. Figure S8: 1H-NMR spectrum in D2O of doxorubicin with peak assignment. Figure S9: NOESY spectrum of doxorubicin mixed with Cross-PEI-800. Figure S10: UV-Vis spectra of doxorubicin (DOX), PEI-800, Cross-PEI-800 in pure form and in mixed solutions. Figure S11: Titration of doxorubicin (DOX) with Cross-PEI-800. Figure S12: Fluorescence spectra of the doxorubicin mixed with Cross-PEI 800. Figure S13: 1H-NMR spectrum of Gatifloxacin. Figure S14: 1H-NMR spectrum of Cross-PEI-800 mixed with Gatifloxacin (5 mg + 5 mg). Figure S15: Comparation between 1H-NMR spectra of gatifloxacine, Cross-PEI-800, gatifloxacine + Cross-PEI-800. Figure S16: NOESY spectrum of Gatifloxacin mixed with Cross-PEI-800. Figure S17: UV-Vis spectra of Gatifloxacin with and without crosslinked polymer. Figure S18: Fluorescence spectra of gatifloxacine and gatifloxacine mixed with Cross-PEI-800. Figure S19: 1H-NMR spectrum in D2O of sinomenine. Figure S20: 1H-NMR spectrum of sinomenine in the presence of Cross-PEI-800 (5mg + 5mg). Figure S21: NOESY spectrum of sinomenine in the presence of Cross-PEI-800 (5mg + 5mg). Figure S22: UV-Vis and emission spectra of SIN pure and in the presence of Cross-PEI-800. Figure S23: 1H-NMR spectrum of Camptothecine in the presence of Cross-PEI-800. Figure S24: 1H-NMR spectrum of Camptothecine in the presence of Cross-PEI-800 enlarged. Figure S25: 1H-NMR spectrum of Camptothecine in the presence of Cross-PEI-800. Figure S26: NOESY spectrum of Camptothecine in the presence of Cross-PEI-800. Figure S27: UV-Vis spectra of Camptothecin pure and in the presence of Cross-PEI-800, PEI-800. Figure S28: Emission spectra of Camptothecin. Figure S29: Titration of Camptothecin with Cross-PEI-800. Figure S30: 1H-NMR spectra of Cross-PEI-800 and celastrol in the presence of Cross-PEI-800. Figure S31: 1H-NMR spectrum of celastrol in the presence of Cross-PEI-800. Figure S32: 1H-NMR of celastrol in the presence of Cross-PEI-800 (second dosage of polymer). Figure S33: NOESY spectrum of celastrol in the presence of Cross-PEI-800. Figure S34: UV-Vis spectra of celastrol solution, pure and in the presence of Polymer Cross-PEI-800. Figure S35: Emission spectra of celastrol pure and in the presence of Cross-PEI-800, PEI-800. Figure S36: UV-Vis titration of celastrol with Cross-PEI-800, PEI-800. Figure S37: Water solubility measures of celastrol pure and in the presence of Cross-PEI-800. Figure S38: UV-Vis spectra of celastrol mixed with Cross-PEI-800 after 10 days. Figure S39: UV-Vis spectra of doxorubicin and celastrol in the presence of Cross-PEI-800 and addition of NH4Cl. Figure S40: UV-Vis spectrum of Armed-CTV in methanol. Table S1: Cytotoxicity of polymer Cross-PEI-800 against normal and cancer cell lines.
Author Contributions
C.C. conceived and designed the study for this manuscript, designed and provided all necessary resources for the synthesis of the compounds and the analysis. Y.M.N. performed the organic synthesis, NMR analysis, UV-Vis studies, and emission spectra. P.C. and F.A. designed and directed the experiments on celastrol. C.C., P.C. and V.K.W.W. wrote the bulk of manuscript, and the other authors contributed to parts of their respective segments. J.P.L.N. revised the entire manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Ministry of Science and Technology in Taiwan, grant numbers MOST 107-2113-M-039-008-MY3, MOST 110-2113-M-039-001-and the China Medical University internal grant CMU109-N-25. P.C. acknowledges funding from FDCT grant from the Macao Science and Technology Development Fund (Project code 0087/2020/A).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data and analyses are available in the manuscript and in the Supplementary Materials.
