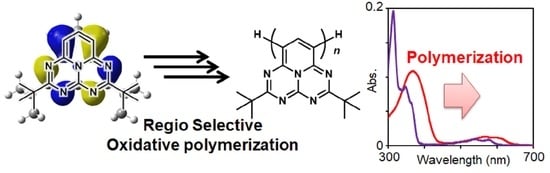
Development of Long Wavelength Light-Absorptive Homopolymers Based on Pentaazaphenalene by Regioselective Oxidative Polymerization
Abstract
:1. Introduction
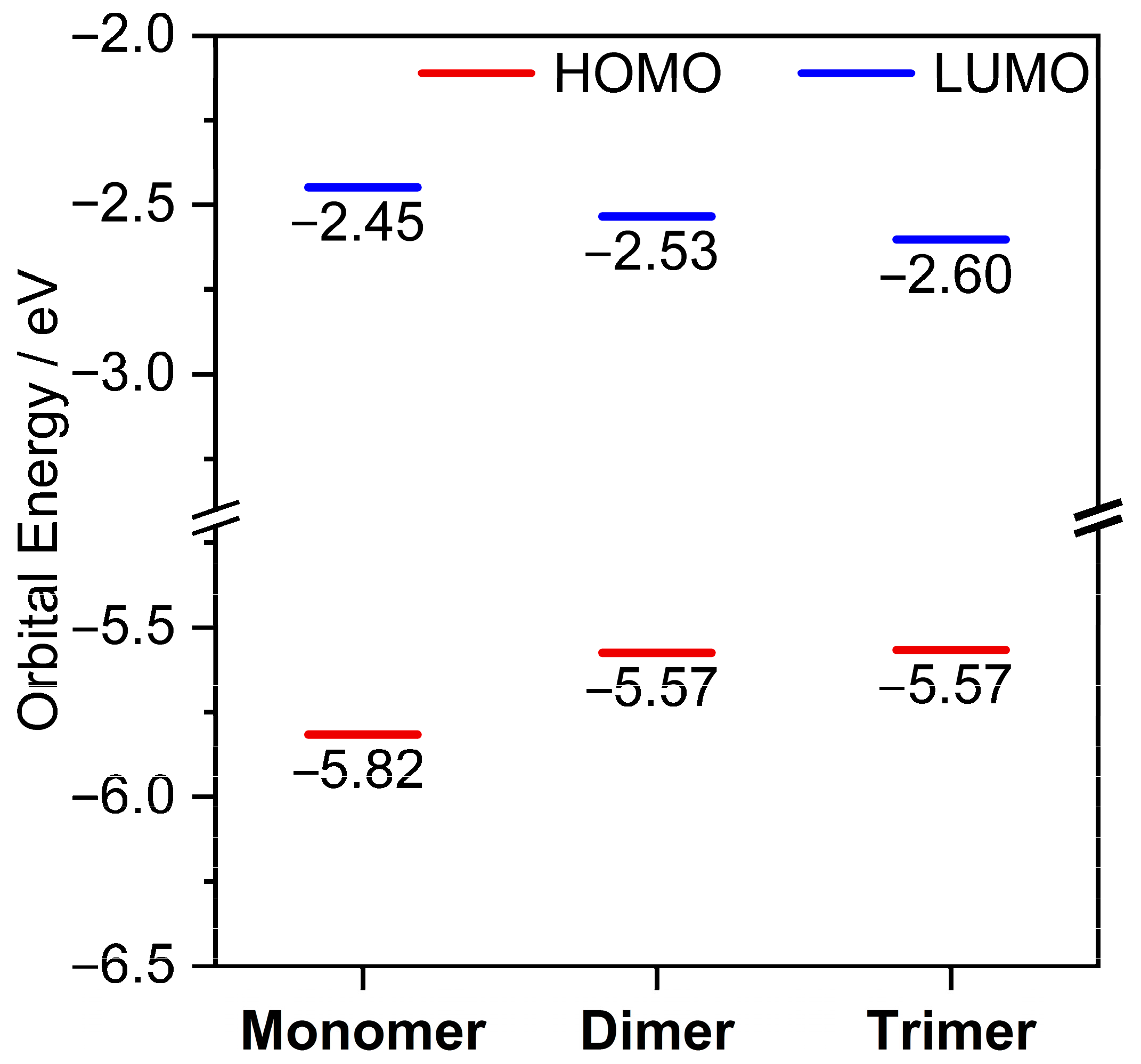
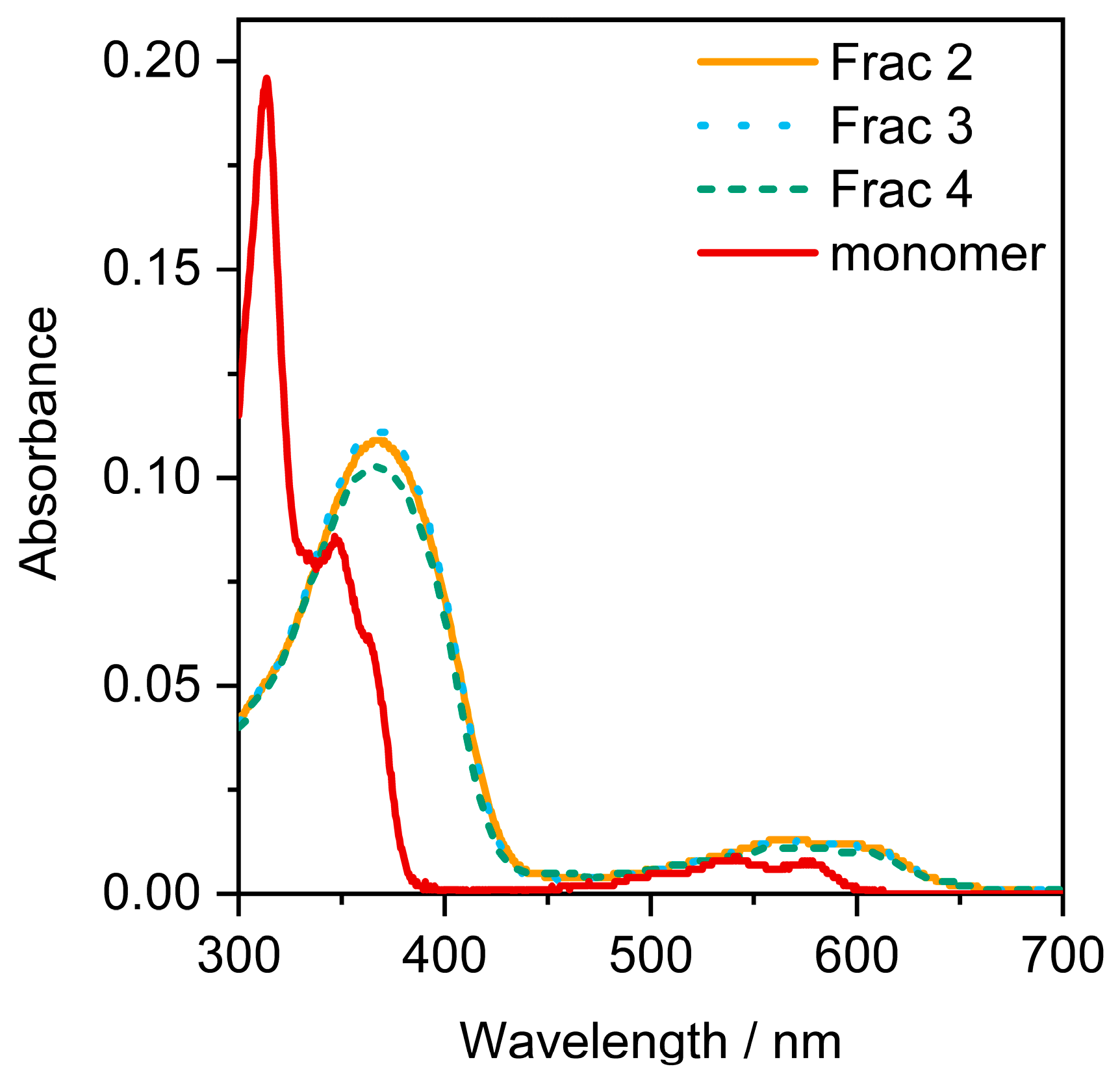
2. Results and Discussion
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yamazawa, C.; Hirano, Y.; Imoto, H.; Tsutsumi, N.; Naka, K. Superior light-resistant Dithieno[3,2-b:2′,3′-d]arsole-based polymers exhibiting ultrastable amplified spontaneous emission. Chem. Commun. 2021, 57, 1595–1598. [Google Scholar] [CrossRef]
- Ishijima, K.; Tanaka, S.; Imoto, H.; Naka, K. 2,3-Diarylbenzo[b]arsole: Structural modification and polymerization for tuning of photophysical properties. Chem. Eur. J. 2021, 27, 4676–4682. [Google Scholar] [CrossRef]
- Tanaka, S.; Enoki, T.; Imoto, H.; Ooyama, Y.; Ohshita, J.; Kato, T.; Naka, K. Highly efficient singlet oxygen generation and high oxidation resistance enhanced by arsole-polymer-based photosensitizer: Application as a recyclable photooxidation catalyst. Macromolecules 2020, 53, 2006–2013. [Google Scholar] [CrossRef]
- Matsumura, Y.; Ishidoshiro, M.; Irie, Y.; Imoto, H.; Naka, K.; Tanaka, K.; Inagi, S.; Tomita, I. Arsole-containing π-conjugated polymer by post-element-transformation-technique. Angew. Chem. Int. Ed. 2016, 55, 15040–15043. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Liu, J.; Jäkle, F. Electron-deficient conjugated materials via p–π* conjugation with boron: Extending monomers to oligomers, macrocycles, and polymers. Chem.-Eur. J. 2021, 27, 2973–2986. [Google Scholar] [CrossRef]
- Baser-Kirazli, N.; Lalancette, R.A.; Jäkle, F. Tuning the donor–π–acceptor character of arylborane–arylamine macrocycles. Organometallics 2021, 40, 520–528. [Google Scholar] [CrossRef]
