Development and Progression of Polymer Electrolytes for Batteries: Influence of Structure and Chemistry
Abstract
:1. Introduction
2. Polymer Electrolytes
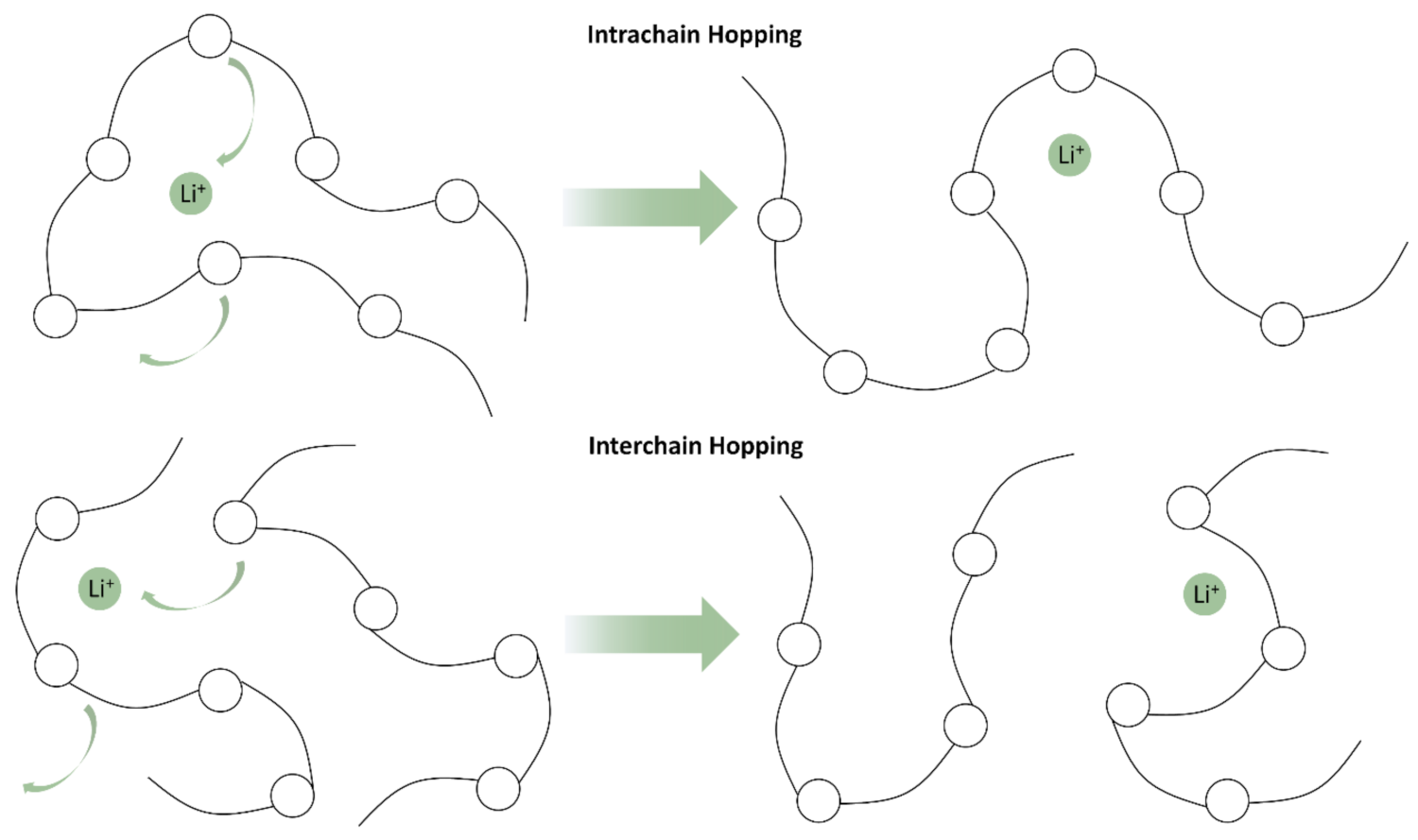
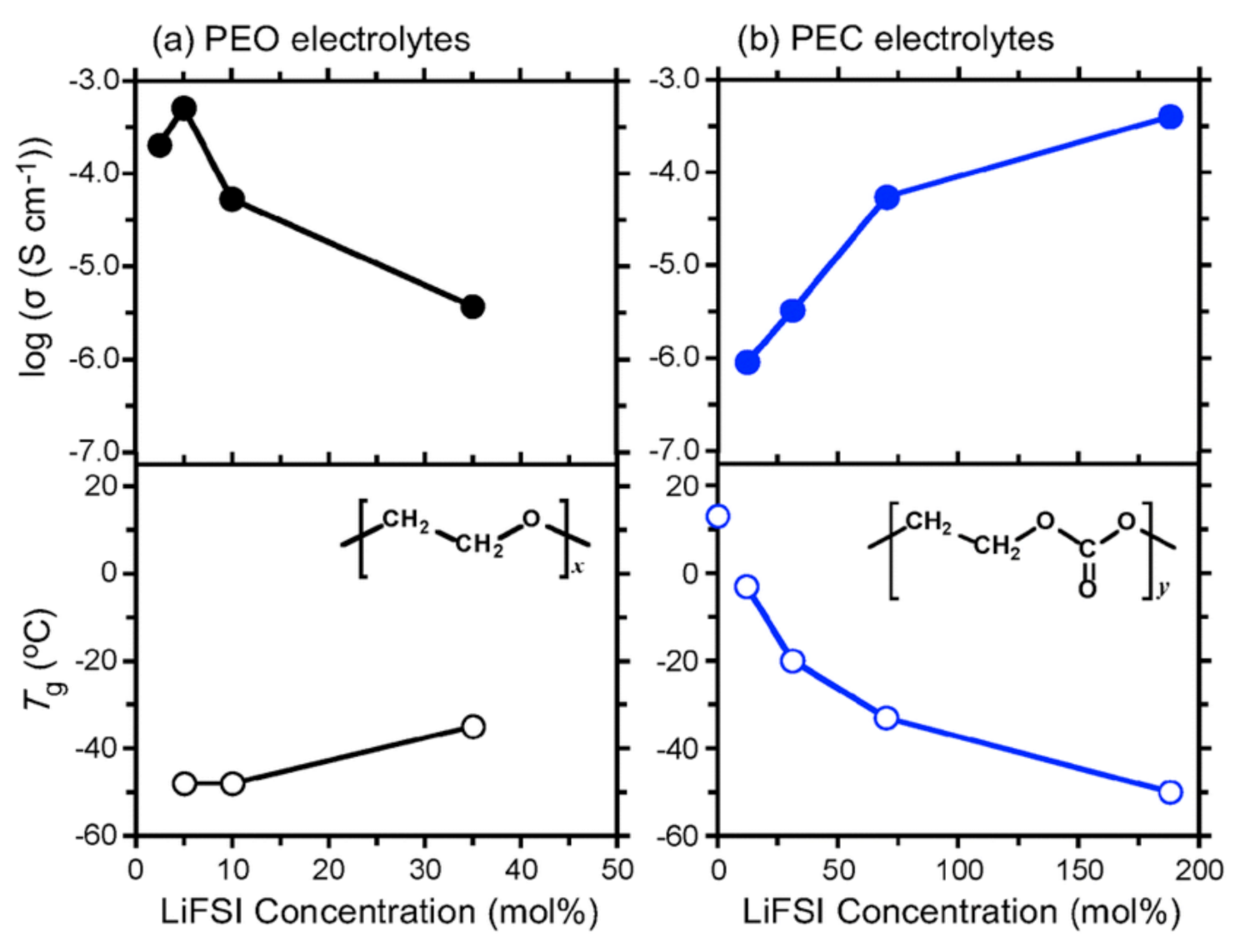
2.1. Polyethylene Oxides—PEOs
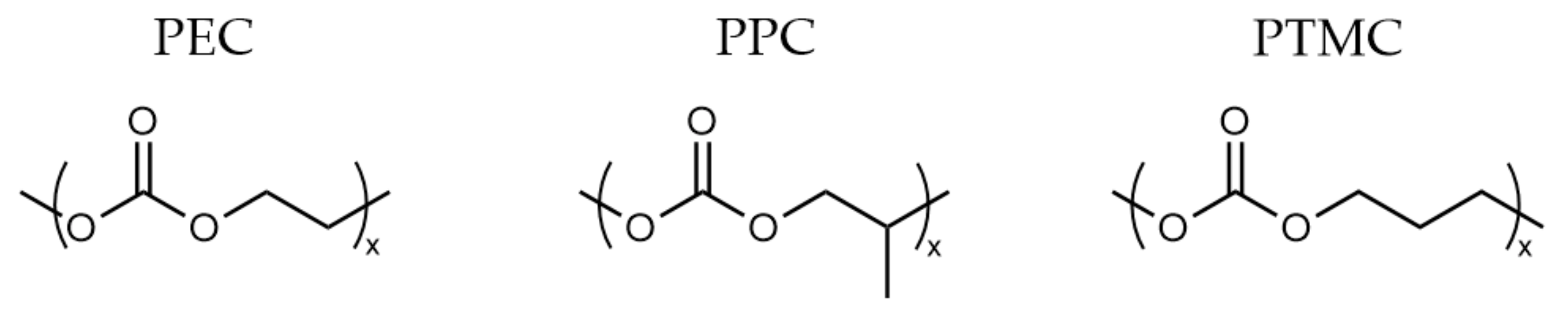
2.2. Polycarbonates
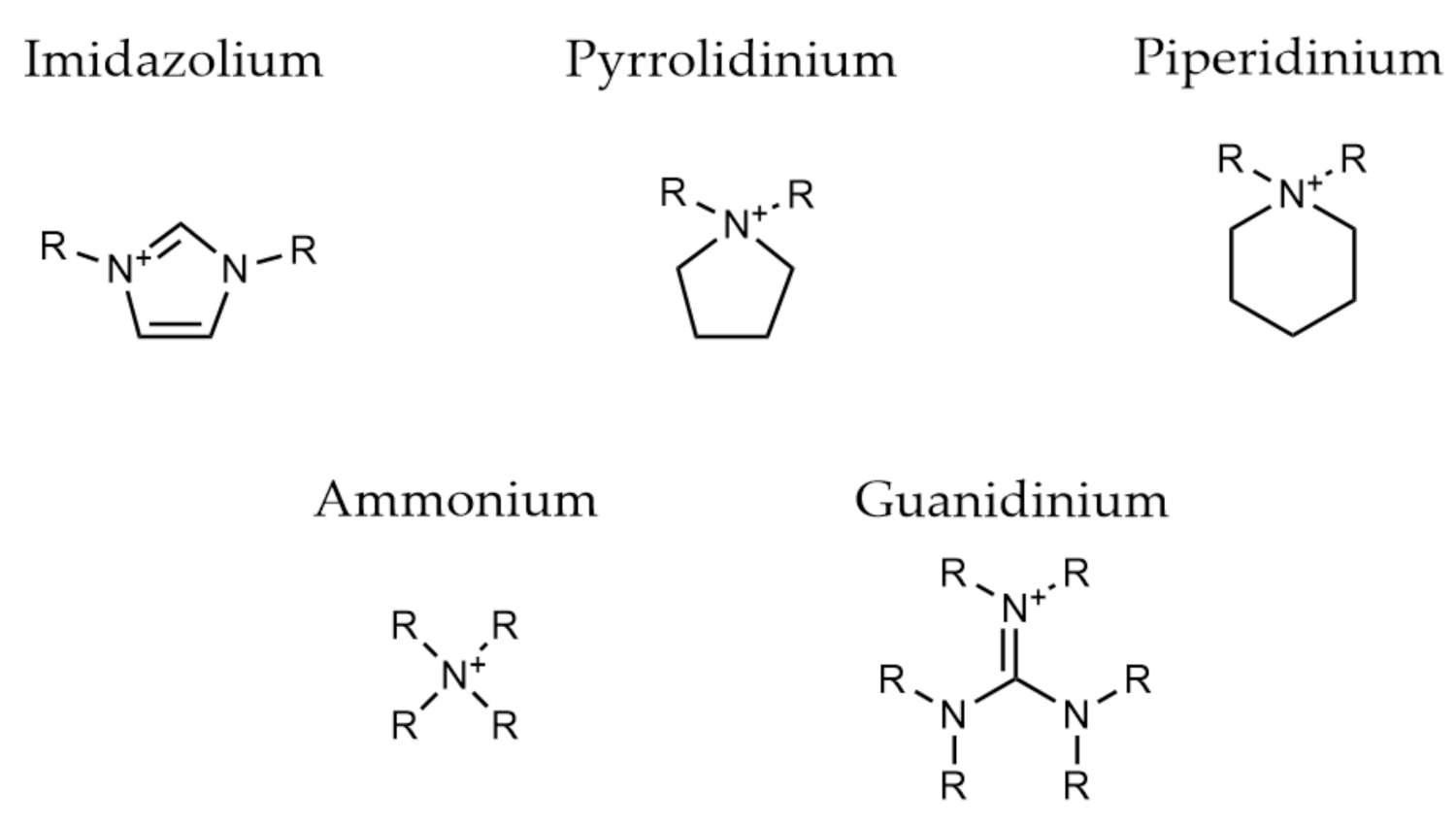
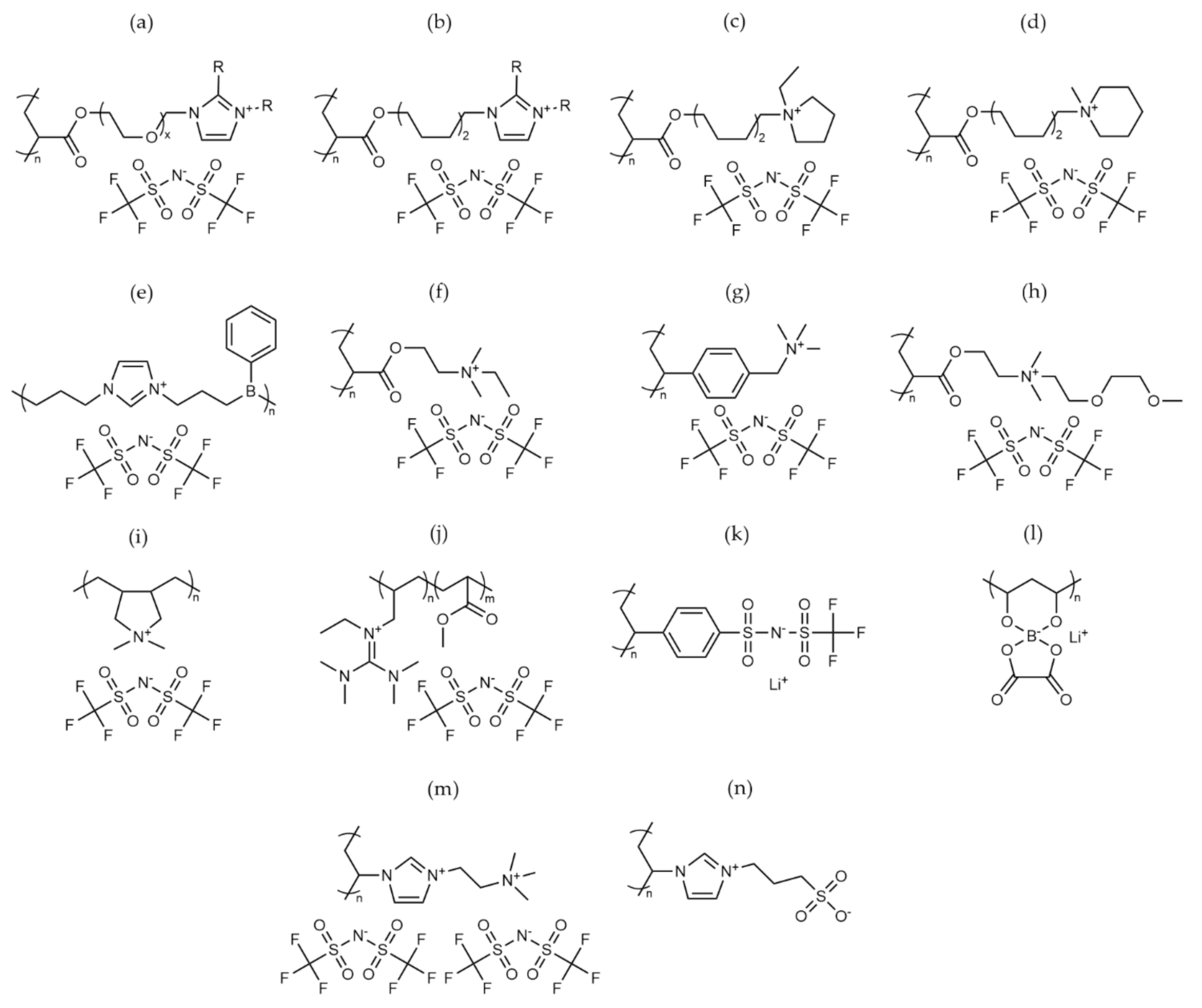
2.3. Ionic Polymers
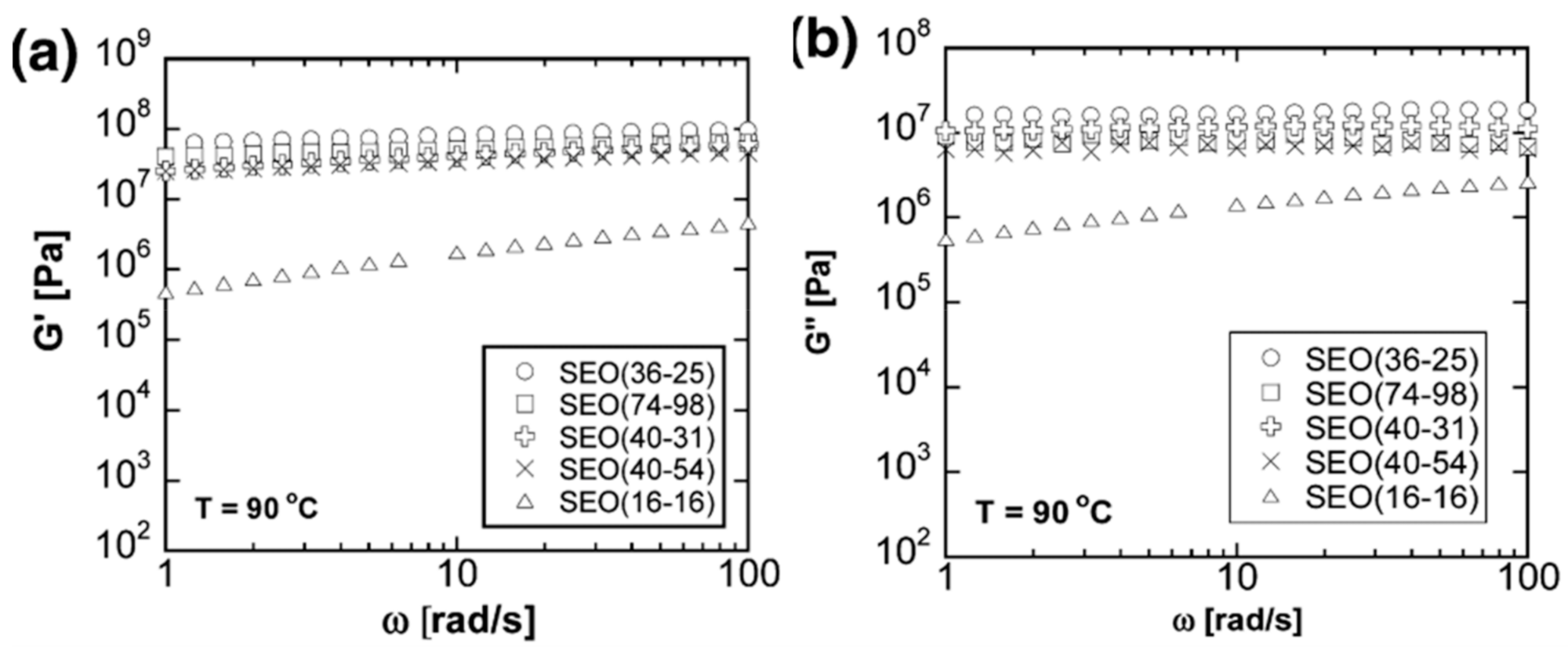
2.4. Block Copolymers
2.5. Ionic Block Copolymers
3. Summary
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Full Name | Acronym |
| Solid Polymer Electrolyte | SPE |
| Gel Polymer Electrolyte | GPC |
| Composite Polymer Electrolyte | CPE |
| Ionic Polymer | IP |
| Block Copolymer | BCP |
| Ionic Block Copolymer | IBCP |
| Reversible Addition–Fragmentation Chain Transfer | RAFT |
| Glass Transition Temperature | Tg |
| Melt Temperature | Tm |
| Lithium Iron Phosphate | LFP |
| Polyethylene Oxide | PEO |
| Polyethylene Glycol | PEG |
| Polymethylene Oxide | PMO |
| Polypropylene Oxide | PPO |
| Ethylene Carbonate | EC |
| Propylene Carbonate | PC |
| Polymethylmethacrylate | PMMA |
| Polyvinylidene fluoride | PVDF |
| Polyethylene Carbonate | PEC |
| Polypropylene Carbonate | PPC |
| Polytrimethylene Carbonate | PTMC |
| Polydiallyldmiethylammonium | PDADMA |
| Polyvinyl alcohol | PVA |
| Polystyrene | PS |
| Bis(trifluoromethanesulfonyl)imide | TFSI |
| Bis(fluorosulfonyl)imide | FSI |
References
- Wu, F.; Maier, J.; Yu, Y. Guidelines and trends for next-generation rechargeable lithium and lithium-ion batteries. Chem. Soc. Rev. 2020, 49, 1569–1614. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Liu, Y.; Lin, D.; Pei, A.; Cui, Y. Materials for lithium-ion battery safety. Sci. Adv. 2018, 4, eaas9820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masias, A.; Marcicki, J.; Paxton, W.A. Opportunities and Challenges of Lithium Ion Batteries in Automotive Applications. ACS Energy Lett. 2021, 6, 621–630. [Google Scholar] [CrossRef]
- Chen, T.; Jin, Y.; Lv, H.; Yang, A.; Liu, M.; Chen, B.; Xie, Y.; Chen, Q. Applications of Lithium-Ion Batteries in Grid-Scale Energy Storage Systems. Trans. Tianjin Univ. 2020, 26, 208–217. [Google Scholar] [CrossRef] [Green Version]
- Mauger, A.; Julien, C.M.; Paolella, A.; Armand, M.; Zaghib, K. Building Better Batteries in the Solid State: A Review. Materials (Basel) 2019, 12, 3892. [Google Scholar] [CrossRef] [Green Version]
- Boaretto, N.; Garbayo, I.; Valiyaveettil-SobhanRaj, S.; Quintela, A.; Li, C.; Casas-Cabanas, M.; Aguesse, F. Lithium solid-state batteries: State-of-the-art and challenges for materials, interfaces and processing. J. Power Sources 2021, 502, 229919. [Google Scholar] [CrossRef]
- Long, L.; Wang, S.; Xiao, M.; Meng, Y. Polymer electrolytes for lithium polymer batteries. J. Mater. Chem. A 2016, 4, 10038–10069. [Google Scholar] [CrossRef]
- Zhang, X.; Daigle, J.-C.; Zaghib, K. Comprehensive Review of Polymer Architecture for All-Solid-State Lithium Rechargeable Batteries. Materials 2020, 13, 2488. [Google Scholar] [CrossRef]
- Zhou, D.; Shanmukaraj, D.; Tkacheva, A.; Armand, M.; Wang, G. Polymer Electrolytes for Lithium-Based Batteries: Advances and Prospects. Chem 2019, 5, 2326–2352. [Google Scholar] [CrossRef]
- Aldalur, I.; Wang, X.; Santiago, A.; Goujon, N.; Echeverría, M.; Martínez-Ibáñez, M.; Piszcz, M.; Howlett, P.C.; Forsyth, M.; Armand, M.; et al. Nanofiber-reinforced polymer electrolytes toward room temperature solid-state lithium batteries. J. Power Sources 2020, 448, 227424. [Google Scholar] [CrossRef]
- Wang, X.; Kerr, R.; Chen, F.; Goujon, N.; Pringle, J.M.; Mecerreyes, D.; Forsyth, M.; Howlett, P.C. Toward High-Energy-Density Lithium Metal Batteries: Opportunities and Challenges for Solid Organic Electrolytes. Adv. Mater. 2020, 32, 1905219. [Google Scholar] [CrossRef]
- Jiang, Y.; Yan, X.; Ma, Z.; Mei, P.; Xiao, W.; You, Q.; Zhang, Y. Development of the PEO Based Solid Polymer Electrolytes for All-Solid State Lithium Ion Batteries. Polymers 2018, 10, 1237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mindemark, J.; Lacey, M.J.; Bowden, T.; Brandell, D. Beyond PEO—Alternative host materials for Li+-conducting solid polymer electrolytes. Prog. Polym. Sci. 2018, 81, 114–143. [Google Scholar] [CrossRef]
