Fiber Nanoarchitectonics for Pre-Treatments in Facile Detection of Short-Chain Fatty Acids in Waste Water and Faecal Samples
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
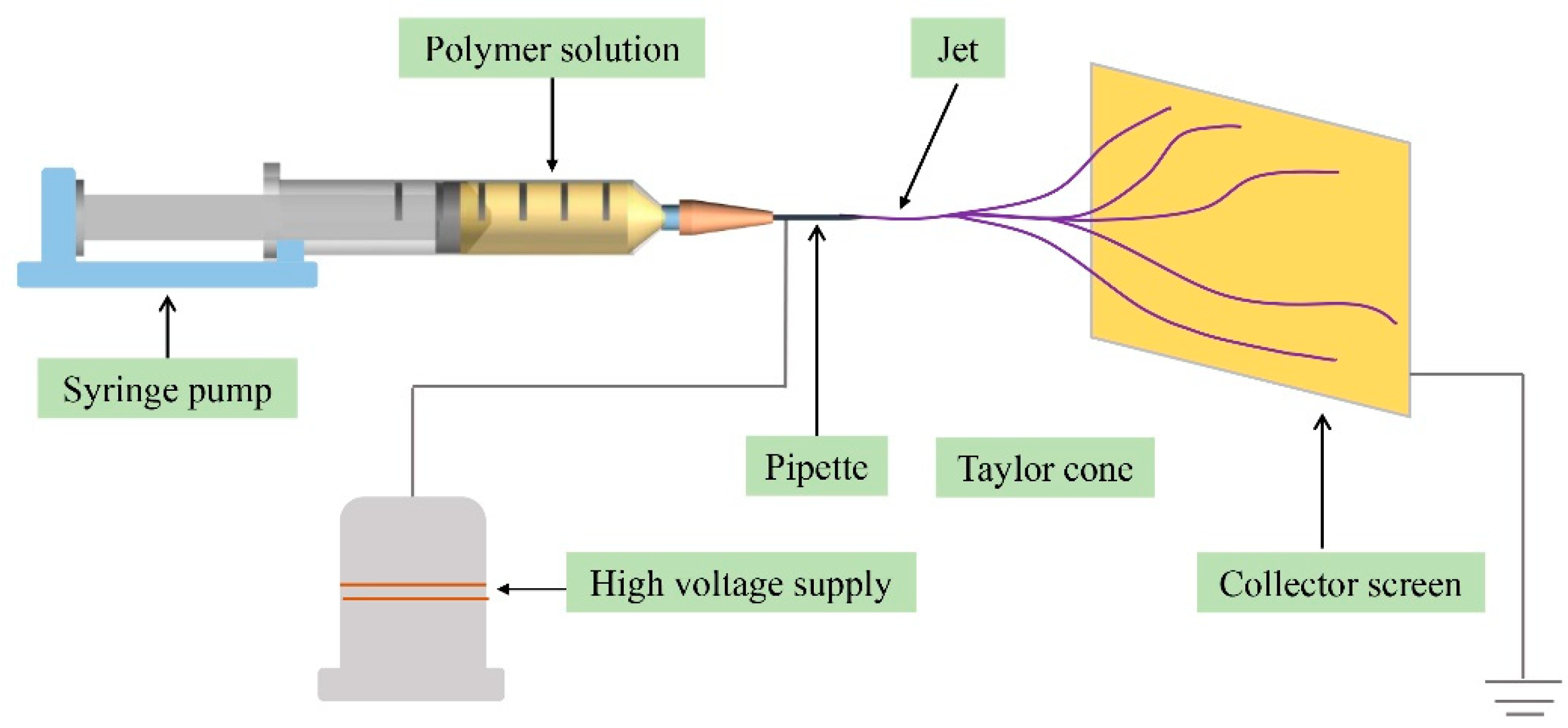
2.2. Apparatus
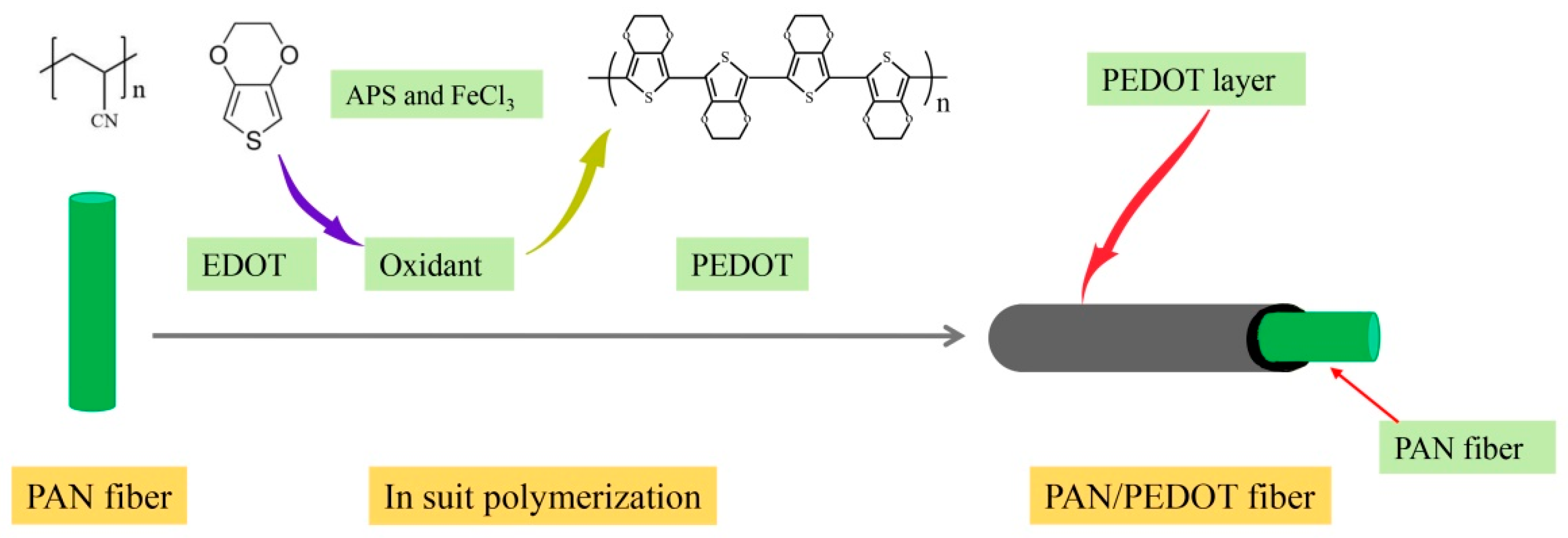
2.3. Fabrication of PAN/PEDOT and PS/PPY Nanofiber
2.4. Preparation of Standard Stock Solution and Samples
2.5. Experimental Conditions of GC–MS Analysis
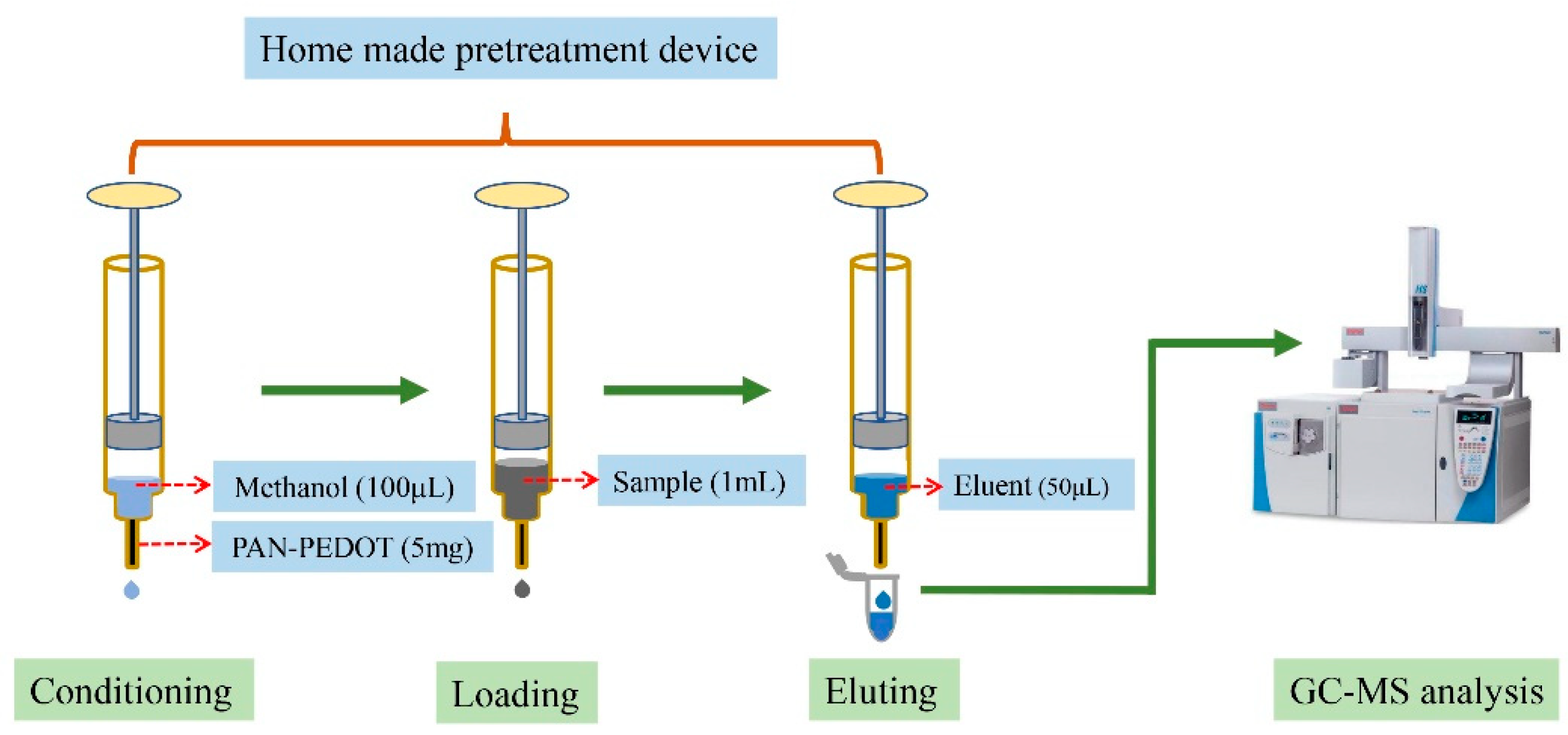
2.6. Method Validation and Application
3. Results
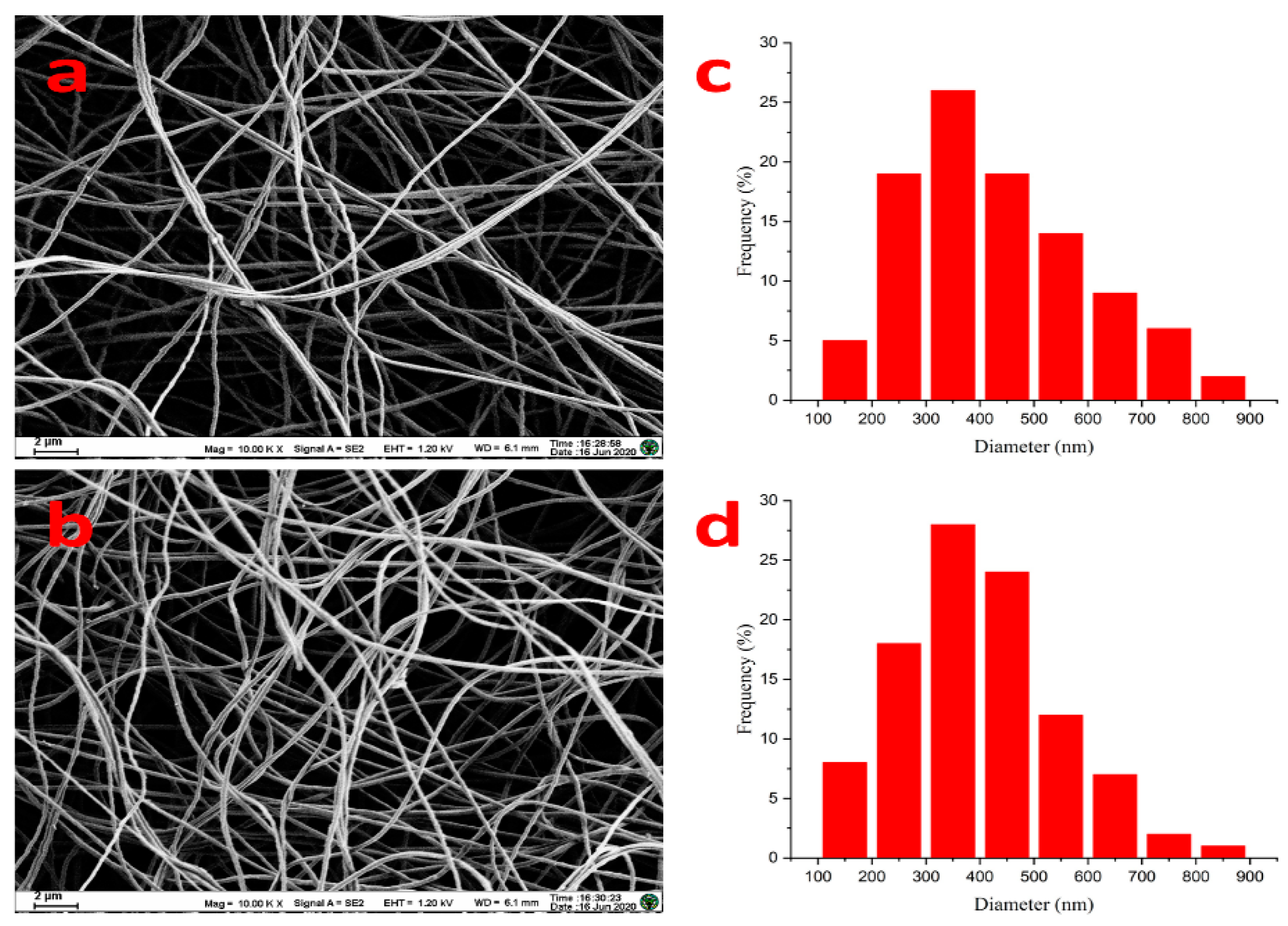
3.1. Characterization of the PAN/PEDOT Nanofiber
3.2. GC–MS Detection of SCFAs
3.3. Validation of the Method
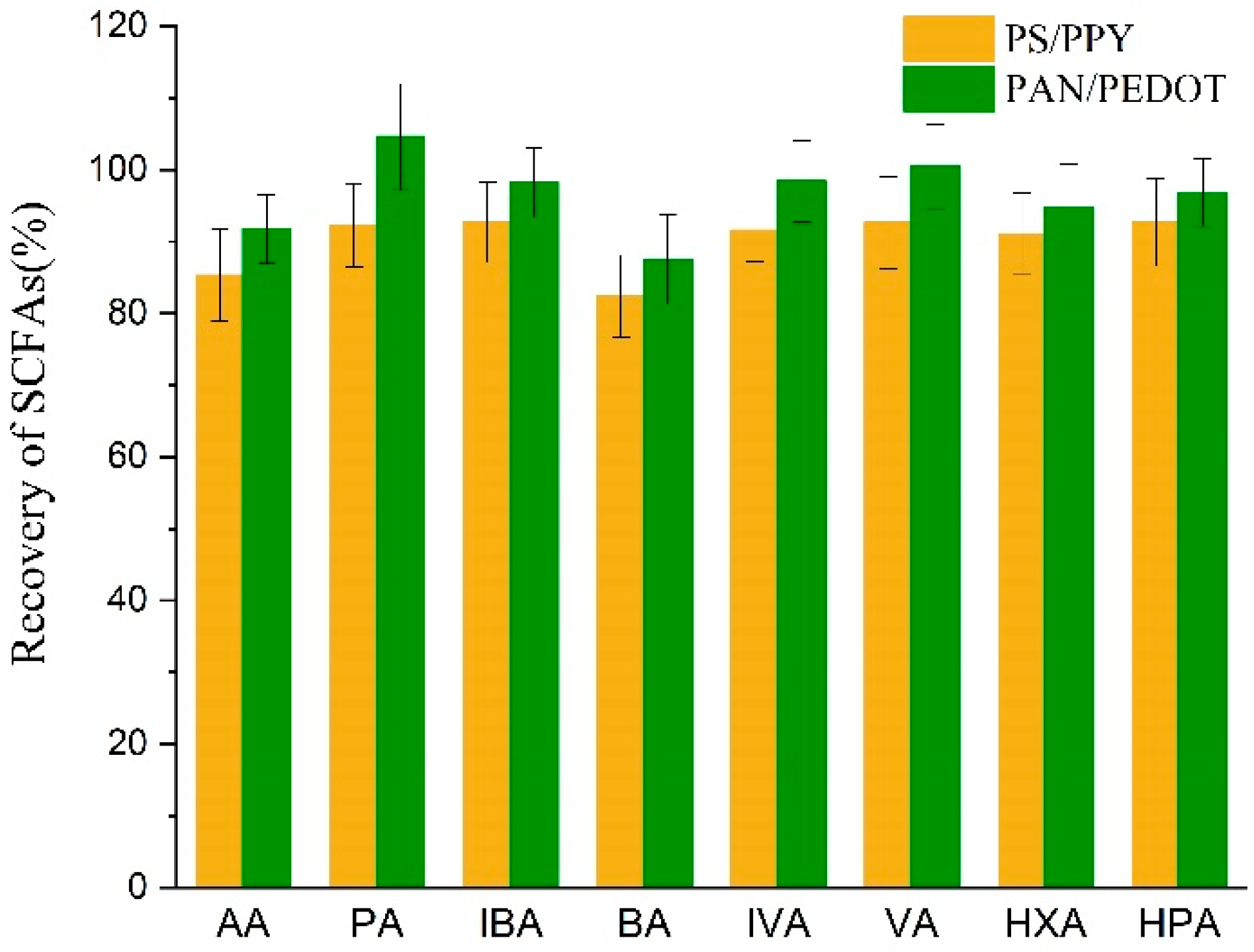
3.4. Comparison with Other Methods
3.5. Determination of SCFAs in Real Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tangerman, A.; Nagengast, F.M. A gas chromatographic analysis of fecal short-chain fatty acids, using the direct injection method. Anal. Biochem. 1996, 236, 1–8. [Google Scholar] [CrossRef]
- Dong, B.; Gao, P.; Zhang, D.; Chen, Y.G.; Dai, L.L.; Dai, X.H. A new process to improve short-chain fatty acids and bio-methane generation from waste activated sludge. J. Environ. Sci. 2016, 43, 159–168. [Google Scholar] [CrossRef]
- Elefsiniotis, P.; Wareham, D.G.; Smith, M.O. Use of volatile fatty acids from an acid-phase digester for denitrification. J. Biotechnol. 2004, 114, 289–297. [Google Scholar] [CrossRef]
- Horgan, C.J.; Coats, E.R.; Loge, F.J. Assessing the Effects of Solids Residence Time and Volatile Fatty Acid Augmentation on Biological Phosphorus Removal Using Real Wastewater. Water Environ. Res. 2010, 82, 216–226. [Google Scholar] [CrossRef]
