Recent Advances in Chitin and Chitosan/Graphene-Based Bio-Nanocomposites for Energetic Applications
Abstract
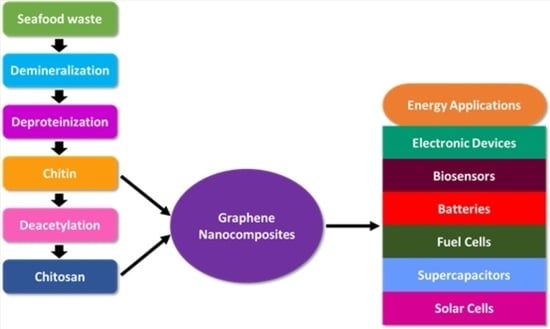
:1. Introduction
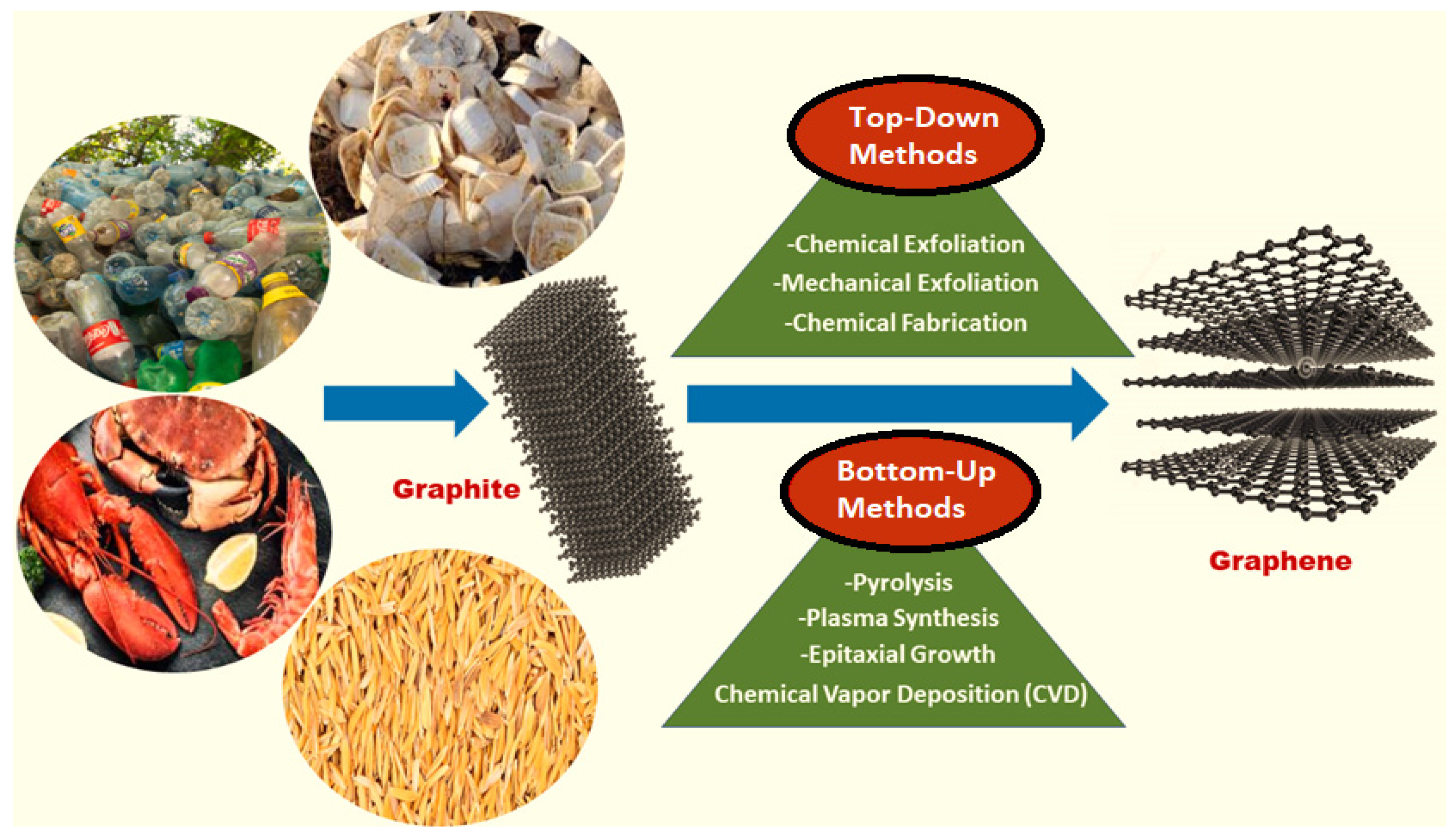
2. Graphene and Graphene Oxide
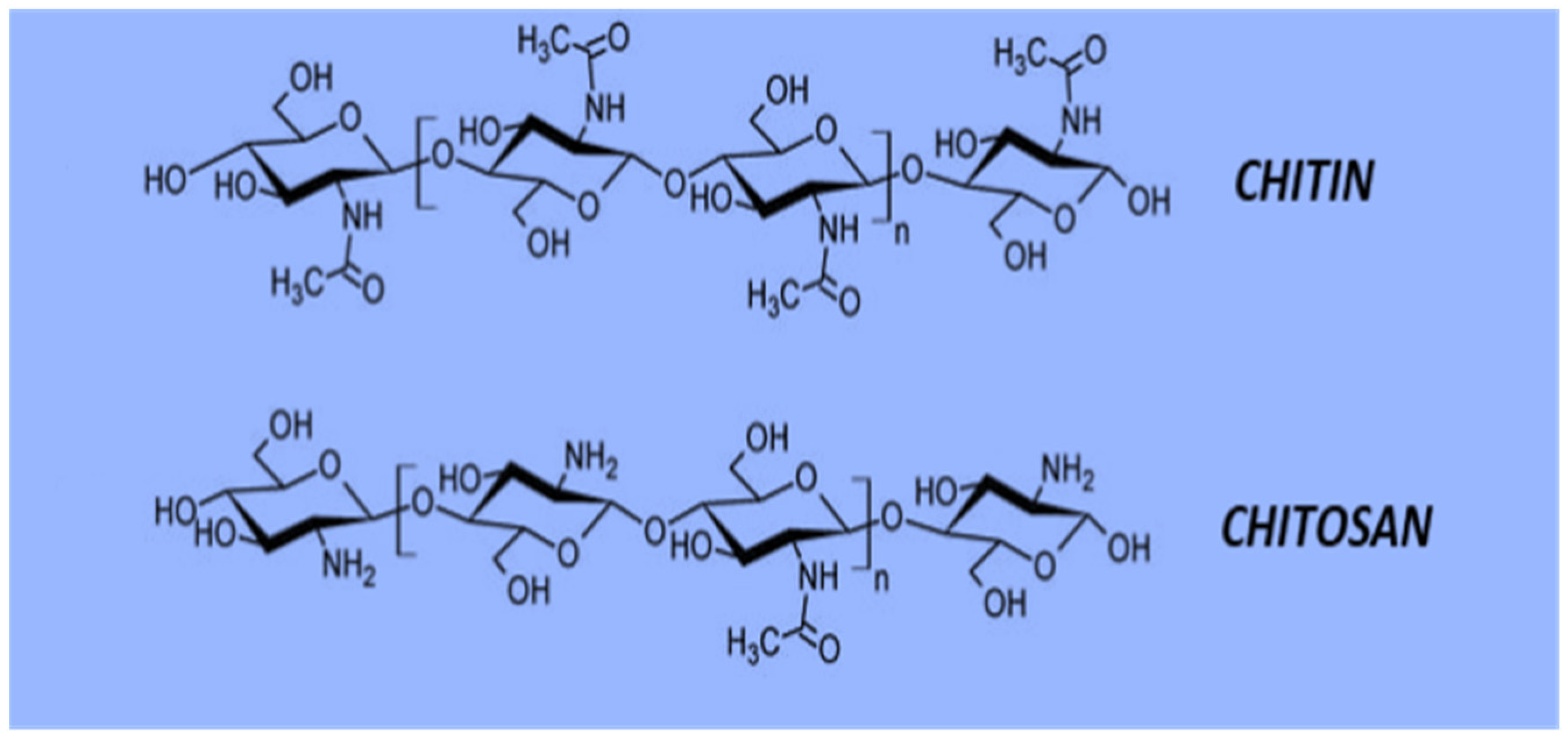
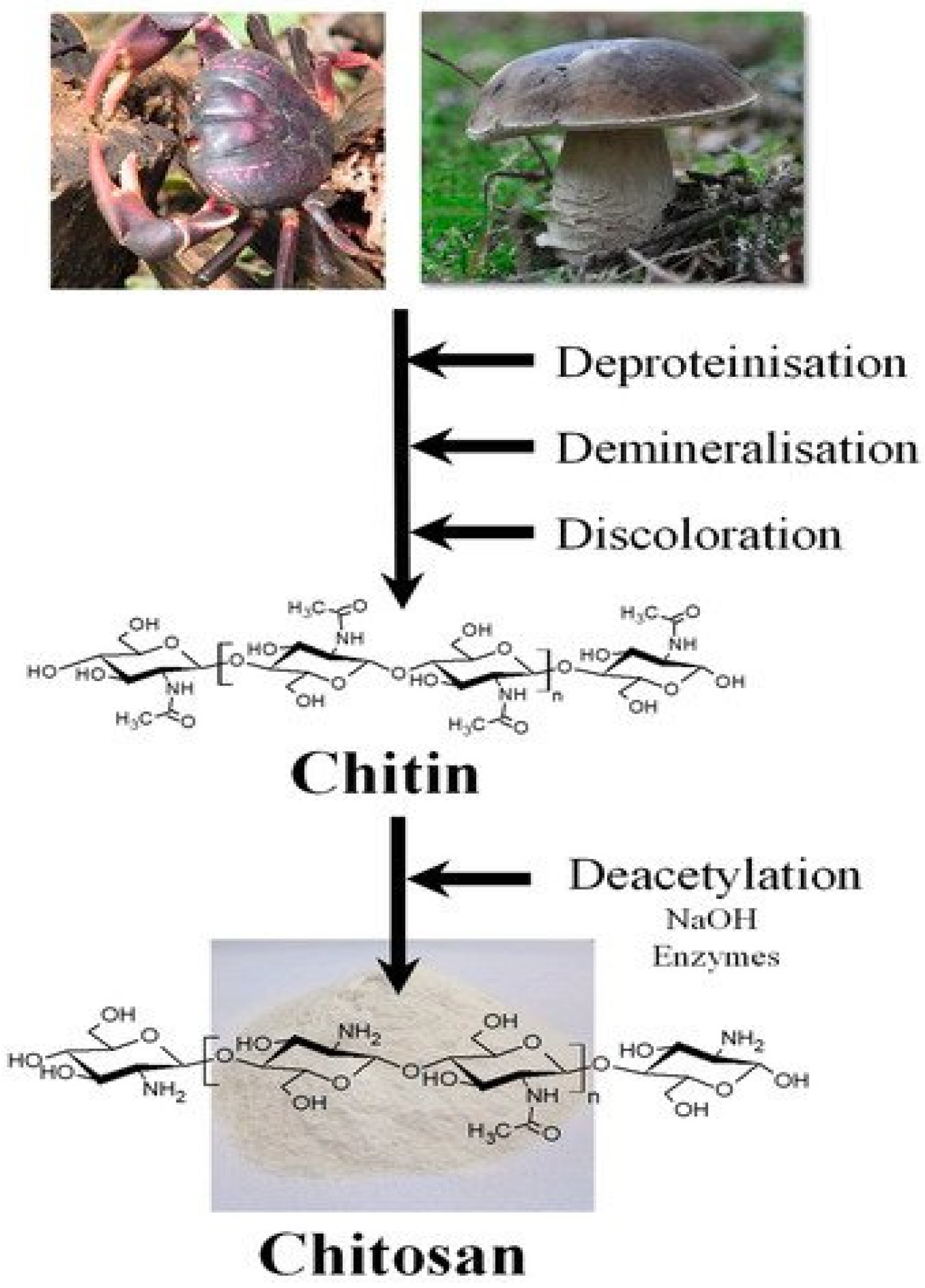
Chitin and Chitosan; Structural Analysis
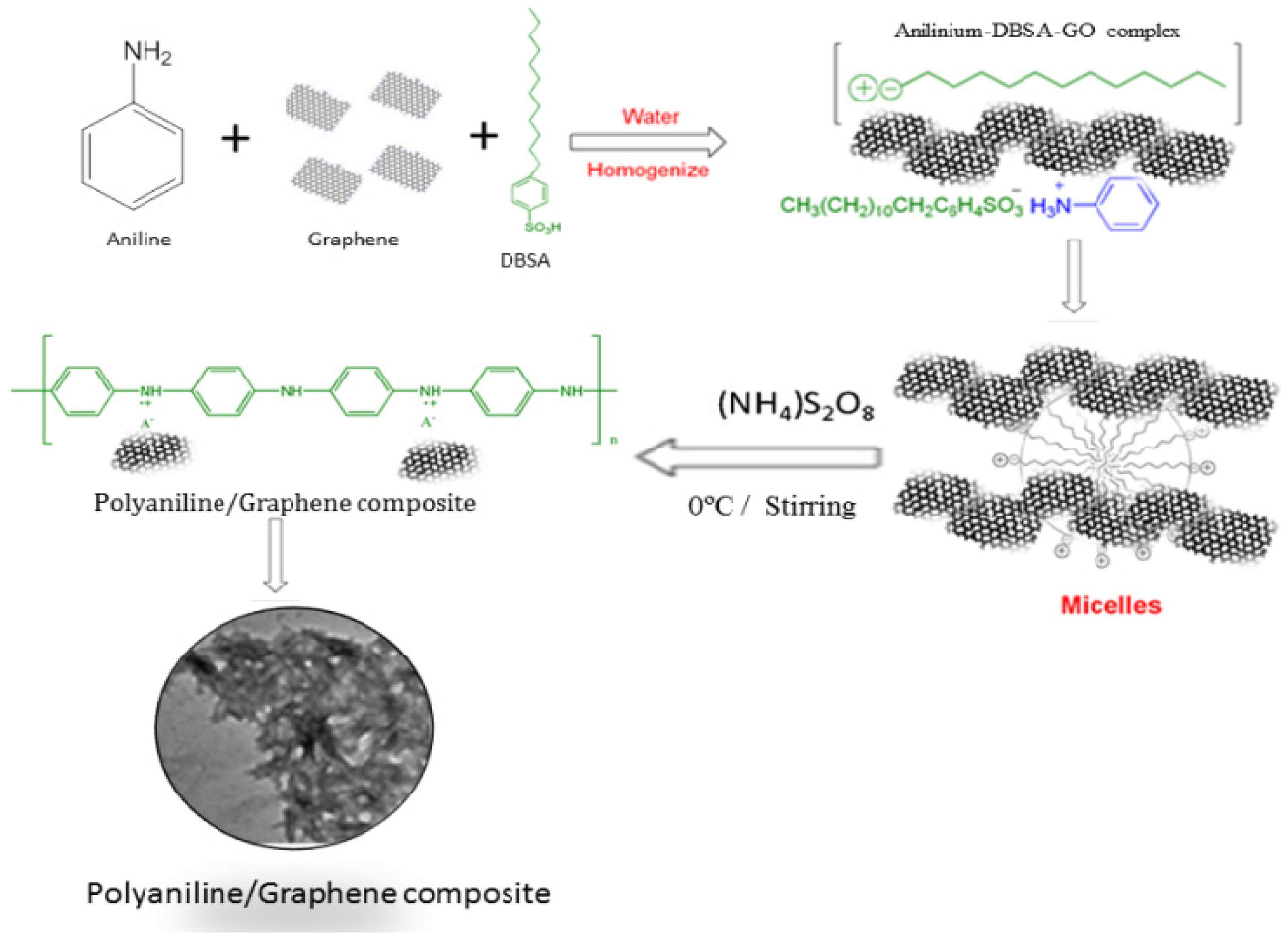
3. Fabrication of Graphene Nanocomposites
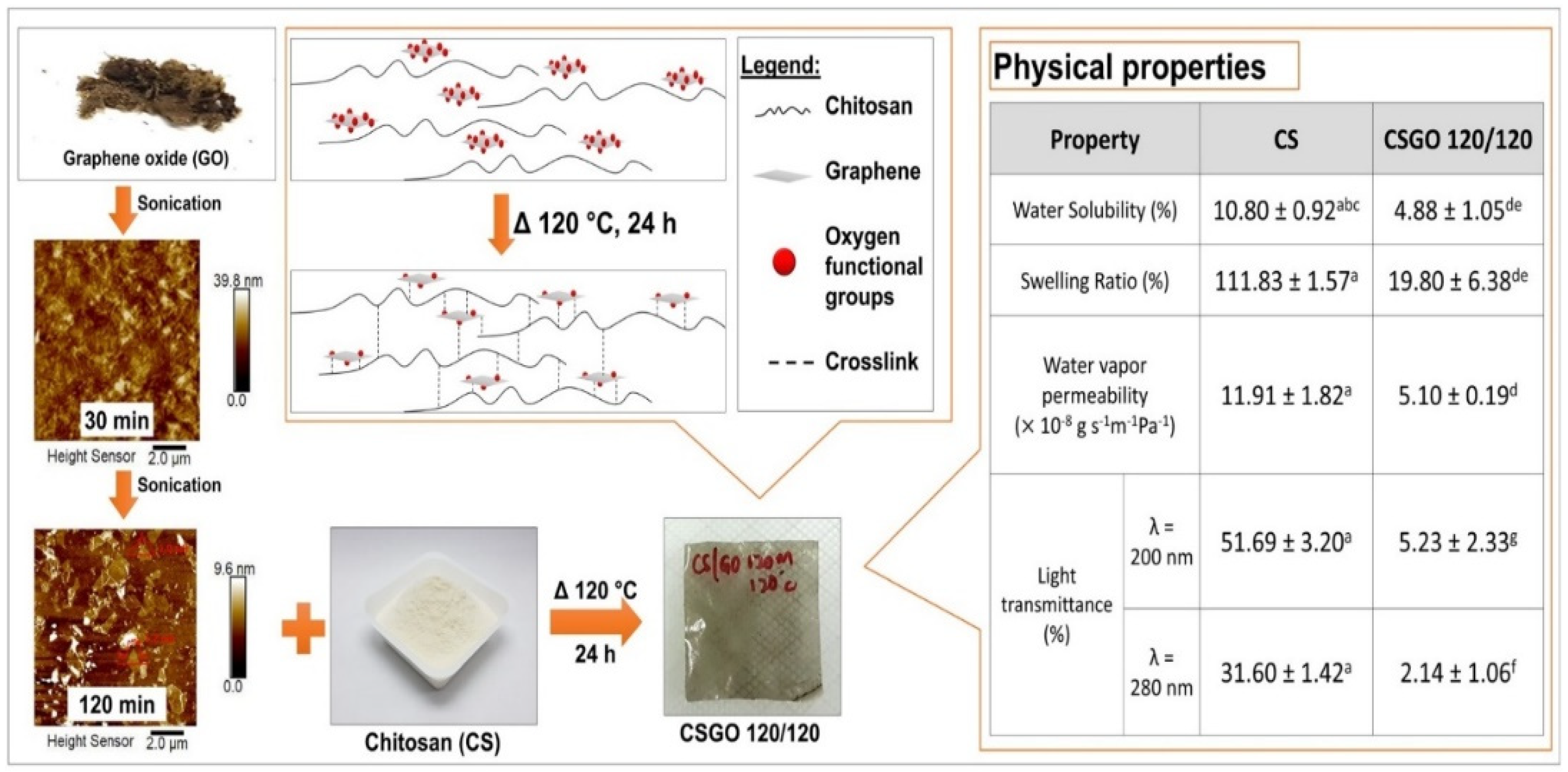
Chitin and Chitosan Graphene Bio-Nanocomposites
4. Energetic Applications of Chitin and Chitosan
4.1. Electrical Devices
4.2. Biosensors
4.3. Batteries and Electrochemistry
4.4. Fuel Cells
4.5. Supercapacitors
4.6. Solar Cells
5. Limitations and Challenges
- The current limitations in the medicinal fields are caused by low solubility and pH, which have led to instable physiological changes among nanocomposites.
- The hygiene and safety of synthesized bio-nanocomposites remain uncertain as the European Food Safety Authority (EFSA) denies them, despite possessing an approval for food contact from the Food Development Authority (FDA).
- Low colloidal stability makes chitin- and chitosan-based bio-nanocomposites unsuitable for large-scale drug delivery.
- Elevated elasticity of chitosan-based bio-nanocomposites restricts their use and applications.
- Despite showing satisfactory effectiveness in several medicinal applications, there are numerous issues such as drug release, loading efficacy and capacity, rate of degradation, and functionalization.
- Finally, industrial processing centers continue to face financial challenges in establishing a solid commercial viability of sustainable biopolymers in the real world.
Future Recommendations
- Nanotechnology has great potential in agro-economics to improve agricultural areas. In this regard, nano-chitin or nano-chitosan might be powerful tools for delivering environmentally benign nano-chemicals or nano-agro-fertilizers.