- Karuppasamy, K.; Theerthagiri, J.; Vikraman, D.; Yim, C.-J.; Hussain, S.; Sharma, R.; Maiyalagan, T.; Qin, J.; Kim, H.-S. Ionic Liquid-Based Electrolytes for Energy Storage Devices: A Brief Review on Their Limits and Applications. Polymers 2020, 12, 918. [Google Scholar] [CrossRef] [Green Version]
- Forsyth, M.; Porcarelli, L.; Wang, X.; Goujon, N.; Mecerreyes, D. Innovative Electrolytes Based on Ionic Liquids and Polymers for Next-Generation Solid-State Batteries. Acc. Chem. Res. 2019, 52, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Young, W.-S.; Kuan, W.-F.; Epps, T.H., III. Block copolymer electrolytes for rechargeable lithium batteries. J. Polym. Sci. Part B: Polym. Phys. 2014, 52, 1–16. [Google Scholar] [CrossRef]
- Kambe, Y.; Arges, C.G.; Patel, S.; Stoykovish, M.P.; Nealey, P.F. Ion Conduction in Microphase-Separated Block Copolymer Electrolytes. Electrochem. Soc. Interface 2017, 26, 61–67. [Google Scholar] [CrossRef]
- Fenton, D.E.; Parker, J.M.; Wright, P.V. Complexes of alkali metal ions with poly(ethylene oxide). Polymer 1973, 14, 589. [Google Scholar] [CrossRef]
- Armand, M. Fast Ion Transport in Solids. In Proceedings of the International Conference on Fast Ion Transport in Solids, Electrodes and Electrolytes, Lake Geneva, WI, USA, 21–25 May 1979; Vashita, P., Mundy, J.N., Shenoy, G.K., Eds.; North Holland: Amsterdam, The Netherlands, 1979. [Google Scholar]
- Armand, M. Polymer solid electrolytes—An overview. Solid State Ion. 1983, 9–10, 745–754. [Google Scholar] [CrossRef]
- Sun, J.; MacFarlane, D.R.; Forsyth, M. Mechanical properties of polyether-plasticiser-salt systems as polymer electrolytes. Solid State Ion. 1996, 85, 137–141. [Google Scholar] [CrossRef]
- Bruce, P.G.; Lisowska-Oleksiak, A.; Vincent, C.A. The electrical double layer at the interface between mercury and a polyether electrolyte: Part 1: Tetraethylene glycol dimethyl ether solutions. J. Electroanal. Chem. 1994, 364, 163–169. [Google Scholar] [CrossRef]
- Croce, F.; Appetecchi, G.B.; Persi, L.; Scrosati, B. Nanocomposite polymer electrolytes for lithium batteries. Nature 1998, 394, 456–458. [Google Scholar] [CrossRef]
- Wieczorek, W.; Such, K.; Wyciślik, H.; Płocharski, J. Modifications of crystalline structure of peo polymer electrolytes with ceramic additives. Solid State Ion. 1989, 36, 255–257. [Google Scholar] [CrossRef]
- Bohnke, O.; Frand, G.; Rezrazi, M.; Rousselot, C.; Truche, C. Fast ion transport in new lithium electrolytes gelled with PMMA. 1. Influence of polymer concentration. Solid State Ion. 1993, 66, 97–104. [Google Scholar] [CrossRef]
- Appetecchi, G.B.; Croce, F.; Scrosati, B. Kinetics and stability of the lithium electrode in poly(methylmethacrylate)-based gel electrolytes. Electrochim. Acta 1995, 40, 991–997. [Google Scholar] [CrossRef]
- Dautzenberg, G.; Croce, F.; Passerini, S.; Scrosati, B. Characterization of PAN-Based Gel Electrolytes. Electrochemical Stability and Lithium Cyclability. Chem. Mater. 1994, 6, 538–542. [Google Scholar] [CrossRef]
- Croce, F.; Brown, S.D.; Greenbaum, S.G.; Slane, S.M.; Salomon, M. Lithium-7 NMR and ionic conductivity studies of gel electrolytes based on polyacrylonitrile. Chem. Mater. 1993, 5, 1268–1272. [Google Scholar] [CrossRef]
- Chew, S.Y.; Sun, J.; Wang, J.; Liu, H.; Forsyth, M.; MacFarlane, D.R. Lithium-polymer battery based on an ionic liquid–polymer electrolyte composite for room temperature applications. Electrochim. Acta 2008, 53, 6460–6463. [Google Scholar] [CrossRef]
- Shaplov, A.S.; Marcilla, R.; Mecerreyes, D. Recent Advances in Innovative Polymer Electrolytes based on Poly(ionic liquid)s. Electrochim. Acta 2015, 175, 18–34. [Google Scholar] [CrossRef]
- Wang, X.; Chen, F.; Girard, G.M.A.; Zhu, H.; MacFarlane, D.R.; Mecerreyes, D.; Armand, M.; Howlett, P.C.; Forsyth, M. Poly(Ionic Liquid)s-in-Salt Electrolytes with Co-coordination-Assisted Lithium-Ion Transport for Safe Batteries. Joule 2019, 3, 2687–2702. [Google Scholar] [CrossRef]