- Dong, Y.; Qu, Y.P.; He, W.H.; Du, Y.; Liu, J.; Han, X.Y.; Feng, Y.J. A 90-liter stackable baffled microbial fuel cell for brewery wastewater treatment based on energy self-sufficient mode. Bioresour. Technol. 2015, 195, 66–72. [Google Scholar] [CrossRef]
- Luo, J.Y.; Feng, L.Y.; Chen, Y.G.; Sun, H.; Shen, Q.T.; Li, X.; Chen, H. Alkyl polyglucose enhancing propionic acid enriched short-chain fatty acids production during anaerobic treatment of waste activated sludge and mechanisms. Water Res. 2015, 73, 332–341. [Google Scholar] [CrossRef]
- Bielawska, K.; Dziakowska, I.; Roszkowska-Jakimiec, W. Chromatographic determination of fatty acids in biological material. Toxicol. Mech. Methods 2010, 20, 526–537. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, S.M.; Makarem, S.; Alexovič, M.; Tabani, H. Simultaneous separation and quantification of acidic and basic dye specimens via a dual gel electro-membrane extraction from real environmental samples. J. Iran Chem. Soc. 2021, 18, 2091–2099. [Google Scholar] [CrossRef]
- Moghaddam, A.Z.; Bameri, A.E.; Ganjali, M.R.; Alexovič, M.; Jazi, M.E.; Tabani, H. A low-voltage electro-membrane extraction for quantification of imatinib and sunitinib in biological fluids. Bioanalysis 2021, 13, 1401–1413. [Google Scholar] [CrossRef]
- Cuervo, A.; Salazar, N.; Ruas-Madiedo, P.; Gueimonde, M.; González, S. Fiber from a regular diet is directly associated with fecal short-chain fatty acid concentrations in the elderly. Nutr. Res. 2013, 33, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.F.; Pujos-Guillot, E.; Martin, J.F.; Galan, P.; Juste, C.; Jia, W.; Sebedio, J.L. Metabolite analysis of human fecal water by gas chromatography/mass spectrometry with ethyl chloroformate derivatization. Anal. Biochem. 