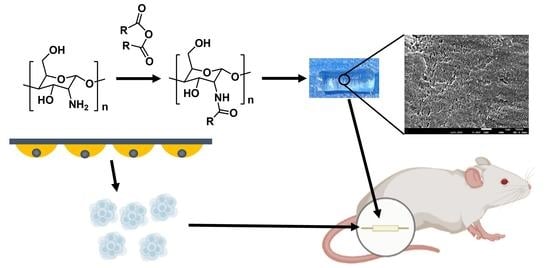
Chitin Nerve Conduits with Three-Dimensional Spheroids of Mesenchymal Stem Cells from SD Rats Promote Peripheral Nerve Regeneration
Abstract
:1. Introduction
2. Materials and Methods
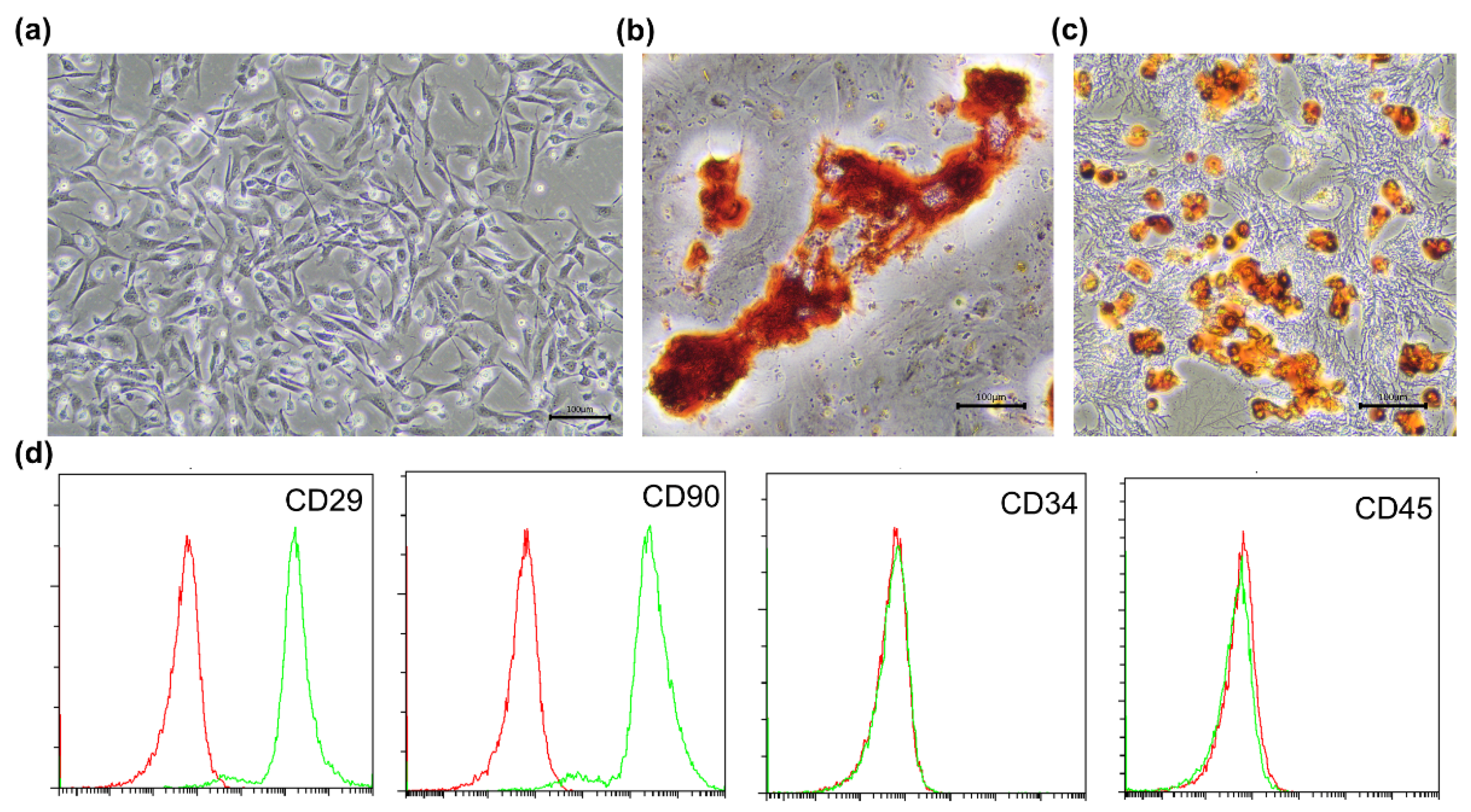
2.1. Isolation and Culture of BMSCs
2.2. Identification of BMSCs
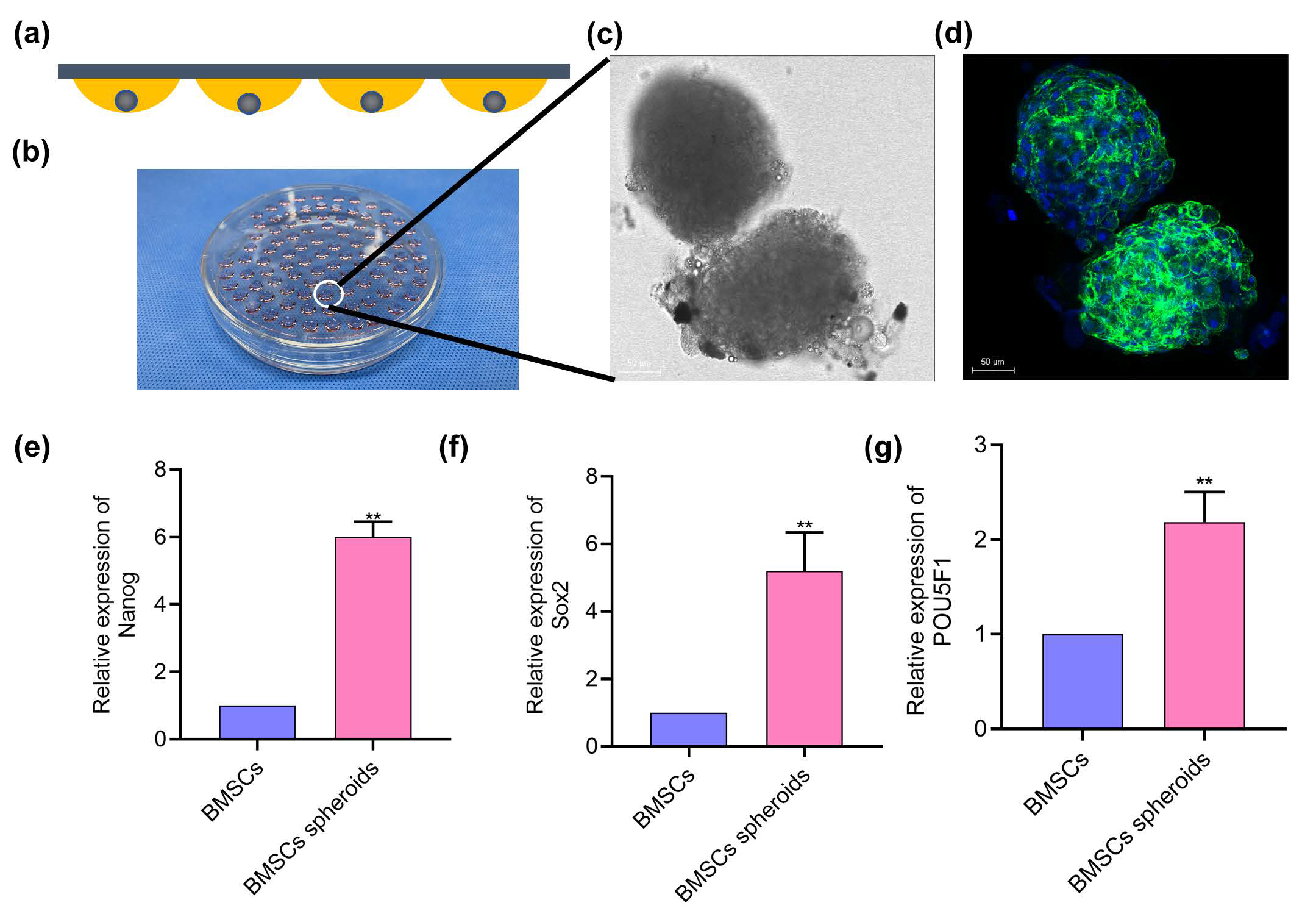
2.3. Spheroids Formation
2.4. Morphological Observation of Cell Spheroids
2.5. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.6. Fabrication of the Chitin Nerve Conduits
2.7. Characterization of Chitin Nerve Conduits
2.7.1. Scanning Electron Microscopy
2.7.2. Water Contact Angle Measurement
2.7.3. Fourier Transform Infrared Spectroscopy
2.7.4. Tensile Stress Test
2.7.5. Live/Dead Staining
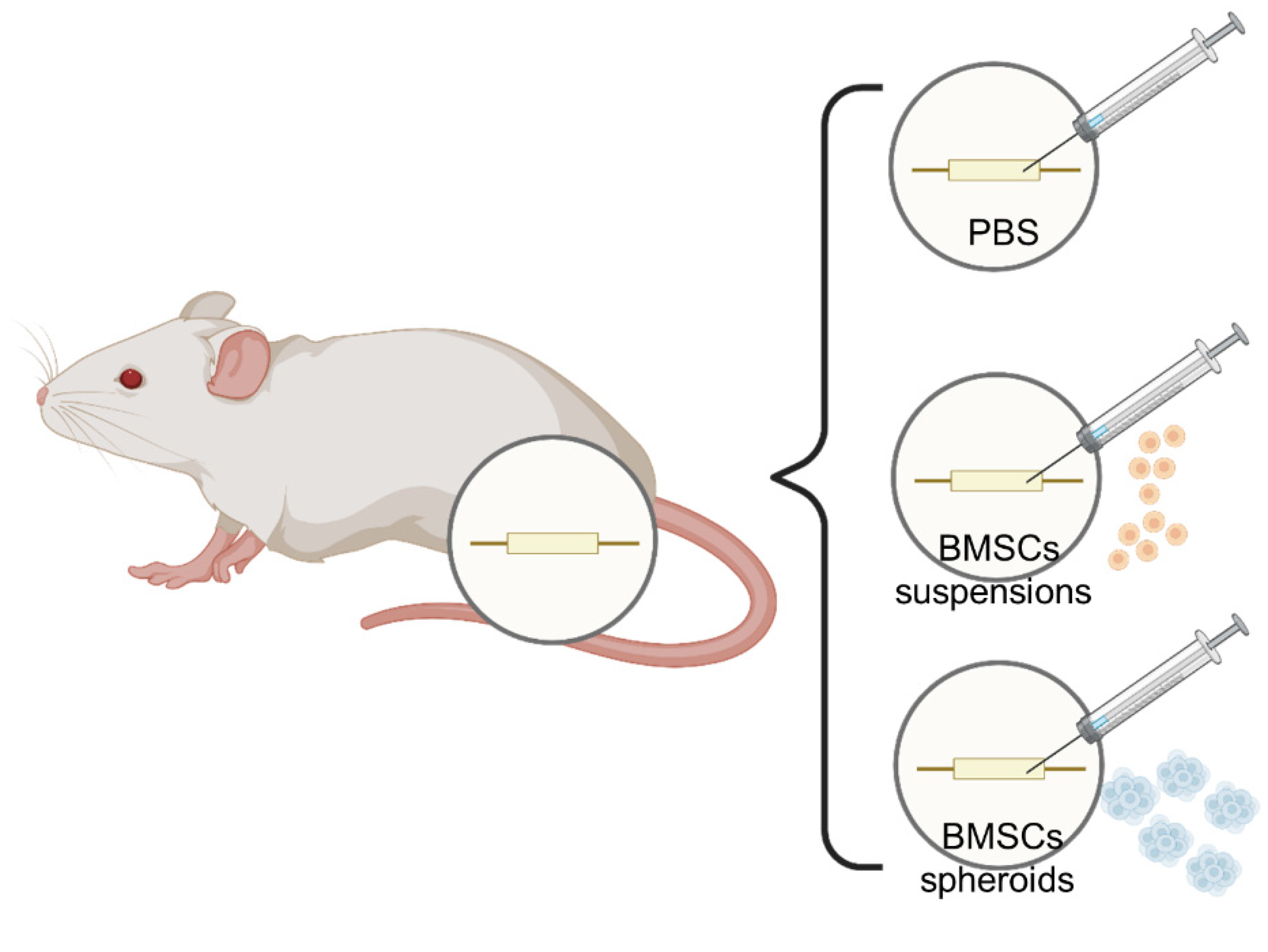
2.8. Rat Model of Sciatic Nerve Injury and Cell Transplantation
2.9. Electrophysiological Examination
2.10. Neurohistological Analysis
2.11. Statistical Analysis
3. Results
3.1. Phenotype Identification of BMSCs
3.2. BMSCs Spheroids Exhibited Enhanced Stemness Related Potential
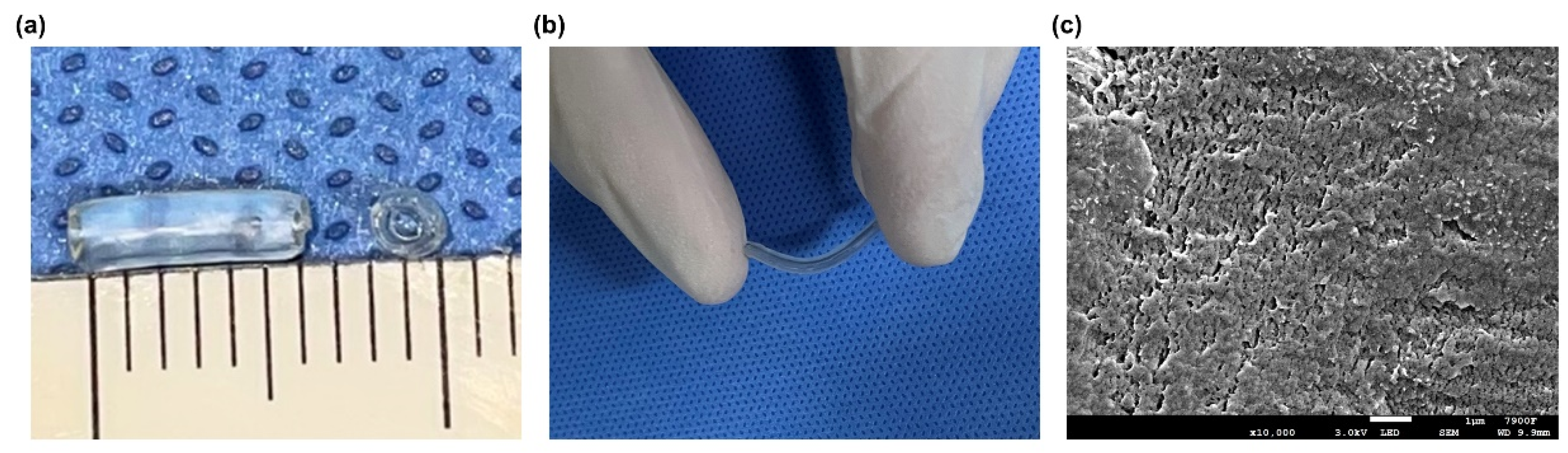
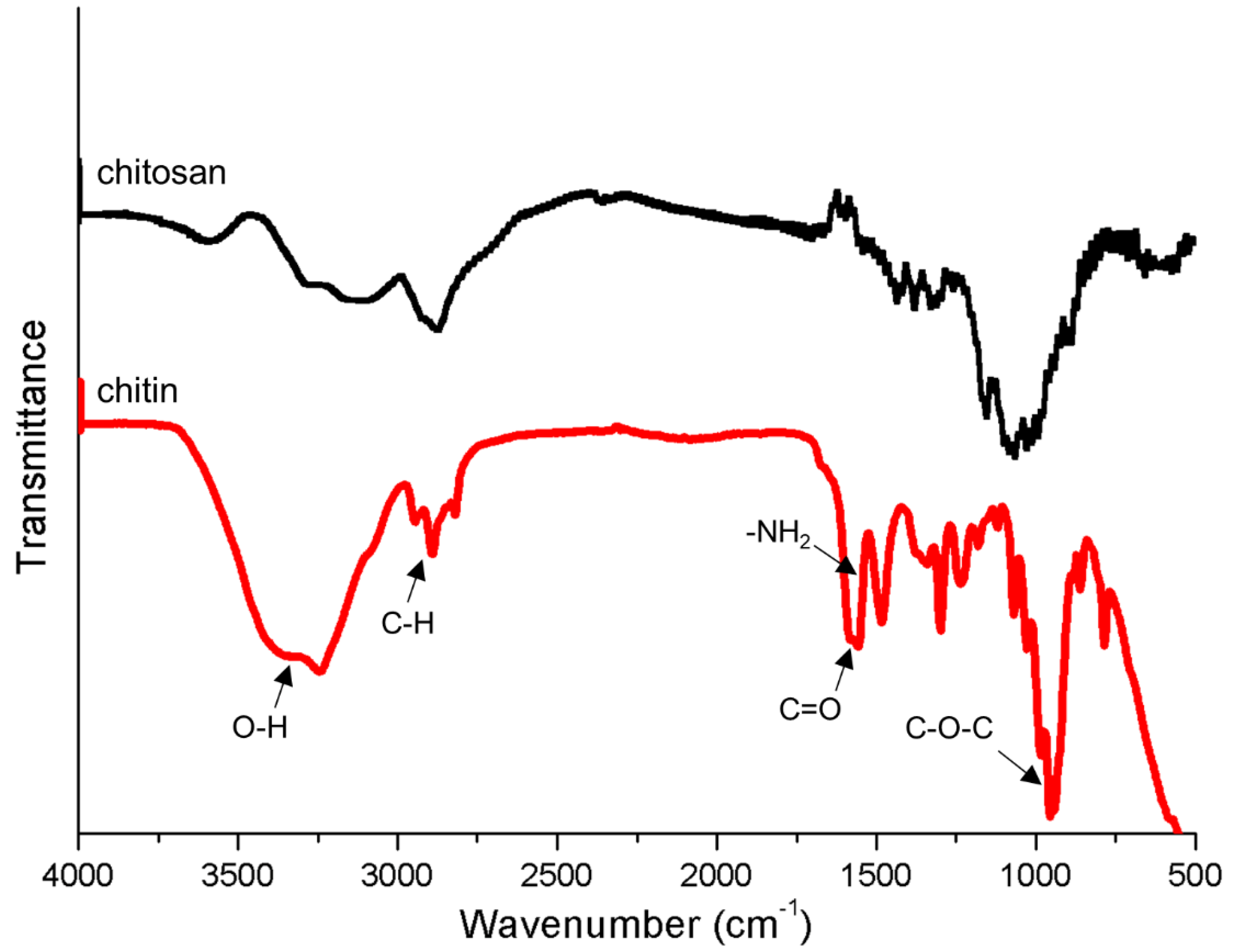
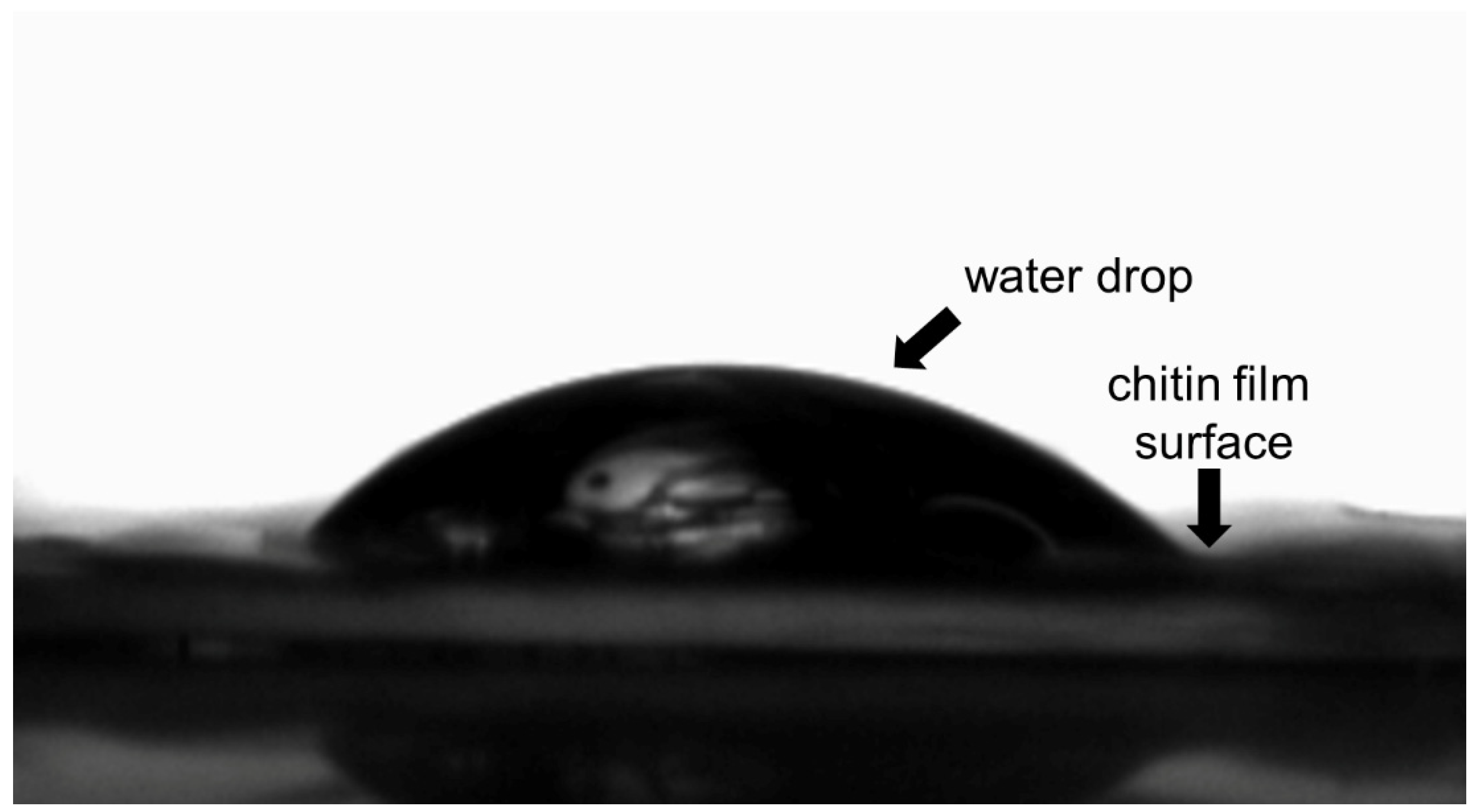
3.3. Characteristics of Chitin Conduits
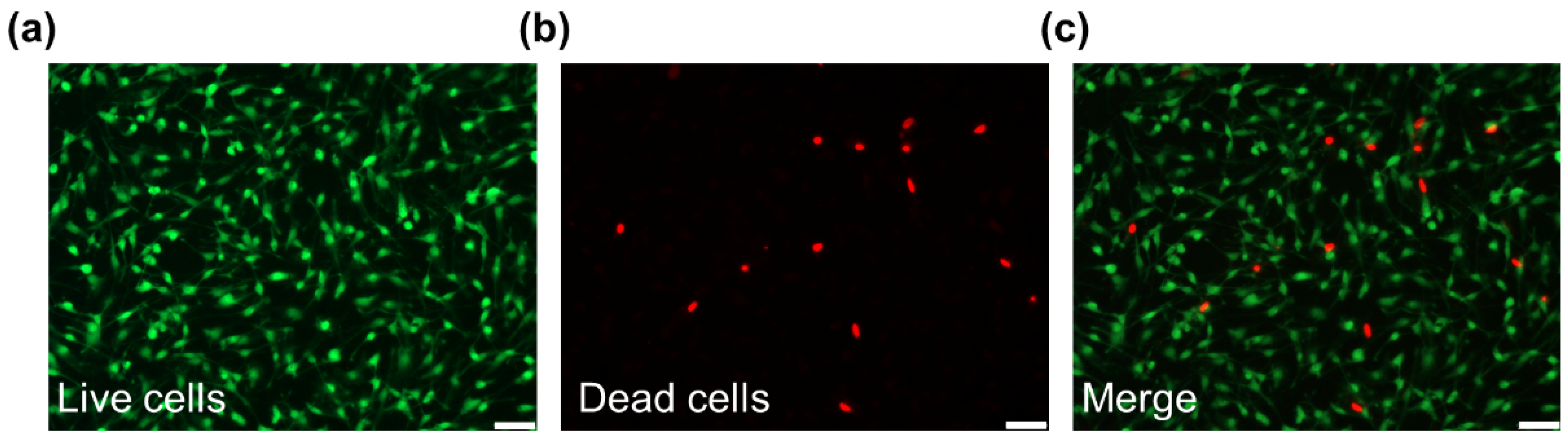
3.4. Live/dead Staining
3.5. Effect on the Recovery of Hindlimb Motor Function
3.6. Electrophysiological Parameters of Injured Sciatic Nerves
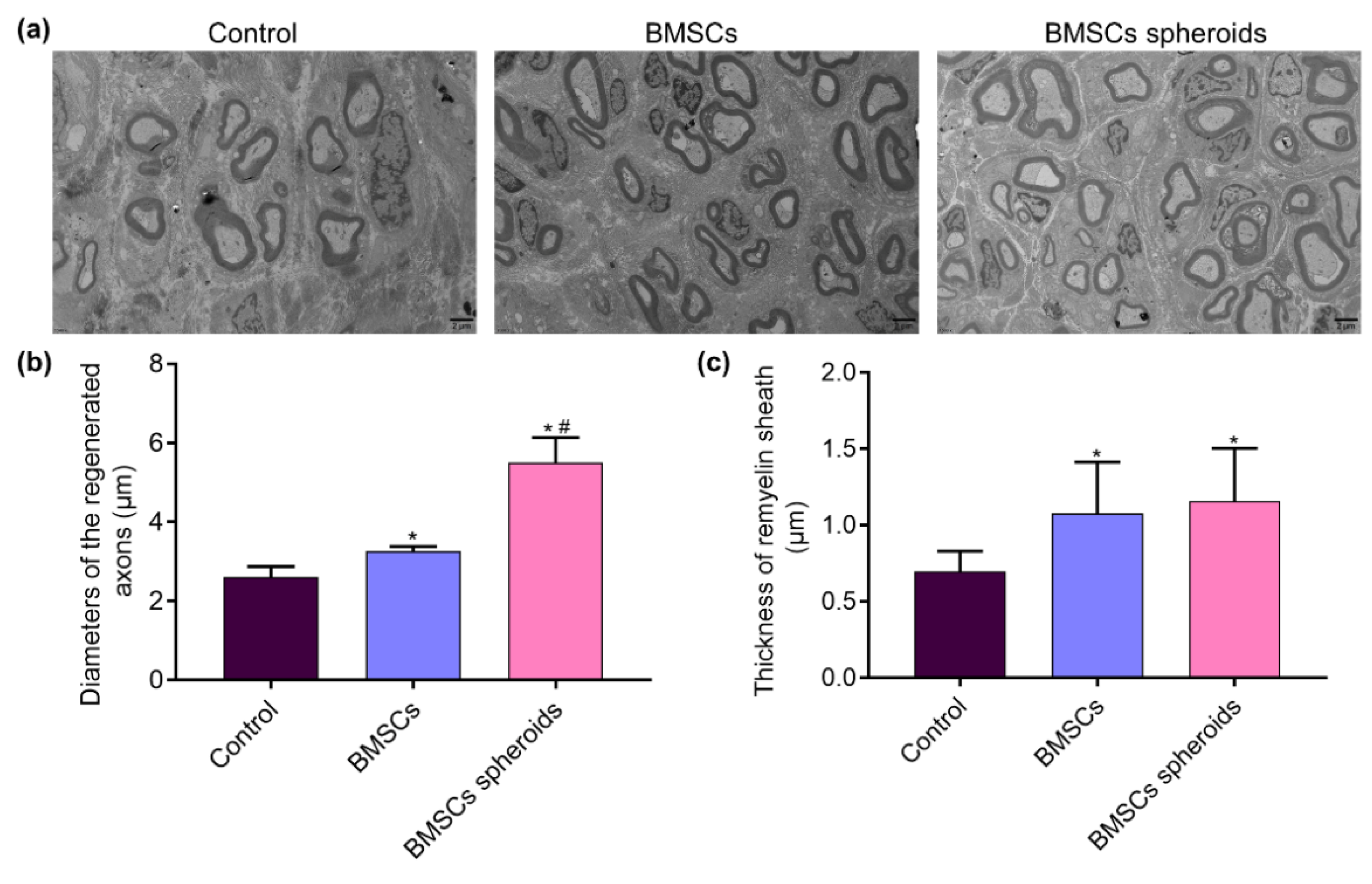
3.7. Histological Evaluation of Regenerative Nerves
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, C.; Liu, S.Y.; Pi, W.; Zhang, P.X. Cortical plasticity and nerve regeneration after peripheral nerve injury. Neural Regen. Res. 2021, 16, 1518–1523. [Google Scholar] [CrossRef]