- Their components can be used to grow crops, manage pests, increase fish output, produce meat, preserve seeds, improve the immune system of crops and develop crops with high drought and salinity resistance, among other elements.
- There are very few in vivo studies demonstrating the formulation and conjugation of chitosan-based nano-carriers with antibodies as well as the assessment of long-term toxicity of the nano-carriers. Thus, future research can be conducted on studies of antibodies coupled with chitosan-based nano-carriers.
- As metal oxides have shown unique semiconducting, optical and photocatalytic characteristics, chitosan/metal oxide bio-nanocomposites could bring a remarkable change for wound healing and other future regeneration studies.
- Chitin and chitosan can be an attractive future research choice as heterogeneous bio-nanocatalysts and kinetic studies.
- Due to their physiological pH, chitosan-based bio-nanocomposites have limited solubility. In this situation, researchers should concentrate on developing novel chitosan-based bio-nanocomposite materials with improved solubility and aggregation.
- Conventional acid and alkali treatments should be replaced with novel biological methods for chitosan extraction. In competitive industrial situations, eco-friendly and cost-effective extraction methods must also be established.
- Crabs, shrimps, insects and fungi are acquiring enormous demand in many industrial areas due to their remarkable characteristics. As these natural resources become more popular, there is a growing worry that they will become extinct. The researchers’ mission should be focused on identifying alternate sources of energy in order to restore ecological equilibrium.
- Researchers should concentrate on introducing nanomaterials with high degrees of elasticity for novel electronic devices.
