Ultrastructural and Physicochemical Characterization of a Non-Crosslinked Type 1 Bovine Derived Collagen Membrane
Abstract
:1. Introduction
2. Materials and Methods
2.1. Surface Morphology Analysis
2.2. Scanning Electron Microscopy and Transmission Electron Microscopy
- (a)
- fixation in 2.5 wt% glutaraldehyde diluted in 0.1 M cacodylate buffer solution (overnight);
- (b)
- washing in 3 baths in cacodylate buffer solution (0.1 M), for 15 min each bath;
- (c)
- dehydration in 30 vol% acetone bath (15 min), 50 vol% acetone, 70 vol% acetone (15 min), 90 vol% acetone (15 min), 100 vol% acetone (15 min), and 100 vol% acetone (15 min);
- (d)
- infiltration in acetone + epon mixture (2:1) for 2 h; acetone + epon (1:1) for 2 h; acetone + epon mixture (1:2) for 2 h, infiltration in pure Epon (overnight);
- (e)
- inclusion in Epon and polymerization between 48 and 72 h at 60 °C;
- (f)
- plate cuts with a thickness of 1 micrometer and staining with toluidine blue;
- (g)
- cutting with ultramicrotome to obtain 70 nm slides, which were collected on 300 mesh copper grids;
- (h)
- contrasting of the slides with uranyl acetate (for 20–30 min); and
- (i)
- TEM observation.
2.3. Surface Roughness and Wettability
2.4. Tensile Test
- (1)
- Maximal load (N) at the extreme loading point.
- (2)
- Tensile strength (maximum stress, MPa) calculated as the maximal load divided by the cross-sectional area of each specimen.
- (3)
- Maximal strain (%) calculated at the point of maximal load.
2.5. Measurement of Thermodynamic Properties Using DSC
2.6. Immunohistochemical Analysis (In Vitro)
- (a)
- deparaffinize the sections in 3 xylol baths (5 min each bath);
- (b)
