Flammability and Thermal Kinetic Analysis of UiO-66-Based PMMA Polymer Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of UiO-66
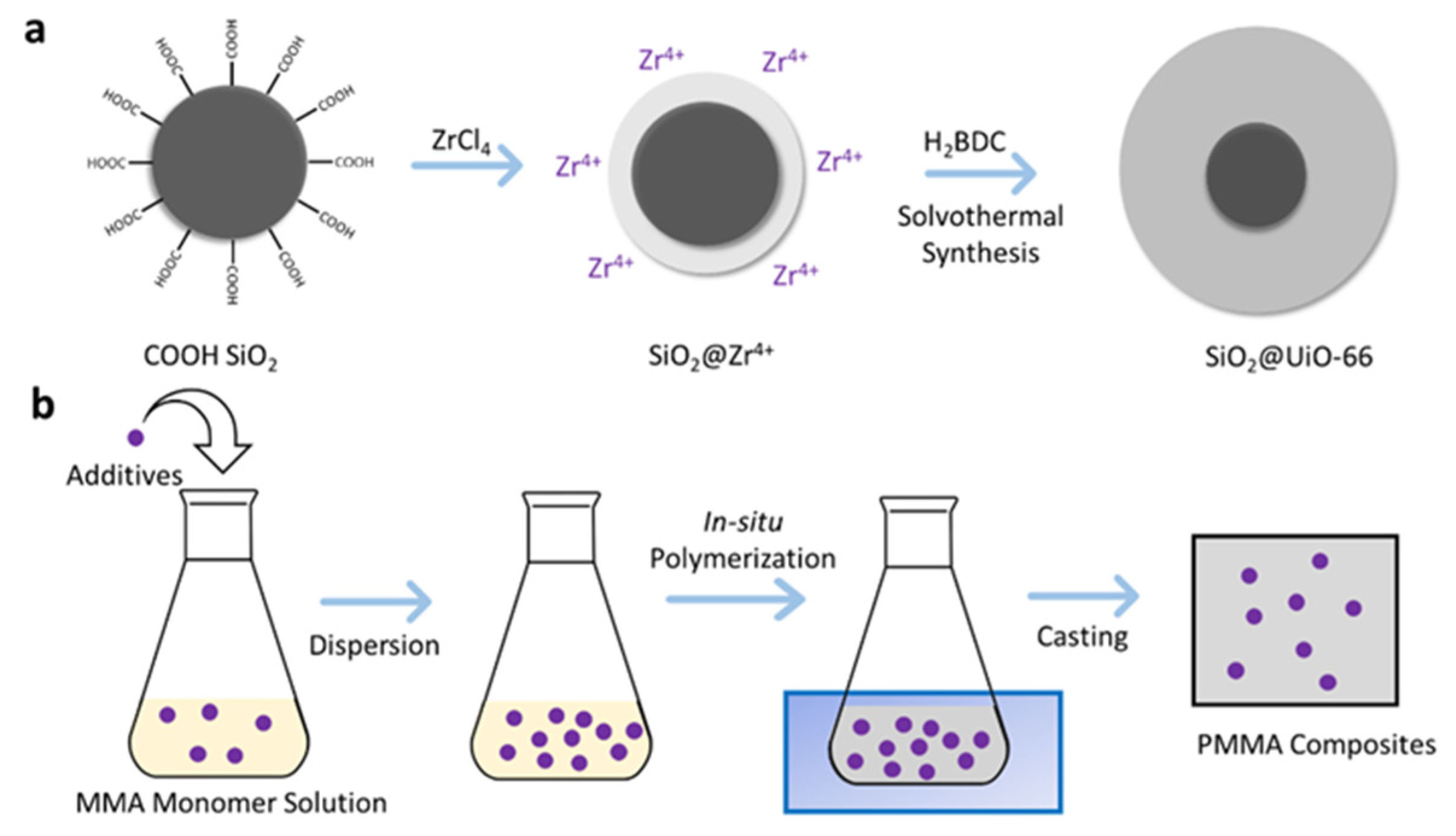
2.3. Synthesis of SiO2@UiO-66 Composite
2.4. Preparation of PMMA and Its Composites
2.5. Characterization and Measurements
3. Results and Discussion
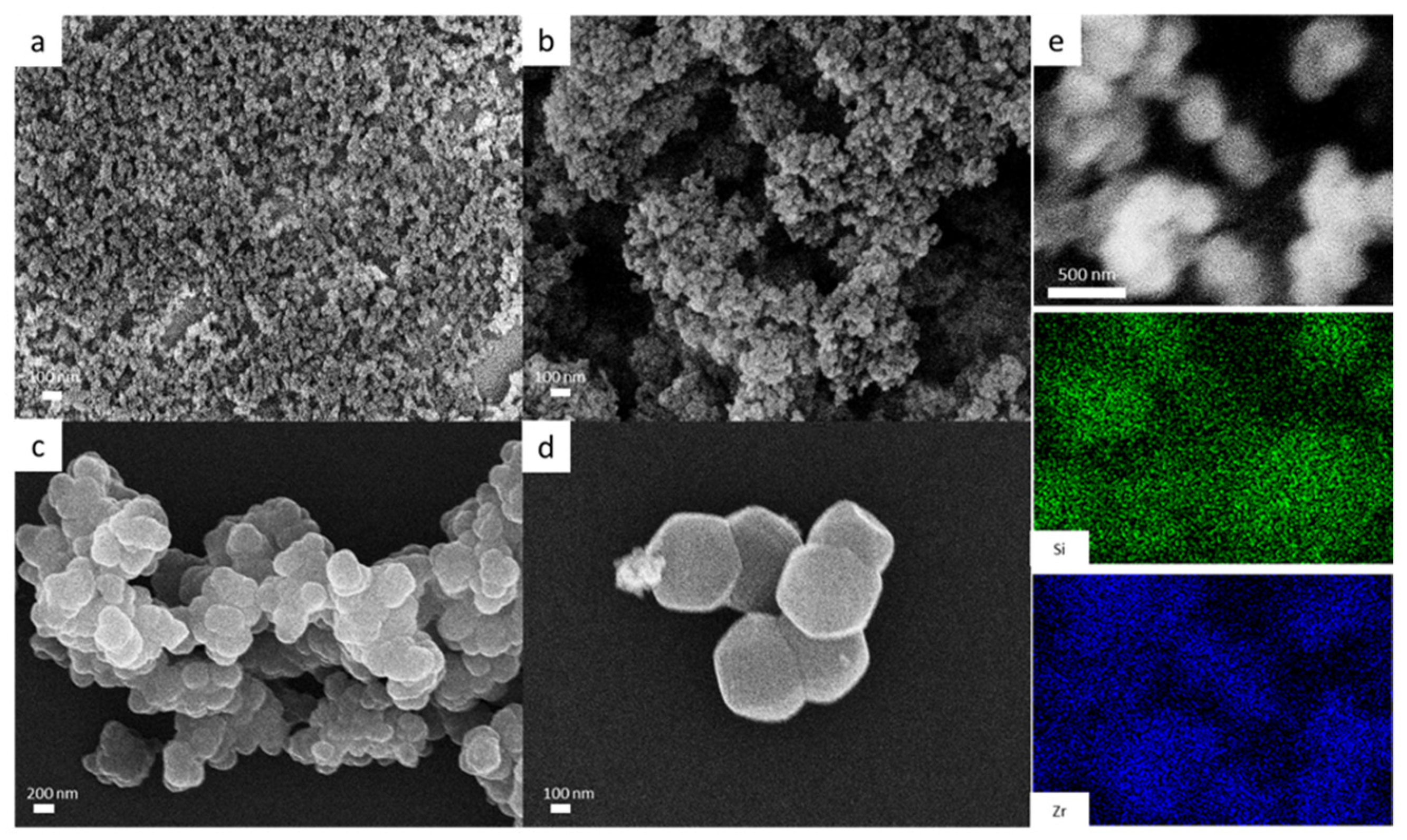
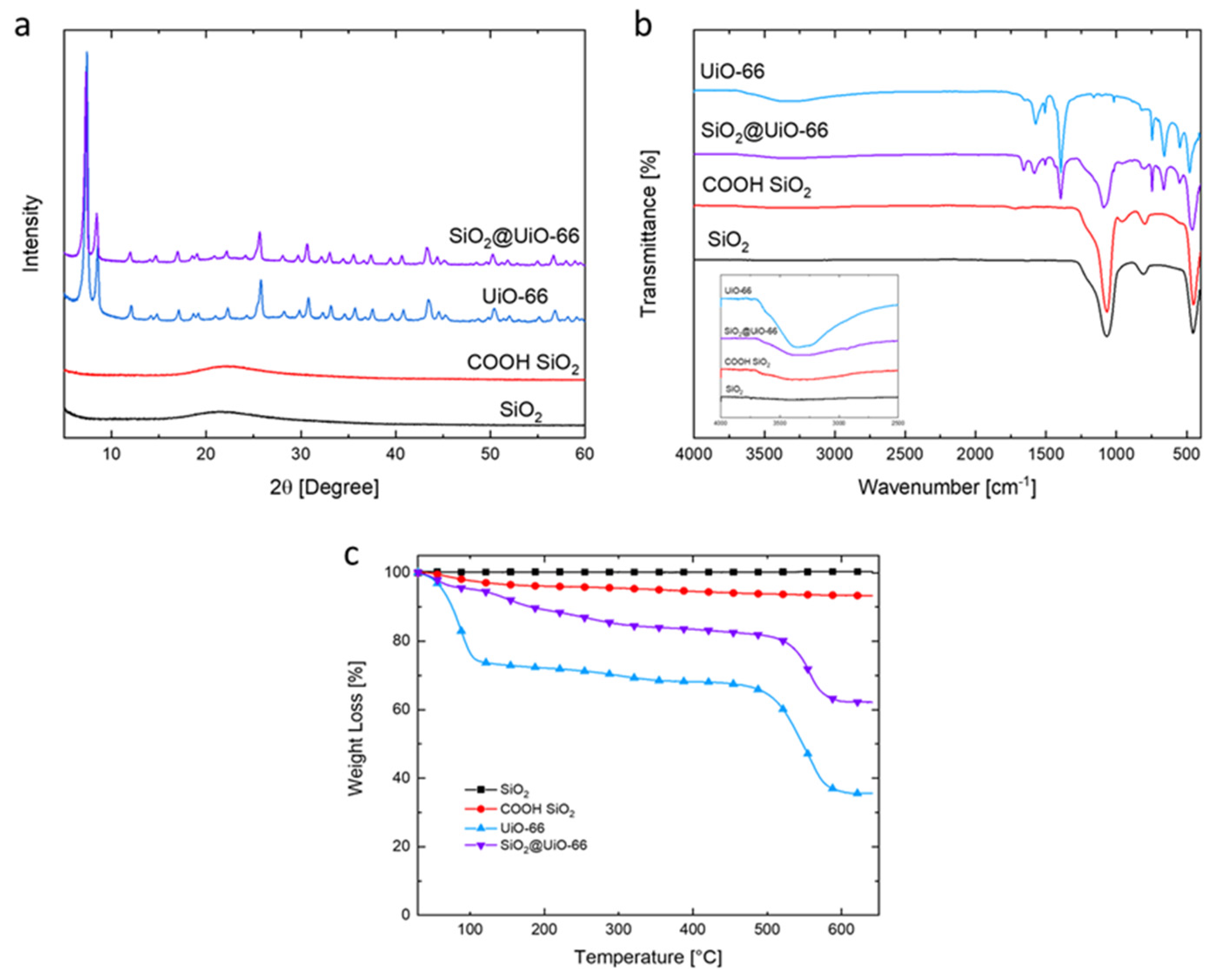
3.1. Characterization of PMMA Additives
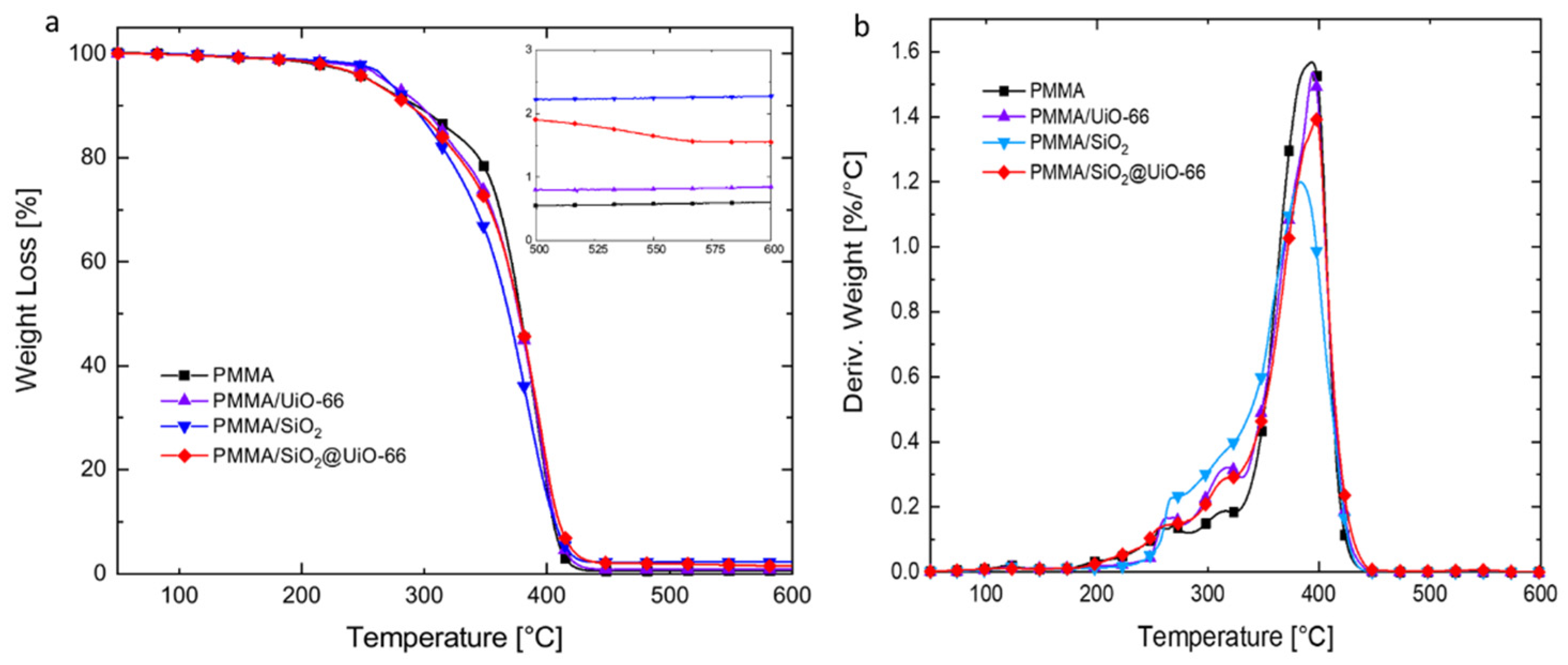
3.2. Thermal Properties of PMMA and Its Composites
3.3. Flammability of PMMA and Its Composites
3.4. Effect of UiO-66 on Thermal Decomposition Kinetics of PMMA
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, Q.-L.; Xu, Q. Metal-organic framework composites. Chem. Soc. Rev. 2014, 43, 5468–5512. [Google Scholar] [CrossRef]
- Kuppler, R.J.; Timmons, D.J.; Fang, Q.-R.; Li, J.-R.; Makal, T.A.; Young, M.D.; Yuan, D.; Zhao, D.; Zhuang, W.; Zhou, H.-C. Potential applications of metal-organic frameworks. Coord. Chem. Rev. 2009, 253, 3042–3066. [Google Scholar] [CrossRef]
- Nabipour, H.; Wang, X.; Song, L.; Hu, Y. Metal-organic frameworks for flame retardant polymers application: A critical review. Compos. Part A Appl. Sci. Manuf. 2020, 139, 106113. [Google Scholar] [CrossRef]
- Hou, Y.; Xu, Z.; Chu, F.; Gui, Z.; Song, L.; Hu, Y.; Hu, W. A review on metal-organic hybrids as flame retardants for enhancing fire safety of polymer composites. Compos. B Eng. 2021, 221, 109014. [Google Scholar] [CrossRef]