- Zhang, P.; Han, N.; Wang, T.; Xue, F.; Kou, Y.; Wang, Y.; Yin, X.; Lu, L.; Tian, G.; Gong, X.; et al. Biodegradable conduit small gap tubulization for peripheral nerve mutilation: A substitute for traditional epineurial neurorrhaphy. Int. J. Med. Sci. 2013, 10, 171–175. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.X.; Li-Ya, A.; Kou, Y.H.; Yin, X.F.; Xue, F.; Han, N.; Wang, T.B.; Jiang, B.G. Biological conduit small gap sleeve bridging method for peripheral nerve injury: Regeneration law of nerve fibers in the conduit. Neural Regen. Res. 2015, 10, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Sarker, M.D.; Naghieh, S.; McInnes, A.D.; Schreyer, D.J.; Chen, X. Regeneration of peripheral nerves by nerve guidance conduits: Influence of design, biopolymers, cells, growth factors, and physical stimuli. Prog. Neurobiol. 2018, 171, 125–150. [Google Scholar] [CrossRef] [PubMed]
- Alvites, R.D.; Branquinho, M.V.; Sousa, A.C.; Amorim, I.; Magalhães, R.; João, F.; Almeida, D.; Amado, S.; Prada, J.; Pires, I.; et al. Combined Use of Chitosan and Olfactory Mucosa Mesenchymal Stem/Stromal Cells to Promote Peripheral Nerve Regeneration In Vivo. Stem Cells Int. 2021, 2021, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Boecker, A.; Daeschler, S.C.; Kneser, U.; Harhaus, L. Relevance and Recent Developments of Chitosan in Peripheral Nerve Surgery. Front. Cell. Neurosci. 2019, 13, 104. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Wang, Y.; Gong, J.; Yang, L.; Niu, C.; Ni, X.; Wang, Y.; Peng, S.; Gu, X.; Sun, C.; et al. Chitosan degradation products facilitate peripheral nerve regeneration by improving macro-phage-constructed microenvironments. Biomaterials 2017, 134, 64–77. [Google Scholar] [CrossRef]
- Younes, I.; Rinaudo, M. Chitin and Chitosan Preparation from Marine Sources. Structure, Properties and Applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, L.; Yang, X.; Deng, L.; Ying, D.; Lu, A.; Zhang, L.; Yu, A.; Duan, B. Biocompatible Chitin Hydrogel Incorporated with PEDOT Nanoparticles for Peripheral Nerve Repair. ACS Appl. Mater. Interfaces 2021, 13, 16106–16117. [Google Scholar] [CrossRef]