Acknowledgments
We thank Ali Adan Nasim for their support in cytotoxicity studies.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bhalla, V. Supramolecular chemistry: From molecule to molecular machines. Resonance 2018, 23, 277–290. [Google Scholar] [CrossRef]
- James, T.D. Specialty grand challenges in supramolecular chemistry. Front. Chem. 2017, 5, 83. [Google Scholar] [CrossRef]
- Lehn, J.-M. Supramolecular Chemistry—Scope and Perspectives Molecules, Supermolecules, and Molecular Devices (Nobel Lecture). Angew. Chem. Int. Ed. Engl. 1988, 27, 89–112. [Google Scholar] [CrossRef]
- Maiya, B.G. Molecular recognition. J. Porphyr. Phthalocyanines 2000, 4, 393–397. [Google Scholar] [CrossRef]
- Sutherland, I.O. Tilden lecture. Molecular recognition by synthetic receptors. Chem. Soc. Rev. 1986, 15, 63–91. [Google Scholar] [CrossRef]
- Tibbitt, M.W.; Dahlman, J.E.; Langer, R. Emerging frontiers in drug delivery. J. Am. Chem. Soc. 2016, 138, 704–717. [Google Scholar] [CrossRef]
- Peter, F. Guengerich mechanisms of drug toxicity and relevance to pharmaceutical development. Drug Metab. Pharmacokinet. 2011, 26, 3–14. [Google Scholar] [CrossRef]
- Langer, R. Drug delivery and targeting. Nature 1998, 392, 5–10. [Google Scholar] [CrossRef]
- Chen, F.; Li, Y.; Lin, X.; Qiu, H.; Yin, S. Polymeric systems containing supramolecular coordination complexes for drug delivery. Polymers 2021, 13, 370. [Google Scholar] [CrossRef]
- Zakeri, A.; Kouhbanani, M.A.J.; Beheshtkhoo, N.; Beigi, V.; Mousavi, S.M.; Hashemi, S.A.R.; Karimi Zade, A.; Amani, A.M.; Savardashtaki, A.; Mirzaei, E.; et al. Polyethylenimine-based nanocarriers in co-delivery of drug and gene: A developing horizon. Nano Rev. Exp. 2018, 9, 1488497. [Google Scholar] [CrossRef] [PubMed]
- Bieber, T.; Elsässer, H.P. Preparation of a low molecular weight polyethylenimine for efficient cell transfection. Biotechniques 2001, 30, 74–81. [Google Scholar] [CrossRef]
- Bonner, D.K.; Zhao, X.; Buss, H.; Langer, R.; Hammond, P.T. Crosslinked linear polyethylenimine enhances delivery of DNA to the cytoplasm. J. Control. Release 2013, 167, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Boussif, O.; Lezoualc’h, F.; Zanta, M.A.; Mergny, M.D.; Scherman, D.; Demeneix, B.; Behr, J.P. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: Polyethylenimine. Proc. Natl. Acad. Sci. USA 1995, 92, 7297–7301. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhang, X.; Zhang, X.; Yang, B.; Li, Z.; Zhang, Q.; Huang, Z.; Wei, Y. Fabrication of cross-linked fluorescent polymer nanoparticles and their cell imaging applications. J. Mater. Chem. C 2015, 3, 1854–1860. [Google Scholar] [CrossRef]
- Chen, S.; Jin, T. Poly-cross-linked PEI through aromatically conjugated imine linkages as a new class of pH-responsive nucleic acids packing cationic polymers. Front. Pharmacol. 2016, 7, 15. [Google Scholar] [CrossRef]