- Pan, Y.-T.; Zhang, Z.; Yang, R. The rise of MOFs and their derivatives for flame retardant polymeric materials: A critical review. Compos. B Eng. 2020, 199, 108265. [Google Scholar] [CrossRef]
- Korobeinichev, O.P.; Paletsky, A.A.; Gonchikzhapov, M.B.; Glaznev, R.K.; Gerasimov, I.E.; Naganovsky, Y.K.; Shundrina, I.K.; Snegirev, A.Y.; Vinu, R. Kinetics of thermal decomposition of PMMA at different heating rates and in a wide temperature range. Thermochim. Acta 2019, 671, 17–25. [Google Scholar] [CrossRef]
- Bhargava, A.; van Hees, P.; Andersson, B. Pyrolysis modeling of PVC and PMMA using a distributed reactivity model. Polym. Degrad. Stab. 2016, 129, 199–211. [Google Scholar] [CrossRef]
- Luche, J.; Rogaume, T.; Richard, F.; Guillaume, E. Characterization of thermal properties and analysis of combustion behavior of PMMA in a cone calorimeter. Fire Saf. J. 2011, 46, 451–461. [Google Scholar] [CrossRef]
- Bai, Y.; Dou, Y.; Xie, L.-H.; Rutledge, W.; Li, J.-R.; Zhou, H.-C. Zr-based metal-organic frameworks: Design, synthesis, structure, and applications. Chem. Soc. Rev. 2016, 45, 2327–2367. [Google Scholar] [CrossRef]
- Yang, D.; Hu, Y.; Song, L.; Nie, S.; He, S.; Cai, Y. Catalyzing carbonization function of α-ZrP based intumescent fire retardant polypropylene nanocomposites. Polym. Degrad. Stab. 2008, 93, 2014–2018. [Google Scholar] [CrossRef]
