Synthesis and Characterization of White-Light Luminescent End-Capped Polyimides Based on FRET and Excited State Intramolecular Proton Transfer
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Imide Compound in PMMA Matrix
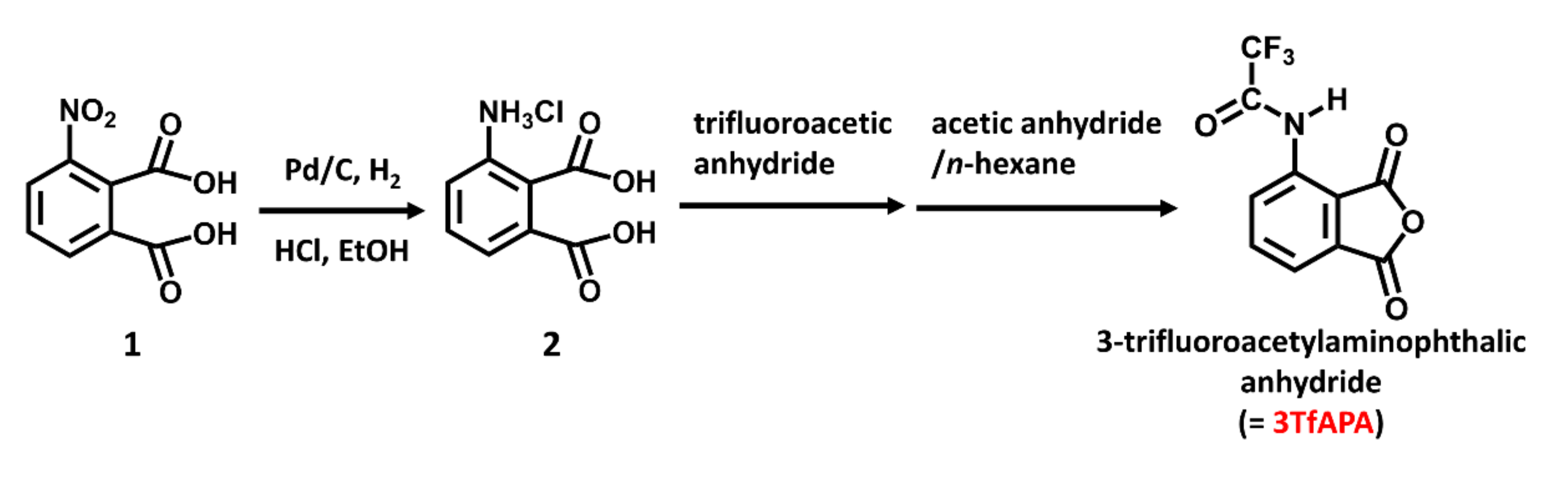
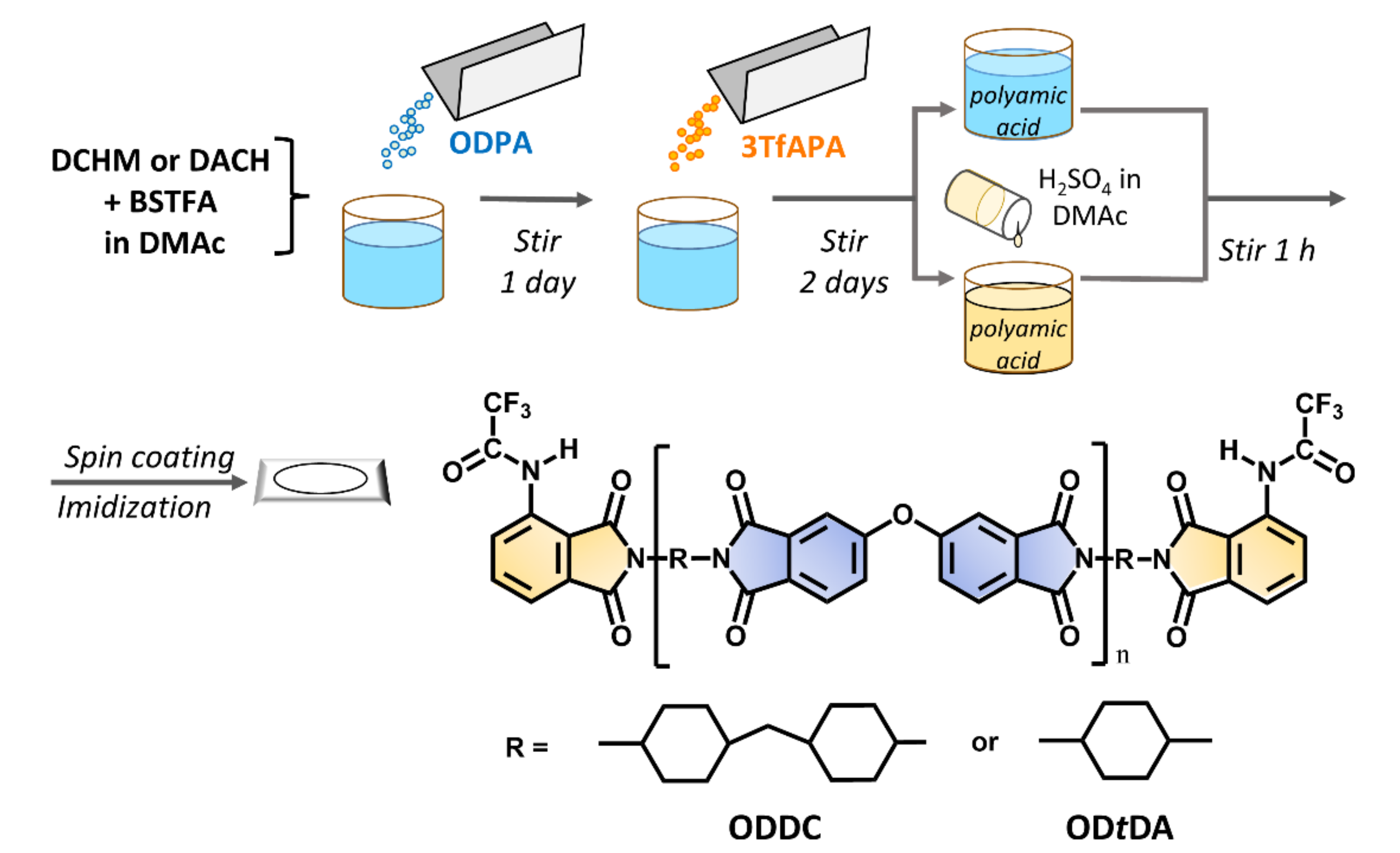
2.3. Synthesis of ESIPT Anhydride and Film Preparation of End-Capped Polyimides
2.4. Measurememts
2.4.1. UV–Vis Absorption and Excitation/Emission Spectroscopy
2.4.2. Time-Resolved Luminescence Measurements
2.4.3. Other Measurements
2.5. Quantum Chemical Calculations
2.6. Crystallography
3. Results and Discussion
3.1. Structure and Properties of Imide Compound (3TfAPI)
3.1.1. TD-DFT Calculation
3.1.2. Crystal Structure
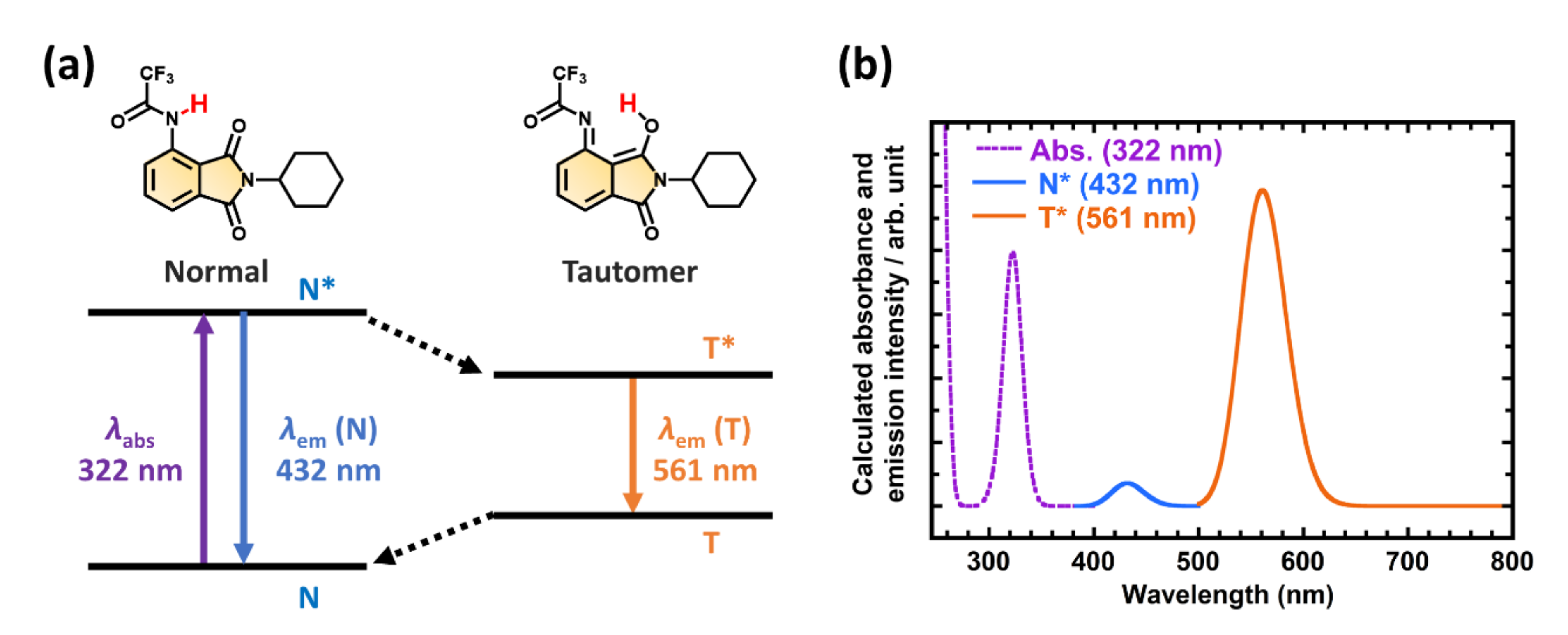
3.1.3. Optical Properties of 3TfAPI
3.2. End-Capped Polyimides (PIs)
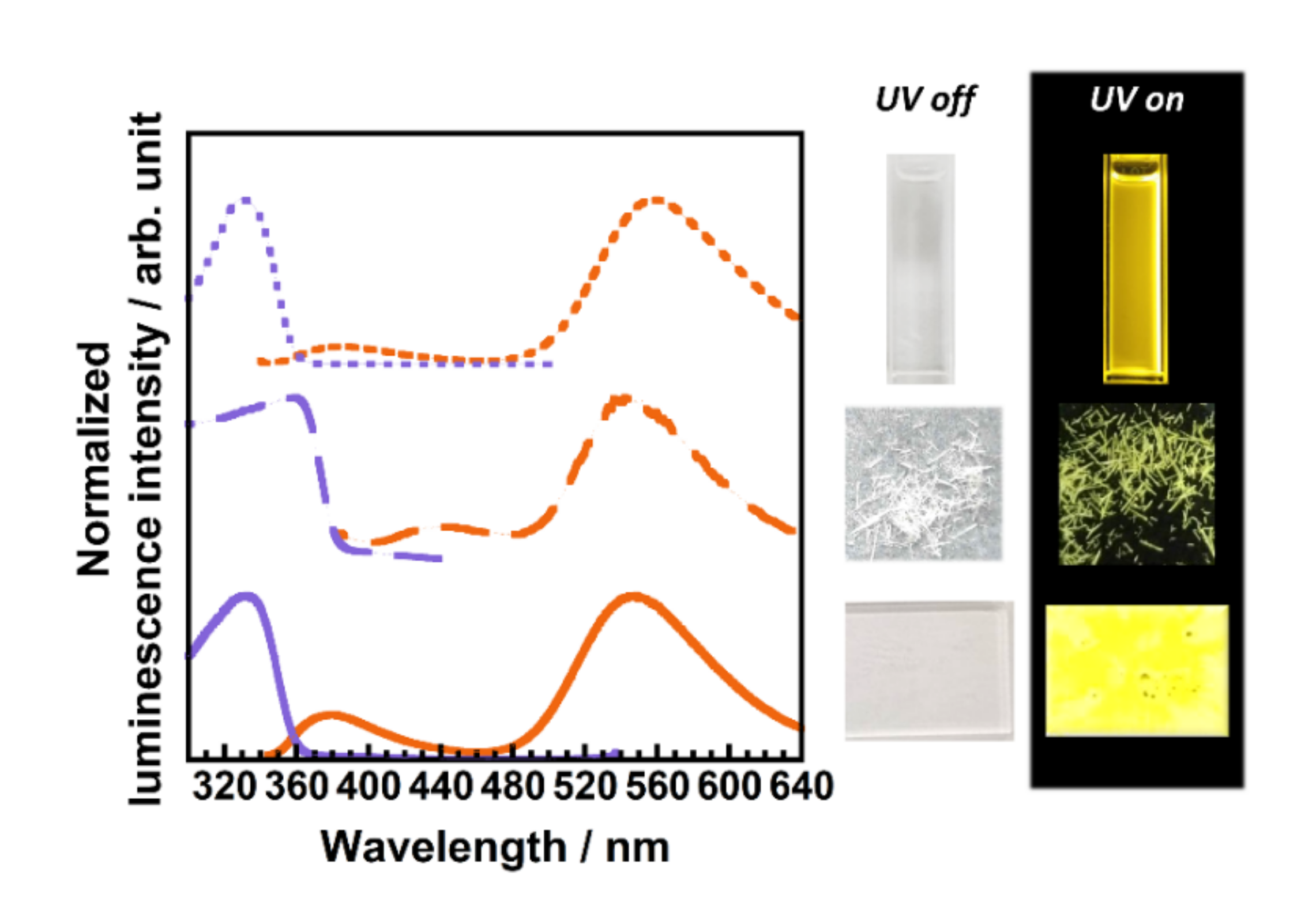
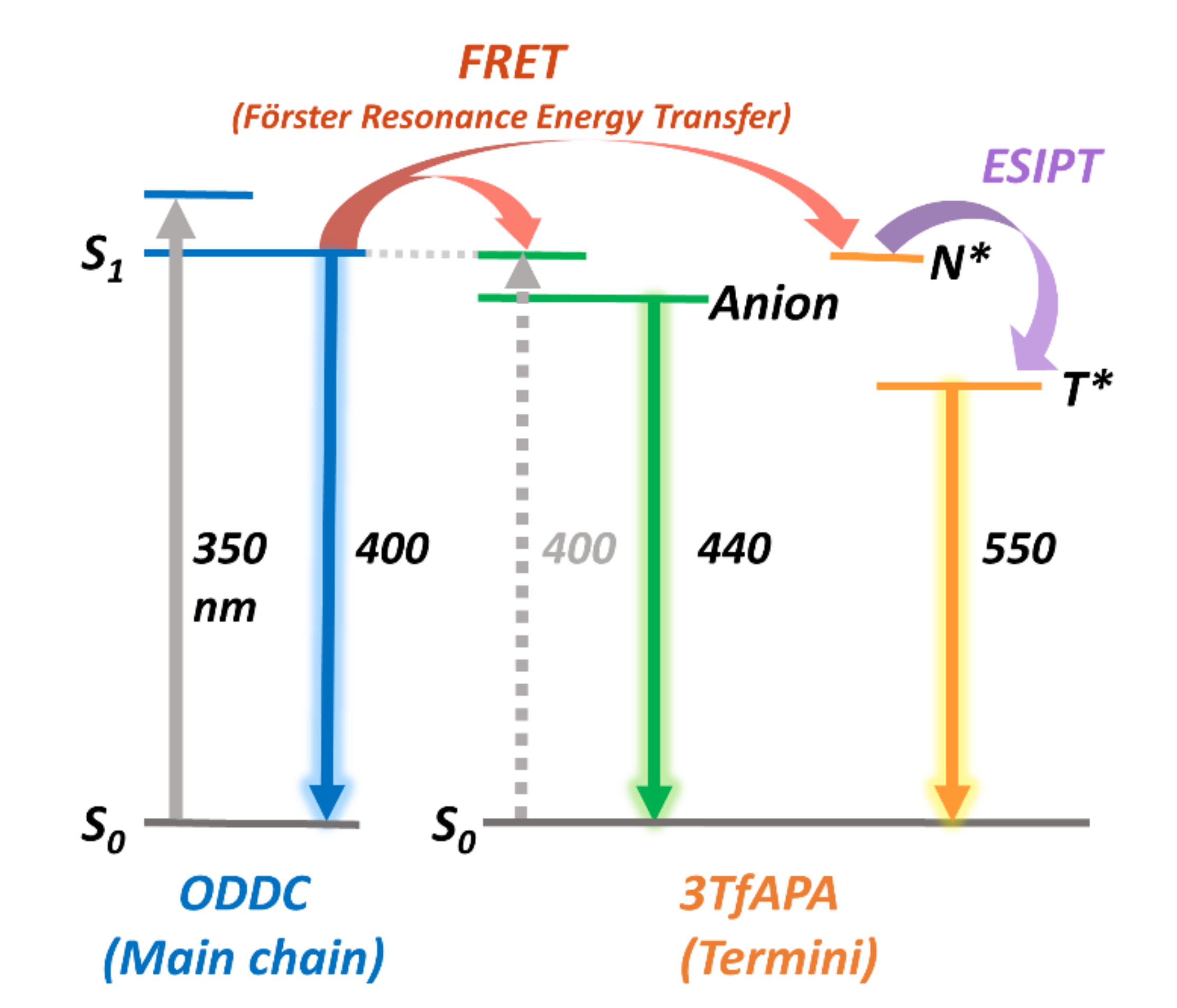
3.2.1. Optical Properties of ODDC-TfA
3.2.2. Optical Properties of ODtDA-TfA
3.2.3. Thermal Stability of the End-Capped PIs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Park, Y.W. Editorial for the Conducting Polymers for Carbon Electronics themed issue. Chem. Soc. Rev. 2010, 39, 2352–2353. [Google Scholar] [CrossRef]
- Abbel, R.; Grenier, C.; Pouderoijen, M.J.; Stouwdam, J.W.; Leclère, P.E.L.G.; Sijbesma, R.P.; Meijer, E.W.; Schenning, A.P.H.J. White-light emitting hydrogen-bonded supramolecular copolymers based on #-conjugated oligomers. J. Am. Chem. Soc. 2009, 131, 833–843. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Liao, W.M.; Yin, S.Y.; Sun, S.S.; Su, C.Y. Single-Phase White-Light-Emitting and Photoluminescent Color-Tuning Coordination Assemblies. Chem. Rev. 2018, 118, 8889–8935. [Google Scholar] [CrossRef]