- Zheng, F.; Li, R.; He, Q.; Koral, K.; Tao, J.; Fan, L.; Xiang, R.; Ma, J.; Wang, N.; Yin, Y.; et al. The electrostimulation and scar inhibition effect of chitosan/oxidized hydroxyethyl cellulose/reduced graphene oxide/asiaticoside liposome based hydrogel on peripheral nerve regeneration in vitro. Mater. Sci. Eng. C 2020, 109, 110560. [Google Scholar] [CrossRef]
- El Knidri, H.; Belaabed, R.; Addaou, A.; Laajeb, A.; Lahsini, A. Extraction, chemical modification and characterization of chitin and chitosan. Int. J. Biol. Macromol. 2018, 120, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Muxika, A.; Etxabide, A.; Uranga, J.; Guerrero, P.; de la Caba, K. Chitosan as a bioactive polymer: Processing, properties and applications. Int. J. Biol. Macromol. 2017, 105, 1358–1368. [Google Scholar] [CrossRef]
- Ylostalo, J.H. 3D Stem Cell Culture. Cells 2020, 9, 2178. [Google Scholar] [CrossRef]
- Ahmad, T.; Shin, Y.M.; Lee, J.; Shin, H.J.; Perikamana, S.K.M.; Shin, H. Agglomeration of human dermal fibroblasts with ECM mimicking nano-fragments and their effects on proliferation and cell/ECM interactions. J. Ind. Eng. Chem. 2018, 67, 80–91. [Google Scholar] [CrossRef]
- Guven, S.; Chen, P.; Inci, F.; Tasoglu, S.; Erkmen, B.; Demirci, U. Multiscale assembly for tissue engineering and regenerative medicine. Trends Biotechnol. 2015, 33, 269–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, M.; Ma, C.; Ding, S.; Zhu, Y.; Shi, G.; Zhu, A. Rational Design of Stimuli-Responsive Polymers Modified Nanopores for Selective and Sensitive Determination of Salivary Glucose. Anal. Chem. 2019, 91, 14029–14035. [Google Scholar] [CrossRef]