2009, 393, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Douny, C.; Dufourny, S.; Brose, F.; Verachtert, P.; Rondia, P.; Lebrun, S.; Marzorati, M.; Everaert, N.; Ddecenserie, V.; Scippo, M.L. Development of an analytical method to detect short-chain fatty acids by SPME-GC–MS in samples coming from an in vitro gastrointestinal model. J. Chromatogr. B 2019, 1124, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.J.; Xie, J.W.; Liu, W.Y.; Xia, Y.N. Electrospun Nanofibers: New Concepts, Materials, and Applications. Acc. Chem. Res. 2017, 50, 1976–1987. [Google Scholar] [CrossRef]
- McCann, J.T.; Li, D.; Xia, Y.N. Electrospinning of nanofibers with core-sheath, hollow, or porous structures. J. Mater. Chem. 2005, 15, 735–738. [Google Scholar] [CrossRef]
- Greenfeld, I.; Sui, X.M.; Wagner, H.D. Stiffness, Strength, and Toughness of Electrospun Nanofibers: Effect of Flow-Induced Molecular Orientation. Macromolecules 2016, 49, 6518–6530. [Google Scholar] [CrossRef]
- Li, C.M.; Vepari, C.; Jin, H.J.; Kim, H.J.; Kaplan, D.L. Electrospun silk-BMP-2 scaffolds for bone tissue engineering. Biomaterials 2006, 27, 3115–3124. [Google Scholar] [CrossRef]
- Zainab, G.; Babar, A.A.; Iqbal, N.; Wang, X.F.; Yu, J.Y.; Ding, B. Amine-impregnated porous nanofiber membranes for CO2 capture. Compos. Commun. 2018, 10, 45–51. [Google Scholar] [CrossRef]
- Wu, D.P.; Han, D.W.; Stecki, A.J. Immunoassay on Free-Standing Electrospun Membranes. ACS Appl. Mater. Interfaces 2010, 2, 252–258. [Google Scholar] [CrossRef]
- Qi, D.J.; Kang, X.J.; Chen, L.Q.; Zhang, Y.Y.; Wei, H.M.; Gu, Z.Z. Electrospun polymer nanofibers as a solid-phase extraction sorbent for the determination of trace pollutants in environmental water. Anal. Bioanal. Chem. 2008, 390, 929–938. [Google Scholar] [CrossRef]