- Park, S.; Kwon, J.E.; Kim, S.H.; Seo, J.; Chung, K.; Park, S.-Y.; Jang, D.-J.; Medina, B.M.; Gierschner, J.; Park, S.Y. A White-Light-Emitting Molecule: Frustrated Energy Transfer between Constituent Emitting Centers. J. Am. Chem. Soc. 2009, 131, 14043–14049. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Li, X.; Hou, Q.; Peng, J.; Yang, W.; Cao, Y. High-efficiency white-light emission from a single copolymer: Fluorescent blue, green, and red chromophores on a conjugated polymer backbone. Adv. Mater. 2007, 19, 1113–1117. [Google Scholar] [CrossRef]
- Ki, W.; Li, J. A semiconductor bulk material that emits direct white light. J. Am. Chem. Soc. 2008, 130, 8114–8115. [Google Scholar] [CrossRef]
- Cui, Y.; Yue, Y.; Qian, G.; Chen, B. Luminescent functional metal-organic frameworks. Chem. Rev. 2012, 112, 1126–1162. [Google Scholar] [CrossRef]
- Nara, M.; Orita, R.; Ishige, R.; Ando, S. White-Light Emission and Tunable Luminescence Colors of Polyimide Copolymers Based on FRET and Room-Temperature Phosphorescence. ACS Omega 2020, 5, 14831–14841. [Google Scholar] [CrossRef]
- Richards, B.S. Luminescent layers for enhanced silicon solar cell performance: Down-conversion. Sol. Energy Mater. Sol. Cells 2006, 90, 1189–1207. [Google Scholar] [CrossRef]
- Van Sark, W.G.J.H.M.; Barnham, K.W.J.; Slooff, L.H.; Chatten, A.J.; Büchtemann, A.; Meyer, A.; McCormack, S.J.; Koole, R.; Farrell, D.J.; Bose, R.; et al. Luminescent Solar Concentrators—A review of recent results. Opt. Express 2008, 16, 21773. [Google Scholar] [CrossRef] [PubMed]
- Currie, M.J.; Mapel, J.K.; Heidel, T.D.; Goffri, S.; Baldo, M.A. High-Efficiency Organic Solar Concentrators for Photovoltaics. Science 2008, 321, 226–228. [Google Scholar] [CrossRef]
- Haines, C.; Chen, M.; Ghiggino, K.P. The effect of perylene diimide aggregation on the light collection efficiency of luminescent concentrators. Sol. Energy Mater. Sol. Cells 2012, 105, 287–292. [Google Scholar] [CrossRef]
- McKenna, B.; Evans, R.C. Towards Efficient Spectral Converters through Materials Design for Luminescent Solar Devices. Adv. Mater. 2017, 29, 1606491. [Google Scholar] [CrossRef]
- Rafiee, M.; Chandra, S.; Ahmed, H.; McCormack, S.J. An overview of various configurations of Luminescent Solar Concentrators for photovoltaic applications. Opt. Mater. 2019, 91, 212–227. [Google Scholar] [CrossRef]