- Amjad, M.W.; Amin, M.C.I.M.; Katas, H.; Butt, A.M. Doxorubicin-loaded cholic acid-polyethyleneimine micelles for targeted delivery of antitumor drugs: Synthesis, characterization, and evaluation of their in vitro cytotoxicity. Nanoscale Res. Lett. 2012, 7, 687. [Google Scholar] [CrossRef]
- Dong, D.W.; Tong, S.W.; Qi, X.R. Comparative studies of polyethylenimine-doxorubicin conjugates with pH-sensitive and pH-insensitive linkers. J. Biomed. Mater. Res. Part A 2013, 101, 1336–1344. [Google Scholar] [CrossRef]
- Coluccini, C.; Ng, Y.M.; Reyes, Y.I.A.; Chen, H.Y.T.; Khung, Y.L. Functionalization of Polyethyleneimine with hollow cyclotriveratrylene and its subsequent supramolecular interaction with doxorubicin. Molecules 2020, 25, 5455. [Google Scholar] [CrossRef] [PubMed]
- Lindsey, A. The structure of cyclotriveratrylene (10,15-Dihydr0-2,3,7,8,12,13- hexamethoxy-5H-tribenzo[a,d,g]cyclononene) and related compounds. J. Chem. Soc. 1965, 39, 1685–1692. [Google Scholar] [CrossRef]
- Hardie, M.J. Recent advances in the chemistry of cyclotriveratrylene. Chem. Soc. Rev. 2010, 39, 516–527. [Google Scholar] [CrossRef]
- Zhong, Z.; Ikeda, A.; Shinkai, S.; Sakamoto, S.; Yamaguchi, K. Creation of novel chiral cryptophanes by a self-assembling method utilizing a pyridyl-Pd(II) interaction. Org. Lett. 2001, 3, 1085–1087. [Google Scholar] [CrossRef]
- Ahmad, R.; Hardie, M.J. Variable Ag(I) coordination modes in silver cobalt(III) bis(dicarbollide) supramolecular assemblies with cyclotriveratrylene host molecules. Cryst. Growth Des. 2003, 3, 493–499. [Google Scholar] [CrossRef]
- Hardie, M.J.; Sumby, C.J. Interwoven 2-D coordination network prepared from the molecular host tris(isonicotinoyl)cyclotriguaiacylene and silver(I) cobalt(III) bis(dicarbollide). Inorg. Chem. 2004, 43, 6872–6874. [Google Scholar] [CrossRef] [PubMed]
- Sumby, C.J.; Hardie, M.J. Disentangling disorder in the three-dimensional coordination network of {Ag3[Tris(2-pyridylmethyl)cyclotriguaiacylene]2}(PF6)3. Cryst. Growth Des. 2005, 5, 1321–1324. [Google Scholar] [CrossRef]
- Zhang, S.; Echegoyen, L. Selective anion sensing by a tris-amide CTV derivative: 1H NMR titration, self-assembled monolayers, and impedance spectroscopy. J. Am. Chem. Soc. 2005, 127, 2006–2011. [Google Scholar] [CrossRef]
- Sumby, C.J.; Fisher, J.; Prior, T.J.; Hardie, M.J. Tris(pyridylmethylamino)cyclotriguaiacylene cavitands: An investigation of the solution and solid-state behaviour of metallo-supramolecular cages and cavitand-based coordination polymers. Chem. A Eur. J. 2006, 12, 2945–2959. [Google Scholar] [CrossRef] [PubMed]
- Ronson, T.K.; Fisher, J.; Harding, L.P.; Hardie, M.J. Star-burst prisms with cyclotriveratrylene-type ligands: A [Pd 6L8]12+ stella octangular structure. Angew. Chem. Int. Ed. 2007, 46, 9086–9088. [Google Scholar] [CrossRef]
- Bardelang, D.; Camerel, F.; Ziessel, R.; Schmutz, M.; Hannon, M.J. New organogelators based on cyclotriveratrylene platforms bearing 2-dimethylacetal-5-carbonylpyridine fragments. J. Mater. Chem. 2008, 18, 489–494. [Google Scholar] [CrossRef]