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Haupt, K.; Medina Rangel, P.X.; Bui, B.T.S. Molecularly imprinted polymers: Antibody mimics for bioimaging and therapy. Chem. Rev. 2020, 120, 9554–9582. [Google Scholar] [CrossRef] [PubMed]
- Saska, S.; Pilatti, L.; Blay, A.; Shibli, J.A. Bioresorbable Polymers: Advanced Materials and 4D Printing for Tissue Engineering. Polymers 2021, 13, 563. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; He, Y.; Zhang, F.; Liu, Y.; Leng, J. A review of shape memory polymers and composites: Mechanisms, materials, and applications. Adv. Mater. 2021, 33, 2000713. [Google Scholar] [CrossRef]
- Yu, Y.; Shen, M.; Song, Q.; Xie, J. Biological activities and pharmaceutical applications of polysaccharide from natural resources: A review. Carbohydr. Polym. 2018, 183, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, A.K.; Shukla, S.K.; Bhanu, S.; Kankane, S. Responsive polymers in controlled drug delivery. Prog. Polym. Sci. 2008, 33, 1088–1118. [Google Scholar] [CrossRef]
- Balint, R.; Cassidy, N.J.; Cartmell, S.H. Conductive polymers: Towards a smart biomaterial for tissue engineering. Acta Biomater. 2014, 10, 2341–2353. [Google Scholar] [CrossRef]
- Zhu, Y.; Romain, C.; Williams, C.K. Sustainable polymers from renewable resources. Nature 2016, 540, 354–362. [Google Scholar] [CrossRef]
- Wang, X.; Yao, C.; Wang, F.; Li, Z. Cellulose-based nanomaterials for energy applications. Small 2017, 13, 1702240. [Google Scholar] [CrossRef]
- Xue, G.; Xu, Y.; Ding, T.; Li, J.; Yin, J.; Fei, W.; Cao, Y.; Yu, J.; Yuan, L.; Gong, L.; et al. Water-evaporation-induced electricity with nanostructured carbon materials. Nat. Nanotechnol. 2017, 12, 317–321. [Google Scholar] [CrossRef]
- Inagaki, M.; Toyoda, M.; Soneda, Y.; Morishita, T. Nitrogen-doped carbon materials. Carbon 2018, 132, 104–140. [Google Scholar] [CrossRef]
- Mohan, M.; Sharma, V.K.; Kumar, E.A.; Gayathri, V. Hydrogen storage in carbon materials—A review. Energy Storage 2019, 1, e35. [Google Scholar] [CrossRef]
- Papageorgiou, D.G.; Kinloch, I.A.; Young, R.J. Mechanical properties of graphene and graphene-based nanocomposites. Prog. Mater. Sci. 2017, 90, 75–127. [Google Scholar] [CrossRef]
- Wu, J.B.; Lin, M.L.; Cong, X.; Liu, H.N.; Tan, P.H. Raman spectroscopy of graphene-based materials and its applications in related devices. Chem. Soc. Rev. 2018, 47, 1822–1873. [Google Scholar] [CrossRef] [Green Version]
- Rinaudo, M. Main properties and current applications of some polysaccharides as biomaterials. Polym. Int. 2008, 57, 397–430. [Google Scholar] [CrossRef]
- Bertolino, V.; Cavallaro, G.; Milioto, S.; Lazzara, G. Polysaccharides/Halloysite nanotubes for smart bionanocomposite materials. Carbohydr. Polym. 2020, 245, 116502. [Google Scholar] [CrossRef]
- Malerba, M.; Cerana, R. Chitin- and Chitosan-Based Derivatives in Plant Protection against Biotic and Abiotic Stresses and in Recovery of Contaminated Soil and Water. Polysaccharides 2020, 1, 21–30. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Pacheco, N.; Naal-Ek, M.G.; Ayora-Talavera, T.; Shirai, K.; Román-Guerrero, A.; Fabela-Morón, M.F.; Cuevas-Bernardino, J.C. Effect of bio-chemical chitosan and gallic acid into rheology and physicochemical properties of ternary edible films. Int. J. Biol. Macromol. 2019, 125, 149–158. [Google Scholar] [CrossRef]
- Anitha, A.; Sowmya, S.; Kumar, P.S.; Deepthi, S.; Chennazhi, K.P.; Ehrlich, H.; Tsurkan, M.; Jayakumar, R. Chitin and chitosan in selected biomedical applications. Prog. Polym. Sci. 2014, 39, 1644–1667. [Google Scholar] [CrossRef]
- López-Mata, M.A.; Ruiz-Cruz, S.; Silva-Beltrán, N.P.; Ornelas-Paz, J.D.J.; Ocaño-Higuera, V.M.; Rodríguez-Félix, F.; Cira-Chávez, L.A.; Del-Toro-Sánchez, C.L.; Shirai, K. Physicochemical and antioxidant properties of chitosan films incorporated with cinnamon oil. Int. J. Polym. Sci. 2015, 2015, 1–8. [Google Scholar] [CrossRef]
- Espadín, A.; De Dios, L.T.; Ruvalcaba, E.; Valadez-García, J.; Velasquillo, C.; Bustos-Jaimes, I.; Vázquez-Torres, H.; Gimeno, M.; Shirai, K. Production and characterization of a nanocomposite of highly crystalline nanowhiskers from biologically extracted chitin in enzymatic poly (ε-caprolactone). Carbohydr. Polym. 2018, 181, 684–692. [Google Scholar] [CrossRef]
- Sirajudheen, P.; Poovathumkuzhi, N.C.; Vigneshwaran, S.; Chelaveettil, B.M.; Meenakshi, S. Applications of chitin and chitosan based biomaterials for the adsorptive removal of textile dyes from water-A comprehensive review. Carbohydr. Polym. 2021, 273, 118604. [Google Scholar] [CrossRef]
- Safarzadeh, M.; Sadeghi, S.; Azizi, M.; Rastegari-Pouyani, M.; Pouriran, R.; Hoseini, M.H.M. Chitin and chitosan as tools to combat COVID-19: A triple approach. Int. J. Biol. Macromol. 2021, 183, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Babaei-Ghazvini, A.; Acharya, B.; Korber, D.R. Antimicrobial Biodegradable Food Packaging Based on Chitosan and Metal/Metal-Oxide Bio-Nanocomposites: A Review. Polymers 2021, 13, 2790. [Google Scholar] [CrossRef] [PubMed]
- Vedula, S.S.; Yadav, G.D. Chitosan-Based Membranes Preparation and Applications: Challenges and Opportunities. J. Indian Chem. Soc. 2021, 98, 100017. [Google Scholar] [CrossRef]
- Ndlovu, S.P.; Ngece, K.; Alven, S.; Aderibigbe, B.A. Gelatin-Based Hybrid Scaffolds: Promising Wound Dressings. Polymers 2021, 13, 2959. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Alamry, K.A. Recent advances of emerging green chitosan-based biomaterials with potential biomedical applications: A review. Carbohydr. Res. 2021, 506, 108368. [Google Scholar] [CrossRef] [PubMed]
- Pal, P.; Pal, A.; Nakashima, K.; Yadav, B.K. Applications of chitosan in environmental remediation: A review. Chemosphere 2020, 266, 128934. [Google Scholar] [CrossRef]
- Jariwala, D.; Sangwan, V.K.; Lauhon, L.J.; Marks, T.J.; Hersam, M.C. Carbon nanomaterials for electronics, optoelectronics, photovoltaics, and sensing. Chem. Soc. Rev. 2013, 42, 2824–2860. [Google Scholar] [CrossRef] [Green Version]
- Ikram, R.; Jan, B.M.; Ahmad, W. An overview of industrial scalable production of graphene oxide and analytical approaches for synthesis and characterization. J. Mater. Res. Technol. 2020, 9, 11587–11610. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.E.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [Green Version]
- Ikram, R.; Jan, B.M.; Ahmad, W. Advances in synthesis of graphene derivatives using industrial wastes precursors; prospects and challenges. J. Mater. Res. Technol. 2020, 9, 15924–15951. [Google Scholar] [CrossRef]
- Azizi-Lalabadi, M.; Jafari, S.M. Bio-nanocomposites of graphene with biopolymers; fabrication, properties, and applications. Adv. Colloid Interface Sci. 2021, 292, 102416. [Google Scholar] [CrossRef]
- Tiwari, S.K.; Sahoo, S.; Wang, N.; Huczko, A. Graphene research and their outputs: Status and prospect. J. Sci. Adv. Mater. Devices 2020, 5, 10–29. [Google Scholar] [CrossRef]
- Olabi, A.G.; Abdelkareem, M.A.; Wilberforce, T.; Sayed, E.T. Application of graphene in energy storage device–A review. Renew. Sustain. Energy Rev. 2021, 135, 110026. [Google Scholar] [CrossRef]
- Balandin, A.A. Phononics of graphene and related materials. ACS Nano 2020, 14, 5170–5178. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Shi, H.; Das, P.; Qin, J.; Li, Y.; Wang, X.; Su, F.; Wen, P.; Li, S.; Lu, P.; et al. The chemistry and promising applications of graphene and porous graphene materials. Adv. Funct. Mater. 2020, 30, 1909035. [Google Scholar] [CrossRef]
- Tarcan, R.; Todor-Boer, O.; Petrovai, I.; Leordean, C.; Astilean, S.; Botiz, I. Reduced graphene oxide today. J. Mater. Chem. C 2020, 8, 1198–1224. [Google Scholar] [CrossRef]
- Ahmad, W.; Ur Rahman, A.; Ahmad, I.; Yaseen, M.; Mohamed Jan, B.; Stylianakis, M.M.; Kenanakis, G.; Ikram, R. Oxidative Desulfurization of Petroleum Distillate Fractions Using Manganese Dioxide Supported on Magnetic Reduced Graphene Oxide as Catalyst. Nanomaterials 2021, 11, 203. [Google Scholar] [CrossRef] [PubMed]
- Ikram, R.; Mohamed Jan, B.; Vejpravova, J.; Choudhary, M.I.; Zaman Chowdhury, Z. Recent Advances of Graphene-Derived Nanocomposites in Water-Based Drilling Fluids. Nanomaterials 2020, 10, 2004. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Fang, S.; Hu, Y.H. 3D Graphene materials: From understanding to design and synthesis control. Chem. Rev. 2020, 120, 10336–10453. [Google Scholar] [CrossRef]
- Kumar, R.; Sahoo, S.; Joanni, E.; Singh, R.K.; Maegawa, K.; Tan, W.K.; Kawamura, G.; Kar, K.K.; Matsuda, A. Heteroatom doped graphene engineering for energy storage and conversion. Mater. Today 2020, 39, 47–65. [Google Scholar] [CrossRef]
- Wang, W.; Meng, Q.; Li, Q.; Liu, J.; Zhou, M.; Jin, Z.; Zhao, K. Chitosan derivatives and their application in biomedicine. Int. J. Mol. Sci. 2020, 21, 487. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.R.; Muzzarelli, R.; Muzzarelli, C.; Sashiwa, H.; Domb, A.J. Chitosan chemistry and pharmaceutical perspectives. Chem. Rev. 2004, 104, 6017–6084. [Google Scholar] [CrossRef]
- Shariatinia, Z. Pharmaceutical applications of chitosan. Adv. Colloid Interface Sci. 2019, 263, 131–194. [Google Scholar] [CrossRef]
- Sahariah, P.; Masson, M. Antimicrobial chitosan and chitosan derivatives: A review of the structure—Activity relationship. Biomacromolecules 2017, 18, 3846–3868. [Google Scholar] [CrossRef]