- hydrate the sections (100%, 90%, 70% ethanol, distilled water—5 min each bath);
- (c)
- incubate the sections in 3% hydrogen peroxide diluted in distilled water, for 15 min, protected from light to inhibit endogenous peroxidase;
- (d)
- wash in 3 baths of TBS (Tris Buffered Saline) pH 7.4 buffer (5 min each bath);
- (e)
- perform antigen retrieval in Tris/EDTA (ethylenediaminetetraacetic acid) buffer pH 9.0 at 95 °C, for 20 min;
- (f)
- cool and wash in 3 baths of TBS buffer pH 7.4 (5 min each bath); and
- (g)
- block non-specific sites with 3% TBS/BSA for 20 min.
3. Results
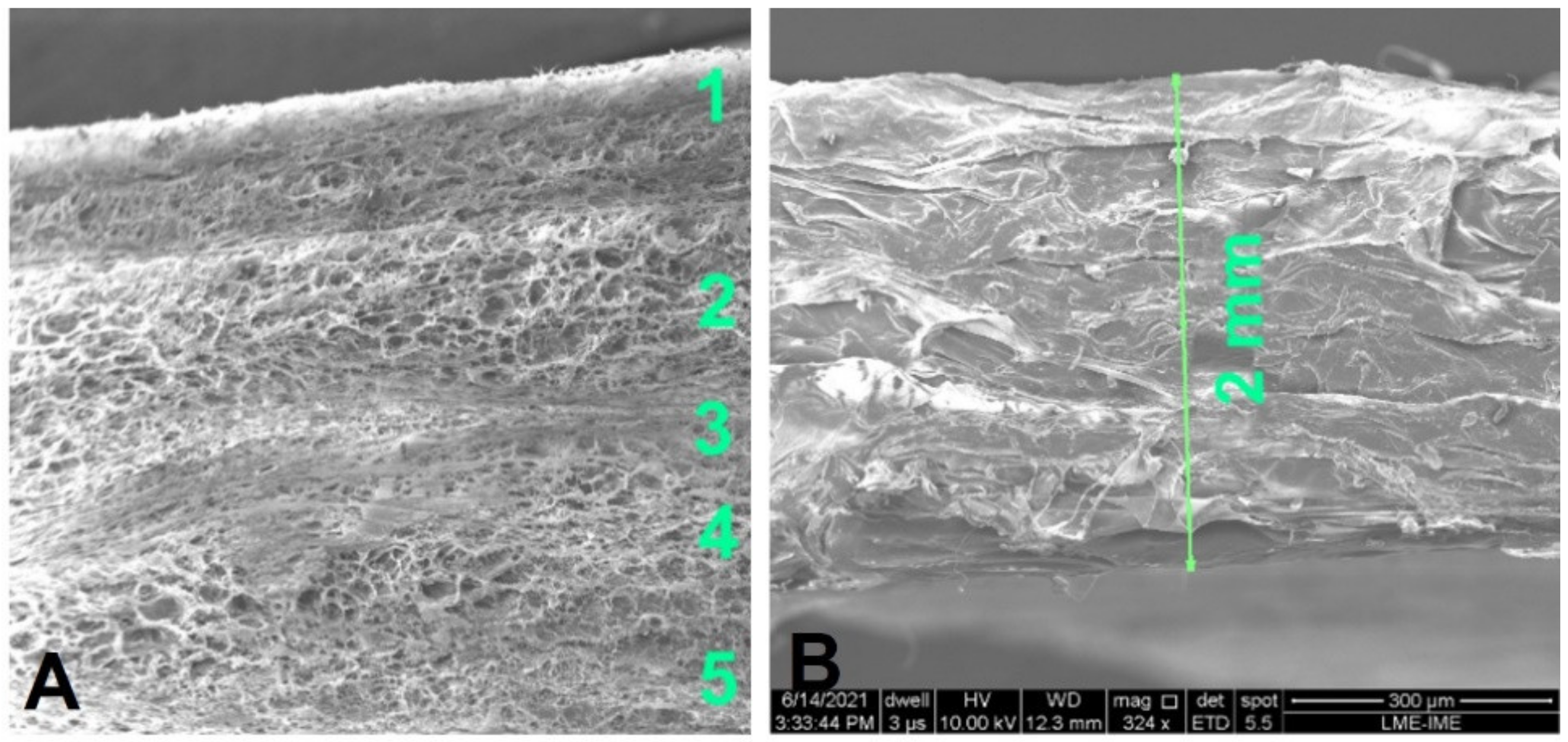
3.1. Surface Morphology
3.2. Roughness and Wettability
- Ra (average roughness): Ra is average value of the amplitudes in relation to a reference line. This parameter quantifies the average vertical distance between the five highest peaks and five major valleys.
- Rsk: asymmetry factor (skewness).
- Rms (root mean square roughness): Rms is the root-mean-square deviation of all points from a plane fit to the test part surface.Rku: flattening profile (kurtosis).
- PV: the height between the lowest and the highest point on the test area.
- Rpk: maximum height of the highest peak of surface roughness, situated above the midline.
- Rk: the core height of the profile along the Y-axis of the BAC curve generated by placing a 40% line on the curve at the minimum slope point and extending the lines to the 0% and 100% points.
- R3z (average roughness of the third peak). In each module, the distances between the third highest peak and the third deepest valley are plotted. R3z is the height of this peak plus the depth of the valley.