- Percec, V.; Imam, M.R.; Peterca, M.; Wilson, D.A.; Heiney, P.A. Self-assembly of dendritic crowns into chiral supramolecular spheres. J. Am. Chem. Soc. 2009, 131, 1294–1304. [Google Scholar] [CrossRef] [PubMed]
- Westcott, A.; Sumby, C.J.; Walshaw, R.D.; Hardie, M.J. Metallo-gels and organo-gels with tripodal cyclotriveratrylene-type and 1,3,5-substituted benzene-type ligands. New J. Chem. 2009, 33, 902–912. [Google Scholar] [CrossRef]
- Little, M.A.; Halcrow, M.A.; Harding, L.P.; Hardie, M.J. Ag(I) organometallic coordination polymers and capsule with Tris-allyl cyclotriveratrylene derivatives. Inorg. Chem. 2010, 49, 9486–9496. [Google Scholar] [CrossRef] [PubMed]
- Cai, F.; Shen, J.-S.; Wang, J.-H.; Zhang, H.; Zhao, J.-S.; Zeng, E.-M.; Jiang, Y.-B. Hydrogelators of cyclotriveratrylene derivatives. Org. Biomol. Chem. 2012, 10, 1418–1423. [Google Scholar] [CrossRef] [PubMed]
- Chighine, K.; Léonce, E.; Boutin, C.; Desvaux, H.; Berthault, P. Xe ultra-fast Z spectroscopy enables micromolar detection of biosensors on a 1 T benchtop spectrometer. Magn. Reson. 2021, 2, 409–420. [Google Scholar] [CrossRef]
- Dumartin, M.L.; Givelet, C.; Meyrand, P.; Bibal, B.; Gosse, I. A fluorescent cyclotriveratrylene: Synthesis, emission properties and acetylcholine recognition in water. Org. Biomol. Chem. 2009, 7, 2725–2728. [Google Scholar] [CrossRef]
- Konarev, D.V.; Khasanov, S.S.; Vorontsov, I.I.; Saito, G.; Antipin, Y. The formation of a single-bonded (C 70 2) 2 dimer in a new ionic multicomponent complex of cyclotriveratrylene. Chem. Commun. 2002, 2, 2548–2549. [Google Scholar] [CrossRef]
- Steed, J.W.; Junk, P.C.; Atwood, J.L.; Barnes, M.J.; Raston, C.L.; Burkhalter, R.S. Ball-and-socket nanostructures—New supramolecular chemistry based on cyclotriveratrylene. J. Am. Chem. Soc. 1994, 116, 10346–10347. [Google Scholar] [CrossRef]
- Felder, D.; Heinrich, B.; Guillon, D.; Nicoud, J.-F.; Nierengarten, J.-F. A Liquid crystalline supramolecular complex of C60 with a cyclotriveratrylene derivative. Chem. A Eur. J. 2000, 6, 3501–3507. [Google Scholar] [CrossRef]
- Huerta, E.; Cequier, E.; de Mendoza, J. Preferential separation of fullerene[84] from fullerene mixtures by encapsulation. Chem. Commun. 2007, 47, 5016–5018. [Google Scholar] [CrossRef] [PubMed]
- Nierengarten, J.F.; Oswald, L.; Eckert, J.F.; Nicoud, J.F.; Armaroli, N. Complexation of fullerenes with dendritic cyclotriveratrylene derivatives. Tetrahedron Lett. 1999, 40, 5681–5684. [Google Scholar] [CrossRef]
- Rio, Y. Water soluble supramolecular cyclotriveratrylene–[60] fullerene. Tetrahedron Lett. 2002, 43, 4321–4324. [Google Scholar] [CrossRef]
- Atwood, J.L.; Barnes, M.J.; Gardinerb, M.G.; Raston, C.L. Cyclotriveratrylene polarisation assisted aggregation of C60. Chem. Commun. 1996, 1449–1450. [Google Scholar] [CrossRef]
- Huerta, E.; Isla, H.; Pérez, E.M.; Bo, C.; Martín, N.; Mendoza, J. De Tripodal exTTF-CTV hosts for fullerenes. J. Am. Chem. Soc. 2010, 132, 5351–5353. [Google Scholar] [CrossRef]