- Li, C.; Jiao, G.; Wu, W.; Wang, H.; Ren, S.; Zhang, L.; Zhou, H.; Liu, H.; Chen, Y. Exosomes from Bone Marrow Mesenchymal Stem Cells Inhibit Neuronal Apoptosis and Promote Motor Function Recovery via the Wnt/β-catenin Signaling Pathway. Cell Transplant. 2019, 28, 1373–1383. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Xiao, L.; Peng, J.; Qian, Y.Q.; Huang, C.C. Exosomes derived from bone marrow mesenchymal stem cells improve osteoporosis through promoting osteoblast proliferation via MAPK pathway. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 3962–3970. [Google Scholar]
- Bhang, S.H.; Lee, S.; Shin, J.Y.; Lee, T.J.; Kim, B.S. Transplantation of Cord Blood Mesenchymal Stem Cells as Spheroids Enhances Vascularization. Tissue Eng. Part A 2012, 18, 2138–2147. [Google Scholar] [CrossRef]
- Lin, Y.J.; Lee, Y.W.; Chang, C.W.; Huang, C.C. 3D Spheroids of Umbilical Cord Blood MSC-Derived Schwann Cells Promote Peripheral Nerve Regeneration. Front. Cell Dev. Biol. 2020, 8, 604946. [Google Scholar] [CrossRef]
- Rao, F.; Yuan, Z.; Zhang, D.; Yu, F.; Li, M.; Li, D.; Jiang, B.; Wen, Y.; Zhang, P. Small-Molecule SB216763-Loaded Microspheres Repair Peripheral Nerve Injury in Small Gap Tubulization. Front. Neurosci. 2019, 13, 489. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wang, L.; Belwal, T.; Jiang, Y.; Li, D.; Xu, Y.; Luo, Z.; Li, L. Chitosan-based melatonin bilayer coating for maintaining quality of fresh-cut products. Carbohydr. Polym. 2020, 235, 115973. [Google Scholar] [CrossRef] [PubMed]
- Khoury, J.; Selezneva, I.; Pestov, S.; Tarassov, V.; Ermakov, A.; Mikheev, A.; Lazov, M.; Kirkpatrick, S.R.; Shashkov, D.; Smolkov, A. Surface bioactivation of PEEK by neutral atom beam technology. Bioact. Mater. 2019, 4, 132–141. [Google Scholar] [CrossRef]