- Wakita, J.; Sekino, H.; Sakai, K.; Urano, Y.; Ando, S. Molecular Design, Synthesis, and Properties of Highly Fluorescent Polyimides. J. Phys. Chem. B 2009, 113, 15212–15224. [Google Scholar] [CrossRef] [PubMed]
- Pyo, S.M.; Shin, T.J.; Kim, S.I.; Ree, M. Photoluminescences from Aromatic Polyimides in Thin Films: Effects of Precursor Origin and Imidization History. Mol. Cryst. Liq. Cryst. Sci. Technol. Sect. A Mol. Cryst. Liq. Cryst. 1998, 316, 353–358. [Google Scholar] [CrossRef]
- Hasegawa, M.; Horie, K. Photophysics, photochemistry, and optical properties of polyimides. Prog. Polym. Sci. 2001, 26, 259–335. [Google Scholar] [CrossRef]
- Matsuda, S.; Urano, Y.; Park, J.; Ha, C.; Ando, S. Preparation and Characterization of Organic Electroluminescent Devices Using Fluorescent Polyimides as a Light-Emitting Layer. J. Photopolym. Sci. Technol. 2004, 17, 241–246. [Google Scholar] [CrossRef][Green Version]
- Yokota, R.; Yamamoto, S.; Yano, S.; Sawaguchi, T.; Hasegawa, M.; Yamaguchi, H.; Ozawa, H.; Sato, R. Molecular design of heat resistant polyimides having excellent processability and high glass transition temperature. High Perform. Polym. 2001, 13, S61–S72. [Google Scholar] [CrossRef]
- Wakita, J.; Inoue, S.; Kawanishi, N.; Ando, S. Excited-state intramolecular proton transfer in imide compounds and its application to control the emission colors of highly fluorescent polyimides. Macromolecules 2010, 43, 3594–3605. [Google Scholar] [CrossRef]
- Liang, N.; Fujiwara, E.; Nara, M.; Ishige, R.; Ando, S. Colorless Copolyimide Films Exhibiting Large Stokes-Shifted Photoluminescence Applicable for Spectral Conversion. ACS Appl. Polym. Mater. 2021, 3, 3911–3921. [Google Scholar] [CrossRef]
- Takizawa, K.; Wakita, J.; Sekiguchi, K.; Ando, S. Variations in aggregation structures and fluorescence properties of a semialiphatic fluorinated polyimide induced by very high pressure. Macromolecules 2012, 45, 4764–4771. [Google Scholar] [CrossRef]