- He, X.M.; Zhu, G.T.; Yin, J.; Zhao, Q.; Yuan, B.F.; Feng, Y.Q. Electrospun polystyrene/oxidized carbon nanotubes film as both sorbent for thin film microextraction and matrix for matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. J. Chromatogr. A 2014, 1351, 29–36. [Google Scholar] [CrossRef]
- Kawashima, H.; Shinotsuka, M.; Nakano, M.; Goto, H. Fabrication of conductive paper coated with PEDOT: Preparation and characterization. J. Coat. Technol. Res. 2012, 9, 467–474. [Google Scholar] [CrossRef][Green Version]
- Kurra, N.; Hota, M.K.; Alshareef, H.N. Conducting polymer micro-supercapacitors for flexible energy storage and Ac line-filtering. Nano Energy 2015, 13, 500–508. [Google Scholar] [CrossRef]
- Lachman, N.; Xu, H.P.; Zhou, Y.; Ghaffari, M.; Lin, M.R.; Bhattacharyya, D.; Ugur, A.; Gleason, K.K.; Zhang, Q.M.; Wardle, B.L. Tailoring thickness of conformal conducting polymer decorated aligned carbon nanotube electrodes for energy storage. Adv. Mater. Interfaces 2014, 1, 1400076. [Google Scholar] [CrossRef]
- Wang, W.T.; Xu, G.W.; Cui, X.T.; Sheng, G.; Luo, X.L. Enhanced catalytic and dopamine sensing properties of electrochemically reduced conducting polymer nanocomposite doped with pure graphene oxide. Biosens. Bioelectron. 2014, 58, 153–156. [Google Scholar] [CrossRef]
- Zhang, S.M.; Cicoira, F. Water Enabled Healing of Conducting Polymer Films. Adv. Mater. 2017, 29, 1703098. [Google Scholar] [CrossRef]
- Richardson-Burns, S.M.; Hendricks, J.L.; Foster, B.; Povlich, L.K.; Kim, D.H.; Martin, D.C. Polymerization of the conducting polymer poly(3,4-ethylenedioxythiophene) (PEDOT) around living neural cells. Biomaterials 2007, 28, 1539–1552. [Google Scholar] [CrossRef]