- Boonsongrit, Y.; Mueller, B.W.; Mitrevej, A. Characterization of drug–chitosan interaction by 1H NMR, FTIR and isothermal titration calorimetry. Eur. J. Pharm. Biopharm. 2008, 69, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, H.; Xie, S.; Wang, N.; Wu, S.; Duan, Y.; Zhang, M.; Shui, L. Fabrication of Photo-Crosslinkable Poly(Trimethylene Carbonate)/Polycaprolactone Nanofibrous Scaf-folds for Tendon Regeneration. Int. J. Nanomed. 2020, 15, 6373–6383. [Google Scholar] [CrossRef]
- Wang, Z.; Hui, A.; Zhao, H.; Ye, X.; Zhang, C.; Wang, A.; Zhang, C. A Novel 3D-bioprinted Porous Nano Attapulgite Scaffolds with Good Performance for Bone Regeneration. Int. J. Nanomed. 2020, 15, 6945–6960. [Google Scholar] [CrossRef] [PubMed]
- Rao, F.; Wang, Y.; Zhang, D.; Lu, C.; Cao, Z.; Sui, J.; Wu, M.; Zhang, Y.; Pi, W.; Wang, B.; et al. Aligned chitosan nanofiber hydrogel grafted with peptides mimicking bioactive brain-derived neurotrophic factor and vascular endothelial growth factor repair long-distance sciatic nerve defects in rats. Theranostics 2020, 10, 1590–1603. [Google Scholar] [CrossRef]
- Rao, F.; Zhang, D.; Fang, T.; Lu, C.; Wang, B.; Ding, X.; Wei, S.; Zhang, Y.; Pi, W.; Xu, H.; et al. Exosomes from Human Gingiva-Derived Mesenchymal Stem Cells Combined with Biodegradable Chitin Conduits Promote Rat Sciatic Nerve Regeneration. Stem Cells Int. 2019, 2019, 1–12. [Google Scholar] [CrossRef]
- Palombella, S.; Guiotto, M.; Higgins, G.C.; Applegate, L.L.; Raffoul, W.; Cherubino, M.; Hart, A.; Riehle, M.O.; Di Summa, P.G. Human platelet lysate as a potential clinical-translatable supplement to support the neurotrophic properties of human adipose-derived stem cells. Stem Cell Res. Ther. 2020, 11, 1–14. [Google Scholar] [CrossRef]
- Zhang, P.; Yin, X.; Kou, Y.; Han, N.; Wang, T.; Tian, G.; Lu, L.; Jiang, B. Peripheral nerve mutilation through biodegradable conduit small gap tubulisation: A multicentre randomised trial. Lancet 2015, 386, S40. [Google Scholar] [CrossRef]