- Ma, M.; Lu, L.; Li, H.; Xiong, Y.; Dong, F. Functional metal organic framework/SiO2 nanocomposites: From versatile synthesis to advanced applications. Polymers 2019, 11, 1823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Yan, Z.; Gao, J.; Tong, P.; Liu, W.; Zhang, L. Metal-organic framework UiO-66 modified magnetite@silica core-shell magnetic microspheres for magnetic solid-phase extraction of domoic acid from shellfish samples. J. Chromatogr. A 2015, 1400, 10–18. [Google Scholar] [CrossRef]
- Shen, R.; Hatanaka, L.C.; Ahmed, L.; Agnew, R.J.; Mannan, M.S.; Wang, Q. Cone calorimeter analysis of flame retardant poly (methyl methacrylate)-silica nanocomposites. J. Therm. Anal. Calorim. 2017, 128, 1443–1451. [Google Scholar] [CrossRef]
- Rantuch, P.; Martinka, J.; Ház, A. The evaluation of torrefied wood using a cone calorimeter. Polymers 2021, 13, 1748. [Google Scholar] [CrossRef] [PubMed]
- Lazar, S.; Shen, R.; Quan, Y.; Palen, B.; Wang, Q.; Ellison, C.J.; Grunlan, J.C. Mixed solvent synthesis of polydopamine nanospheres for sustainable multilayer flame retardant nanocoating. Polym. Chem. 2021, 12, 2389–2396. [Google Scholar] [CrossRef]
- Guo, J.Z.; Song, K.; Liu, C. (Eds.) Polymer-Based Multifunctional Nanocomposites and Their Applications; Elsevier Science Publishing: Philadelphia, PA, USA, 2018; ISBN 9780128150672. [Google Scholar]
- Ng, Y.H.; Zope, I.S.; Dasari, A.; Tan, K.H. Correlating the performance of a fire-retardant coating across different scales of testing. Polymers 2020, 12, 2271. [Google Scholar] [CrossRef]