- Abidian, M.R.; Corey, J.M.; Kipke, D.R.; Martin, D.C. Conducting polymer nanotubes improve electrical properties, mechanical adhesion, neural attachment, and neurite outgrowth of neural electrodes. Small 2010, 6, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hsieh, C.H.; Lai, C.W.; Chang, Y.F.; Chan, H.Y.; Tsai, C.F.; Ho, J.A.A.; Wu, L.C. Tyramine detection using PEDOT: PSS/AuNPs/1-methyl-4-mercaptopyridine modified screen-printed carbon electrode with molecularly imprinted polymer solid phase extraction. Biosens. Bioelectron. 2017, 87, 142–149. [Google Scholar] [CrossRef]
- Tian, T.; Zheng, S.L.; Ye, B.F.; Qu, B.; Zhao, Y.J.; Kang, X.J.; Gu, Z.Z. Poly-3, 4-ethylenedioxythiophene nanoclusters for high effective solid phase extraction. J. Chromatogr. A. 2013, 1275, 17–24. [Google Scholar] [CrossRef]
- Zhao, R.S.; Chu, L.L.; Wang, Y.; Song, Y.; Liu, P.; Li, C.; Huang, J.J.; Kang, X.J. Application of packed-fiber solid-phase extraction coupled with GC–MS for the determination of short-chain fatty acids in children’s urine. Clin. Chim. Acta 2017, 468, 120–125. [Google Scholar] [CrossRef]
- Mohammadi, A.; Ameli, A.; Alizadeh, N. Headspace solid-phase microextraction using a dodecylsulfate-doped polypyrrole film coupled to ion mobility spectrometry for the simultaneous determination of atrazine and ametryn in soil and water samples. Talanta 2009, 78, 1107–1114. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhong, M.; Chen, J. Electrodeposited polyaniline as a fiber coating for solid-phase microextraction of organochlorine pesticides from water. J. Sep. Sci. 2008, 31, 2839–2845. [Google Scholar] [CrossRef]
- Zhang, S.M.; Wang, H.B.; Zhu, M.J. A sensitive GC/MS detection method for analyzing microbial metabolites short chain fatty acids in fecal and serum samples. Talanta 2019, 196, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Banel, A.; Jakimska, A.; Wasielewska, M.; Wolska, L.; Zygmunt, B. Determination of SCFAs in water using GC-FID. Selection of the separation system. Anal. Chim. Acta 2012, 716, 24–27. [Google Scholar] [CrossRef] [PubMed]