- Gon, M.; Tanaka, K.; Chujo, Y. Discovery of functional luminescence properties based on flexible and bendable boron-fused azomethine/azobenzene complexes with O,N,O-type tridentate ligands. Chem. Rec. 2021, 21, 1358–1373. [Google Scholar] [CrossRef]
- Ochi, J.; Tanaka, K.; Chujo, Y. Recent progresses in the development of solid-state luminescent o-carboranes with stimuli responsivity. Angew. Chem. Int. Ed. 2020, 132, 9841–9855. [Google Scholar] [CrossRef] [PubMed]
- Gon, M.; Tanaka, K.; Chujo, Y. Concept of excitation-driven boron complexes and their applications for functional luminescent materials. Bull. Chem. Soc. Jpn. 2019, 92, 7–18. [Google Scholar] [CrossRef] [Green Version]
- Ito, S.; Gon, M.; Tanaka, K.; Chujo, Y. Molecular design and applications of luminescent materials composed of group 13 elements with aggregation-induced emission property. Natl. Sci. Rev. 2021, 8, nwab049. [Google Scholar] [CrossRef]
- Yoshii, R.; Yamane, H.; Tanaka, K.; Chujo, Y. Synthetic strategy for low-band gap oligomers and homopolymers using characteristics of thiophene-fused boron dipyrromethene. Macromolecules 2014, 47, 3755–3760. [Google Scholar] [CrossRef]
- Zhu, X.; Tsuji, H.; López Navarrete, J.T.; Casado, J.; Nakamura, E. Carbon-bridged oligo(phenylenevinylene)s: Stable π-systems with high responsiveness to doping and excitation. J. Am. Chem. Soc. 2012, 134, 19254–19259. [Google Scholar] [CrossRef]
- Chujo, Y.; Tanaka, K. New polymeric materials based on element-blocks. Bull. Chem. Soc. Jpn. 2015, 88, 633–643. [Google Scholar] [CrossRef] [Green Version]
- Gon, M.; Tanaka, K.; Chujo, Y. Recent progress in the development of advanced element-block materials. Polym. J. 2018, 50, 109–126. [Google Scholar] [CrossRef] [Green Version]