- Xu, Q.; Jin, C.; Majlingova, A.; Restas, A. Discuss the heat release capacity of polymer derived from microscale combustion calorimeter. J. Therm. Anal. Calorim. 2018, 133, 649–657. [Google Scholar] [CrossRef]
- Gao, B.; Huang, M.; Zhang, Z.; Yang, Q.; Su, B.; Yang, Y.; Ren, Q.; Bao, Z. Hybridization of metal–organic framework and monodisperse spherical silica for chromatographic separation of xylene isomers. Chin. J. Chem. Eng. 2019, 27, 818–826. [Google Scholar] [CrossRef]
- Yang, F.; Xie, S.; Wang, G.; Yu, C.W.; Liu, H.; Liu, Y. Investigation of a modified metal-organic framework UiO-66 with nanoscale zero-valent iron for removal of uranium (VI) from aqueous solution. Environ. Sci. Pollut. Res. Int. 2020, 27, 20246–20258. [Google Scholar] [CrossRef]
- Yang, P.; Liu, Q.; Liu, J.; Zhang, H.; Li, Z.; Li, R.; Liu, L.; Wang, J. Interfacial growth of a metal–organic framework (UiO-66) on functionalized graphene oxide (GO) as a suitable seawater adsorbent for extraction of uranium(vi). J. Mater. Chem. A 2017, 5, 17933–17942. [Google Scholar] [CrossRef]
- Guo, W.; Nie, S.; Kalali, E.N.; Wang, X.; Wang, W.; Cai, W.; Song, L.; Hu, Y. Construction of SiO2@UiO-66 core–shell microarchitectures through covalent linkage as flame retardant and smoke suppressant for epoxy resins. Compos. B Eng. 2019, 176, 107261. [Google Scholar] [CrossRef]