- Kim, J.J.; Evans, G.R. Applications of Biomaterials in Plastic Surgery. Clin. Plast. Surg. 2012, 39, 359–376. [Google Scholar] [CrossRef]
- Ao, Q.; Wang, A.; Cao, W.; Zhang, L.; Kong, L.; He, Q.; Gong, Y.; Zhang, X. Manufacture of multimicrotubule chitosan nerve conduits with novel molds and characterization in vitro. J. Biomed. Mater. Res. A 2006, 77, 11–18. [Google Scholar] [CrossRef]
- Yao, L.; de Ruiter, G.C.; Wang, H.; Knight, A.M.; Spinner, R.J.; Yaszemski, M.J.; Windebank, A.J.; Pandit, A. Controlling dispersion of axonal regeneration using a multichannel collagen nerve conduit. Biomaterials 2010, 31, 5789–5797. [Google Scholar] [CrossRef]
- Hamed, I.; Özogul, F.; Regenstein, J.M. Industrial applications of crustacean by-products (chitin, chitosan, and chitooligosaccharides): A review. Trends Food Sci. Technol. 2016, 48, 40–50. [Google Scholar] [CrossRef]
- Ahmad, S.I.; Ahmad, R.; Khan, M.S.; Kant, R.; Shahid, S.; Gautam, L.; Hasan, G.M.; Hassan, M.I. Chitin and its derivatives: Structural properties and biomedical applications. Int. J. Biol. Macromol. 2020, 164, 526–539. [Google Scholar] [CrossRef] [PubMed]
- Topp, K.S.; Boyd, B.S. Structure and biomechanics of peripheral nerves: Nerve responses to physical stresses and implications for physical therapist practice. Phys. Ther. 2006, 86, 92–109. [Google Scholar] [CrossRef]
- Kurwale, N.S.; Suri, V.; Srivastava, A.; Suri, A.; Mohanti, S.; Yadav, P.; Sharma, M.C.; Sarkar, C. Role of bone marrow derived pluripotent stem cells in peripheral nerve repair in adult rats: A morphometric evaluation. J. Neurosci. Rural. Pr. 2015, 6, 152–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.; Gao, T.; Zhao, Y.; Mao, Y.; Sheng, Z.; Lan, Q. Nicotine exerts neuroprotective effects by attenuating local inflammatory cytokine production following crush injury to rat sciatic nerves. Eur. Cytokine Netw 2019, 30, 59–66. [Google Scholar] [PubMed]