- Gon, M.; Ito, S.; Tanaka, K.; Chujo, Y. Design strategies and recent results for near-infrared-emissive materials based on element-block π-conjugated polymers. Bull. Chem. Soc. Jpn. 2021, 94, 2290–2302. [Google Scholar] [CrossRef]
- Tanaka, K.; Chujo, Y. Modulation of the solid-state luminescent properties of conjugated polymers by changing the connecting points of flexible boron element-blocks. Polym. J. 2020, 52, 555–566. [Google Scholar] [CrossRef]
- Ito, S.; Gon, M.; Tanaka, K.; Chujo, Y. Recent developments in stimuli-responsive luminescent polymers composed of boron compounds. Polym. Chem. 2021, 12, 6372–6380. [Google Scholar] [CrossRef]
- Yeo, H.; Tanaka, K.; Hirose, M.; Chujo, Y. Construction of multi-n-heterocycle-containing organic solvent-soluble polymers with 1,3,4,6,9b-pentaazaphenalene. Polym. J. 2014, 46, 688–693. [Google Scholar] [CrossRef]
- Watanabe, H.; Kawano, Y.; Tanaka, K.; Chujo, Y. Enhancing light-absorption and luminescent properties of non-emissive 1,3,4,6,9b-pentaazaphenalene through perturbation of forbidden electronic transition by boron complexation. Asian J. Org. Chem. 2020, 9, 259–266. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, H.; Tanaka, K.; Chujo, Y. The effect of the substituent positions on self-assembly behaviors of liquid-crystalline 1,3,4,6,9b-pentaazaphenalene derivatives. Bull. Chem. Soc. Jpn 2021, 94, 1854–1858. [Google Scholar] [CrossRef]
- Watanabe, H.; Ochi, J.; Tanaka, K.; Chujo, Y. Tuning the NIR absorption properties of 1,3,4,6,9b-pentaazaphenalene derivatives through the spatially separated frontier molecular orbitals. Eur. J. Org. Chem. 2020, 2020, 777–783. [Google Scholar] [CrossRef]
- Watanabe, H.; Hirose, M.; Tanaka, K.; Tanaka, K.; Chujo, Y. Color tuning of alternating conjugated polymers composed of pentaazaphenalene by modulating their unique electronic structures involving isolated-LUMOs. Polym. Chem. 2016, 7, 3674–3680. [Google Scholar] [CrossRef]