- Fu, Y.-Y.; Yang, C.-X.; Yan, X.-P. Fabrication of ZIF-8@SiO2 core-shell microspheres as the stationary phase for high-performance liquid chromatography. Chemistry 2013, 19, 13484–13491. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Zhuo, Q.; Liu, X.; Sun, Z.; Wu, Z.; Fan, H. Hydrophobic silica aerogel reinforced with carbon nanotube for oils removal. J. Porous Mater. 2014, 21, 967–973. [Google Scholar] [CrossRef]
- Zhang, X.; Han, Q.; Ding, M. One-pot synthesis of UiO-66@SiO2 shell–core microspheres as stationary phase for high performance liquid chromatography. RSC Adv. 2015, 5, 1043–1050. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, H.-Y.; Wang, L.; Zhang, Y.; Zhao, J. Ru/UiO-66 catalyst for the reduction of nitroarenes and tandem reaction of alcohol oxidation/Knoevenagel condensation. ACS Omega 2018, 3, 4199–4212. [Google Scholar] [CrossRef]
- Shi, X.; Dai, X.; Cao, Y.; Li, J.; Huo, C.; Wang, X. Degradable poly(lactic acid)/metal–organic framework nanocomposites exhibiting good mechanical, flame retardant, and dielectric properties for the fabrication of disposable electronics. Ind. Eng. Chem. Res. 2017, 56, 3887–3894. [Google Scholar] [CrossRef]
- Ferriol, M.; Gentilhomme, A.; Cochez, M.; Oget, N.; Mieloszynski, J.L. Thermal degradation of poly(methyl methacrylate) (PMMA): Modelling of DTG and TG curves. Polym. Degrad. Stab. 2003, 79, 271–281. [Google Scholar] [CrossRef]