- Mobaraki, M.; Faraji, A.; Zare, M.; Dolati, P.; Ataei, M.; Dehghan Manshadi, H.R. Molecular mechanisms of cardiotoxicity: A review on the major side-effects of doxorubicin. Indian J. Pharm. Sci. 2017, 79, 335–344. [Google Scholar] [CrossRef]
- Jawad, B.; Poudel, L.; Podgornik, R.; Steinmetz, N.F.; Ching, W.Y. Molecular mechanism and binding free energy of doxorubicin intercalation in DNA. Phys. Chem. Chem. Phys. 2019, 21, 3877–3893. [Google Scholar] [CrossRef]
- Thorn, C.F.; Oshiro, C.; Marsh, S.; Hernandez-Boussard, T.; McLeod, H.; Klein, T.E.; Altman, R.B. Doxorubicin pathways: Pharmacodynamics and adverse effects. Pharmacogenet. Genom. 2011, 21, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Wolfson, J.S.; Hooper, D.C. Fluoroquinolone antimicrobial agents. Clin. Microbiol. Rev. 1989, 2, 378–424. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.X.; Peng, C.; Zhang, H.; Qin, L.P. Sinomenium acutum: A review of chemistry, pharmacology, pharmacokinetics, and clinical use. Pharm. Biol. 2012, 50, 1053–1061. [Google Scholar] [CrossRef]
- Das, B.; Krishnaiah, M.; Katta Venkateswarlu, R. Das Camptothecins: Some Recent Chemical Studies. Nat. Prod. Commun. 2006, 1, 255–263. [Google Scholar]
- Cai, X.H.; Jin, J.; He, M.H. Advances in structural modifications of celastrol. Arkivoc 2016, 172–182. [Google Scholar] [CrossRef]
- Salminen, A.; Lehtonen, M.; Paimela, T.; Kaarniranta, K. Celastrol: Molecular targets of thunder god vine. Biochem. Biophys. Res. Commun. 2010, 394, 439–442. [Google Scholar] [CrossRef]
- Coghi, P.; Ng, J.P.L.; Kadioglu, O.; Law, B.Y.K.; Qiu, A.C.; Saeed, M.E.M.; Chen, X.; Ip, C.K.; Efferth, T.; Liu, L.; et al. Synthesis, computational docking and biological evaluation of celastrol derivatives as dual inhibitors of SERCA and P-glycoprotein in cancer therapy. Eur. J. Med. Chem. 2021, 15, 113676. [Google Scholar] [CrossRef]
- Sun, D.; Ding, J.; Xiao, C.; Chen, J.; Zhuang, X.; Chen, X. Preclinical evaluation of antitumor activity of acid-sensitive PEGylated doxorubicin. ACS Appl. Mater. Interfaces 2014, 6, 21202–21214. [Google Scholar] [CrossRef] [PubMed]
- Krasnovskaya, O.O.; Malinnikov, V.M.; Dashkova, N.S.; Gerasimov, V.M.; Grishina, I.V.; Kireev, I.I.; Lavrushkina, S.V.; Panchenko, P.A.; Zakharko, M.A.; Ignatov, P.A.; et al. Thiourea modified doxorubicin: A perspective pH-sensitive prodrug. Bioconjug. Chem. 2019, 30, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Dogan, I.; Pannala, V.R.; Kootala, S.; Hilborn, J.; Ossipov, D. A hyaluronic acid-camptothecin nanoprodrug with cytosolic mode of activation for targeting cancer. Polym. Chem. 2013, 4, 4621–4630. [Google Scholar] [CrossRef]
- Shi, J.; Li, J.; Xu, Z.; Chen, L.; Luo, R.; Zhang, C.; Gao, F.; Zhang, J.; Fu, C. Celastrol: A review of useful strategies overcoming its limitation in anticancer application. Front. Pharmacol. 2020, 11, 1726. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Woodle, M.C.; Mixson, A.J. Advances in delivery systems for doxorubicin. J. Nanomed. Nanotechnol. 2018, 9, 519. [Google Scholar] [CrossRef]