- Watanabe, H.; Hirose, M.; Tanaka, K.; Chujo, Y. Development of Emissive Aminopentaazaphenalene Derivatives Employing a Design Strategy for Obtaining Luminescent Conjugated Molecules by Modulating the Symmetry of Molecule Orbitals with Substituent Effects. Chem. Commun. 2017, 53, 5036–5039. [Google Scholar] [CrossRef]
- Watanabe, H.; Tanaka, K.; Chujo, Y. Independently tuned frontier orbital energy levels of 1,3,4,6,9b-pentaazaphenalene derivatives by the conjugation effect. J. Org. Chem. 2019, 84, 2768–2778. [Google Scholar] [CrossRef]
- Tanaka, K.; Chujo, Y. New idea for narrowing an energy gap by selective perturbation for one frontier molecular orbital. Chem. Lett. 2021, 50, 269–279. [Google Scholar] [CrossRef]
- Meier, H.; Stalmach, U.; Kolshorn, H. Effective conjugation length and UV/vis spectra of oligomers. Acta Polymer. 1997, 48, 379–384. [Google Scholar] [CrossRef]
- Collison, C.J.; Rothberg, L.J.; Treemaneekarn, V.; Li, Y. Conformational effects on the photophysics of conjugated polymers: A two species model for meh− ppv spectroscopy and dynamics. Macromolecules 2001, 34, 2346–2352. [Google Scholar] [CrossRef]
- Ma, J.; Li, S.; Jiang, Y. A Time-dependent DFT study on band gaps and effective conjugation lengths of polyacetylene, polyphenylene, polypentafulvene, polycyclopentadiene, polypyrrole, polyfuran, polysilole, polyphosphole, and polythiophene. Macromolecules 2002, 35, 1109–1115. [Google Scholar] [CrossRef]
- Izumi, T.; Kobashi, S.; Takimiya, K.; Aso, Y.; Otsubo, T. Synthesis and spectroscopic properties of a series of β-blocked long oligothiophenes up to the 96-mer: revaluation of effective conjugation length. J. Am. Chem. Soc. 2003, 125, 5286–5287. [Google Scholar] [CrossRef]
- Morin, J.-F.; Leclerc, M.; Adès, D.; Siove, A. Polycarbazoles: 25 Years of progress. Macromol. Rapid Commun. 2005, 26, 761–778. [Google Scholar] [CrossRef]