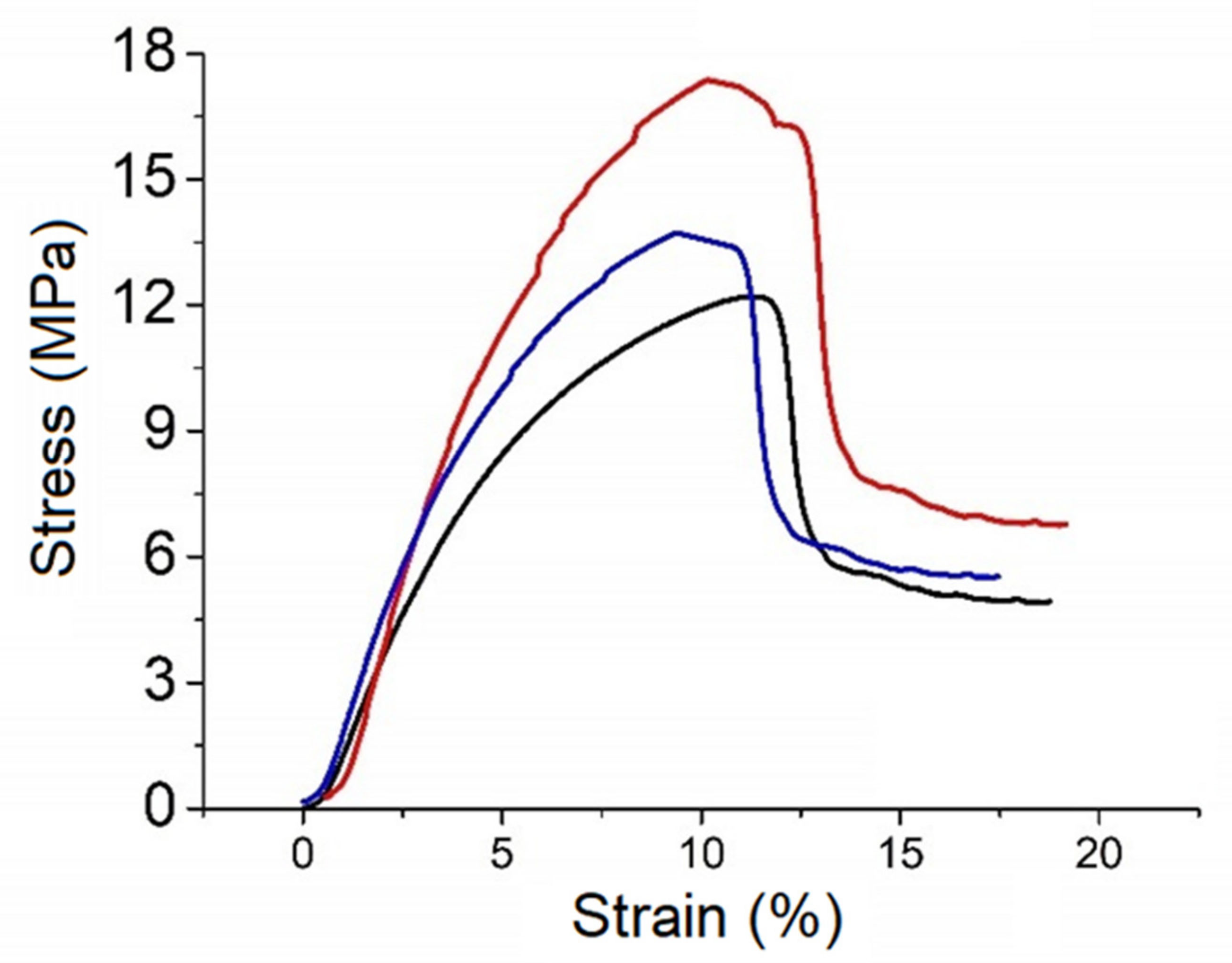
3.3. Tensile Tests
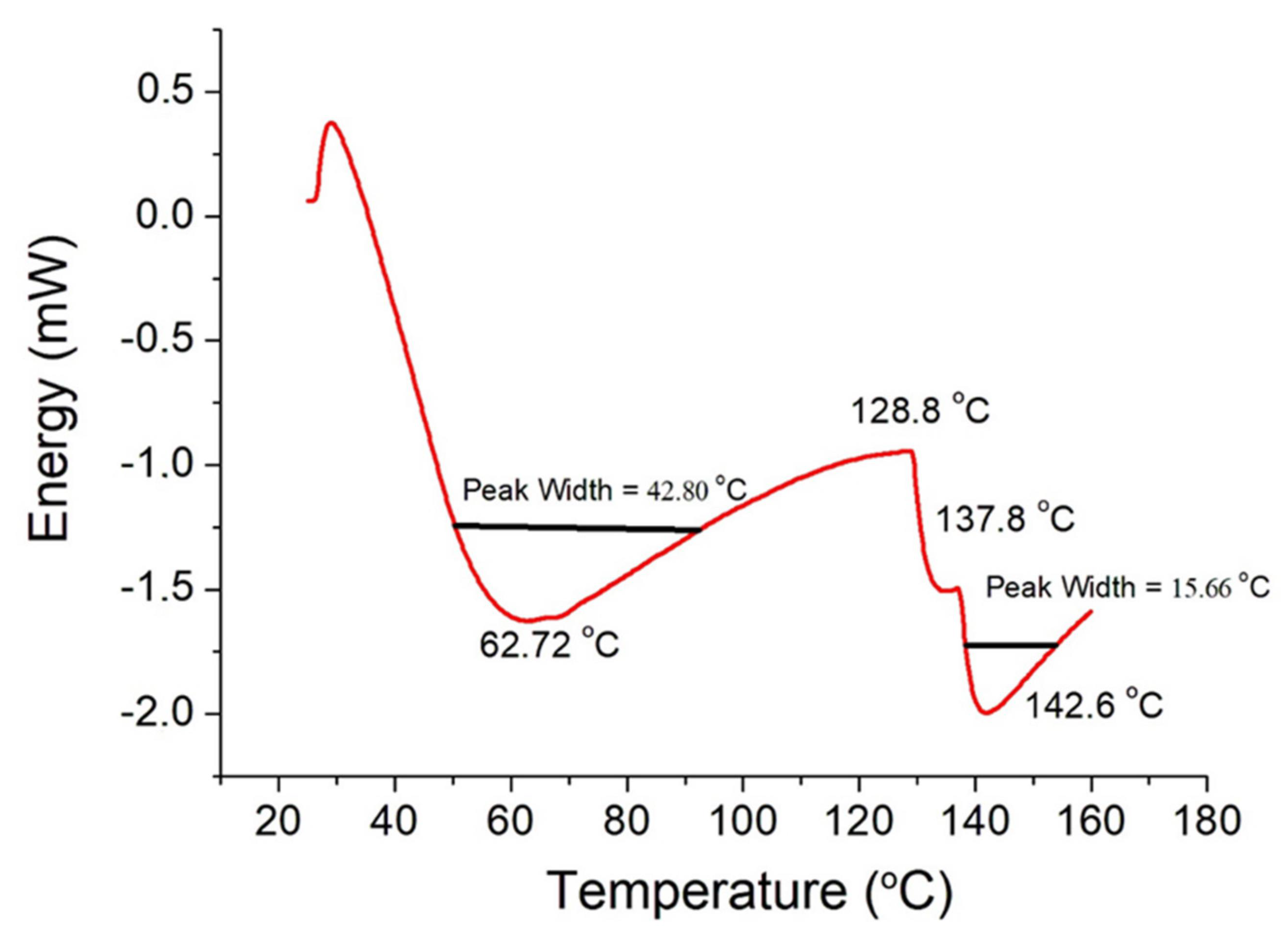
3.4. DSC
3.5. Immunohistochemistry
4. Discussion
5. Conclusions
- The collagen fibers are structured in five layers.
- The elasticity is adequate for complex procedure (Young modulus = 3.58 GPa).
- It has good wettability (60s = 55.89o).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Birkenfeld, F.; Behrens, E.; Kern, M.; Gassling, V.; Wiltfang, J. Mechanical properties of collagen membranes: Are they sufficient for orbital floor reconstructions? J. Cranio-Maxillofac. Surg. 2014, 43, 260–263. [Google Scholar] [CrossRef] [PubMed]
- Chia-Lai, P.-J.; Orlowska, A.; Al-Maawi, S.; Dias, A.; Zhang, Y.; Wang, X.; Zender, N.; Sader, R.; Kirkpatrick, C.J.; Ghanaati, S. Sugar-based collagen membrane cross-linking increases barrier capacity of membranes. Clin. Oral Investig. 2017, 22, 1851–1863. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M. Processing of collagen based biomaterials and the resulting materials properties. Biomed. Eng. Online 2019, 18, 1–74. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Zheng, C.; Luo, X.; Wang, X.; Jiang, H. Recent advances of collagen-based biomaterials: Multi-hierarchical structure, modification and biomedical applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 99, 1509–1522. [Google Scholar] [CrossRef]
- Rudolf, J.-L.; Moser, C.; Sculean, A.; Eick, S. In-vitro antibiofilm activity of chlorhexidine digluconate on polylactide-based and collagen-based membranes. BMC Oral Health 2019, 19, 291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bottino, M.C.; Thomas, V. Membranes for Periodontal Regeneration—A Materials Perspective. Front. Oral Biol. 2015, 17, 90–100. [Google Scholar] [CrossRef]
- Brum, I.S.; Elias, C.N.; de Carvalho, J.J.; Pires, J.L.S.; Pereira, M.J.S.; de Biasi, R.S. Properties of a bovine collagen type I membrane for guided bone regeneration applications. e-Polymers 2021, 21, 210–221. [Google Scholar] [CrossRef]