- Manuel Stephan, A.; Nahm, K.S. Review on composite polymer electrolytes for lithium batteries. Polymer 2006, 47, 5952–5964. [Google Scholar] [CrossRef] [Green Version]
- Kwon, D.-S.; Kim, H.J.; Shim, J. Dendrite-Suppressing Polymer Materials for Safe Rechargeable Metal Battery Applications: From the Electro-Chemo-Mechanical Viewpoint of Macromolecular Design. Macromol. Rapid Commun. 2021, 42, 2100279. [Google Scholar] [CrossRef]
- Quartarone, E.; Mustarelli, P. Electrolytes for solid-state lithium rechargeable batteries: Recent advances and perspectives. Chem. Soc. Rev. 2011, 40, 2525–2540. [Google Scholar] [CrossRef]
- Hallinan, D.T., Jr.; Balsara, N.P. Polymer Electrolytes. Annu. Rev. Mater. Res. 2013, 43, 503–525. [Google Scholar] [CrossRef]
- Zheng, Y.; Pan, Q.; Clites, M.; Byles, B.W.; Pomerantseva, E.; Li, C.Y. High-Capacity All-Solid-State Sodium Metal Battery with Hybrid Polymer Electrolytes. Adv. Energy Mater. 2018, 8, 1801885. [Google Scholar] [CrossRef]
- Qiao, L.; Judez, X.; Rojo, T.; Armand, M.; Zhang, H. Review—Polymer Electrolytes for Sodium Batteries. J. Electrochem. Soc. 2020, 167, 070534. [Google Scholar] [CrossRef]
- Qi, X.; Ma, Q.; Liu, L.; Hu, Y.-S.; Li, H.; Zhou, Z.; Huang, X.; Chen, L. Sodium Bis(fluorosulfonyl)imide/Poly(ethylene oxide) Polymer Electrolytes for Sodium-Ion Batteries. ChemElectroChem 2016, 3, 1741–1745. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, L.; Lu, Y.; Wagemaker, M.; Chen, L.; Hu, Y.S. Revealing an Interconnected Interfacial Layer in Solid-State Polymer Sodium Batteries. Angew. Chem. 2019, 58, 17026–17032. [Google Scholar] [CrossRef]
- Croce, F.; Curini, R.; Martinelli, A.; Persi, L.; Ronci, F.; Scrosati, B.; Caminiti, R. Physical and Chemical Properties of Nanocomposite Polymer Electrolytes. J. Phys. Chem. B 1999, 103, 10632–10638. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, J.; Yue, L.; Wang, Q.; Chai, J.; Liu, Z.; Zhou, X.; Li, H.; Guo, Y.; Cui, G.; et al. Safety-Reinforced Poly(Propylene Carbonate)-Based All-Solid-State Polymer Electrolyte for Ambient-Temperature Solid Polymer Lithium Batteries. Adv. Energy Mater. 2015, 5, 1501082. [Google Scholar] [CrossRef]
- Wang, X.; Girard, G.M.A.; Zhu, H.; Yunis, R.; MacFarlane, D.R.; Mecerreyes, D.; Bhattacharyya, A.J.; Howlett, P.C.; Forsyth, M. Poly(ionic liquid)s/Electrospun Nanofiber Composite Polymer Electrolytes for High Energy Density and Safe Li Metal Batteries. ACS Appl. Energy Mater. 2019, 2, 6237–6245. [Google Scholar] [CrossRef]
- Zhu, Y.S.; Wang, X.J.; Hou, Y.Y.; Gao, X.W.; Liu, L.L.; Wu, Y.P.; Shimizu, M. A new single-ion polymer electrolyte based on polyvinyl alcohol for lithium ion batteries. Electrochim. Acta 2013, 87, 113–118. [Google Scholar] [CrossRef]
- Lu, F.; Gao, X.; Wu, A.; Sun, N.; Shi, L.; Zheng, L. Lithium-Containing Zwitterionic Poly(Ionic Liquid)s as Polymer Electrolytes for Lithium-Ion Batteries. J. Phys. Chem. C 2017, 121, 17756–17763. [Google Scholar] [CrossRef]
- Singh, M.; Odusanya, O.; Wilmes, G.M.; Eitouni, H.B.; Gomez, E.D.; Patel, A.J.; Chen, V.L.; Park, M.J.; Fragouli, P.; Iatrou, H.; et al. Effect of Molecular Weight on the Mechanical and Electrical Properties of Block Copolymer Electrolytes. Macromolecules 2007, 40, 4578–4585. [Google Scholar] [CrossRef]
- Kuan, W.-F.; Remy, R.; Mackay, M.E.; Epps, T.H., III. Controlled ionic conductivity via tapered block polymer electrolytes. RSC Adv. 2015, 5, 12597–12604. [Google Scholar] [CrossRef] [Green Version]
- Bouchet, R.; Maria, S.; Meziane, R.; Aboulaich, A.; Lienafa, L.; Bonnet, J.-P.; Phan, T.N.T.; Bertin, D.; Gigmes, D.; Devaux, D.; et al. Single-ion BAB triblock copolymers as highly efficient electrolytes for lithium-metal batteries. Nat. Mater. 2013, 12, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Goujon, N.; Huynh, T.V.; Barlow, K.J.; Kerr, R.; Vezzù, K.; Di Noto, V.; O’Dell, L.A.; Chiefari, J.; Howlett, P.C.; Forsyth, M. Enabling High Lithium Conductivity in Polymerized Ionic Liquid Block Copolymer Electrolytes. Batter. Supercaps 2018, 2, 132–138. [Google Scholar] [CrossRef]
- Manuel Stephan, A. Review on gel polymer electrolytes for lithium batteries. Eur. Polym. J. 2006, 42, 21–42. [Google Scholar] [CrossRef]