- Scortichini, S.; Boarelli, M.C.; Silvi, S.; Fiorini, D. Development and Validation of a GC-FID Method for the Analysis of Short Chain Fatty Acids in Rat and Human Faeces and in Fermentation Fluids. J. Chromatogr. B 2020, 1143, 121972. [Google Scholar] [CrossRef]
- Ábalos, M.; Bayona, J.M.; Pawliszyn, J. Development of a headspace solid-phase microextraction procedure for the determination of free volatile fatty acids in waste waters. J. Chromatogr. A 2000, 873, 107–115. [Google Scholar] [CrossRef]
- Zhao, G.H.; Nyman, M.; Jönsson, J.A. Rapid determination of short-chain fatty acids in colonic contents and faeces of humans and rats by acidified water-extraction and direct-injection gas chromatography. Biomed. Chromatogr. 2006, 20, 674–682. [Google Scholar] [CrossRef] [PubMed]

| SCFAs | Retention Time | Quantitative Ion (m/z) |
|---|---|---|
| AA | 8.41 | 43.1, 60.1 |
| PA | 10.73 | 57.1,73.1 |
| IBA | 11.71 | 41.1, 43.1 |
| BA | 13.85 | 42.1, 60.1 |
| IVA | 15.31 | 60.1, 87.1 |
| VA | 17.77 | 60.1, 73.1 |
| HXA | 21.58 | 73.1, 87.1 |
| HPA | 25.24 | 60.1 |
| SCFAs | R2 | Linear Range (μmol/L) | LOD (μmol/L) | LOQ (μmol/L) | Intra-Day RSD (%, n = 5) | Intra-Day RSD (%, n = 5) | Recovery b (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.1 a | 1 a | 10 a | 0.1 a | 1 a | 10 a | ||||||
| AA | 0.9967 | 3.33–3331 | 0.87 | 2.87 | 9.7 | 8.6 | 7.9 | 10.2 | 9.7 | 7.1 | 91.7 |
| PA | 0.9980 | 2.70–1351 | 0.61 | 2.02 | 7.5 | 8.1 | 6.5 | 8.9 | 11.5 | 7.8 | 104.6 |
| IBA | 0.9969 | 2.27–1135 | 0.56 | 1.85 | 8.5 | 7.7 | 7.2 | 7.6 | 7.4 | 6.9 | 98.2 |