- Higuchi, J.; Fortunato, G.; Woźniak, B.; Chodara, A.; Domaschke, S.; Męczyńska-Wielgosz, S.; Kruszewski, M.; Dommann, A.; Łojkowski, W. Polymer Membranes Sonocoated and Electrosprayed with Nano-Hydroxyapatite for Periodontal Tissues Regeneration. Nanomaterials 2019, 9, 1625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caballé-Serrano, J.; Munar-Frau, A.; Delgado, L.; Pérez, R.; Hernndez-Alfaro, F. Physicochemical characterization of barrier membranes for bone regeneration. J. Mech. Behav. Biomed. Mater. 2019, 97, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Deng, J.; Sun, X.; Qu, Y.; Man, Y. Collagen Membrane and Immune Response in Guided Bone Regeneration: Recent Progress and Perspectives. Tissue Eng. Part B Rev. 2017, 23, 421–435. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, L.; Liu, R.; Lin, Y.; Chen, S.; Lu, S.; Lin, Z.; Chen, Z.; Wu, C.; Xiao, Y. The osteoimmunomodulatory property of a barrier collagen membrane and its manipulation via coating nanometer-sized bioactive glass to improve guided bone regeneration. Biomater. Sci. 2018, 6, 1007–1019. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Tang, C.; Wang, M.; Zhao, Z.; Shokoohi-Tabrizi, H.A.; Shi, B.; Andrukhov, O.; Rausch-Fan, X. In vitro biocompatibility of biohybrid polymers membrane evaluated in human gingival fibroblasts. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 2590–2598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Georgiev, G.P.; Kotov, G.; Iliev, A.; Slavchev, S.; Ovtscharoff, W.; Landzhov, B. A comparative study of the epiligament of the medial collateral and the anterior cruciate ligament in the human knee. Immunohistochemical analysis of collagen type I and V and procollagen type III. Ann. Anat.—Anat. Anz. 2019, 224, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Rujirachotiwat, A.; Suttamanatwong, S. Curcumin Promotes Collagen Type I, Keratinocyte Growth Factor-1, and Epidermal Growth Factor Receptor Expressions in the In Vitro Wound Healing Model of Human Gingival Fibroblasts. Eur. J. Dent. 2020, 15, 063–070. [Google Scholar] [CrossRef]