- Li, A.; Xu, W.; Chen, R.; Liu, Y.; Li, W. Fabrication of zeolitic imidazolate frameworks on layered double hydroxide nanosheets to improve the fire safety of epoxy resin. Compos. Part A Appl. Sci. Manuf. 2018, 112, 558–571. [Google Scholar] [CrossRef]
- Hu, Y.-H.; Chen, C.-Y.; Wang, C.-C. Viscoelastic properties and thermal degradation kinetics of silica/PMMA nanocomposites. Polym. Degrad. Stab. 2004, 84, 545–553. [Google Scholar] [CrossRef]
- Schartel, B.; Hull, T.R. Development of fire-retarded materials—Interpretation of cone calorimeter data. Fire Mater. 2007, 31, 327–354. [Google Scholar] [CrossRef]
- Ahmed, L.; Zhang, B.; Shen, R.; Agnew, R.J.; Park, H.; Cheng, Z.; Mannan, M.S.; Wang, Q. Fire reaction properties of polystyrene-based nanocomposites using nanosilica and nanoclay as additives in cone calorimeter test. J. Therm. Anal. Calorim. 2018, 132, 1853–1865. [Google Scholar] [CrossRef]
- Basahel, S.N.; Ali, T.T.; Mokhtar, M.; Narasimharao, K. Influence of crystal structure of nanosized ZrO2 on photocatalytic degradation of methyl orange. Nanoscale Res. Lett. 2015, 10, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Yang, Y.; Shen, R.; Parker, T.; Zhang, Y.; Wang, Z.; Wang, Q. Thermal behavior and kinetics study of carbon/epoxy resin composites. Polym. Compos. 2019, 40, 4530–4546. [Google Scholar] [CrossRef]
- Snegirev, A.Y.; Talalov, V.A.; Stepanov, V.V.; Korobeinichev, O.P.; Gerasimov, I.E.; Shmakov, A.G. Autocatalysis in thermal decomposition of polymers. Polym. Degrad. Stab. 2017, 137, 151–161. [Google Scholar] [CrossRef]