- Cheung, R.C.F.; Ng, T.B.; Wong, J.H.; Chan, W.Y. Chitosan: An update on potential biomedical and pharmaceutical applications. Mar. Drugs 2015, 13, 5156–5186. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.; Nam, J.P.; Nah, J.W. Application of chitosan and chitosan derivatives as biomaterials. J. Ind. Eng. Chem. 2016, 33, 1–10. [Google Scholar] [CrossRef]
- Zhang, L.; Zeng, Y.; Cheng, Z. Removal of heavy metal ions using chitosan and modified chitosan: A review. J. Mol. Liq. 2016, 214, 175–191. [Google Scholar] [CrossRef]
- Cavallaro, G.; Micciulla, S.; Chiappisi, L.; Lazzara, G. Chitosan-based smart hybrid materials: A physico-chemical perspective. J. Mater. Chem. B 2021, 9, 594–611. [Google Scholar] [CrossRef] [PubMed]
- Karimi-Maleh, H.; Ayati, A.; Davoodi, R.; Tanhaei, B.; Karimi, F.; Malekmohammadi, S.; Orooji, Y.; Fu, L.; Sillanpää, M. Recent advances in using of chitosan-based adsorbents for removal of pharmaceutical contaminants: A review. J. Clean. Prod. 2021, 125880. [Google Scholar] [CrossRef]
- Si, Z.; Hou, Z.; Vikhe, Y.S.; Thappeta, K.R.V.; Marimuthu, K.; De, P.P.; Ng, O.T.; Li, P.; Zhu, Y.; Pethe, K.; et al. Antimicrobial effect of a novel chitosan derivative and its synergistic effect with antibiotics. ACS Appl. Mater. Interfaces 2021, 13, 3237–3245. [Google Scholar] [CrossRef] [PubMed]
- Brasselet, C.; Pierre, G.; Dubessay, P.; Dols-Lafargue, M.; Coulon, J.; Maupeu, J.; Vallet-Courbin, A.; de Baynast, H.; Doco, T.; Michaud, P.; et al. Modification of Chitosan for the Generation of Functional Derivatives. Appl. Sci. 2019, 9, 1321. [Google Scholar] [CrossRef] [Green Version]
- Zhou, D.Y.; Wu, Z.X.; Yin, F.W.; Song, S.; Li, A.; Zhu, B.W.; Yu, L.L. Chitosan and Derivatives: Bioactivities and Application in Foods. Annu. Rev. Food Sci. Technol. 2021, 12, 407–432. [Google Scholar] [CrossRef]
- Simsir, H.; Eltugral, N.; Karagoz, S. Hydrothermal carbonization for the preparation of hydrochars from glucose, cellulose, chitin, chitosan and wood chips via low-temperature and their characterization. Bioresour. Technol. 2017, 246, 82–87. [Google Scholar] [CrossRef]
- Hu, K.; Kulkarni, D.D.; Choi, I.; Tsukruk, V.V. Graphene-polymer nanocomposites for structural and functional applications. Prog. Polym. Sci. 2014, 39, 1934–1972. [Google Scholar] [CrossRef]
- Sun, X.; Huang, C.; Wang, L.; Liang, L.; Cheng, Y.; Fei, W.; Li, Y. Recent progress in graphene/polymer nanocomposites. Adv. Mater. 2021, 33, 2001105. [Google Scholar] [CrossRef]
- Cui, Y.; Kundalwal, S.I.; Kumar, S. Gas barrier performance of graphene/polymer nanocomposites. Carbon 2016, 98, 313–333. [Google Scholar] [CrossRef] [Green Version]
- Guan, L.Z.; Zhao, L.; Wan, Y.J.; Tang, L.C. Three-dimensional graphene-based polymer nanocomposites: Preparation, properties and applications. Nanoscale 2018, 10, 14788–14811. [Google Scholar] [CrossRef] [PubMed]
- Sanes, J.; Sánchez, C.; Pamies, R.; Avilés, M.D.; Bermúdez, M.D. Extrusion of polymer nanocomposites with graphene and graphene derivative nanofillers: An overview of recent developments. Materials 2020, 13, 549. [Google Scholar] [CrossRef] [Green Version]
- Singh, K.; Ohlan, A.; Dhawan, S.K. Polymer-graphene nanocomposites: Preparation, characterization, properties, and applications. In Nanocomposites—New Trends and Developments; Ebrahimi, F., Ed.; InTech: Rijeka, Croatia, 2012; Chapter 3; pp. 37–72. [Google Scholar]
- Papageorgiou, D.G.; Li, Z.; Liu, M.; Kinloch, I.A.; Young, R.J. Mechanisms of mechanical reinforcement by graphene and carbon nanotubes in polymer nanocomposites. Nanoscale 2020, 12, 2228–2267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.; Zhang, K.; Wang, Y.; Wang, Y.; Yang, Y. Thermoelectric effect induced electricity in stretchable graphene-polymer nanocomposites for ultrasensitive self-powered strain sensor system. Nano Energy 2019, 56, 25–32. [Google Scholar] [CrossRef]
- Xia, X.; Wang, Y.; Zhong, Z.; Weng, G.J. A frequency-dependent theory of electrical conductivity and dielectric permittivity for graphene-polymer nanocomposites. Carbon 2017, 111, 221–230. [Google Scholar] [CrossRef]
- Huang, G.; Chen, W.; Wu, T.; Guo, H.; Fu, C.; Xue, Y.; Wang, K.; Song, P. Multifunctional graphene-based nano-additives toward high-performance polymer nanocomposites with enhanced mechanical, thermal, flame retardancy and smoke suppressive properties. Chem. Eng. J. 2021, 410, 127590. [Google Scholar] [CrossRef]
- Wang, J.; Jin, X.; Li, C.; Wang, W.; Wu, H.; Guo, S. Graphene and graphene derivatives toughening polymers: Toward high toughness and strength. Chem. Eng. J. 2019, 370, 831–854. [Google Scholar] [CrossRef]
- Govindaraj, P.; Fox, B.; Aitchison, P.; Hameed, N. A review on graphene polymer nanocomposites in harsh operating conditions. Ind. Eng. Chem. Res. 2019, 58, 17106–17129. [Google Scholar] [CrossRef]
- Zhang, M.; Li, Y.; Su, Z.; Wei, G. Recent advances in the synthesis and applications of graphene–polymer nanocomposites. Polym. Chem. 2015, 6, 6107–6124. [Google Scholar] [CrossRef]
- Kang, H.; Tang, Y.; Yao, L.; Yang, F.; Fang, Q.; Hui, D. Fabrication of graphene/natural rubber nanocomposites with high dynamic properties through convenient mechanical mixing. Compos. Part B Eng. 2017, 112, 1–7. [Google Scholar] [CrossRef]
- Bhattacharya, M. Polymer nanocomposites—A comparison between carbon nanotubes, graphene, and clay as nanofillers. Materials 2016, 9, 262. [Google Scholar] [CrossRef]
- Shen, X.; Zheng, Q.; Kim, J.K. Rational design of two-dimensional nanofillers for polymer nanocomposites toward multifunctional applications. Prog. Mater. Sci. 2021, 115, 100708. [Google Scholar] [CrossRef]
- Khodakarami, M.; Bagheri, M. Recent advances in synthesis and application of polymer nanocomposites for water and wastewater treatment. J. Clean. Prod. 2021, 296, 126404. [Google Scholar] [CrossRef]
- Chaurasia, A.; Parashar, A. Effect of BNNR on mechanical properties of polyethylene nanocomposites. Mater. Today Proc. 2021. [Google Scholar] [CrossRef]
- Silva, M.; Pinho, I.S.; Covas, J.A.; Alves, N.M.; Paiva, M.C. 3D printing of graphene-based polymeric nanocomposites for biomedical applications. Funct. Compos. Mater. 2021, 2, 1–21. [Google Scholar]
- Salimikia, I.; Heydari, R.; Yazdankhah, F. Polyaniline/graphene oxide nanocomposite as a sorbent for extraction and determination of nicotine using headspace solid-phase microextraction and gas chromatography–flame ionization detector. J. Iran. Chem. Soc. 2018, 15, 1593–1601. [Google Scholar] [CrossRef]
- Heydari, M.; Saraji, M.; Jafari, M.T. Electrochemically prepared three-dimensional reduced graphene oxide-polyaniline nanocomposite as a solid-phase microextraction coating for ethion determination. Talanta 2020, 209, 120576. [Google Scholar] [CrossRef] [PubMed]
- Suvina, V.; Krishna, S.M.; Nagaraju, D.H.; Melo, J.S.; Balakrishna, R.G. Polypyrrole-reduced graphene oxide nanocomposite hydrogels: A promising electrode material for the simultaneous detection of multiple heavy metal ions. Mater. Lett. 2018, 232, 209–212. [Google Scholar] [CrossRef]
- Park, I.H.; Lee, J.Y.; Ahn, S.J.; Choi, H.J. Melt Rheology and Mechanical Characteristics of Poly (Lactic Acid)/Alkylated Graphene Oxide Nanocomposites. Polymers 2020, 12, 2402. [Google Scholar] [CrossRef]
- Jasmi, F.; Azeman, N.H.; Bakar, A.A.A.; Zan, M.S.D.; Badri, K.H.; Su’ait, M.S. Ionic conductive polyurethane-graphene nanocomposite for performance enhancement of optical fiber Bragg grating temperature sensor. IEEE Access 2018, 6, 47355–47363. [Google Scholar] [CrossRef]
- Jin, K.; Zhang, W.; Wang, Y.; Guo, X.; Chen, Z.; Li, L.; Zhang, Y.; Wang, Z.; Chen, J.; Sun, L.; et al. In–situ hybridization of polyaniline nanofibers on functionalized reduced graphene oxide films for high-performance supercapacitor. Electrochim. Acta 2018, 285, 221–229. [Google Scholar] [CrossRef]
- Wan, Y.; Li, J.; Yang, Z.; Ao, H.; Xiong, L.; Luo, H. Simultaneously depositing polyaniline onto bacterial cellulose nanofibers and graphene nanosheets toward electrically conductive nanocomposites. Curr. Appl. Phys. 2018, 18, 933–940. [Google Scholar] [CrossRef]
- Lu, B.; Yuan, X.; Ren, Y.; Shi, Q.; Wang, S.; Dong, J.; Nan, Z.D. Cost-effective three dimensional Ag/polymer dyes/graphene-carbon spheres hybrids for high performance nonenzymatic sensor and its application in living cell H2O2 detection. Bioelectrochemistry 2018, 123, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, Q.; Peng, C.; Shu, N.; Niu, L.; Zhu, Y. To increase electrochemical performance of electrode material by attaching activated carbon particles on reduced graphene oxide sheets for supercapacitor. J. Power Sources 2020, 450, 227611. [Google Scholar] [CrossRef]
- Hossain, M.F.; Park, J.Y. Plain to point network reduced graphene oxide-activated carbon composites decorated with platinum nanoparticles for urine glucose detection. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abd Muain, M.F.; Cheo, K.H.; Omar, M.N.; Hamzah, A.S.A.; Lim, H.N.; Salleh, A.B.; Tan, W.S.; Tajudin, A.A. Gold nanoparticle-decorated reduced-graphene oxide targeting anti hepatitis B virus core antigen. Bioelectrochemistry 2018, 122, 199–205. [Google Scholar] [CrossRef]
- Van Pham, T. One-step hydrothermal synthesis and photocatalytic activity of SnO2/rGO nanocomposites: Effects of pH values. Commun. Phys. 2021, 31. [Google Scholar]
- Lawal, A.T. Graphene-based nano composites and their applications. A review. Biosens. Bioelectron. 2019, 141, 111384. [Google Scholar] [CrossRef]
- Borah, M.J.; Devi, A.; Saikia, R.A.; Deka, D. Biodiesel production from waste cooking oil catalyzed by in-situ decorated TiO2 on reduced graphene oxide nanocomposite. Energy 2018, 158, 881–889. [Google Scholar] [CrossRef]