- Kanosue, K.; Ando, S. Fluorescence emissions of imide compounds and end-capped polyimides enhanced by intramolecular double hydrogen bonds. Phys. Chem. Chem. Phys. 2015, 17, 30659–30669. [Google Scholar] [CrossRef]
- Kanosue, K.; Shimosaka, T.; Wakita, J.; Ando, S. Polyimide and Imide Compound Exhibiting Bright Red Fluorescence with Very Large Stokes Shifts via Excited-State Intramolecular Proton Transfer. Macromolecules 2015, 48, 1777–1785. [Google Scholar] [CrossRef]
- Orita, R.; Franckevičius, M.; Vyšniauskas, A.; Gulbinas, V.; Sugiyama, H.; Uekusa, H.; Kanosue, K.; Ishige, R.; Ando, S. Enhanced fluorescence of phthalimide compounds induced by the incorporation of electron-donating alicyclic amino groups. Phys. Chem. Chem. Phys. 2018, 20, 16033–16044. [Google Scholar] [CrossRef]
- Kanosue, K.; Augulis, R.; Peckus, D.; Karpicz, R.; Tamulevičius, T.; Tamulevičius, S.; Gulbinas, V.; Ando, S. Polyimide and Imide Compound Exhibiting Bright Red Fluorescence with Very Large Stokes Shifts via Excited-State Intramolecular Proton Transfer II. Ultrafast Proton Transfer Dynamics in the Excited State. Macromolecules 2016, 49, 1848–1857. [Google Scholar] [CrossRef]
- Fujiwara, E.; Fukudome, H.; Takizawa, K.; Ishige, R.; Ando, S. Pressure-Induced Variations of Aggregation Structures in Colorless and Transparent Polyimide Films Analyzed by Optical Microscopy, UV–Vis Absorption, and Fluorescence Spectroscopy. J. Phys. Chem. B 2018, 122, 8985–8997. [Google Scholar] [CrossRef] [PubMed]
- Liang, N.; Fujiwara, E.; Nara, M.; Ishige, R.; Ando, S. Photoluminescence Properties of Novel Fluorescent Polyimide Based on Excited State Intramolecular Proton Transfer at The End Groups. J. Photopolym. Sci. Technol. 2019, 32, 449–455. [Google Scholar] [CrossRef]
- Fujiwara, E.; Orita, R.; Vyšniauskas, A.; Franckevičius, M.; Ishige, R.; Gulbinas, V.; Ando, S. Ultrafast Spectroscopic Analysis of Pressure-Induced Variations of Excited-State Energy and Intramolecular Proton Transfer in Semi-Aliphatic Polyimide Films. J. Phys. Chem. B 2021, 125, 2425–2434. [Google Scholar] [CrossRef]