- Meng, H.A.; Xue, M.; Xia, T.A.; Zhao, Y.L.; Tamanoi, F.; Stoddart, J.F.; Zink, J.I.; Nel, A.E. Autonomous in vitro anticancer drug release from mesoporous silica nanoparticles by pH-sensitive nanovalves. J. Am. Chem. Soc. 2010, 132, 12690. [Google Scholar] [CrossRef]
- Lao, J.; Madani, J.; Puértolas, T.; Álvarez, M.; Hernández, A.; Pazo-Cid, R.; Artal, Á.; Antón Torres, A. Liposomal doxorubicin in the treatment of breast cancer patients: A review. J. Drug Deliv. 2013, 2013, 456409. [Google Scholar] [CrossRef]
- Bao, Y.; Yin, M.; Hu, X.; Zhuang, X.; Sun, Y.; Guo, Y.; Tan, S.; Zhang, Z. A safe, simple and efficient doxorubicin prodrug hybrid micelle for overcoming tumor multidrug resistance and targeting delivery. J. Control. Release 2016, 235, 182–194. [Google Scholar] [CrossRef]
- Tan, M.L.; Choong, P.F.M.; Dass, C.R. Review: Doxorubicin delivery systems based on chitosan for cancer therapy. J. Pharm. Pharmacol. 2009, 61, 131–142. [Google Scholar] [CrossRef]
- Marcianes, P.; Negro, S.; Barcia, E.; Montejo, C.; Fernández-Carballido, A. Potential active targeting of gatifloxacin to macrophages by means of surface-modified PLGA microparticles destined to treat tuberculosis. AAPS PharmSciTech 2020, 21, 15. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Qiu, N.; Ou, Y.; Wei, Q.; Tang, W.; Zheng, M.; Xing, Y.; Li, J.J.; Ling, Y.; Li, J.; et al. N-Demethylsinomenine, an active metabolite of sinomenine, attenuates chronic neuropathic and inflammatory pain in mice. Sci. Rep. 2021, 11, 9300. [Google Scholar] [CrossRef]
- Webber, M.J.; Langer, R. Drug delivery by supramolecular design. Chem. Soc. Rev. 2017, 46, 6600–6620. [Google Scholar] [CrossRef]
- Yang, J.; Chatelet, B.; Dufaud, V.; Hérault, D.; Jean, M.; Vanthuyne, N.; Mulatier, J.C.; Pitrat, D.; Guy, L.; Dutasta, J.P.; et al. Enantio- and substrate-selective recognition of chiral neurotransmitters with C3-symmetric switchable receptors. Org. Lett. 2020, 22, 891–895. [Google Scholar] [CrossRef]
- Yaouba, S.; Valkonen, A.; Coghi, P.; Gao, J.; Guantai, E.M.; Derese, S.; Wong, V.K.W.; Erdélyi, M.; Yenesew, A. Crystal structures and cytotoxicity of ent-kaurane-type diterpenoids from two Aspilia species. Molecules 2018, 23, 3199. [Google Scholar] [CrossRef]
- Wei, Q.; Seward, G.K.; Hill, P.A.; Patton, B.; Dimitrov, I.E.; Kuzma, N.N.; Dmochowski, I.J. Designing 129Xe NMR biosensors for matrix metalloproteinase detection. J. Am. Chem. Soc. 2006, 128, 13274–13283. [Google Scholar] [CrossRef] [PubMed]
- Riggle, B.A.; Wang, Y.; Dmochowski, I.J. A “Smart” 129Xe NMR biosensor for pH-dependent cell labeling. J. Am. Chem. Soc. 2015, 137, 5542–5548. [Google Scholar] [CrossRef]