- Anichini, C.; Samorì, P. Graphene-Based Hybrid Functional Materials. Small 2021, 17, 2100514. [Google Scholar] [CrossRef]
- Chen, T.; Zhou, Y.; Zhang, J.; Cao, Y. Two-dimensional MnO2/reduced graphene oxide nanosheet as a high-capacity and high-rate cathode for lithium-ion batteries. Int. J. Electrochem. Sci. 2018, 13, 8575–8588. [Google Scholar] [CrossRef]
- Chou, C.T.; Wang, F.H. Fabrication and characterization of transparent conducting reduced graphene oxide/Ag nanowires/ZnO: Ga composite thin films on flexible substrates. J. Vac. Sci. Technol. A Vac. Surf. Film. 2018, 36, 05G504. [Google Scholar] [CrossRef]
- Gulati, U.; Chinna Rajesh, U.; Rawat, D.S. Reduced Graphene oxide supported copper oxide nanocomposites from a renewable copper mineral precursor: A green approach for decarboxylative C (sp3)–H activation of proline amino acid to afford value-added synthons. ACS Sustain. Chem. Eng. 2018, 6, 10039–10051. [Google Scholar] [CrossRef]
- Huang, W.; Ding, S.; Chen, Y.; Hao, W.; Lai, X.; Peng, J.; Tu, J.; Cao, Y.; Li, X. 3D NiO hollow sphere/reduced graphene oxide composite for high-performance glucose biosensor. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Mao, P.; Yan, F.; Gao, C.; Liu, Y.; Ding, J.; Wu, W.; Liu, Y. Flower-like Fe2O3/reduced graphene oxide composite for electrochemical energy storage. Synth. Met. 2016, 222, 198–204. [Google Scholar] [CrossRef] [Green Version]
- Qian, L.; Wei, Z.; Yu, T.; Chang, B.; Wang, Z.; Liu, Y.; Sun, H.; Huang, W. Boosting the Electrocatalytic Hydrogen Evolution and Sodium-Storage Properties of Co9S8 Nanoparticles via the Encapsulation with Nitrogen-Doped Few-Layer Graphene Networks. Sustain. Energy Fuels 2021, 5, 4618–4627. [Google Scholar] [CrossRef]
- Xuan, X.; Kim, J.Y.; Hui, X.; Das, P.S.; Yoon, H.S.; Park, J.Y. A highly stretchable and conductive 3D porous graphene metal nanocomposite based electrochemical-physiological hybrid biosensor. Biosens. Bioelectron. 2018, 120, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Zheng, H.; Huo, D.; Wu, F.; Shao, L.; Zheng, P.; Jiang, Y.; Zheng, X.; Qiu, X.; Liu, Y.; et al. N-doped graphene-based copper nanocomposite with ultralow electrical resistivity and high thermal conductivity. Sci. Rep. 2018, 8, 1–7. [Google Scholar]
- Liu, Z.; Guo, Y. Sensitive determination of trace 4-nitrophenol in water based on thio-β-cyclodextrin functionalized graphene/copper nanospheres. J. Electroanal. Chem. 2018, 825, 57–64. [Google Scholar] [CrossRef]
- Fan, H.; Wang, L.; Zhao, K.; Li, N.; Shi, Z.; Ge, Z.; Jin, Z. Fabrication, mechanical properties, and biocompatibility of graphene-reinforced chitosan composites. Biomacromolecules 2010, 11, 2345–2351. [Google Scholar] [CrossRef]
- Sayyar, S.; Murray, E.; Thompson, B.C.; Chung, J.; Officer, D.L.; Gambhir, S.; Spinks, G.M.; Wallace, G.G. Processable conducting graphene/chitosan hydrogels for tissue engineering. J. Mater. Chem. B 2015, 3, 481–490. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Lian, H.T.; Yin, J.F.; Sun, X.Y. Dopamine molecularly imprinted electrochemical sensor based on graphene–Chitosan composite. Electrochim. Acta 2012, 75, 108–114. [Google Scholar] [CrossRef]
- Kim, D.S.; Dhand, V.; Rhee, K.Y.; Park, S.J. Study on the effect of silanization and improvement in the tensile behavior of graphene-chitosan-composite. Polymers 2015, 7, 527–551. [Google Scholar] [CrossRef] [Green Version]
- Fan, L.; Luo, C.; Li, X.; Lu, F.; Qiu, H.; Sun, M. Fabrication of novel magnetic chitosan grafted with graphene oxide to enhance adsorption properties for methyl blue. J. Hazard. Mater. 2012, 215, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Geng, L.; Lin, Y.; Chen, S.; Shi, S.; Cai, Y.; Li, L.; Peng, X. Superior strength and toughness of graphene/chitosan fibers reinforced by interfacial complexation. Compos. Sci. Technol. 2020, 194, 108174. [Google Scholar] [CrossRef]
- Krystyjan, M.; Khachatryan, G.; Grabacka, M.; Krzan, M.; Witczak, M.; Grzyb, J.; Woszczak, L. Physicochemical, Bacteriostatic, and Biological Properties of Starch/Chitosan Polymer Composites Modified by Graphene Oxide, Designed as New Bionanomaterials. Polymers 2021, 13, 2327. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.; Chang, X.; Zhang, Y.; Huang, Y.; Yao, Y.; Guo, Z. Graphene oxide cross-linked chitosan nanocomposite membrane. Appl. Surf. Sci. 2013, 280, 989–992. [Google Scholar] [CrossRef]
- Lee, J.H.; Marroquin, J.; Rhee, K.Y.; Park, S.J.; Hui, D. Cryomilling application of graphene to improve material properties of graphene/chitosan nanocomposites. Compos. Part B Eng. 2013, 45, 682–687. [Google Scholar] [CrossRef]
- Yang, X.; Tu, Y.; Li, L.; Shang, S.; Tao, X.M. Well-dispersed chitosan/graphene oxide nanocomposites. ACS Appl. Mater. Interfaces 2010, 2, 1707–1713. [Google Scholar] [CrossRef]
- Delbecq, F. Supramolecular gels from lipopeptide gelators: Template improvement and strategies for the in-situ preparation of inorganic nanomaterials and for the dispersion of carbon nanomaterials. Adv. Colloid Interface Sci. 2014, 209, 98–108. [Google Scholar] [CrossRef]
- Hejjaji, E.M.; Smith, A.M.; Morris, G.A. Designing chitosan-tripolyphosphate microparticles with desired size for specific pharmaceutical or forensic applications. Int. J. Biol. Macromol. 2017, 95, 564–573. [Google Scholar] [CrossRef]
- Terzopoulou, Z.; Kyzas, G.Z.; Bikiaris, D.N. Recent advances in nanocomposite materials of graphene derivatives with polysaccharides. Materials 2015, 8, 652–683. [Google Scholar] [CrossRef] [Green Version]
- Layek, R.K.; Samanta, S.; Nandi, A.K. Graphene sulphonic acid/chitosan nano biocomposites with tunable mechanical and conductivity properties. Polymer 2012, 53, 2265–2273. [Google Scholar] [CrossRef]
- Han, D.; Yan, L. Supramolecular hydrogel of chitosan in the presence of graphene oxide nanosheets as 2D cross-linkers. ACS Sustain. Chem. Eng. 2014, 2, 296–300. [Google Scholar] [CrossRef]
- Suri, A.; Khandegar, V.; Kaur, P.J. Ofloxacin exclusion using novel HRP immobilized chitosan cross-link with graphene-oxide nanocomposite. Groundw. Sustain. Dev. 2021, 12, 100515. [Google Scholar] [CrossRef]
- Khan, Y.H.; Islam, A.; Sarwar, A.; Gull, N.; Khan, S.M.; Munawar, M.A.; Zia, S.; Sabir, A.; Shafiq, M.; Jamil, T. Novel green nano composites films fabricated by indigenously synthesized graphene oxide and chitosan. Carbohydr. Polym. 2016, 146, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.P.; Zawawi, R.M.; ZA, N.H. Effect of sonication time and heat treatment on the structural and physical properties of chitosan/graphene oxide nanocomposite films. Food Packag. Shelf Life 2021, 28, 100663. [Google Scholar]
- Cobos, M.; González, B.; Fernández, M.J.; Fernández, M.D. Study on the effect of graphene and glycerol plasticizer on the properties of chitosan-graphene nanocomposites via in situ green chemical reduction of graphene oxide. Int. J. Biol. Macromol. 2018, 114, 599–613. [Google Scholar] [CrossRef]
- Kumar, S.; Wani, M.Y.; Koh, J.; Gil, J.M.; Sobral, A.J. Carbon dioxide adsorption and cycloaddition reaction of epoxides using chitosan–graphene oxide nanocomposite as a catalyst. J. Environ. Sci. 2018, 69, 77–84. [Google Scholar] [CrossRef]
- Huang, J.; Jacobsen, J.; Larsen, S.W.; Genina, N.; Van de Weert, M.; Müllertz, A.; Nielsen, H.M.; Mu, H. Graphene oxide as a functional excipient in buccal films for delivery of clotrimazole: Effect of molecular interactions on drug release and antifungal activity in vitro. Int. J. Pharm. 2020, 589, 119811. [Google Scholar] [CrossRef]
- Zhang, Q.; Cheng, W.; Wu, D.; Yang, Y.; Feng, X.; Gao, C.; Meng, L.; Shen, X.; Zhang, Y.; Tang, X. An electrochemical method for determination of amaranth in drinks using functionalized graphene oxide/chitosan/ionic liquid nanocomposite supported nanoporous gold. Food Chem. 2022, 367, 130727. [Google Scholar] [CrossRef]
- da Silva, S.B.; Batista, G.L.; Santin, C.K. Chitosan for Sensors and Electrochemical Applications. In Chitin and Chitosan; Lambertus, A.M., van den, B., Boeriu, C.G., Eds.; Wiley: Hoboken, NJ, USA, 2019; Chapter 18; pp. 461–476. [Google Scholar]
- Vinodh, R.; Sasikumar, Y.; Kim, H.J.; Atchudan, R.; Yi, M. Chitin and Chitosan Based Biopolymer Derived Electrode Materials for Supercapacitor Applications: A Critical Review. J. Ind. Eng. Chem. 2021, in press. [Google Scholar] [CrossRef]
- Wang, C.; Esker, A.R. Nanocrystalline chitin thin films. Carbohydr. Polym. 2014, 102, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Lizundia, E.; Nguyen, T.D.; Winnick, R.J.; MacLachlan, M.J. Biomimetic photonic materials derived from chitin and chitosan. J. Mater. Chem. C 2021, 9, 796–817. [Google Scholar] [CrossRef]
- Kim, S.J.; Hong, B.M.; Park, W.H. The effects of chitin/chitosan nanowhiskers on the thermal, mechanical and dye adsorption properties of electrospun PVA nanofibrous membranes. Cellulose 2020, 27, 5771–5783. [Google Scholar] [CrossRef]
- Saleh, T.A.; Parthasarathy, P.; Irfan, M. Advanced functional polymer nanocomposites and their use in water ultra-purification. Trends Environ. Anal. Chem. 2019, 24, e00067. [Google Scholar] [CrossRef]
- Singh, R.P.; Singh, P.; Singh, K.R. Introduction to Composite Materials: Nanocomposites and Their Potential Applications. In Composite Materials; CRC Press: Boca Raton, FL, USA, 2021; pp. 1–28. [Google Scholar]
- Pagliaro, M.; Ciriminna, R.; Yusuf, M.; Eskandarinezhad, S.; Wani, I.A.; Ghahremani, M.; Nezhad, Z.R. Application of nanocellulose composites in the environmental engineering as a catalyst, flocculants, and energy storages: A review. J. Compos. Compd. 2021, 3, 114–128. [Google Scholar]