- Yen, H.J.; Wu, J.H.; Wang, W.C.; Liou, G.S. High-efficiency photoluminescence wholly aromatic triarylamine-based polyimide nanofiber with aggregation-induced emission enhancement. Adv. Opt. Mater. 2013, 1, 668–676. [Google Scholar] [CrossRef]
- Lv, C.; Liu, W.; Luo, Q.; Yi, H.; Yu, H.; Yang, Z.; Zou, B.; Zhang, Y. A highly emissive AIE-active luminophore exhibiting deep-red to near-infrared piezochromism and high-quality lasing. Chem. Sci. 2020, 11, 4007–4015. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Long, Y.; Chen, X.; Yang, T.; Zhao, J.; Meng, Y.; Chi, Z.; Liu, S.; Chen, X.; Aldred, M.P.; et al. Preserving High-Efficiency Luminescence Characteristics of an Aggregation-Induced Emission-Active Fluorophore in Thermostable Amorphous Polymers. ACS Appl. Mater. Interfaces 2020, 12, 34198–34207. [Google Scholar] [CrossRef] [PubMed]
- Doi, M.; Muto, K.; Nara, M.; Liang, N.; Sano, K. Photoluminescence Properties of Copolyimides Containing Naphthalene Core and Analysis of Excitation Energy Transfer between the Dianhydride Moieties. J. Photopolym. Sci. Technol. 2021, 34, 423–430. [Google Scholar]
- Hu, X. Synthesis of novel hyperbranched polybenzo-bisthiazole amide with donor-acceptor (D-A) architecture, high fluorescent quantum yield and large stokes shift. Polymers 2017, 9, 304. [Google Scholar] [CrossRef]
- Porcu, P.; Vonlanthen, M.; Ruiu, A.; González-Méndez, I.; Rivera, E. Energy transfer in dendritic systems having pyrene peripheral groups as donors and different acceptor groups. Polymers 2018, 10, 1062. [Google Scholar] [CrossRef]
- Sanju, K.S.; Neelakandan, P.P.; Ramaiah, D. DNA-assisted white light emission through FRET. Chem. Commun. 2011, 47, 1288–1290. [Google Scholar] [CrossRef] [PubMed]
- Tseng, H.W.; Liu, J.Q.; Chen, Y.A.; Chao, C.M.; Liu, K.M.; Chen, C.L.; Lin, T.C.; Hung, C.H.; Chou, Y.L.; Lin, T.C.; et al. Harnessing excited-state intramolecular proton-transfer reaction via a series of amino-type hydrogen-bonding molecules. J. Phys. Chem. Lett. 2015, 6, 1477–1486. [Google Scholar] [CrossRef]