- Cowie, J.M.G.; Cree, S.H. Electrolytes Dissolved in Polymers. Annu. Rev. Phys. Chem. 1989, 40, 85–113. [Google Scholar] [CrossRef]
- Sutton, P.; Airoldi, M.; Porcarelli, L.; Olmedo-Martínez, J.L.; Mugemana, C.; Bruns, N.; Mecerreyes, D.; Steiner, U.; Gunkel, I. Tuning the Properties of a UV-Polymerized, Cross-Linked Solid Polymer Electrolyte for Lithium Batteries. Polymers 2020, 12, 595. [Google Scholar] [CrossRef] [Green Version]
- Bruce, P.G.; Gray, F.M. Polymer Electrolytes II: Physical properties. In Solid State Electrochemistry; Bruce, P.G., Ed.; Cambridge University Press: Cambridge, UK, 1995; pp. 119–162. [Google Scholar]
- Nagasubramanian, G.; Shen, D.H.; Surampudi, S.; Wang, Q.; Prakash, G.K.S. Lithium superacid salts for secondary lithium batteries. Electrochim. Acta 1995, 40, 2277–2280. [Google Scholar] [CrossRef]
- Angell, C.A. Polymer electrolytes—Some principles, cautions, and new practices. Electrochim. Acta 2017, 250, 368–375. [Google Scholar] [CrossRef]
- Yue, L.; Ma, J.; Zhang, J.; Zhao, J.; Dong, S.; Liu, Z.; Cui, G.; Chen, L. All solid-state polymer electrolytes for high-performance lithium ion batteries. Energy Storage Mater. 2016, 5, 139–164. [Google Scholar] [CrossRef]
- Rosen, S.L. Fundamental Principles of Polymeric Materials, 2nd ed.; Wiley: New York, NY, USA, 1993. [Google Scholar]
- Rahman, M.; Brazel, C.S. The plasticizer market: An assessment of traditional plasticizers and research trends to meet new challenges. Prog. Polym. Sci. 2004, 29, 1223–1248. [Google Scholar] [CrossRef]
- Bandara, L.R.A.K.; Dissanayake, M.A.K.L.; Mellander, B.E. Ionic conductivity of plasticized(PEO)-LiCF3SO3 electrolytes. Electrochim. Acta 1998, 43, 1447–1451. [Google Scholar] [CrossRef]
- Niedzicki, L.; Kasprzyk, M.; Kuziak, K.; Żukowska, G.Z.; Armand, M.; Bukowska, M.; Marcinek, M.; Szczeciński, P.; Wieczorek, W. Modern generation of polymer electrolytes based on lithium conductive imidazole salts. J. Power Sources 2009, 192, 612–617. [Google Scholar] [CrossRef]
- Xue, Z.; He, D.; Xie, X. Poly(ethylene oxide)-based electrolytes for lithium-ion batteries. J. Mater. Chem. A 2015, 3, 19218–19253. [Google Scholar] [CrossRef]
- Sivakkumar, S.R.; MacFarlane, D.R.; Forsyth, M.; Kim, D.-W. Ionic Liquid-Based Rechargeable Lithium Metal-Polymer Cells Assembled with Polyaniline/Carbon Nanotube Composite Cathode. J. Electrochem. Soc. 2007, 154, A834. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; MacFarlane, D.R.; Forsyth, M. Lithium polyelectrolyte–ionic liquid systems. Solid State Ion. 2002, 147, 333–339. [Google Scholar] [CrossRef]
- Shin, J.-H.; Henderson, W.A.; Passerini, S. Ionic liquids to the rescue? Overcoming the ionic conductivity limitations of polymer electrolytes. Electrochem. Commun. 2003, 5, 1016–1020. [Google Scholar] [CrossRef]
- Tsuchida, E.; Ohno, H.; Tsunemi, K.; Kobayashi, N. Lithium ionic conduction in poly (methacrylic acid)-poly (ethylene oxide) complex containing lithium perchlorate. Solid State Ion. 1983, 11, 227–233. [Google Scholar] [CrossRef]
- Prasanth, R.; Shubha, N.; Hng, H.H.; Srinivasan, M. Effect of poly(ethylene oxide) on ionic conductivity and electrochemical properties of poly(vinylidenefluoride) based polymer gel electrolytes prepared by electrospinning for lithium ion batteries. J. Power Sources 2014, 245, 283–291. [Google Scholar] [CrossRef]
- Correia, D.M.; Costa, C.M.; Nunes-Pereira, J.; Silva, M.M.; Botelho, G.; Ribelles, J.L.G.; Lanceros-Méndez, S. Physicochemical properties of poly(vinylidene fluoride-trifluoroethylene)/poly(ethylene oxide) blend membranes for lithium ion battery applications: Influence of poly(ethylene oxide) molecular weight. Solid State Ion. 2014, 268, 54–67. [Google Scholar] [CrossRef]
- Raghavan, P.; Manuel, J.; Zhao, X.; Kim, D.-S.; Ahn, J.-H.; Nah, C. Preparation and electrochemical characterization of gel polymer electrolyte based on electrospun polyacrylonitrile nonwoven membranes for lithium batteries. J. Power Sources 2011, 196, 6742–6749. [Google Scholar] [CrossRef]