- Brotin, T.; Dutasta, J.P. Xe@cryptophane complexes with C2 symmetry: Synthesis and investigations by 129Xe NMR of the consequences of the size of the host cavity for xenon encapsulation. Eur. J. Org. Chem. 2003, 2003, 973–984. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, R.; Lu, X.; Lu, W.; Zhang, C.; Liang, W. Pegylated phospholipids-based self-assembly with water-soluble drugs. Pharm. Res. 2010, 27, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Al-Abdullah, E.S. Gatifloxacin; Elsevier: Amsterdam, The Netherlands, 2012; Volume 37, p. 2200. ISBN 978012397. [Google Scholar]
- Aura, R.; Silvia, I.; Eleonora, M.; Gabriel, H. Quinolone antibacterials: Commentary and considerations regarding UV spectra and chemical structure. Acta Medica Marisiensis 2015, 61, 328–336. [Google Scholar] [CrossRef]
- Dong, J.W.; Li, X.J.; Cai, L.; Shi, J.Y.; Li, Y.F.; Yang, C.; Li, Z.J. Simultaneous determination of alkaloids dicentrine and sinomenine in Stephania epigeae by 1H NMR spectroscopy. J. Pharm. Biomed. Anal. 2018, 160, 330–335. [Google Scholar] [CrossRef]
- Ezell, E.L.; Smith, L.L. 1H- and 13C-NMR Spectra of Camptothecin and Derivatives. J. Nat. Prod. 1991, 54, 1645–1650. [Google Scholar] [CrossRef]
- Subramanian, N.; Sundaraganesan, N.; Sudha, S.; Aroulmoji, V.; Sockalingam, G.D.; Bergamin, M. Experimental and theoretical investigation of the molecular and electronic structure of anticancer drug camptothecin. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 78, 1058–1067. [Google Scholar] [CrossRef] [PubMed]
- Zolotarskaya, O.Y.; Wagner, A.F.; Beckta, J.M.; Valerie, K.; Wynne, K.J.; Yang, H. Synthesis of water-soluble camptothecin-polyoxetane conjugates via click chemistry. Mol. Pharm. 2012, 9, 3403–3408. [Google Scholar] [CrossRef][Green Version]
- Zhang, D.; Chen, Z.; Hu, C.; Yan, S.; Li, Z.; Lian, B.; Xu, Y.; Ding, R.; Zeng, Z.; Zhang, X.-K.; et al. Celastrol binds to its target protein via specific noncovalent interactions and reversible covalent bonds. Chem. Commun. 2018, 54, 12871–12874. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, W.; Wang, M.; Zhang, X.; Zhang, H.; Tong, X.; Xiao, Y. Competitive profiling of celastrol targets in human cervical cancer HeLa cells via quantitative chemical proteomics. Mol. Biosyst. 2017, 13, 83–91. [Google Scholar] [CrossRef]
- Dawn, A.; Chandra, H.; Ade-Browne, C.; Yadav, J.; Kumari, H. Multifaceted supramolecular interactions from C-Methylresorcin[4]arene lead to an enhancement in in vitro antibacterial activity of Gatifloxacin. Chem. A Eur. J. 2017, 23, 18171–18179. [Google Scholar] [CrossRef]
- Lekar, A.V.; Borisenko, S.N.; Vetrova, E.V.; Borisenko, R.N.; Borisenko, N.I. Mass Spectrometry study of non-covalent complexes of bioflavonoids with cyclotriveratrylene synthesized in subcritical water. Am. J. Anal. Chem. 2013, 4, 464–468. [Google Scholar] [CrossRef][Green Version]
- Fernandes, T.S.; Santos, E.C.S.; Madriaga, V.G.C.; Bessa, I.A.A.; Nascimento, V.; Garcia, F.; Ronconi, C.M. A self-assembled AMF-responsive nanoplatform based on Pillar[5]arene and superparamagnetic nanoparticles for controlled release of doxorubicin. J. Braz. Chem. Soc. 2019, 30, 2452–2463. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).