- Fujii, M.; Namba, M.; Yamaji, M.; Okamoto, H. Solvent-induced multicolour fluorescence of amino-substituted 2,3-naphthalimides studied by fluorescence and transient absorption measurements. Photochem. Photobiol. Sci. 2016, 15, 842–850. [Google Scholar] [CrossRef]
- Okamoto, H.; Itani, K.; Yamaji, M.; Konishi, H.; Ota, H. Excited-state intramolecular proton transfer (ESIPT) fluorescence from 3-amidophthalimides displaying RGBY emission in the solid state. Tetrahedron Lett. 2018, 59, 388–391. [Google Scholar] [CrossRef]
- Wang, L.; Fujii, M.; Namba, M.; Yamaji, M.; Okamoto, H. Fluorescence properties of amido-substituted 2,3-naphthalimides: Excited-state intramolecular proton transfer (ESIPT) fluorescence and responses to Ca2+ ions. Tetrahedron Lett. 2019, 60, 151189. [Google Scholar] [CrossRef]
- Serdiuk, I.E. White Light from a Single Fluorophore: A Strategy Utilizing Excited- State Intramolecular Proton-Transfer Phenomenon and Its Verification. J. Phys. Chem. C 2017, 121, 5277–5286. [Google Scholar] [CrossRef]
- Benelhadj, K.; Muzuzu, W.; Massue, J.; Retailleau, P.; Charaf-Eddin, A.; Laurent, A.D.; Jacquemin, D.; Ulrich, G.; Ziessel, R. White Emitters by Tuning the Excited-State Intramolecular Proton-Transfer Fluorescence Emission in 2-(2′-Hydroxybenzofuran)benzoxazole Dyes. Chem. A Eur. J. 2014, 20, 12843–12857. [Google Scholar] [CrossRef]
- Duarte, L.G.T.A.; Germino, J.C.; Berbigier, J.F.; Barboza, C.A.; Faleiros, M.M.; De Alencar Simoni, D.; Galante, M.T.; De Holanda, M.S.; Rodembusch, F.S.; Atvars, T.D.Z. White-light generation from all-solution-processed OLEDs using a benzothiazole-salophen derivative reactive to the ESIPT process. Phys. Chem. Chem. Phys. 2019, 21, 1172–1182. [Google Scholar] [CrossRef]
- Kundu, A.; Hariharan, P.S.; Prabakaran, K.; Moon, D.; Anthony, S.P. Aggregation induced emission of excited-state intramolecular proton transfer compounds: Nanofabrication mediated white light emitting nanoparticles. Cryst. Growth Des. 2016, 16, 3400–3408. [Google Scholar] [CrossRef]