- Omran, A.A.B.; Mohammed, A.A.; Sapuan, S.M.; Ilyas, R.A.; Asyraf, M.R.M.; Rahimian Koloor, S.S.; Petrů, M. Micro- and nanocellulose in polymer composite materials: A review. Polymers 2021, 13, 231. [Google Scholar] [CrossRef] [PubMed]
- Jafarzadeh, S.; Nafchi, A.M.; Salehabadi, A.; Oladzad-Abbasabadi, N.; Jafari, S.M. Application of bio-nanocomposite films and edible coatings for extending the shelf life of fresh fruits and vegetables. Adv. Colloid Interface Sci. 2021, 291, 102405. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Cui, Y.; Barnes, M.; Satam, C.; Zhang, S.; Chowdhury, R.A.; Adumbumkulath, A.; Sahin, O.; Miller, C.; Sajadi, S.M.; et al. Multifunctional Bio-Nanocomposite Coatings for Perishable Fruits. Adv. Mater. 2020, 32, 1908291. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.D.; Doan, T.C.D.; Huynh, T.M.; Nguyen, V.N.P.; Dinh, H.H.; Dang, D.M.T.; Dang, C.M. An electrochemical sensor based on polyvinyl alcohol/chitosan-thermally reduced graphene composite modified glassy carbon electrode for sensitive voltammetric detection of lead. Sens. Actuators B Chem. 2021, 345, 130443. [Google Scholar] [CrossRef]
- Xie, P.; Yuan, W.; Liu, X.; Peng, Y.; Yin, Y.; Li, Y.; Wu, Z. Advanced carbon nanomaterials for state-of-the-art flexible supercapacitors. Energy Storage Mater. 2020, 36, 56–76. [Google Scholar] [CrossRef]
- Li, W.; Song, B.; Zhang, S.; Zhang, F.; Liu, C.; Zhang, N.; Yao, H.; Shi, Y. Using 3-Isocyanatopropyltrimethoxysilane to decorate graphene oxide with nano-titanium dioxide for enhancing the anti-corrosion properties of epoxy coating. Polymers 2020, 12, 837. [Google Scholar] [CrossRef] [Green Version]
- Veeralingam, S.; Badhulika, S. 2D-SnSe2 nanoflakes on paper with 1D-NiO gate insulator based MISFET as multifunctional NIR photo switch and flexible temperature sensor. Mater. Sci. Semicond. Process. 2020, 105, 104738. [Google Scholar] [CrossRef]
- Park, S.G.; Lee, K.H.; Lee, J.H.; Bang, G.; Kim, J.; Park, H.J.; Oh, M.S.; Lee, S.; Kim, Y.H.; Kim, Y.M.; et al. Improved polaronic transport under a strong Mott–Hubbard interaction in Cu-substituted NiO. Inorg. Chem. Front. 2020, 7, 853–858. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajjadi, M.; Iravani, S.; Varma, R.S. Starch, cellulose, pectin, gum, alginate, chitin and chitosan derived (nano) materials for sustainable water treatment: A review. Carbohydr. Polym. 2020, 251, 116986. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Zhang, C.; Zhao, G.; Stoll, S.; Ren, F.; Leng, X. Antioxidant capacities of the selenium nanoparticles stabilized by chitosan. J. Nanobiotechnology 2017, 15, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magerusan, L.; Pogacean, F.; Coros, M.; Socaci, C.; Pruneanu, S.; Leostean, C.; Pana, I.O. Green methodology for the preparation of chitosan/graphene nanomaterial through electrochemical exfoliation and its applicability in Sunset Yellow detection. Electrochim. Acta 2018, 283, 578–589. [Google Scholar] [CrossRef]
- Kamran, U.; Park, S.J. Tuning ratios of KOH and NaOH on acetic acid-mediated chitosan-based porous carbons for improving their textural features and CO2 uptakes. J. CO2 Util. 2020, 40, 101212. [Google Scholar] [CrossRef]
- Al-Mokaram, A.; Amir, M.A.; Yahya, R.; Abdi, M.M.; Mahmud, H.N.M.E. The development of non-enzymatic glucose biosensors based on electrochemically prepared polypyrrole–chitosan–titanium dioxide nanocomposite films. Nanomaterials 2017, 7, 129. [Google Scholar] [CrossRef] [Green Version]
- Gupta, R.; Goddard, N.J. A study of diffraction-based chitosan leaky waveguide (LW) biosensors. Analyst 2021, 146, 4964–4971. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.; Zhu, Z.; Song, R.; Li, Z.; Chen, C. An ion-imprinted sensor based on chitosan-graphene oxide composite polymer modified glassy carbon electrode for environmental sensing application. Electrochim. Acta 2019, 317, 93–101. [Google Scholar] [CrossRef]
- Fartas, F.M.; Abdullah, J.; Yusof, N.A.; Sulaiman, Y.; Saiman, M.I. Biosensor based on tyrosinase immobilized on graphene-decorated gold nanoparticle/chitosan for phenolic detection in aqueous. Sensors 2017, 17, 1132. [Google Scholar] [CrossRef] [Green Version]
- Casteleijn, M.G.; Richardson, D.; Parkkila, P.; Granqvist, N.; Urtti, A.; Viitala, T. Spin coated chitin films for biosensors and its analysis are dependent on chitin-surface interactions. Colloids Surf. A Physicochem. Eng. Asp. 2018, 539, 261–272. [Google Scholar] [CrossRef]
- Saleviter, S.; Fen, Y.W.; Omar, N.A.S.; Zainudin, A.A.; Daniyal, W.M.E.M.M. Optical and structural characterization of immobilized 4-(2-pyridylazo) resorcinol in chitosan-graphene oxide composite thin film and its potential for Co2+ sensing using surface plasmon resonance technique. Results Phys. 2018, 11, 118–122. [Google Scholar] [CrossRef]
- Ali, F.I.; Mahmoud, S.T.; Awwad, F.; Greish, Y.E.; Abu-Hani, A.F. Low power consumption and fast response H2S gas sensor based on a chitosan-CuO hybrid nanocomposite thin film. Carbohydr. Polym. 2020, 236, 116064. [Google Scholar] [CrossRef]
- Borgohain, R.; Boruah, P.K.; Baruah, S. Heavy-metal ion sensor using chitosan capped ZnS quantum dots. Sens. Actuators B Chem. 2016, 226, 534–539. [Google Scholar] [CrossRef]
- Conder, J.; Vaulot, C.; Marino, C.; Villevieille, C.; Ghimbeu, C.M. Chitin and chitosan—Structurally related precursors of dissimilar hard carbons for Na-ion battery. ACS Appl. Energy Mater. 2019, 2, 4841–4852. [Google Scholar] [CrossRef]
- Chai, L.; Qu, Q.; Zhang, L.; Shen, M.; Zhang, L.; Zheng, H. Chitosan, a new and environmental benign electrode binder for use with graphite anode in lithium-ion batteries. Electrochim. Acta 2013, 105, 378–383. [Google Scholar] [CrossRef]
- Ma, L.; Zhou, X.; Xu, L.; Xu, X.; Zhang, L.; Chen, W. Chitosan-assisted fabrication of ultrathin MoS2/graphene heterostructures for Li-ion battery with excellent electrochemical performance. Electrochim. Acta 2015, 167, 39–47. [Google Scholar] [CrossRef]
- Chen, D.; Zhan, W.; Fu, X.; Zhu, M.; Lan, J.; Sui, G.; Yang, X. High-conductivity 1T-MoS 2 catalysts anchored on a carbon fiber cloth for high-performance lithium–sulfur batteries. Mater. Chem. Front. 2021, 5, 6941–6950. [Google Scholar] [CrossRef]
- Kim, S.; Cho, M.; Lee, Y. Multifunctional chitosan–RGO network binder for enhancing the cycle stability of Li–S batteries. Adv. Funct. Mater. 2020, 30, 1907680. [Google Scholar] [CrossRef]
- Jin, H.; Zhu, M.; Liu, J.; Gan, L.; Gong, Z.; Long, M. Alkaline chitosan solution as etching phase to design Si@ SiO2@ N-Carbon anode for Lithium-ion battery. Appl. Surf. Sci. 2021, 541, 148436. [Google Scholar] [CrossRef]
- Wang, D.; Du, G.; Han, D.; Su, Q.; Ding, S.; Zhang, M.; Zhao, W.; Xu, B. Porous flexible nitrogen-rich carbon membranes derived from chitosan as free-standing anodes for potassium-ion and sodium-ion batteries. Carbon 2021, 181, 1–8. [Google Scholar] [CrossRef]
- Sim, G.S.; Santhoshkumar, P.; Park, J.W.; Ho, C.W.; Shaji, N.; Kim, H.K.; Nanthagopal, M.; Lee, C.W. Chitosan-derived nitrogen-doped carbon on Li2ZnTi3O8/TiO2 composite as an anode material for lithium-ion batteries. Ceram. Int. 2021, in press. [Google Scholar] [CrossRef]
- Liu, C.; Li, R.; Liu, W.; Shen, G.; Chen, D. Chitosan-Assisted Fabrication of a Network C@ V2O5 Cathode for High-Performance Zn-Ion Batteries. ACS Appl. Mater. Interfaces 2021, 13, 37194–37200. [Google Scholar] [CrossRef]
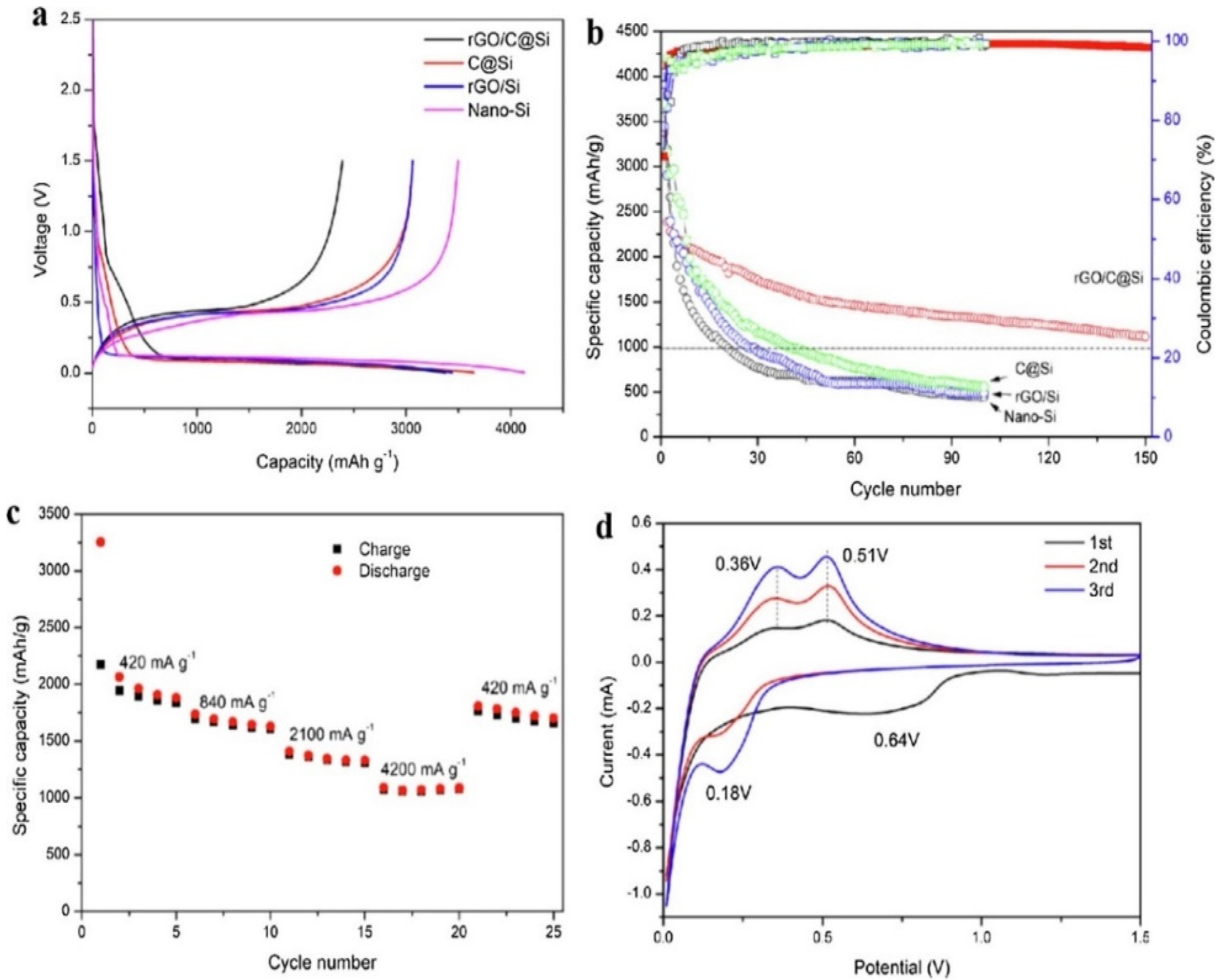
- Yu, L.; Liu, J.; He, S.; Huang, C.; Gong, Z.; Gan, L.; Long, M. N-doped rGO/C@ Si composites using sustainable chitosan as the carbon source for lithium-ion batteries. Appl. Surf. Sci. 2020, 501, 144136. [Google Scholar] [CrossRef]
- Yang, Z.; Jia, D.; Wu, Y.; Song, D.; Sun, X.; Wang, C.; Yang, L.; Zhang, Y.; Gao, J.; Ohsaka, T.; et al. Novel Lithium-Chalcogenide Batteries Combining S, Se and C Characteristics Supported by Chitosan-Derived Carbon Intertwined with CNTs. Chem. Eng. J. 2021, 427, 131790. [Google Scholar] [CrossRef]
- Nasirinezhad, M.; Ghaffarian, S.R.; Tohidian, M. Eco-friendly polyelectrolyte nanocomposite membranes based on chitosan and sulfonated chitin nanowhiskers for fuel cell applications. Iran. Polym. J. 2021, 30, 355–367. [Google Scholar] [CrossRef]
- Li, S.W.; He, H.; Zeng, R.J.; Sheng, G.P. Chitin degradation and electricity generation by Aeromonas hydrophila in microbial fuel cells. Chemosphere 2017, 168, 293–299. [Google Scholar] [CrossRef]
- Yang, W.; Wang, X.; Rossi, R.; Logan, B.E. Low-cost Fe–N–C catalyst derived from Fe (III)-chitosan hydrogel to enhance power production in microbial fuel cells. Chem. Eng. J. 2020, 380, 122522. [Google Scholar] [CrossRef]
- Abu-Saied, M.A.; Soliman, E.A.; Al Desouki, E.A. Development of Proton Exchange Membranes Based on Chitosan Blended with Poly (2-Acrylamido-2-Methylpropane Sulfonic Acid) for Fuel Cells applications. Mater. Today Commun. 2020, 25, 101536. [Google Scholar] [CrossRef]
- ul Haque, S.; Nasar, A.; Rahman, M.M. Applications of chitosan (CHI)-reduced graphene oxide (rGO)-polyaniline (PAni) conducting composite electrode for energy generation in glucose biofuel cell. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Gorgieva, S.; Osmić, A.; Hribernik, S.; Božič, M.; Svete, J.; Hacker, V.; Wolf, S.; Genorio, B. Efficient Chitosan/Nitrogen-Doped Reduced Graphene Oxide Composite Membranes for Direct Alkaline Ethanol Fuel Cells. Int. J. Mol. Sci. 2021, 22, 1740. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, V.; Mohebbi-Kalhori, D.; Samimi, A. Start-up investigation of the self-assembled chitosan/montmorillonite nanocomposite over the ceramic support as a low-cost membrane for microbial fuel cell application. Int. J. Hydrog. Energy 2020, 45, 4804–4820. [Google Scholar] [CrossRef]