- Asghar, A.; Abdul Samad, Y.; Singh Lalia, B.; Hashaikeh, R. PEG based quasi-solid polymer electrolyte: Mechanically supported by networked cellulose. J. Membr. Sci. 2012, 421–422, 85–90. [Google Scholar] [CrossRef]
- Cho, S.; Cha, W.; Park, H.-J.; Lee, J.-M.; Kim, E.-B.; Rhee, H.-W.; Jiang, Z.; Strzalka, J.; Kim, H. Effects of siloxane nanoparticles on glass transition temperature and crystallization in PEO-LiPF6 polymer electrolytes. Synth. Met. 2013, 177, 110–113. [Google Scholar] [CrossRef]
- Cui, J.; Zhou, Z.; Jia, M.; Chen, X.; Shi, C.; Zhao, N.; Guo, X. Solid Polymer Electrolytes with Flexible Framework of SiO2 Nanofibers for Highly Safe Solid Lithium Batteries. Polymers 2020, 12, 1324. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Huang, X.; Wei, H.; Fu, J.; Huang, Y.; Tang, X. Enhanced electrochemical properties of polyethylene oxide-based composite solid polymer electrolytes with porous inorganic–organic hybrid polyphosphazene nanotubes as fillers. J. Solid State Electrochem. 2012, 16, 101–107. [Google Scholar] [CrossRef]
- Nan, C.-W.; Fan, L.; Lin, Y.; Cai, Q. Enhanced Ionic Conductivity of Polymer Electrolytes Containing Nanocomposite SiO2 particles. Phys. Rev. Lett. 2003, 91, 266104. [Google Scholar] [CrossRef] [PubMed]
- Croce, F.; Persi, L.; Scrosati, B.; Serraino-Fiory, F.; Plichta, E.; Hendrickson, M.A. Role of the ceramic fillers in enhancing the transport properties of composite polymer electrolytes. Electrochim. Acta 2001, 46, 2457–2461. [Google Scholar] [CrossRef]
- Yuan, C.; Li, J.; Han, P.; Lai, Y.; Zhang, Z.; Liu, J. Enhanced electrochemical performance of poly(ethylene oxide) based composite polymer electrolyte by incorporation of nano-sized metal-organic framework. J. Power Sources 2013, 240, 653–658. [Google Scholar] [CrossRef]
- Motomatsu, J.; Kodama, H.; Furukawa, T.; Tominaga, Y. Dielectric Relaxation Behavior of a Poly(ethylene carbonate)-Lithium Bis-(trifluoromethanesulfonyl) Imide Electrolyte. Macromol. Chem. Phys. 2015, 216, 1660–1665. [Google Scholar] [CrossRef]
- Tominaga, Y.; Yamazaki, K. Fast Li-ion conduction in poly(ethylene carbonate)-based electrolytes and composites filled with TiO2 nanoparticles. Chem. Commun. 2014, 50, 4448–4450. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Uno, T.; Kubo, M.; Itoh, T. Polymer electrolytes based on polycarbonates and their electrochemical and thermal properties. Ionics 2013, 19, 615–622. [Google Scholar] [CrossRef]
- Mespouille, L.; Coulembier, O.; Kawalec, M.; Dove, A.P.; Dubois, P. Implementation of metal-free ring-opening polymerization in the preparation of aliphatic polycarbonate materials. Prog. Polym. Sci. 2014, 39, 1144–1164. [Google Scholar] [CrossRef]
- Sun, B.; Mindemark, J.; Edström, K.; Brandell, D. Polycarbonate-based solid polymer electrolytes for Li-ion batteries. Solid State Ion. 2014, 262, 738–742. [Google Scholar] [CrossRef]
- Sun, B.; Mindemark, J.; Morozov, E.V.; Costa, L.T.; Bergman, M.; Johansson, P.; Fang, Y.; Furó, I.; Brandell, D. Ion transport in polycarbonate based solid polymer electrolytes: Experimental and computational investigations. Phys. Chem. Chem. Phys. 2016, 18, 9504–9513. [Google Scholar] [CrossRef]
- Sun, B.; Mindemark, J.; Edström, K.; Brandell, D. Realization of high performance polycarbonate-based Li polymer batteries. Electrochem. Commun. 2015, 52, 71–74. [Google Scholar] [CrossRef]
- Mindemark, J.; Mogensen, R.; Smith, M.J.; Silva, M.M.; Brandell, D. Polycarbonates as alternative electrolyte host materials for solid-state sodium batteries. Electrochem. Commun. 2017, 77, 58–61. [Google Scholar] [CrossRef]
- Eshetu, G.G.; Mecerreyes, D.; Forsyth, M.; Zhang, H.; Armand, M. Polymeric ionic liquids for lithium-based rechargeable batteries. Mol. Syst. Des. Eng. 2019, 4, 294–309. [Google Scholar] [CrossRef]
- Armand, M.; Endres, F.; MacFarlane, D.R.; Ohno, H.; Scrosati, B. Ionic-liquid materials for the electrochemical challenges of the future. Nat. Mater. 2009, 8, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Park, M.J.; Choi, I.; Hong, J.; Kim, O. Polymer electrolytes integrated with ionic liquids for future electrochemical devices. J. Appl. Polym. Sci. 2013, 129, 2363–2376. [Google Scholar] [CrossRef]