- Tabuchi, A.; Hayakawa, T.; Kuwata, S.; Ishige, R.; Ando, S. Full-colour solvatochromic fluorescence emitted from a semi-aromatic imide compound based on ESIPT and anion formation. Mater. Adv. 2021, 2, 5629–5638. [Google Scholar] [CrossRef]
- Matsumoto, T. Alicyclic polyimides: An approach from monomer synthesis. J. Synth. Org. Chem. 2000, 58, 776–786. [Google Scholar] [CrossRef]
- Oishi, Y.; Ogasawara, K.; Hirahara, H.; Mori, K. Synthesis of Alicyclic Polyimides by the Silylation Method. J. Photopolym. Sci. Technol. 2001, 14, 37–40. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision A.03; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Ando, S.; Fujigaya, T.; Ueda, M. Density Functional Theory Calculations of Photoabsorption Spectra of Organic Molecules in the Vacuum Ultraviolet Region. Jpn. J. Appl. Phys. 2002, 41, L105–L108. [Google Scholar] [CrossRef]
- Ando, S.; Ueda, M. DFT Calculations of Photoabsorption Spectra for Alicyclic and Heterocyclic Compounds in the VUV Region. J. Photopolym. Sci. Technol. 2003, 16, 537–544. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Gilli, G.; Bellucci, F.; Ferretti, V.; Bertolasi, V. Evidence for resonance-assisted hydrogen bonding from crystal-structure correlations on the enol form of the.beta.-diketone fragment. J. Am. Chem. Soc. 1989, 111, 1023–1028. [Google Scholar] [CrossRef]
- Chicha, H.; Abbassi, N.; Rakib, E.M.; Khouili, M.; El Ammari, L.; Spinelli, D. Reduction of 3-nitrophthalic anhydride by SnCl2 in different alcohols: A simple synthesis of alkyl 1,3-dihydro-3-oxo-2,1-benzisoxazole-4- carboxylates. Tetrahedron Lett. 2013, 54, 1569–1571. [Google Scholar] [CrossRef]



| State | λabsa/nm | λema/nm | ν/cm−1 | Φ |
|---|---|---|---|---|
| CHCl3 solution | 330 | 556 | 1.23 × 104 | 0.17 |
| Crystalline | 365 | 542 | 8.95 × 103 | 0.34 |
| In PMMA matrix | 333 | 549 | 1.18 × 104 | 0.19 |
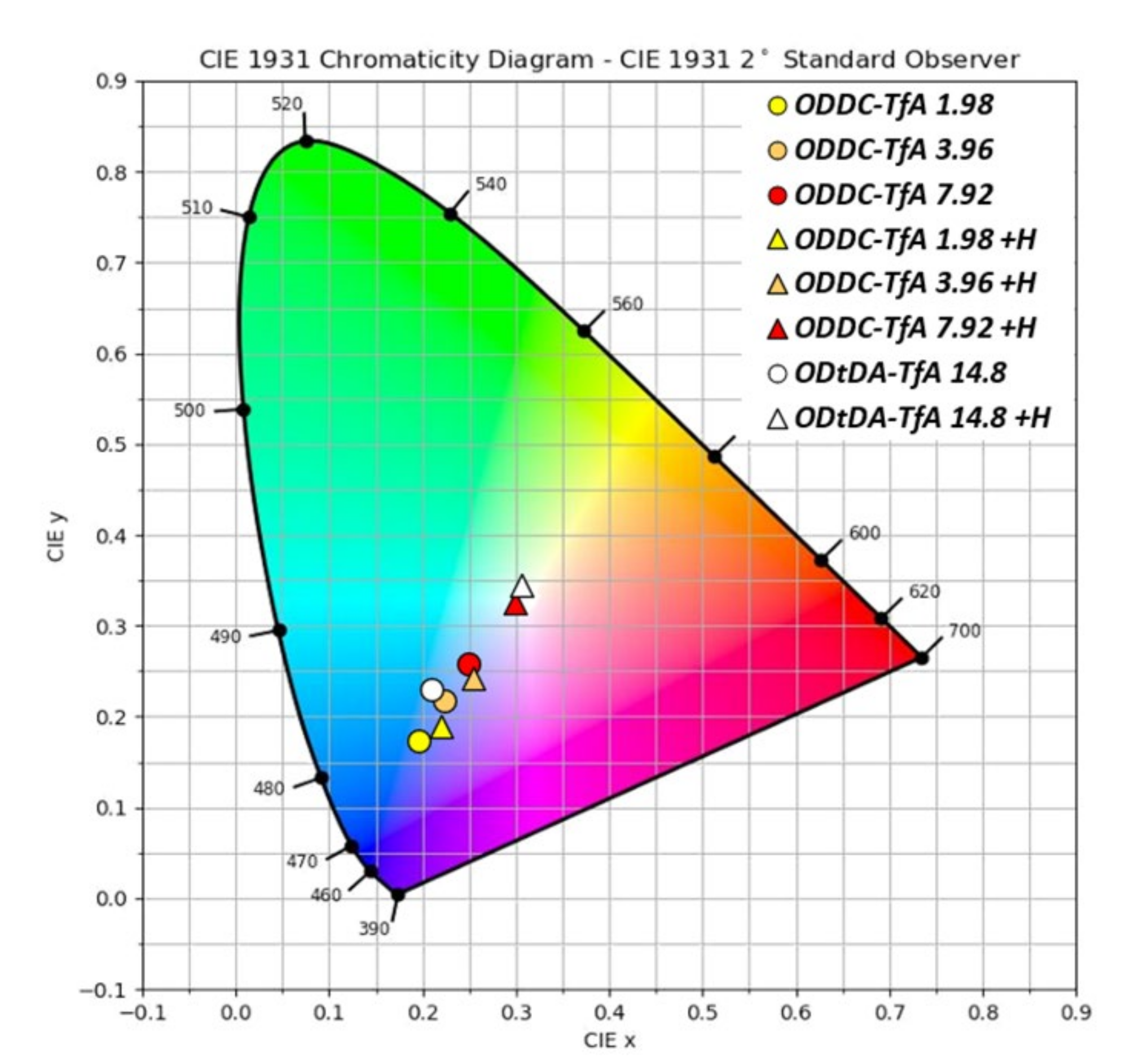