- Zhang, G.; Li, L.; Chen, M.; Yang, F. Chitosan cross-linked poly (aminoanthraquinone)/Prussian blue ternary nitrogen precursor-derived Fe–N–C oxygen reduction catalysts for microbial fuel cells and zinc–air batteries. J. Mater. Chem. A 2020, 8, 9256–9267. [Google Scholar] [CrossRef]
- Vijayalekshmi, V.; Khastgir, D. Eco-friendly methanesulfonic acid and sodium salt of dodecylbenzene sulfonic acid doped cross-linked chitosan based green polymer electrolyte membranes for fuel cell applications. J. Membr. Sci. 2017, 523, 45–59. [Google Scholar] [CrossRef]
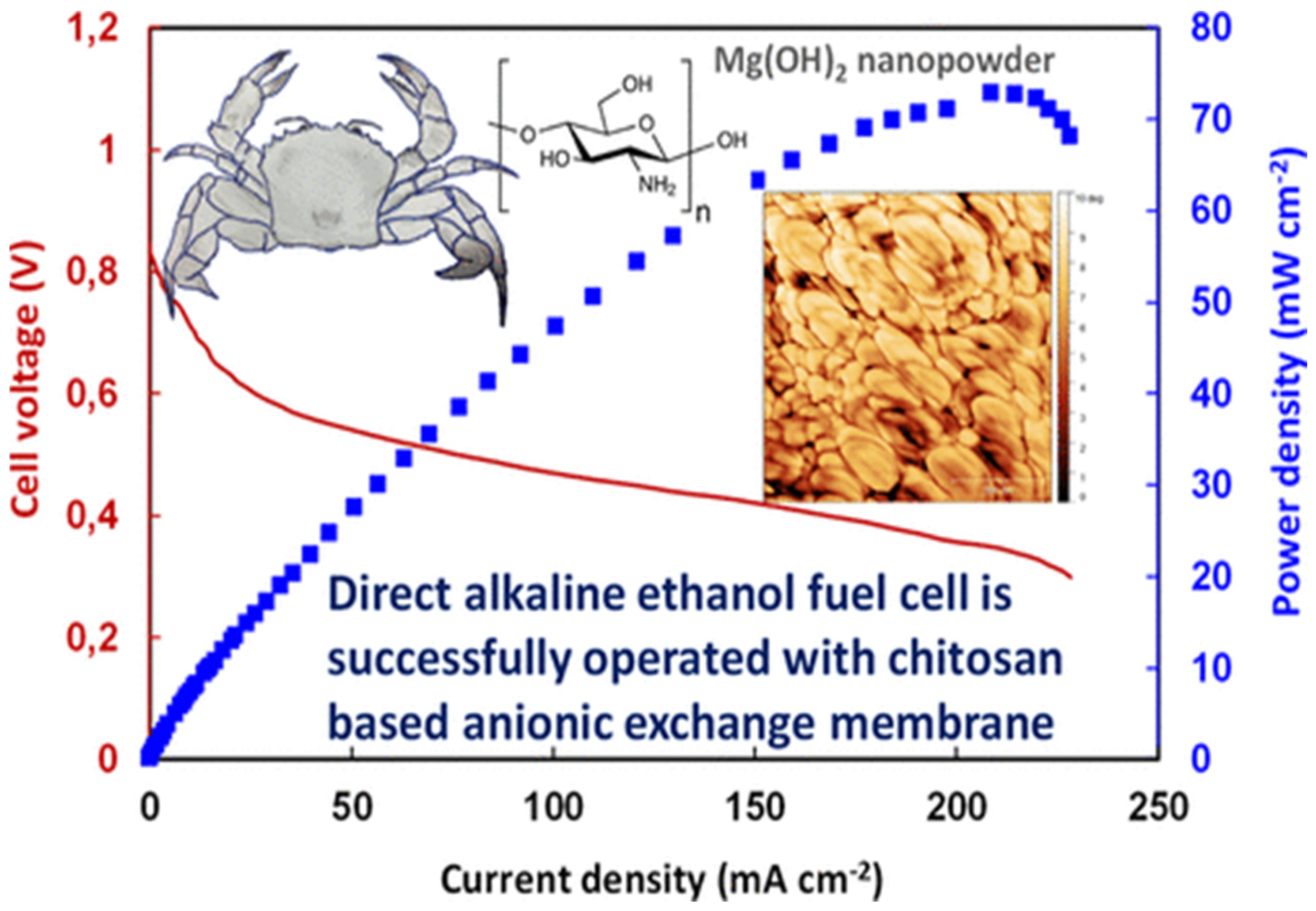
- Kaker, B.; Hribernik, S.; Mohan, T.; Kargl, R.; Stana Kleinschek, K.; Pavlica, E.; Kreta, A.; Bratina, G.; Lue, S.J.; Božič, M. Novel Chitosan–Mg (OH)2-Based Nanocomposite Membranes for Direct Alkaline Ethanol Fuel Cells. ACS Sustain. Chem. Eng. 2019, 7, 19356–19368. [Google Scholar] [CrossRef] [Green Version]
- Tohidian, M.; Ghaffarian, S.R. Polyelectrolyte nanocomposite membranes based on chitosan and surface modified multi-walled carbon nanotubes for use in fuel cell applications. J. Macromol. Sci. 2021, Part A, 1–14. [Google Scholar]
- Zhao, S.; Yang, Y.; Zhong, F.; Niu, W.; Liu, Y.; Zheng, G.; Liu, H.; Wang, J.; Xiao, Z. Fabrication of composite polymer electrolyte membrane using acidic metal-organic frameworks-functionalized halloysite nanotubes modified chitosan. Polymer 2021, 226, 123800. [Google Scholar] [CrossRef]
- Roy, B.K.; Tahmid, I.; Rashid, T.U. Chitosan-Based Materials for Supercapacitor Application—A Review. J. Mater. Chem. A 2021, 9, 17592–17642. [Google Scholar] [CrossRef]
- Cheng, J.; Xu, Q.; Wang, X.; Li, Z.; Wu, F.; Shao, J.; Xie, H. Ultrahigh-surface-area nitrogen-doped hierarchically porous carbon materials derived from chitosan and betaine hydrochloride sustainable precursors for high-performance supercapacitors. Sustain. Energy Fuels 2019, 3, 1215–1224. [Google Scholar] [CrossRef]
- Suneetha, R.B.; Selvi, P.; Vedhi, C. Synthesis, structural and electrochemical characterization of Zn doped iron oxide/grapheneoxide/chitosan nanocomposite for supercapacitor application. Vacuum 2019, 164, 396–404. [Google Scholar] [CrossRef]
- Yuan, M.; Zhang, Y.; Niu, B.; Jiang, F.; Yang, X.; Li, M. Chitosan-derived hybrid porous carbon with the novel tangerine pith-like surface as supercapacitor electrode. J. Mater. Sci. 2019, 54, 14456–14468. [Google Scholar] [CrossRef]
- Chen, K.; Weng, S.; Lu, J.; Gu, J.; Chen, G.; Hu, O.; Jiang, X.; Hou, L. Facile synthesis of chitosan derived heteroatoms-doped hierarchical porous carbon for supercapacitors. Microporous Mesoporous Mater. 2021, 320, 111106. [Google Scholar] [CrossRef]
- Lin, C.H.; Wang, P.H.; Lee, W.N.; Li, W.C.; Wen, T.C. Chitosan with various degrees of carboxylation as hydrogel electrolyte for pseudo solid-state supercapacitors. J. Power Sources 2021, 494, 229736. [Google Scholar] [CrossRef]
- Zhang, K.; Xu, R.; Ge, W.; Qi, M.; Zhang, G.; Xu, Q.H.; Huang, F.; Cao, Y.; Wang, X. Electrostatically self-assembled chitosan derivatives working as efficient cathode interlayers for organic solar cells. Nano Energy 2017, 34, 164–171. [Google Scholar] [CrossRef]
- Ababneh, H.; Hameed, B.H. Chitosan-derived hydrothermally carbonized materials and its applications: A review of recent literature. Int. J. Biol. Macromol. 2021, 186, 314–327. [Google Scholar] [CrossRef]
- Praveen, E.; Peter, I.J.; Kumar, A.M.; Ramachandran, K.; Jayakumar, K. Boosting of power conversion efficiency of 2D ZnO nanostructures-based DSSC by the Lorentz force with chitosan polymer electrolyte. J. Inorg. Organomet. Polym. Mater. 2020, 30, 4927–4943. [Google Scholar] [CrossRef]
- Rahman, N.A.; Hanifah, S.A.; Mobarak, N.N.; Ahmad, A.; Ludin, N.A.; Bella, F.; Su’ait, M.S. Chitosan as a paradigm for biopolymer electrolytes in solid-state dye-sensitised solar cells. Polymer 2021, 230, 124092. [Google Scholar] [CrossRef]
- Pottathara, Y.B.; Reddy, H.; Tiyyagura, Z.A.; Thomas, S. Chitin and Chitosan Composites for Wearable Electronics and Energy Storage Devices. In Handbook of Chitin and Chitosan: Volume 3: Chitin-and Chitosan-Based Polymer Materials for Various Applications; Elsevier: Amsterdam, The Netherlands, 2020; Volume 73, p. 71. [Google Scholar]
- Midya, L.; Chettri, A.; Pal, S. Development of a novel nanocomposite using polypyrrole grafted chitosan-decorated CDs with improved photocatalytic activity under solar light illumination. ACS Sustain. Chem. Eng. 2019, 7, 9416–9421. [Google Scholar] [CrossRef]
- Zulkifli, A.M.; Said, N.I.A.M.; Bakr Aziz, S.; Dannoun, E.M.A.; Hisham, S.; Shah, S.; Abu Bakar, A.; Zainal, Z.H.; Tajuddin, H.A.; Mohammed Hadi, J.; et al. Characteristics of Dye-Sensitized Solar Cell Assembled from Modified Chitosan-Based Gel Polymer Electrolytes Incorporated with Potassium Iodide. Molecules 2020, 25, 4115. [Google Scholar] [CrossRef]
- Yahya, W.Z.N.; Hong, P.Z.; Mohd Zain, W.Z.Z.W.; Mohamed, N.M. Tripropyl Chitosan Iodide-Based Gel Polymer Electrolyte as Quasi Solid-State Dye Sensitized Solar Cells. In Materials Science Forum; Trans Tech Publications Ltd.: Bäch SZ, Switzerland, 2020; Volume 997, pp. 69–76. [Google Scholar]
- Abdulwahid, R.T.; Aziz, S.B.; Brza, M.A.; Kadir, M.F.Z.; Karim, W.O.; Hamsan, H.M.; Asnawi, A.S.; Abdullah, R.M.; Nofal, M.M.; Dannoun, E.M. Electrochemical performance of polymer blend electrolytes based on chitosan: Dextran: Impedance, dielectric properties, and energy storage study. J. Mater. Sci. Mater. Electron. 2021, 32, 1–17. [Google Scholar] [CrossRef]
- Ali, A.; Alzamly, A.; Greish, Y.E.; Bakiro, M.; Nguyen, H.L.; Mahmoud, S.T. A Highly Sensitive and Flexible Metal–Organic Framework Polymer-Based H2S Gas Sensor. ACS Omega 2021, 6, 17690–17697. [Google Scholar] [CrossRef]
- Nazari, F.; Ghoreishi, S.M.; Khoobi, A. Bio-based Fe3O4/chitosan nanocomposite sensor for response surface methodology and sensitive determination of gallic acid. Int. J. Biol. Macromol. 2020, 160, 456–469. [Google Scholar] [CrossRef]
- Hussein, M.S.; Fekry, A.M. Effect of fumed silica/chitosan/poly (vinylpyrrolidone) composite coating on the electrochemical corrosion resistance of Ti–6Al–4V alloy in artificial saliva solution. ACS Omega 2019, 4, 73–78. [Google Scholar] [CrossRef]
- Hwang, J.H.; Pathak, P.; Wang, X.; Rodriguez, K.L.; Park, J.; Cho, H.J.; Lee, W.H. A novel Fe-Chitosan-coated carbon electrode sensor for in situ As (III) detection in mining wastewater and soil leachate. Sens. Actuators B: Chem. 2019, 294, 89–97. [Google Scholar] [CrossRef]
- Khalaf, N.; Ahamad, T.; Naushad, M.; Al-Hokbany, N.; Al-Saeedi, S.I.; Almotairi, S.; Alshehri, S.M. Chitosan polymer complex derived nanocomposite (AgNPs/NSC) for electrochemical non-enzymatic glucose sensor. Int. J. Biol. Macromol. 2020, 146, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, H.; Lai, X.; Chen, Z.; Zeng, X. Conductive and superhydrophobic F-rGO@ CNTs/chitosan aerogel for piezoresistive pressure sensor. Chem. Eng. J. 2020, 386, 123998. [Google Scholar] [CrossRef]
- Dai, H.; Feng, N.; Li, J.; Zhang, J.; Li, W. Chemiresistive humidity sensor based on chitosan/zinc oxide/single-walled carbon nanotube composite film. Sens. Actuators B Chem. 2019, 283, 786–792. [Google Scholar] [CrossRef]
- Chen, T.W.; Chinnapaiyan, S.; Chen, S.M.; Ali, M.A.; Elshikh, M.S.; Mahmoud, A.H. Facile synthesis of copper ferrite nanoparticles with chitosan composite for high-performance electrochemical sensor. Ultrason. Sonochem. 2020, 63, 104902. [Google Scholar] [CrossRef]
- Sadani, K.; Nag, P.; Mukherji, S. LSPR based optical fiber sensor with chitosan capped gold nanoparticles on BSA for trace detection of Hg (II) in water, soil and food samples. Biosens. Bioelectron. 2019, 134, 90–96. [Google Scholar] [CrossRef]
- Qi, P.; Zhang, T.; Shao, J.; Yang, B.; Fei, T.; Wang, R. A QCM humidity sensor constructed by graphene quantum dots and chitosan composites. Sens. Actuators A Phys. 2019, 287, 93–101. [Google Scholar] [CrossRef]
- Chen, S.; Qiu, L.; Cheng, H.M. Carbon-based fibers for advanced electrochemical energy storage devices. Chem. Rev. 2020, 120, 2811–2878. [Google Scholar] [CrossRef]
- Jorge, F.E.; Pimenta, L.T.G.; Marques, M.D.F.V. Preparation and characterization of polyaniline nanocomposites with graphene oxide-zinc oxide hybrids in different morphologies. Mater. Sci. Eng. B 2021, 263, 114851. [Google Scholar] [CrossRef]
- Aswathy, N.R.; Palai, A.K.; Ramadoss, A.; Mohanty, S.; Nayak, S.K. Fabrication of cellulose acetate-chitosan based flexible 3D scaffold-like porous membrane for supercapacitor applications with PVA gel electrolyte. Cellulose 2020, 27, 3871–3887. [Google Scholar] [CrossRef]
- Zhong, S.; Kitta, M.; Xu, Q. Hierarchically Porous Carbons Derived from Metal–Organic Framework/Chitosan Composites for High-Performance Supercapacitors. Chem. Asian J. 2019, 14, 3583–3589. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Fan, Y.; Song, H.; Zhou, X.; Xiong, Y. Poly (ionic liquid)/carboxymethyl chitosan complex-derived nitrogen and sulfur codoped porous carbon for high-performance supercapacitors. Ionics 2019, 25, 4915–4924. [Google Scholar] [CrossRef]