- Wang, H.; Zhang, P.; Yu, J.; Zhang, F.; Dai, W.; Yi, S. Matrix metalloproteinase 7 promoted Schwann cell migration and myelination after rat sciatic nerve injury. Mol. Brain 2019, 12, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Cervellini, I.; Galino, J.; Zhu, N.; Allen, S.; Birchmeier, C.; Bennett, D.L. Sustained MAPK/ERK Activation in Adult Schwann Cells Impairs Nerve Repair. J. Neurosci. 2017, 38, 679–690. [Google Scholar] [CrossRef] [Green Version]
- Miyajima, A.; Tanaka, M.; Itoh, T. Stem/Progenitor Cells in Liver Development, Homeostasis, Regeneration, and Reprogramming. Cell Stem Cell 2014, 14, 561–574. [Google Scholar] [CrossRef] [Green Version]
- Regmi, S.; Raut, P.K.; Pathak, S.; Shrestha, P.; Park, P.-H.; Jeong, J.-H. Enhanced viability and function of mesenchymal stromal cell spheroids is mediated via autophagy induction. Autophagy 2021, 17, 2991–3010. [Google Scholar] [CrossRef]
- Chen, D.-Y.; Wei, H.-J.; Lin, W.-W.; Lin, K.-J.; Huang, C.-C.; Wu, C.-T.; Hwang, S.-M.; Chang, Y.; Sung, H.-W. Intramuscular delivery of 3D aggregates of HUVECs and cbMSCs for cellular cardiomyoplasty in rats with myocardial infarction. J. Control. Release 2013, 172, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-C.; Wei, H.-J.; Lin, K.-J.; Lin, W.-W.; Wang, C.-W.; Pan, W.-Y.; Hwang, S.-M.; Chang, Y.; Sung, H.-W. Multimodality noninvasive imaging for assessing therapeutic effects of exogenously transplanted cell aggregates capable of angiogenesis on acute myocardial infarction. Biomaterials 2015, 73, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.C.; Whitehead, J.; Falahee, P.C.; Zhou, D.; Simon, S.I.; Leach, J.K. Multifactorial Experimental Design to Optimize the Anti-Inflammatory