| BA | 0.9987 | 2.27–1135 | 0.49 | 1.62 | 6.9 | 10.1 | 9.7 | 6.9 | 13.1 | 13.4 | 87.5 |
| IVA | 0.9991 | 1.96–979 | 0.41 | 1.37 | 9.7 | 13.7 | 9.9 | 7.8 | 12.3 | 10.0 | 98.4 |
| VA | 0.9959 | 1.96–979 | 0.34 | 1.14 | 10.0 | 9.3 | 12.0 | 9.5 | 10.9 | 9.9 | 100.5 |
| HXA | 0.9974 | 1.72–861 | 0.38 | 1.26 | 7.4 | 8.7 | 9.2 | 7.8 | 8.9 | 7.2 | 94.7 |
| HPA | 0.9953 | 1.54–768 | 0.40 | 1.33 | 9.9 | 7.8 | 7.4 | 10.7 | 14.1 | 8.9 | 96.8 |
| Detection Method | Extraction or Derivatization | Pretreatment Time | Solvent Used | LOD (μmol/L) | LOQ (μmol/L) | Others | Ref. |
|---|---|---|---|---|---|---|---|
| GC–MS | Both were used | >100 min | 0.4 mL | 0.064–0.067 | 1.605–1.678 | Dehydration was adopted before derivatizatiom | [33] |
| GC–FID | None was used | Not provided | Not provided | 0.096–0.628 | 0.283–1.894 | Two columns were used together | [34] |
| GC–FID | Solvent extraction | >18 min | 3 mL | 0.04–0.64 | 0.14–2.12 | Extraction was repeated three times | [35] |
| GC–FID/MS | SPME | >20 min | 0 | 0.068–11.24 | 0.62–105.58 | [36] | |
| GC–FID | None was used | >33 min | 0 | 0.72–9.04 | 2.38–30.14 | [37] | |
| GC–MS | Ethanol/HCl extraction | <3 min | 0.2 mL | 0.34–0.87 | 1.14–2.87 | This work |
| Sample | AA | PA | IBA | BA | IVA | VA | HXA | HPA |
|---|---|---|---|---|---|---|---|---|
| Waste water 1# (mg/L) | 145.61 | 23.19 | 18.74 | 56.92 | 24.67 | 18.91 | 108.71 | 85.61 |
| Waste water 2# (mg/L) | 238.9 | 57.61 | 37.91 | 102.2 | 75.64 | 29.81 | 46.75 | 24.29 |
| Waste water 3# (mg/L) | 89.32 | 36.9 | 46.1 | 75.4 | 39.7 | 37.8 | 26.51 | 27.84 |
| Waste water 4# (mg/L) | 142.1 | 44.9 | 61.8 | 102.4 | 65.7 | 43.1 | 51.8 | 69.7 |
| Waste water 5# (mg/L) | 135.6 | 98.7 | 74.9 | 164.9 | 58.3 | 76.8 | 21.8 | 10.2 |