- Choosang, J.; Khumngern, S.; Thavarungkul, P.; Kanatharana, P.; Numnuam, A. An ultrasensitive label-free electrochemical immunosensor based on 3D porous chitosan–graphene–ionic liquid–ferrocene nanocomposite cryogel decorated with gold nanoparticles for prostate-specific antigen. Talanta 2021, 224, 121787. [Google Scholar] [CrossRef]
- Hosseini, M.G.; Shahryari, E.; Yardani Sefidi, P. Polyaniline grafted chitosan/GO-CNT/Fe3O4 nanocomposite as a superior electrode material for supercapacitor application. J. Appl. Polym. Sci. 2021, e50976. [Google Scholar] [CrossRef]
- Gawad, S.A.; Nasr, A.; Fekry, A.M.; Filippov, L.O. Electrochemical and hydrogen evolution behaviour of a novel nano-cobalt/nano-chitosan composite coating on a surgical 316L stainless steel alloy as an implant. Int. J. Hydrog. Energy 2021, 46, 18233–18241. [Google Scholar] [CrossRef]
- Zhang, T.W.; Shen, B.; Yao, H.B.; Ma, T.; Lu, L.L.; Zhou, F.; Yu, S.H. Prawn shell derived chitin nanofiber membranes as advanced sustainable separators for Li/Na-ion batteries. Nano Lett. 2017, 17, 4894–4901. [Google Scholar] [CrossRef]
- Kim, J.K.; Kim, D.H.; Joo, S.H.; Choi, B.; Cha, A.; Kim, K.M.; Kwon, T.H.; Kwak, S.K.; Kang, S.J.; Jin, J. Hierarchical chitin fibers with aligned nanofibrillar architectures: A nonwoven-mat separator for lithium metal batteries. ACS Nano 2017, 11, 6114–6121. [Google Scholar] [CrossRef] [PubMed]
- Divya, K.; Rana, D.; Rameesha, L.; Sri Abirami Saraswathi, M.S.; Nagendran, A. Highly selective custom-made chitosan based membranes with reduced fuel permeability for direct methanol fuel cells. J. Appl. Polym. Sci. 2021, 138, 51366. [Google Scholar] [CrossRef]
- Shen, S.; Jia, T.; Jia, J.; Wang, N.; Song, D.; Zhao, J.; Jin, J.; Che, Q. Constructing anhydrous proton exchange membranes through alternate depositing graphene oxide and chitosan on sulfonated poly (vinylidenefluoride) or sulfonated poly (vinylidene fluoride-co-hexafluoropropylene) membranes. Eur. Polym. J. 2021, 142, 110160. [Google Scholar] [CrossRef]
- Chadha, N.; Saini, P. Post synthesis foaming of graphene-oxide/chitosan aerogel for efficient microwave absorbers via regulation of multiple reflections. Mater. Res. Bull. 2021, 143, 111458. [Google Scholar] [CrossRef]
- Youssef, A.M.; Hasanin, M.S.; Abd El-Aziz, M.E.; Turky, G.M. Conducting chitosan/hydroxylethyl cellulose/polyaniline bionanocomposites hydrogel based on graphene oxide doped with Ag-NPs. Int. J. Biol. Macromol. 2021, 167, 1435–1444. [Google Scholar] [CrossRef] [PubMed]
- Aziz, S.B.; Nofal, M.M.; Abdulwahid, R.T.; Kadir, M.F.Z.; Hadi, J.M.; Hussien, M.M.; Kareem, W.O.; Dannoun, E.M.; Saeed, S.R. Impedance, FTIR and Transport Properties of Plasticized Proton Conducting Biopolymer Electrolyte Based on Chitosan for Electrochemical Device Application. Results Phys. 2021, 29, 104770. [Google Scholar] [CrossRef]



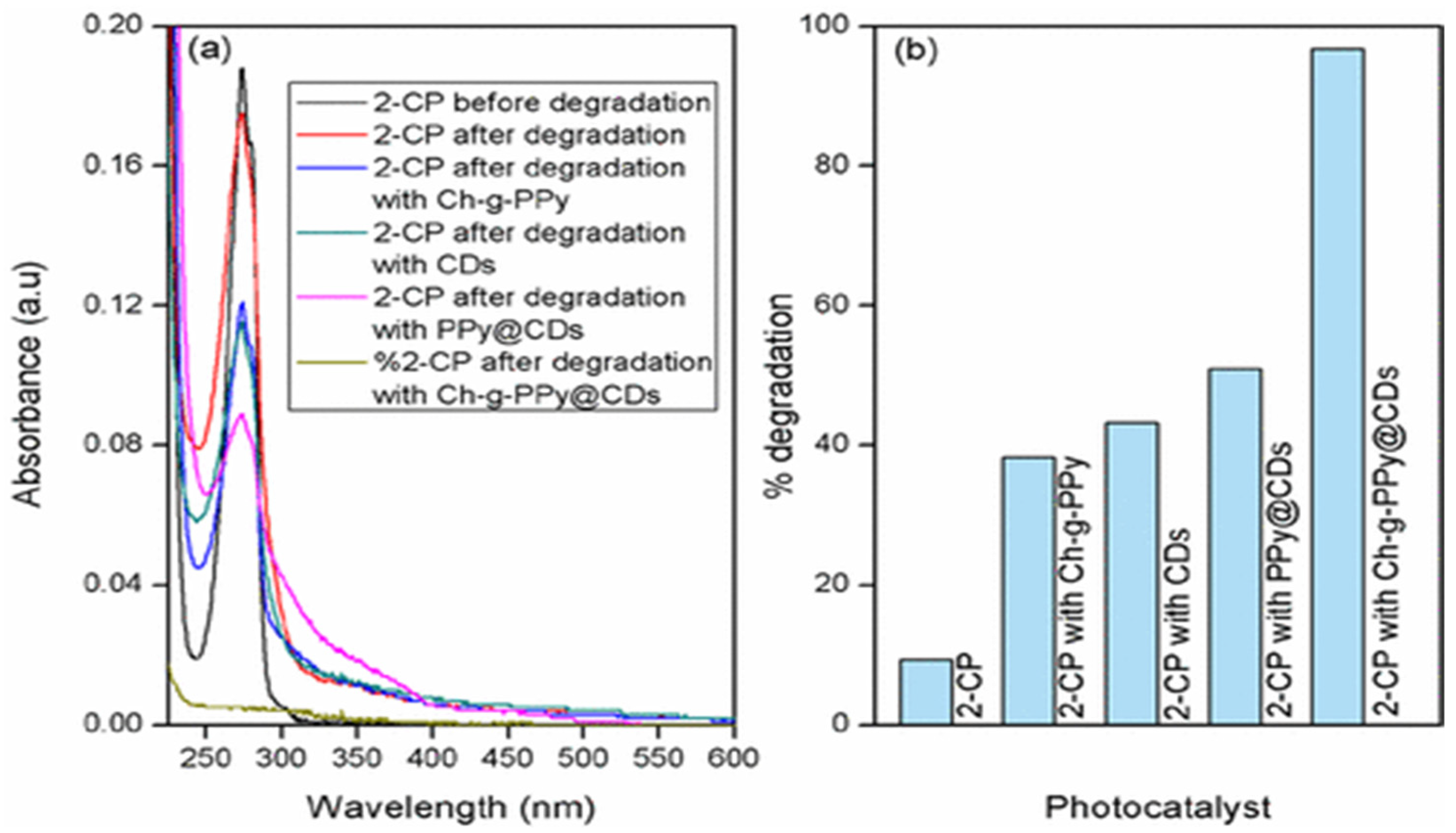
| Counterparts | Manufacturing Methods | Parameters and Conditions | Applications | Ref. |
|---|---|---|---|---|
| Graphene–Polymer Nanocomposites | ||||
| Three-dimensional graphene-based polymer nanocomposite | Three methods were used;
| - |
| [74] |
| Polyaniline/GO nanocomposite | Electrospinning technique |
|
| [75] |
| Polyaniline/GO nanocomposite | Chemical exfoliation |
|
| [76] |
| Hydrogels of conjugate polymer polypyrrole (PPy)/rGO composite | - |
|
| [77] |
| Polylactic acid (PLA)/GO nanocomposite | Solution blending and coagulation |
|
| [78] |
| Polyurethane–GO nanocomposite | - |
|
| [79] |
| Polyaniline nanofibers/functionalized rGO composite films | Hybrid suspension of GO and in situ polymerized polyaniline nanofibers were filtered, followed by hydrothermal treatment | - |
| [80] |
| Bacterial cellulose/graphene/polyaniline nanocomposite | Two-step strategy |
|
| [81] |
| Graphene/Activated Carbon Nanocomposites | ||||
| AG/PMB/GS/GCE | Ag nanocrystals were electrodeposited on different polymer dyes, poly (methylene blue) or poly (4-(2-Pyridylazo)-Resorcinol) (PAR)-modified graphene carbon spheres (GS) hybrids |
|
| [82] |
| rGO/activated carbon nanosheet composite | - |
|
| [83] |
| Glucose-treated rGO–activated carbon (rGO/AC) composites | Hydrothermal technique |
|
| [84] |
| Graphene/Metal Oxide Nanocomposites | ||||
| HBcAG/gold nanoparticles–rGO–enAu nanocomposite | - |
|
| [85] |
| Fe-doped SnO2/rGO nanocomposite | Fe-doped SnO2 was hybridized with different iron concentrations and rGOHydrothermal method | - |
| [86] |
| ZnO–graphene composite | Hydrothermal method |
| [87] | |
| TiO2/rGO nanocomposite | - |
|
| [88] |
| GO–Cu2O nanocomposite | - |
|
| [89] |
| 2D MnO2/rGO nanocomposite | Wet chemical method at low temperature |
|
| [90] |
| rGO/silver nanowires (AgNWs)/Ga-doped zinc oxide (GZO) composite thin films | - |
|
| [91] |
| rGO/CuO nanocomposite | Impregnation of microsized malachite spheres on GO sheets followed by calcination at 300–500 °C for 5 h |
|
| [92] |
| 3D NiO hollow sphere/rGO composite | Coordinating etching and precipitating process by using Cu2O nanosphere/GO composite as a template |
|
| [93] |
| Fe2O3/rGO composite | Hydrothermal method |
|
| [94] |
| Graphene/Metal Nanocomposites | ||||
| rGO/Co9S8 composites | - |
|
| [95] |
| Three-dimensional porous-laser-induced graphene–silver nanocomposite | - |
|
| [96] |
| Nitrogen-doped graphene–copper nanocomposite |
|
| [97] | |
| SH-β-CD-rGO/Cu nanospheres nanocomposite | Chemical deposition of Cu nanospheres on SH-β-CD-rGO |
|
| [98] |
| Composite Materials | Manufacturing Routes | Applications | Outcomes | Ref. |
|---|---|---|---|---|
| Semiconducting chitosan film | Casting method | H2S gas sensor |
| [147,187] |
| Fe3O4/chitosan | Chemical modification | Biosensor for gallic acid (GA) detection |
| [188] |
| Ti–6Al–4V alloy coated with fumed silica/chitosan/poly(vinylpyrrolidone) composite | Artificial saliva solution | Coating for electrochemical corrosion |
| [189] |
| Fe/chitosan-coated carbon electrode | Co-electrodeposition | Sensor for As(III) detection |
| [190] |
| Ag nanoparticles/chitosan-thiourea-formaldehyde | Polymeric metal complexation | Biosensor for non-enzymatic glucose detection |
| [191] |
| F-rGO @ CNTs/chitosan | Freeze-drying and dip-coating | Piezoresistive pressure sensor |
| [192] |
| Chitosan/zinc oxide/single-walled CNTs | Solution casting | Chemiresistive humidity sensor |
| [193] |
| Copper ferrite nanoparticles/chitosan | Ultra-sonication | High-performance electrochemical |
| [194] |
| Localized surface plasmon resonance (LSPR)-based optical fiber/chitosan-capped gold nanoparticles on BSA | Chemical modification | Optical fiber sensor for Hg(II) detection |
| [195] |
| Graphene QDs/chitosan | Ultrasound dispersion | Humidity sensor |
| [196] |
| Polypyrrole/chitin nanofibers/carbon nanotubes | Vacuum filtration with freeze-drying | Supercapacitors |
| [197] |
| Chitin/GO/zinc oxide/polyaniline | Co-polymerisation | Chitin-based polyaniline electrode for Cu(II) detection |
| [198] |
| Chitosan/cellulose acetate/PVA gel | Phase inversion and polymerisation | Supercapacitors |
| [199] |
| MOF-5/chitosan | Chemical modification | High-performance supercapacitors |
| [200] |
| Polyionic liquid/carboxymethyl chitosan | Direct carbonization | Supercapacitors |
| [201] |
| Chitosan/graphene/ionic liquid/ferrocene nanocomposite | Chemical modification and drop-coating | Electrochemical immunosensor |
| [202] |
| Polyaniline-grafted chitosan/GO-CNT/Fe3O4 nanocomposite | Solution mixing evaporation | Electrode material for supercapacitors |
| [203] |
| Nano-cobalt/chitosan composite coating | Implantation | Electrochemical and H2 evolution |
| [204] |
| Chitin from prawn shell/sodium dihydrogen citrate | Chemical extraction and drying in vacuum conditions | Batteries |
| [205] |
| Chitin fiber non-woven separator | Centrifugal jet spinning | Fuel cells |
| [206] |
| Sulfonated chitosan/GO | Casting | Direct methanol fuel cells |
| [207] |
| Chitosan/GO on membrane substrates of sulfonated poly(vinylidenefluoride) | Sulfonation and alternate dipping | High-temperature proton exchange membrane fuel cells |
| [208] |
| Chitosan/GO aerogel | Hydrothermal method | Microwave absorption |
| [209] |
| Chitosan/hydroxyl ethylcellulose/polyaniline loaded with GO doped by silver nanoparticles bio-nanocomposite as a hydrogel | Hydrothermal method | Efficient semiconductor material |
| [210] |
| Chitosan/ammonium thiocyanate | Solution-casting technique | Electric double-layer capacitor |
| [211] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ikram, R.; Mohamed Jan, B.; Abdul Qadir, M.; Sidek, A.; Stylianakis, M.M.; Kenanakis, G. Recent Advances in Chitin and Chitosan/Graphene-Based Bio-Nanocomposites for Energetic Applications. Polymers 2021, 13, 3266. https://doi.org/10.3390/polym13193266
Ikram R, Mohamed Jan B, Abdul Qadir M, Sidek A, Stylianakis MM, Kenanakis G. Recent Advances in Chitin and Chitosan/Graphene-Based Bio-Nanocomposites for Energetic Applications. Polymers. 2021; 13(19):3266. https://doi.org/10.3390/polym13193266
Chicago/Turabian StyleIkram, Rabia, Badrul Mohamed Jan, Muhammad Abdul Qadir, Akhmal Sidek, Minas M. Stylianakis, and George Kenanakis. 2021. "Recent Advances in Chitin and Chitosan/Graphene-Based Bio-Nanocomposites for Energetic Applications" Polymers 13, no. 19: 3266. https://doi.org/10.3390/polym13193266
APA StyleIkram, R., Mohamed Jan, B., Abdul Qadir, M., Sidek, A., Stylianakis, M. M., & Kenanakis, G. (2021). Recent Advances in Chitin and Chitosan/Graphene-Based Bio-Nanocomposites for Energetic Applications. Polymers, 13(19), 3266. https://doi.org/10.3390/polym13193266