- Yamamoto, T. Synthesis of π-Conjugated polymers by organometallic polycondensation. Bull. Chem. Soc. Jpn. 2010, 83, 431–455. [Google Scholar] [CrossRef]
- Scholl, R.; Mansfeld, J. Meso-benzdianthron (helianthron), meso-naphthodianthron, und ein neuer weg zum flavanthren. Berichte Dtsch. Chem. Gesellschaft 1910, 43, 1734–1746. [Google Scholar] [CrossRef] [Green Version]
- King, B.T.; Kroulík, J.; Robertson, C.R.; Rempala, P.; Hilton, C.L.; Korinek, J.D.; Gortari, L.M. Controlling the scholl reaction. J. Org. Chem. 2007, 72, 2279–2288. [Google Scholar] [CrossRef] [PubMed]
- Grzybowski, M.; Skonieczny, K.; Butenschön, H.; Gryko, D.T. Comparison of oxidative aromatic coupling and the scholl reaction. Angew. Chem. Int. Ed. 2013, 52, 9900–9930. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watanabe, H.; Tanaka, K.; Chujo, Y. Development of Long Wavelength Light-Absorptive Homopolymers Based on Pentaazaphenalene by Regioselective Oxidative Polymerization. Polymers 2021, 13, 4021. https://doi.org/10.3390/polym13224021
Watanabe H, Tanaka K, Chujo Y. Development of Long Wavelength Light-Absorptive Homopolymers Based on Pentaazaphenalene by Regioselective Oxidative Polymerization. Polymers. 2021; 13(22):4021. https://doi.org/10.3390/polym13224021
Chicago/Turabian StyleWatanabe, Hiroyuki, Kazuo Tanaka, and Yoshiki Chujo. 2021. "Development of Long Wavelength Light-Absorptive Homopolymers Based on Pentaazaphenalene by Regioselective Oxidative Polymerization" Polymers 13, no. 22: 4021. https://doi.org/10.3390/polym13224021
APA StyleWatanabe, H., Tanaka, K., & Chujo, Y. (2021). Development of Long Wavelength Light-Absorptive Homopolymers Based on Pentaazaphenalene by Regioselective Oxidative Polymerization. Polymers, 13(22), 4021. https://doi.org/10.3390/polym13224021