- Ma, S.; Adayi, A.; Liu, Z.; Li, M.; Wu, M.; Xiao, L.; Sun, Y.; Cai, Q.; Yang, X.; Zhang, X.; et al. Asymmetric Collagen/chitosan Membrane Containing Minocycline-loaded Chitosan Nanoparticles for Guided Bone Regeneration. Sci. Rep. 2016, 6, 31822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pozzolini, M.; Tassara, E.; Dodero, A.; Castellano, M.; Vicini, S.; Ferrando, S.; Aicardi, S.; Cavallo, D.; Bertolino, M.; Petrenko, I.; et al. Potential Biomedical Applications of Collagen Filaments derived from the Marine Demosponges Ircinia oros (Schmidt, 1864) and Sarcotragus foetidus (Schmidt, 1862). Mar. Drugs 2021, 19, 563. [Google Scholar] [CrossRef]
- Bet, M.; Goissis, G.; Vargas, S.; Selistre-De-Araujo, H. Cell adhesion and cytotoxicity studies over polyanionic collagen surfaces with variable negative charge and wettability. Biomaterials 2003, 24, 131–137. [Google Scholar] [CrossRef]
- Ramires, G.A.D.; Helena, J.T.; De Oliveira, J.C.S.; Faverani, L.P.; Bassi, A.P.F. Evaluation of Guided Bone Regeneration in Critical Defects Using Bovine and Porcine Collagen Membranes: Histomorphometric and Immunohistochemical Analyses. Int. J. Biomater. 2021, 2021, 33859694. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, A.; Apel, C.; Sellhaus, B.; Van Neerven, S.; Wessing, B.; Hilgers, R.-D.; Pallua, N. Differences in degradation behavior of two non-cross-linked collagen barrier membranes: Anin vitroandin vivostudy. Clin. Oral Implant. Res. 2013, 25, 1403–1411. [Google Scholar] [CrossRef] [PubMed]
- Ortolani, E.; Quadrini, F.; Bellisario, D.; Santo, L.; Polimeni, A.; Santarsiero, A. Mechanical qualification of collagen membranes used in dentistry. Ann. dell’Istituto Super. Sanita 2015, 51, 229–235. [Google Scholar] [CrossRef]
- Raz, P.; Brosh, T.; Ronen, G.; Tal, H. Tensile Properties of Three Selected Collagen Membranes. BioMed Res. Int. 2019, 2019, 5163603. [Google Scholar] [CrossRef]