| Waste water 6# (mg/L) | 66.7 | 76.4 | 59.7 | 100.2 | 27.9 | 38.7 | 44.5 | 60.8 |
| Waste water 7# (mg/L) | 33.4 | 26.87 | 34.9 | 42.78 | 56.7 | 53.84 | 25.17 | 26.97 |
| Waste water 8# (mg/L) | 105.8 | 69.75 | 51.8 | 63 | 24.8 | 61.83 | 49.8 | 28.62 |
| Fecal 1# (mmol/kg) | 19.22 | 6.57 | 2.14 | 4.52 | 2.97 | 1.62 | 6.18 | 0.05 |
| Fecal 2# (mmol/kg) | 24.12 | 7.34 | 1.97 | 5.31 | 8.65 | 6.24 | 4.21 | 0.1 |
| Fecal 3# (mmol/kg) | 18.79 | 10.34 | 2.53 | 8.97 | 1.07 | 3.79 | 5.61 | 0.02 |
| Fecal 4# (mmol/kg) | 32.48 | 4.79 | 2.0 | 10.32 | 7.98 | 2.18 | 3.97 | 0.07 |
| Fecal 5# (mmol/kg) | 15.73 | 8.51 | 1.82 | 5.64 | 2.62 | 5.31 | 4.91 | 0.05 |
| Fecal 6# (mmol/kg) | 20.01 | 7.1 | 3.04 | 8.59 | 2.80 | 4.09 | 8.2 | 0.09 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, G.; Xie, L.; Xu, S.; Kang, X.; Ma, J. Fiber Nanoarchitectonics for Pre-Treatments in Facile Detection of Short-Chain Fatty Acids in Waste Water and Faecal Samples. Polymers 2021, 13, 3906. https://doi.org/10.3390/polym13223906
Deng G, Xie L, Xu S, Kang X, Ma J. Fiber Nanoarchitectonics for Pre-Treatments in Facile Detection of Short-Chain Fatty Acids in Waste Water and Faecal Samples. Polymers. 2021; 13(22):3906. https://doi.org/10.3390/polym13223906
Chicago/Turabian StyleDeng, Guozhe, Li Xie, Shengjia Xu, Xuejun Kang, and Jizheng Ma. 2021. "Fiber Nanoarchitectonics for Pre-Treatments in Facile Detection of Short-Chain Fatty Acids in Waste Water and Faecal Samples" Polymers 13, no. 22: 3906. https://doi.org/10.3390/polym13223906
APA StyleDeng, G., Xie, L., Xu, S., Kang, X., & Ma, J. (2021). Fiber Nanoarchitectonics for Pre-Treatments in Facile Detection of Short-Chain Fatty Acids in Waste Water and Faecal Samples. Polymers, 13(22), 3906. https://doi.org/10.3390/polym13223906