- Ogihara, W.; Washiro, S.; Nakajima, H.; Ohno, H. Effect of cation structure on the electrochemical and thermal properties of ion conductive polymers obtained from polymerizable ionic liquids. Electrochim. Acta 2006, 51, 2614–2619. [Google Scholar] [CrossRef]
- Hirao, M.; Ito, K.; Ohno, H. Preparation and polymerization of new organic molten salts; N-alkylimidazolium salt derivatives. Electrochim. Acta 2000, 45, 1291–1294. [Google Scholar] [CrossRef]
- Hirao, M.; Ito-Akita, K.; Ohno, H. Polymerization of molten salt monomers having a phenylimidazolium group. Polym. Adv. Technol. 2000, 11, 534–538. [Google Scholar] [CrossRef]
- Yoshizawa, M.; Ohno, H. Synthesis of molten salt-type polymer brush and effect of brush structure on the ionic conductivity. Electrochim. Acta 2001, 46, 1723–1728. [Google Scholar] [CrossRef]
- Ohno, H.; Yoshizawa, M.; Ogihara, W. Development of new class of ion conductive polymers based on ionic liquids. Electrochimica Acta 2004, 50, 255–261. [Google Scholar] [CrossRef]
- Matsumi, N.; Sugai, K.; Miyake, M.; Ohno, H. Polymerized Ionic Liquids via Hydroboration Polymerization as Single Ion Conductive Polymer Electrolytes. Macromolecules 2006, 39, 6924–6927. [Google Scholar] [CrossRef]
- Li, M.; Yang, B.; Wang, L.; Zhang, Y.; Zhang, Z.; Fang, S.; Zhang, Z. New polymerized ionic liquid (PIL) gel electrolyte membranes based on tetraalkylammonium cations for lithium ion batteries. J. Membr. Sci. 2013, 447, 222–227. [Google Scholar] [CrossRef]
- Li, M.; Wang, L.; Yang, B.; Du, T.; Zhang, Y. Facile preparation of polymer electrolytes based on the polymerized ionic liquid poly((4-vinylbenzyl)trimethylammonium bis(trifluoromethanesulfonylimide)) for lithium secondary batteries. Electrochim. Acta 2014, 123, 296–302. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, C.; Zheng, L.; Feng, W.; Zhou, Z.; Nie, J. Solid polymer electrolyte comprised of lithium salt/ether functionalized ammonium-based polymeric ionic liquid with bis(fluorosulfonyl)imide. Electrochim. Acta 2015, 159, 93–101. [Google Scholar] [CrossRef]
- Pappenfus, T.M.; Henderson, W.A.; Owens, B.B.; Mann, K.R.; Smyrl, W.H. Complexes of Lithium Imide Salts with Tetraglyme and Their Polyelectrolyte Composite Materials. J. Electrochem. Soc. 2004, 151, A209. [Google Scholar] [CrossRef]
- Pont, A.-L.; Marcilla, R.; De Meatza, I.; Grande, H.; Mecerreyes, D. Pyrrolidinium-based polymeric ionic liquids as mechanically and electrochemically stable polymer electrolytes. J. Power Sources 2009, 188, 558–563. [Google Scholar] [CrossRef]
- Appetecchi, G.B.; Kim, G.T.; Montanino, M.; Carewska, M.; Marcilla, R.; Mecerreyes, D.; De Meatza, I. Ternary polymer electrolytes containing pyrrolidinium-based polymeric ionic liquids for lithium batteries. J. Power Sources 2010, 195, 3668–3675. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, H.; Girard Gaetan, M.A.; Yunis, R.; MacFarlane, D.R.; Mecerreyes, D.; Bhattacharyya, A.J.; Howlett, P.C.; Forsyth, M. Preparation and characterization of gel polymer electrolytes using poly(ionic liquids) and high lithium salt concentration ionic liquids. J. Mater. Chem. A 2017, 5, 23844–23852. [Google Scholar] [CrossRef]
- Li, M.; Yang, L.; Fang, S.; Dong, S. Novel polymeric ionic liquid membranes as solid polymer electrolytes with high ionic conductivity at moderate temperature. J. Membr. Sci. 2011, 366, 245–250. [Google Scholar] [CrossRef]
- Li, M.; Dong, S.; Fang, S.; Yang, L.; Hirano, S.-I.; Hu, J.; Huang, X. Polymeric ionic liquid membranes as electrolytes for lithium battery applications. J. Appl. Electrochem. 2012, 42, 851–856. [Google Scholar] [CrossRef]
- Chen, Z.; Steinle, D.; Nguyen, H.-D.; Kim, J.-K.; Mayer, A.; Shi, J.; Paillard, E.; Iojoiu, C.; Passerini, S.; Bresser, D. High-energy lithium batteries based on single-ion conducting polymer electrolytes and Li[Ni0.8Co0.1Mn0.1]O2 cathodes. Nano Energy 2020, 77, 105129. [Google Scholar] [CrossRef]
- Feng, S.; Shi, D.; Liu, F.; Zheng, L.; Nie, J.; Feng, W.; Huang, X.; Armand, M.; Zhou, Z. Single lithium-ion conducting polymer electrolytes based on poly[(4-styrenesulfonyl)(trifluoromethanesulfonyl)imide] anions. Electrochim. Acta 2013, 93, 254–263. [Google Scholar] [CrossRef]