- Snegirev, A.Y. Generalized approach to model pyrolysis of flammable materials. Thermochim. Acta 2014, 590, 242–250. [Google Scholar] [CrossRef]
- Holland, B.J.; Hay, J.N. The effect of polymerisation conditions on the kinetics and mechanisms of thermal degradation of PMMA. Polym. Degrad. Stab. 2002, 77, 435–439. [Google Scholar] [CrossRef]
- Lyon, R.E.; Safronava, N. A comparison of direct methods to determine n-th order kinetic parameters of solid thermal decomposition for use in fire models. J. Therm. Anal. Calorim. 2013, 114, 213–227. [Google Scholar] [CrossRef]
- Benhacine, F.; Yahiaoui, F.; Hadj-Hamou, A.S. Thermal stability and kinetic study of isotactic polypropylene/Algerian bentonite nanocomposites prepared via melt blending. J. Polym. 2014, 2014, 426470. [Google Scholar] [CrossRef] [Green Version]

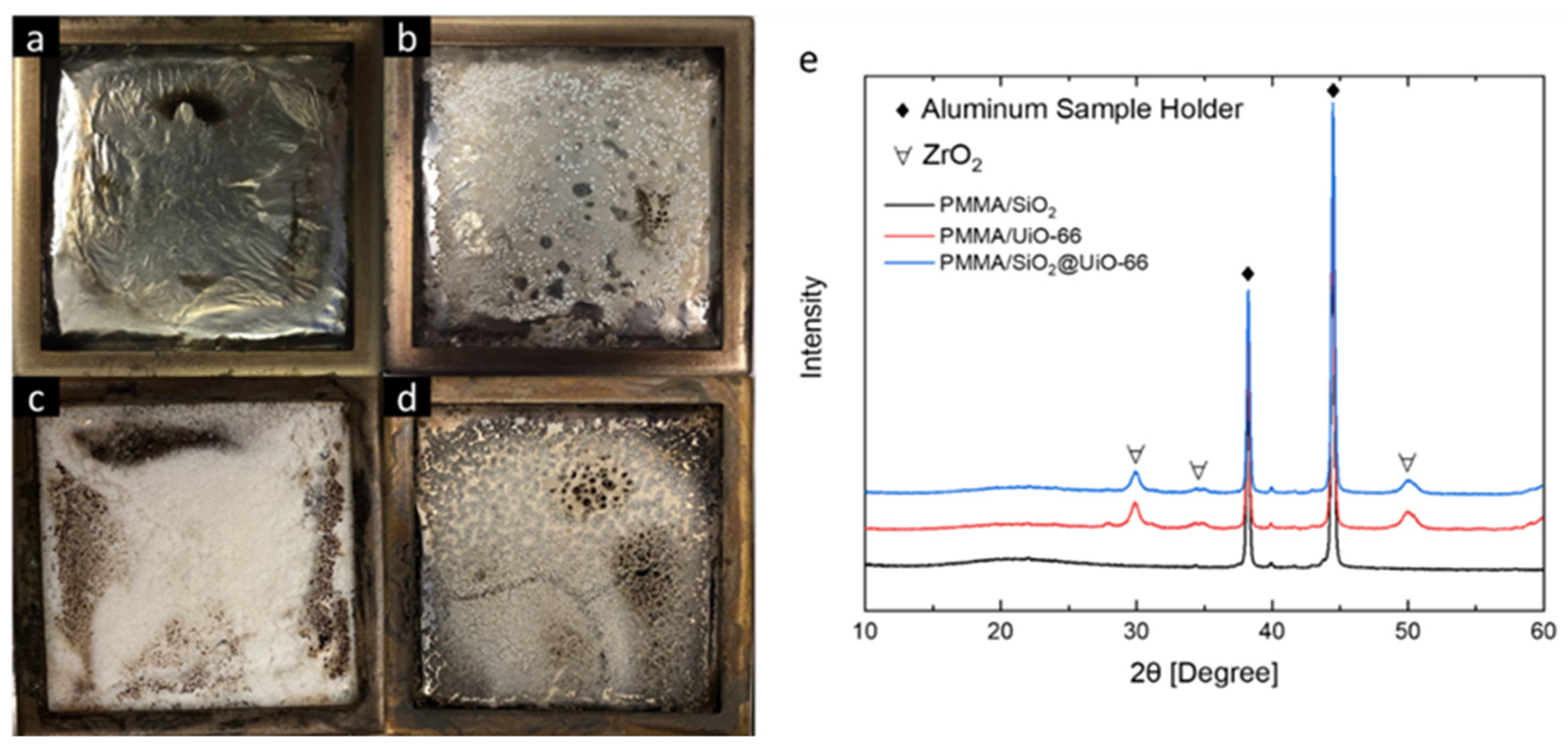
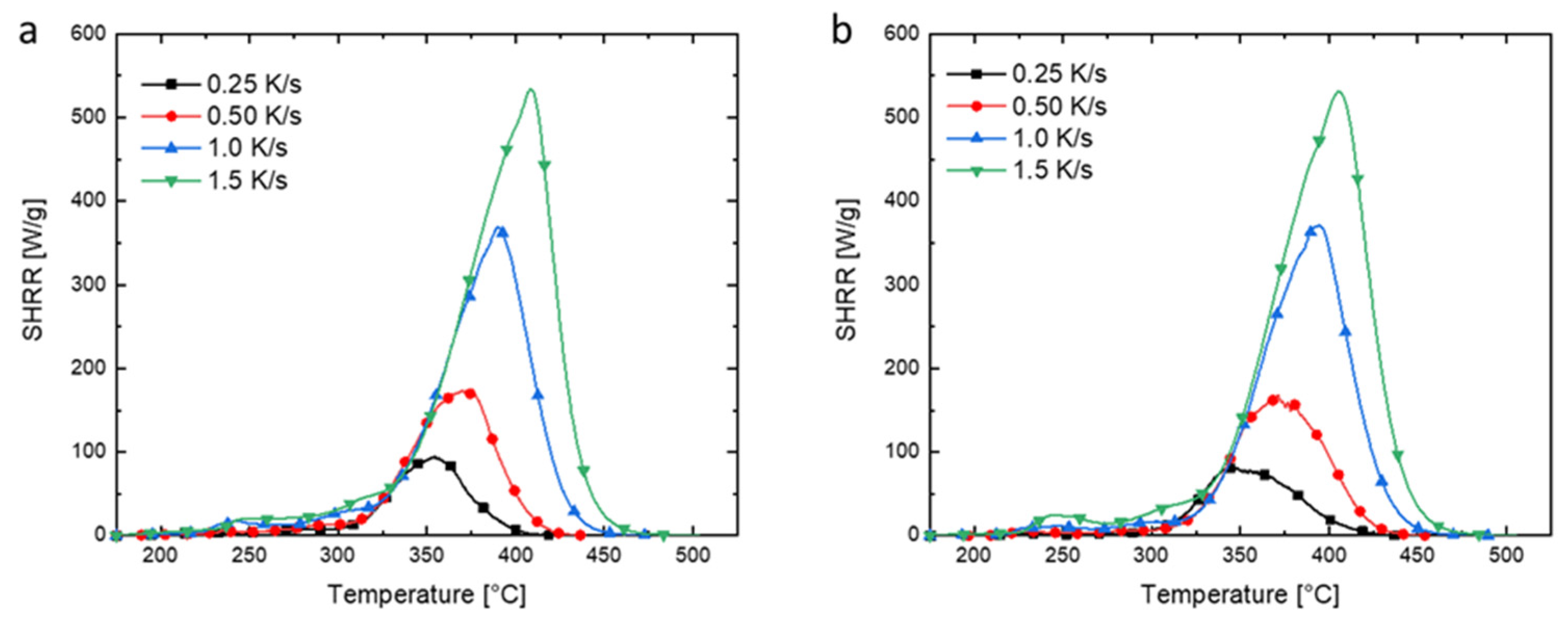
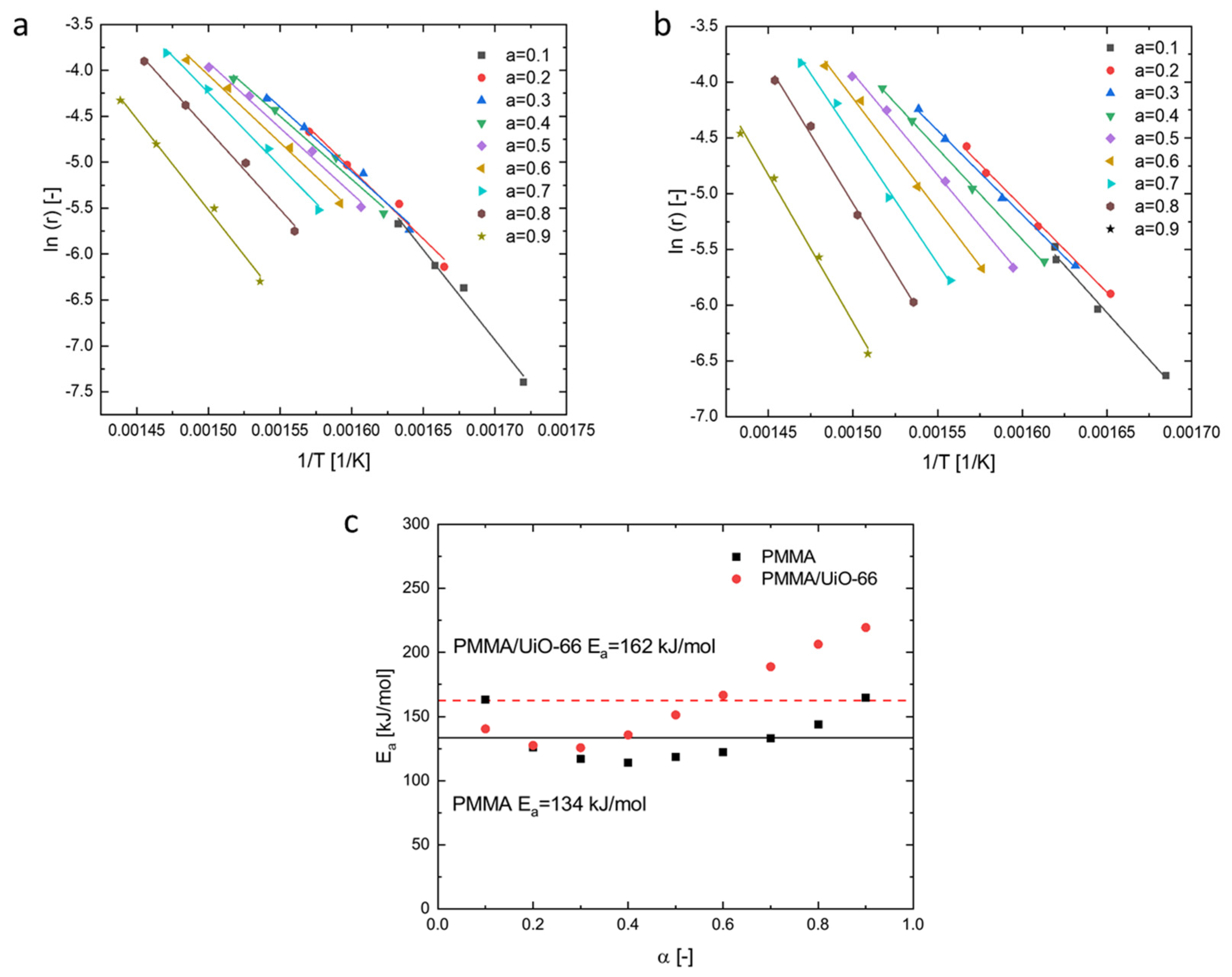
| Samples | Tonset (°C) | Tmax (°C) | Mass Residue at Tmax (wt%) | Peak Mass Loss Rate (wt%/°C) | Residue at 600 °C (wt%) |
|---|---|---|---|---|---|
| PMMA | 293 | 394 | 26.9 | 1.57 | 0.6 |
| PMMA/UiO-66 | 297 | 395 | 25.9 | 1.54 | 0.8 |
| PMMA/SiO2 | 290 | 383 | 33.6 | 1.20 | 2.3 |
| PMMA/SiO2@UiO-66 | 288 | 397 | 24.3 | 1.39 | 1.6 |
| Sample | Time to Ignition (s) | pHRR (kW/m2) | Time to pHRR (s) | Mean HRR (kW/m2) | Average Specific MLR (g/sm2) | Mean CO Yield (kg/kg) | Mean CO2 Yield (kg/kg) | EHC (MJ/kg) | MARHE (kW/m2) | Fuel Load (MJ/kg) |