- Sam, G.; Vadakkekuttical, R.J.; Amol, N.V. In vitro evaluation of mechanical properties of platelet-rich fibrin membrane and scanning electron microscopic examination of its surface characteristics. J. Indian Soc. Periodontol. 2015, 19, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Samouillan, V.; Delaunay, F.; Dandurand, J.; Merbahi, N.; Gardou, J.-P.; Yousfi, M.; Gandaglia, A.; Spina, M.; Lacabanne, C. The Use of Thermal Techniques for the Characterization and Selection of Natural Biomaterials. J. Funct. Biomater. 2011, 2, 230–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nistor, M.-T.; Pamfil, D.; Schick, C.; Vasile, C. Study of the heat-induced denaturation and water state of hybrid hydrogels based on collagen and poly (N-isopropyl acrylamide) in hydrated conditions. Thermochim. Acta 2014, 589, 114–122. [Google Scholar] [CrossRef]
- Ezati, M.; Safavipour, H.; Houshmand, B.; Faghihi, S. Development of a PCL/gelatin/chitosan/β-TCP electrospun composite for guided bone regeneration. Prog. Biomater. 2018, 7, 225–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kopp, J.; Bonnet, M.; Renou, J. Effect of Collagen Crosslinking on Collagen-Water Interactions (A DSC Investigation). Matrix 1990, 9, 443–450. [Google Scholar] [CrossRef]
- Engel, J.; Bächinger, H.P. Structure, Stability and Folding of the Collagen Triple Helix. In Collagen; Brinckmann, J., Notbohm, H., Müller, P.K., Eds.; Topics in Current Chemistry; Springer: Berlin/Heidelberg, Germany, 2005; Volume 247. [Google Scholar] [CrossRef]
- Rochdi, A.; Fouca, L.; Renou, J.P. Effect of Thermal Denaturation on Water–Collagen Interactions: NMR Relaxation and Differential Scanning Calorimetry Analysis. Biopolymers 1999, 50, 690–696. [Google Scholar] [CrossRef]
- León-Mancilla, B.; Araiza-Téllez, M.; Flores-Flores, J.; Piña-Barba, M. Physico-chemical characterization of collagen scaffolds for tissue engineering. J. Appl. Res. Technol. 2016, 14, 77–85. [Google Scholar] [CrossRef]