and Proangiogenic Potential of Mesenchymal Stem Cell Spheroids. Stem Cells 2017, 35, 1493–1504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartosh, T.J.; Ylöstalo, J.H.; Mohammadipoor, A.; Bazhanov, N.; Coble, K.; Claypool, K.; Lee, R.H.; Choi, H.; Prockop, D.J. Aggregation of human mesenchymal stromal cells (MSCs) into 3D spheroids enhances their anti-inflammatory properties. Proc. Natl. Acad. Sci. USA 2010, 107, 13724–13729. [Google Scholar] [CrossRef] [Green Version]
- Huang, G.S.; Dai, L.G.; Yen, B.L.; Hsu, S.H. Spheroid formation of mesenchymal stem cells on chitosan and chitosan-hyaluronan membranes. Biomater. 2011, 32, 6929–6945. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Zhang, M.; Liu, S.-Y.; Zhang, F.-S.; Wan, T.; Ding, Z.-T.; Zhang, P.-X. Chitin Nerve Conduits with Three-Dimensional Spheroids of Mesenchymal Stem Cells from SD Rats Promote Peripheral Nerve Regeneration. Polymers 2021, 13, 3957. https://doi.org/10.3390/polym13223957
Li C, Zhang M, Liu S-Y, Zhang F-S, Wan T, Ding Z-T, Zhang P-X. Chitin Nerve Conduits with Three-Dimensional Spheroids of Mesenchymal Stem Cells from SD Rats Promote Peripheral Nerve Regeneration. Polymers. 2021; 13(22):3957. https://doi.org/10.3390/polym13223957
Chicago/Turabian StyleLi, Ci, Meng Zhang, Song-Yang Liu, Feng-Shi Zhang, Teng Wan, Zhen-Tao Ding, and Pei-Xun Zhang. 2021. "Chitin Nerve Conduits with Three-Dimensional Spheroids of Mesenchymal Stem Cells from SD Rats Promote Peripheral Nerve Regeneration" Polymers 13, no. 22: 3957. https://doi.org/10.3390/polym13223957
APA StyleLi, C., Zhang, M., Liu, S.-Y., Zhang, F.-S., Wan, T., Ding, Z.-T., & Zhang, P.-X. (2021). Chitin Nerve Conduits with Three-Dimensional Spheroids of Mesenchymal Stem Cells from SD Rats Promote Peripheral Nerve Regeneration. Polymers, 13(22), 3957. https://doi.org/10.3390/polym13223957