|---|---|---|---|---|---|---|---|---|---|---|
| PMMA | 19 | 832 | 195 | 307 | 22.5 | 0.0146 | 1.87 | 23.89 | 680.4 | 23.81 |
| PMMA/UiO-66 | 20 | 713 | 275 | 283 | 19.7 | 0.0136 | 1.83 | 23.87 | 537.3 | 23.55 |
| PMMA/SiO2 | 14 | 758 | 220 | 303 | 24.0 | 0.0127 | 2.18 | 23.53 | 551.5 | 23.36 |
| PMMA/SiO2@UiO-66 | 25 | 649 | 228 | 277 | 19.2 | 0.0139 | 1.79 | 23.51 | 504.7 | 23.35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, R.; Yan, T.-H.; Ma, R.; Joseph, E.; Quan, Y.; Zhou, H.-C.; Wang, Q. Flammability and Thermal Kinetic Analysis of UiO-66-Based PMMA Polymer Composites. Polymers 2021, 13, 4113. https://doi.org/10.3390/polym13234113
Shen R, Yan T-H, Ma R, Joseph E, Quan Y, Zhou H-C, Wang Q. Flammability and Thermal Kinetic Analysis of UiO-66-Based PMMA Polymer Composites. Polymers. 2021; 13(23):4113. https://doi.org/10.3390/polym13234113
Chicago/Turabian StyleShen, Ruiqing, Tian-Hao Yan, Rong Ma, Elizabeth Joseph, Yufeng Quan, Hong-Cai Zhou, and Qingsheng Wang. 2021. "Flammability and Thermal Kinetic Analysis of UiO-66-Based PMMA Polymer Composites" Polymers 13, no. 23: 4113. https://doi.org/10.3390/polym13234113
APA StyleShen, R., Yan, T.-H., Ma, R., Joseph, E., Quan, Y., Zhou, H.-C., & Wang, Q. (2021). Flammability and Thermal Kinetic Analysis of UiO-66-Based PMMA Polymer Composites. Polymers, 13(23), 4113. https://doi.org/10.3390/polym13234113