- Zhao, Y.-H.; Chi, Y.-J. Characterization of Collagen from Eggshell Membrane. Biotechnology 2009, 8, 254–258. [Google Scholar] [CrossRef] [Green Version]
- Elgali, I.; Omar, O.; Dahlin, C.; Thomsen, P. Guided bone regeneration: Materials and biological mechanisms revisited. Eur. J. Oral Sci. 2017, 125, 315–337. [Google Scholar] [CrossRef] [PubMed]


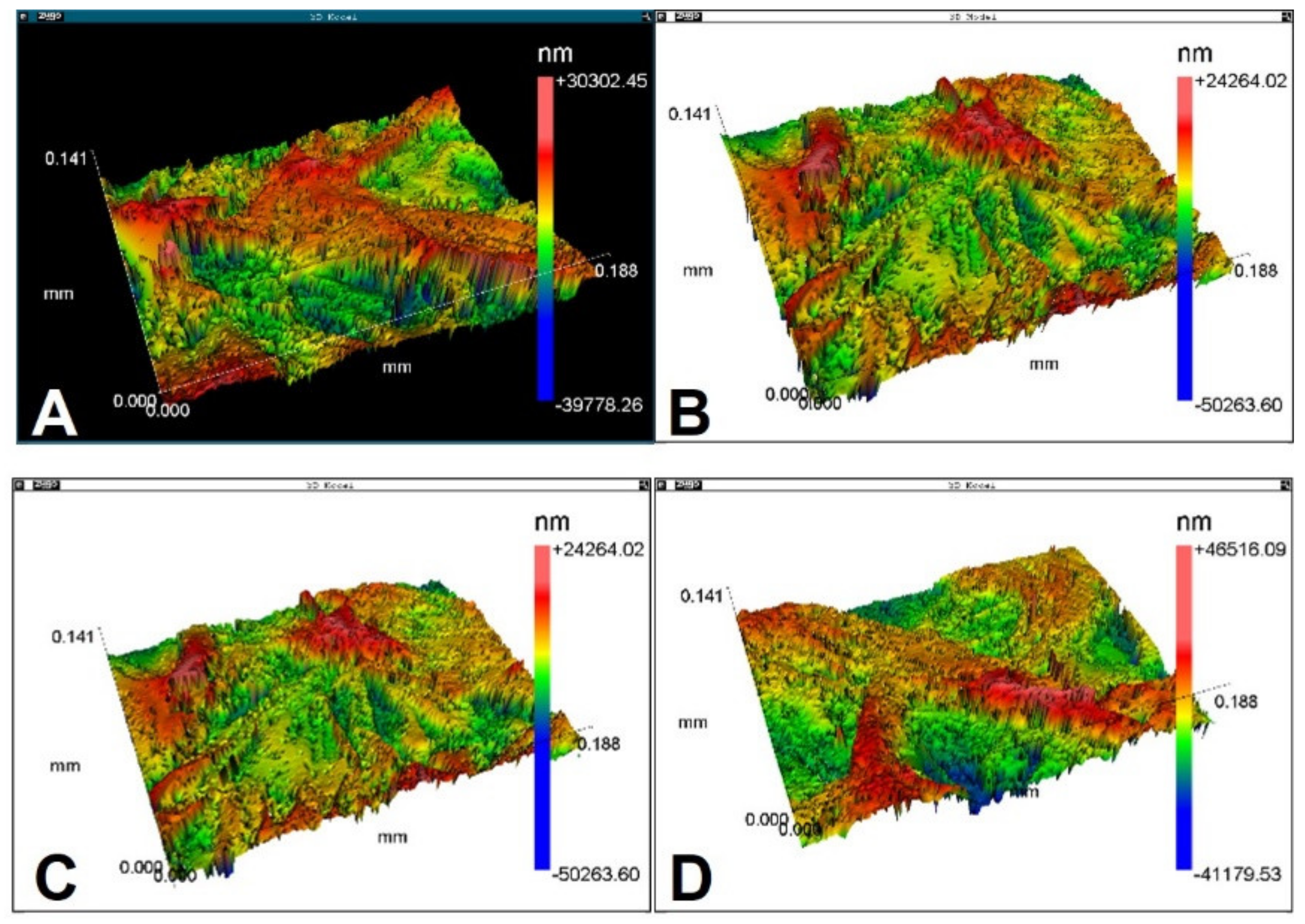


| Sample | Ra (µm) | Rsk (µm) | Rms (µm) | Rku (µm) | PV (µm) | Rpk (µm) | Rk (µm) | R3z (µm) |
|---|---|---|---|---|---|---|---|---|
| 1 | 6.38 | −0.05 | 8.01 | 3.12 | 67.16 | 8.19 | 19.79 | 45.84 |
| 2 | 7.56 | −0.18 | 9.53 | 3.07 | 66.74 | 8.41 | 22.75 | 57.25 |
| 3 | 6.38 | −0.38 | 7.84 | 2.98 | 70.08 | 5.58 | 23.01 | 62.86 |
| 4 | 4.87 | −0.21 | 6.40 | 4.27 | 74.53 | 7.42 | 14.15 | 60.13 |
| 5 | 7.05 | −0.30 | 8.84 | 3.31 | 94.27 | 8.10 | 22.20 | 79.77 |
| 6 | 7.06 | −0.31 | 8.85 | 3.31 | 87.70 | 8.00 | 22.20 | 78.07 |
| Mean | 6.55 | −0.24 | 8.24 | 3.34 | 76.75 | 7.62 | 20.68 | 63.98 |
| StdDev | 0.94 | 0.12 | 1.10 | 0.47 | 11.56 | 1.05 | 3.40 | 12.95 |
| Sample | |||||||
|---|---|---|---|---|---|---|---|
| Time (s) | 1 | 2 | 3 | 4 | 5 | Mean | Standard Deviation |
| 0 | 82.56° | 80.26° | 80.26° | 77.35° | 78.24° | 79.73° | 2.03° |
| 15 | 64.50° | 69.38° | 64.20° | 66.03° | 2.91° | ||
| 30 | 53.71° | 65.89° | 61.06° | 60.22° | 6.13° | ||
| 45 | 49.96° | 62.58° | 60.51° | 57.68° | 6.77° | ||
| 60 | 45.61° | 58.92° | 56.89° | 55.89° | 7.17° | ||
| Parameter | Time after Drop Deposition (s) | ||||
|---|---|---|---|---|---|
| 0 | 15 | 30 | 45 | 60 | |
| Contact Angle (degrees) | 79.73 | 66.03 | 60.22 | 57.68 | 55.89 |
| Wetting Tension (mN/m) | 14.65 | 31.43 | 43.21 | 46.96 | 37.72 |
| Base (mm) | 1.44 | 1.36 | 1.2121 | 1.11 | 0.90 |
| Base Area (mm2) | 1.62 | 1.45 | 1.15 | 0.96 | 0.64 |
| Height (mm) | 0.61 | 0.52 | 0.43 | 0.39 | 0.23 |
| Sessile Volume (µL) | 0.75 | 0.65 | 0.54 | 0.49 | 0.34 |
| Sessile Surface Area (mm2) | 3.05 | 3.04 | 2.89 | 2.78 | 2.55 |