- An, Y.; Zuo, P.; Cheng, X.; Liao, L.; Yin, G. The effects of LiBOB additive for stable SEI formation of PP13TFSI-organic mixed electrolyte in lithium ion batteries. Electrochim. Acta 2011, 56, 4841–4848. [Google Scholar] [CrossRef]
- Yin, K.; Zhang, Z.; Li, X.; Yang, L.; Tachibana, K.; Hirano, S.-I. Polymer electrolytes based on dicationic polymeric ionic liquids: Application in lithium metal batteries. J. Mater. Chem. A 2015, 3, 170–178. [Google Scholar] [CrossRef]
- Tiyapiboonchaiya, C.; Pringle, J.M.; Sun, J.; Byrne, N.; Howlett, P.C.; MacFarlane, D.R.; Forsyth, M. The zwitterion effect in high-conductivity polyelectrolyte materials. Nat. Mater. 2004, 3, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Monroe, C.; Newman, J. The Impact of Elastic Deformation on Deposition Kinetics at Lithium/Polymer Interfaces. J. Electrochem. Soc. 2005, 152, A396. [Google Scholar] [CrossRef]
- Yoshida, H.; Takenaka, M. 1—Physics of block copolymers from bulk to thin films. In Directed Self-Assembly of Block Co-Polymers for Nano-Manufacturing; Gronheid, R., Nealey, P., Eds.; Woodhead Publishing: Sawston, UK, 2015; pp. 3–26. [Google Scholar]
- Giles, J.R.M.; Gray, F.M.; MacCallum, J.R.; Vincent, C.A. Synthesis and characterization of ABA block copolymer-based polymer electrolytes. Polymer 1987, 28, 1977–1981. [Google Scholar] [CrossRef]
- Khan, I.M.; Fish, D.; Delaviz, Y.; Smid, J. ABA triblock comb copolymers with oligo(oxyethylene) side chains as matrix for ion transport. Die Makromol. Chem. 1989, 190, 1069–1078. [Google Scholar] [CrossRef]
- Matsen, M.W. Effect of Architecture on the Phase Behavior of AB-Type Block Copolymer Melts. Macromolecules 2012, 45, 2161–2165. [Google Scholar] [CrossRef] [Green Version]
- Matsen, M.W.; Bates, F.S. Unifying Weak- and Strong-Segregation Block Copolymer Theories. Macromolecules 1996, 29, 1091–1098. [Google Scholar] [CrossRef]
- Swann, J.M.G.; Topham, P.D. Design and Application of Nanoscale Actuators Using Block-Copolymers. Polymers 2010, 2, 454–469. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.-H.; Ye, Y.; Elabd, Y.A.; Winey, K.I. Network Structure and Strong Microphase Separation for High Ion Conductivity in Polymerized Ionic Liquid Block Copolymers. Macromolecules 2013, 46, 5290–5300. [Google Scholar] [CrossRef]
- Sutton, P.; Bennington, P.; Patel, S.N.; Stefik, M.; Wiesner, U.B.; Nealey, P.F.; Steiner, U.; Gunkel, I. Surface Reconstruction Limited Conductivity in Block-Copolymer Li Battery Electrolytes. Adv. Funct. Mater. 2019, 29, 1905977. [Google Scholar] [CrossRef] [Green Version]
- Shen, K.-H.; Brown, J.R.; Hall, L.M. Diffusion in Lamellae, Cylinders, and Double Gyroid Block Copolymer Nanostructures. ACS Macro Lett. 2018, 7, 1092–1098. [Google Scholar] [CrossRef]
- Grubbs, R.B.; Grubbs, R.H. 50th Anniversary Perspective: Living Polymerization—Emphasizing the Molecule in Macromolecules. Macromolecules 2017, 50, 6979–6997. [Google Scholar] [CrossRef]
- Szwarc, M. ‘Living’ Polymers. Nature 1956, 178, 1168–1169. [Google Scholar] [CrossRef]
- Kennedy, J.P.; Feinberg, S.C.; Huang, S.Y. Cationic polymerization with boron halides. V. Synthesis of poly(isobutylene-b-styrene) and poly(styrene-b-isobutylene). J. Polym. Sci. Polym. Chem. Ed. 1978, 16, 243–259. [Google Scholar] [CrossRef]
- Fischer, H. The Persistent Radical Effect: A Principle for Selective Radical Reactions and Living Radical Polymerizations. Chem. Rev. 2001, 101, 3581–3610. [Google Scholar] [CrossRef] [PubMed]
- Perrier, S. 50th Anniversary Perspective: RAFT Polymerization—A User Guide. Macromolecules 2017, 50, 7433–7447. [Google Scholar] [CrossRef]
- Wang, C.; Sakai, T.; Watanabe, O.; Hirahara, K.; Nakanishi, T. All Solid-State Lithium-Polymer Battery Using a Self-Cross-Linking Polymer Electrolyte. J. Electrochem. Soc. 2003, 150, A1166. [Google Scholar] [CrossRef]
- Panday, A.; Mullin, S.; Gomez, E.D.; Wanakule, N.; Chen, V.L.; Hexemer, A.; Pople, J.; Balsara, N.P. Effect of Molecular Weight and Salt Concentration on Conductivity of Block Copolymer Electrolytes. Macromolecules 2009, 42, 4632–4637. [Google Scholar] [CrossRef]