| r | λabsa/nm | λexa/nm | λema/nm | τb/ns | EFRET | Φ | |
|---|---|---|---|---|---|---|---|
| ODDC-TfA | 0 | <400 | 340 | 400 | 10.10 | 0.00 | 0.14 |
| 1.98 | <400 | 350 | 395 436 550 | 6.67 | 0.34 | 0.12 | |
| 3.96 | <400 | 350 | 397 433 550 | 5.50 | 0.46 | 0.12 | |
| 7.92 | <400 | 340 | 400 440 552 | 4.23 | 0.58 | 0.15 | |
| H2SO4-doped ODDC-TfA | 1.98 | <400 | 340 | 400 551 | 4.78 | 0.53 | 0.09 |
| 3.96 | <400 | 340 | 398 549 | 4.45 | 0.56 | 0.10 | |
| 7.92 | <400 | 340 | 400 549 | 3.83 | 0.62 | 0.10 |
| Polyimide | r | λabsa/nm | λex a/nm | λem a/nm | τ b/ns | EFRET | Φ |
|---|---|---|---|---|---|---|---|
| ODtDA-TfA | 0 | <400 | 350 | 400 | 9.05 | 0.00 | 0.29 |
| 14.8 | <400 | 350 | 400 449 550 | 3.86 | 0.57 | 0.23 | |
| H2SO4-doped ODtDA-TfA | 14.8 | <400 | 350 | 420 555 | 3.95 | 0.56 | 0.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tabuchi, A.; Hayakawa, T.; Kuwata, S.; Ishige, R.; Ando, S. Synthesis and Characterization of White-Light Luminescent End-Capped Polyimides Based on FRET and Excited State Intramolecular Proton Transfer. Polymers 2021, 13, 4050. https://doi.org/10.3390/polym13224050
Tabuchi A, Hayakawa T, Kuwata S, Ishige R, Ando S. Synthesis and Characterization of White-Light Luminescent End-Capped Polyimides Based on FRET and Excited State Intramolecular Proton Transfer. Polymers. 2021; 13(22):4050. https://doi.org/10.3390/polym13224050
Chicago/Turabian StyleTabuchi, Atsuko, Teruaki Hayakawa, Shigeki Kuwata, Ryohei Ishige, and Shinji Ando. 2021. "Synthesis and Characterization of White-Light Luminescent End-Capped Polyimides Based on FRET and Excited State Intramolecular Proton Transfer" Polymers 13, no. 22: 4050. https://doi.org/10.3390/polym13224050
APA StyleTabuchi, A., Hayakawa, T., Kuwata, S., Ishige, R., & Ando, S. (2021). Synthesis and Characterization of White-Light Luminescent End-Capped Polyimides Based on FRET and Excited State Intramolecular Proton Transfer. Polymers, 13(22), 4050. https://doi.org/10.3390/polym13224050