| e (mm) | σmax (MPa) | εmax (%) | ||
|---|---|---|---|---|
| This work | 0.31 | 14.43 | 18.46 | |
| Bozkurt A et al. [19] | Bio-gide | 0.48 | 12.3 | 4.7 |
| Ortolani et al. [20] | Collprotect | 0.28 | 13.1 | 16.3 |
| Jason | 0.20 | 13.0 | 17.9 | |
| Raz et al. [21] | Remaix | 0.29 | 10.4 | 7.01 |
| OssixPlus | 0.26 | 5.13 | 6.0 |
| Parameter | ||||
|---|---|---|---|---|
| Tonset (°C) | Tp (°C) | Tendset (°C) | ΔH (J/g) | |
| This work | 41.3 | 62.7 | 84.1 | 42.1 |
| Samouillan [23] | 65.1 | 58.5 | ||
| León-Mancilla [29] | 85 | 90.0 | ||
| Rochdi [28] | 58.6–61.6 | 45.6–78.1 | ||
| Kopp et al. [26] | 75 | 150.0 | 85.0 | |
| Zhao [30] | 60.0–65.0 | |||
| Nistor [24] | 73.0 | 48.0 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva Brum, I.; Elias, C.N.; Nascimento, A.L.R.; de Andrade, C.B.V.; de Biasi, R.S.; de Carvalho, J.J. Ultrastructural and Physicochemical Characterization of a Non-Crosslinked Type 1 Bovine Derived Collagen Membrane. Polymers 2021, 13, 4135. https://doi.org/10.3390/polym13234135
da Silva Brum I, Elias CN, Nascimento ALR, de Andrade CBV, de Biasi RS, de Carvalho JJ. Ultrastructural and Physicochemical Characterization of a Non-Crosslinked Type 1 Bovine Derived Collagen Membrane. Polymers. 2021; 13(23):4135. https://doi.org/10.3390/polym13234135
Chicago/Turabian Styleda Silva Brum, Igor, Carlos Nelson Elias, Ana Lucia Rosa Nascimento, Cherley Borba Vieira de Andrade, Ronaldo Sergio de Biasi, and Jorge José de Carvalho. 2021. "Ultrastructural and Physicochemical Characterization of a Non-Crosslinked Type 1 Bovine Derived Collagen Membrane" Polymers 13, no. 23: 4135. https://doi.org/10.3390/polym13234135
APA Styleda Silva Brum, I., Elias, C. N., Nascimento, A. L. R., de Andrade, C. B. V., de Biasi, R. S., & de Carvalho, J. J. (2021). Ultrastructural and Physicochemical Characterization of a Non-Crosslinked Type 1 Bovine Derived Collagen Membrane. Polymers, 13(23), 4135. https://doi.org/10.3390/polym13234135