- Merz, E.J.; Nielsen, L.E.; Buchdahl, R. Influence of Molecular Weight on the Properties of Polystyrene. Ind. Eng. Chem. 1951, 43, 1396–1401. [Google Scholar] [CrossRef]
- Simone, P.M.; Lodge, T.P. Lyotropic Phase Behavior of Polybutadiene−Poly(ethylene oxide) Diblock Copolymers in Ionic Liquids. Macromolecules 2008, 41, 1753–1759. [Google Scholar] [CrossRef]
- Simone, P.M.; Lodge, T.P. Phase Behavior and Ionic Conductivity of Concentrated Solutions of Polystyrene-Poly(ethylene oxide) Diblock Copolymers in an Ionic Liquid. ACS Appl. Mater. Interfaces 2009, 1, 2812–2820. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Brown, J.R.; Remy, R.A.; Scott, D.M.; Mackay, M.E.; Hall, L.M.; Epps, T.H., III. Determination of Interfacial Mixing in Tapered Block Polymer Thin Films: Experimental and Theoretical Investigations. Macromolecules 2016, 49, 5213–5222. [Google Scholar] [CrossRef]
- Porcarelli, L.; Shaplov, A.S.; Salsamendi, M.; Nair, J.R.; Vygodskii, Y.S.; Mecerreyes, D.; Gerbaldi, C. Single-Ion Block Copoly(ionic liquid)s as Electrolytes for All-Solid State Lithium Batteries. ACS Appl. Mater. Interfaces 2016, 8, 10350–10359. [Google Scholar] [CrossRef] [PubMed]
- Porcarelli, L.; Aboudzadeh, M.A.; Rubatat, L.; Nair, J.R.; Shaplov, A.S.; Gerbaldi, C.; Mecerreyes, D. Single-ion triblock copolymer electrolytes based on poly(ethylene oxide) and methacrylic sulfonamide blocks for lithium metal batteries. J. Power Sources 2017, 364, 191–199. [Google Scholar] [CrossRef]
- Nykaza, J.R.; Savage, A.M.; Pan, Q.; Wang, S.; Beyer, F.L.; Tang, M.H.; Li, C.Y.; Elabd, Y.A. Polymerized ionic liquid diblock copolymer as solid-state electrolyte and separator in lithium-ion battery. Polymer 2016, 101, 311–318. [Google Scholar] [CrossRef] [Green Version]
- Mauger, A.; Armand, M.; Julien, C.M.; Zaghib, K. Challenges and issues facing lithium metal for solid-state rechargeable batteries. J. Power Sources 2017, 353, 333–342. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.-L.; Sun, R.; Willis, C.; Morgan, B.F.; Beyer, F.L.; Elabd, Y.A. Lithium ion conducting polymerized ionic liquid pentablock terpolymers as solid-state electrolytes. Polymer 2019, 161, 128–138. [Google Scholar] [CrossRef]


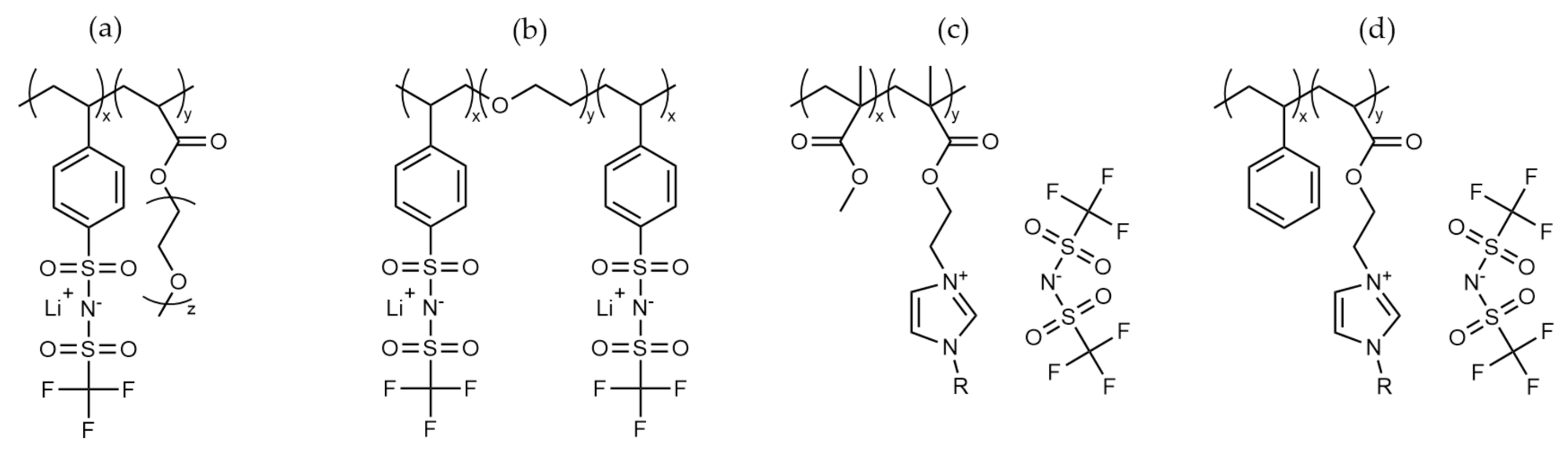
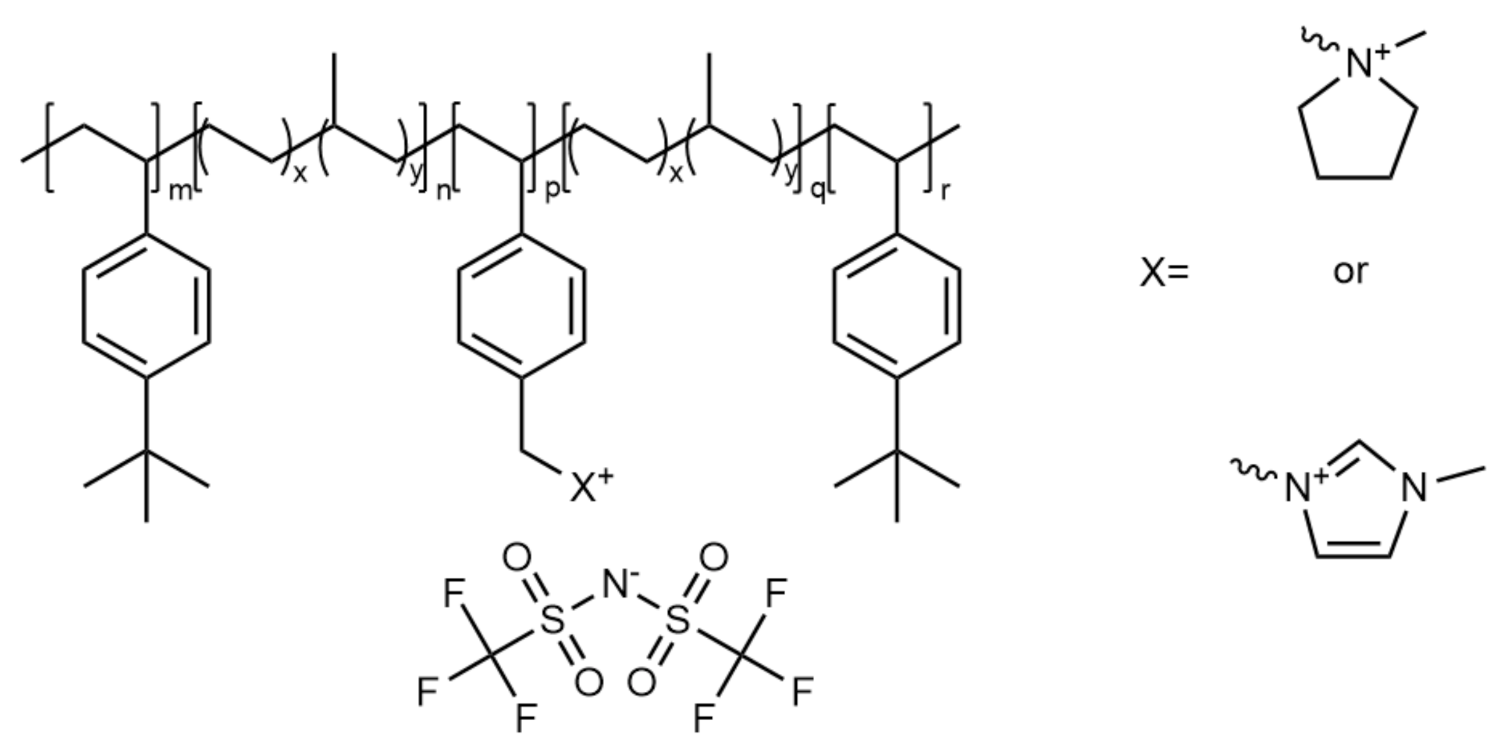
| Polymer | Salt | Additive | Tg (°C) | Ionic Conductivity (Scm−1) | Storage Modulus (Pa) | Reference |
|---|---|---|---|---|---|---|
| PEO | LiClO4 | Al2O3 | - | 10−5 (30 °C) | - | [40] |
| PPC | LiTFSI | Cellulose | 5 | 3.4 × 10−4 (25 °C) | 2.5 × 107 (25 °C) | [41] |
| PDADMA TFSI | LiFSI | C3mpyrFSI PVDF | −50 | 4.5 × 10−4 (25 °C) | 105 (25 °C) | [42] |
| LiPVAOB | - | PC | 100 | 6.1 × 10−6 (25 °C) | - | [43] |
| PVIPS | LiTFSI | - | 1.1 × 10−4 (50 °C) | - | [44] | |
| PS-b-PEO | LiTFSI | - | - | 3.6 × 10−4 (90 °C) | 5.7 × 107 (90 °C) | [45] |
| PS-b-P(SOEM)-b-POEM | LiTFSI | - | −54 | 10−5 (50 °C) | - | [46] |
| PSTFSI-b-PEO-b-PSTFSI | - | - | 1.3 × 10−5 (60 °C) | 9 × 106 (40 °C) | [47] | |
| PS-b-PAEBImTFSI | LiFSI | C3mpyrFSI | −26 | 7.6 × 10−6 (50 °C) | 1.4 × 106 (50 °C) | [48] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rollo-Walker, G.; Malic, N.; Wang, X.; Chiefari, J.; Forsyth, M. Development and Progression of Polymer Electrolytes for Batteries: Influence of Structure and Chemistry. Polymers 2021, 13, 4127. https://doi.org/10.3390/polym13234127
Rollo-Walker G, Malic N, Wang X, Chiefari J, Forsyth M. Development and Progression of Polymer Electrolytes for Batteries: Influence of Structure and Chemistry. Polymers. 2021; 13(23):4127. https://doi.org/10.3390/polym13234127
Chicago/Turabian StyleRollo-Walker, Gregory, Nino Malic, Xiaoen Wang, John Chiefari, and Maria Forsyth. 2021. "Development and Progression of Polymer Electrolytes for Batteries: Influence of Structure and Chemistry" Polymers 13, no. 23: 4127. https://doi.org/10.3390/polym13234127
APA StyleRollo-Walker, G., Malic, N., Wang, X., Chiefari, J., & Forsyth, M. (2021). Development and Progression of Polymer Electrolytes for Batteries: Influence of Structure and Chemistry. Polymers, 13(23), 4127. https://doi.org/10.3390/polym13234127








