A Review of the Effects of Collagen Treatment in Clinical Studies
Abstract
:1. Introduction
2. Source
3. Effects of Treatment
3.1. Skin Regeneration
3.2. Bone Defects
3.3. Sarcopenia
3.4. Wound Healing
3.5. Dental Therapy
3.6. Gastroesophageal Reflux
3.7. Osteoarthritis
3.8. Rheumatoid Arthritis
4. Discussion
4.1. Treatment of Comorbid Diseases and Preventing Complications
4.2. Collagen and COVID-19
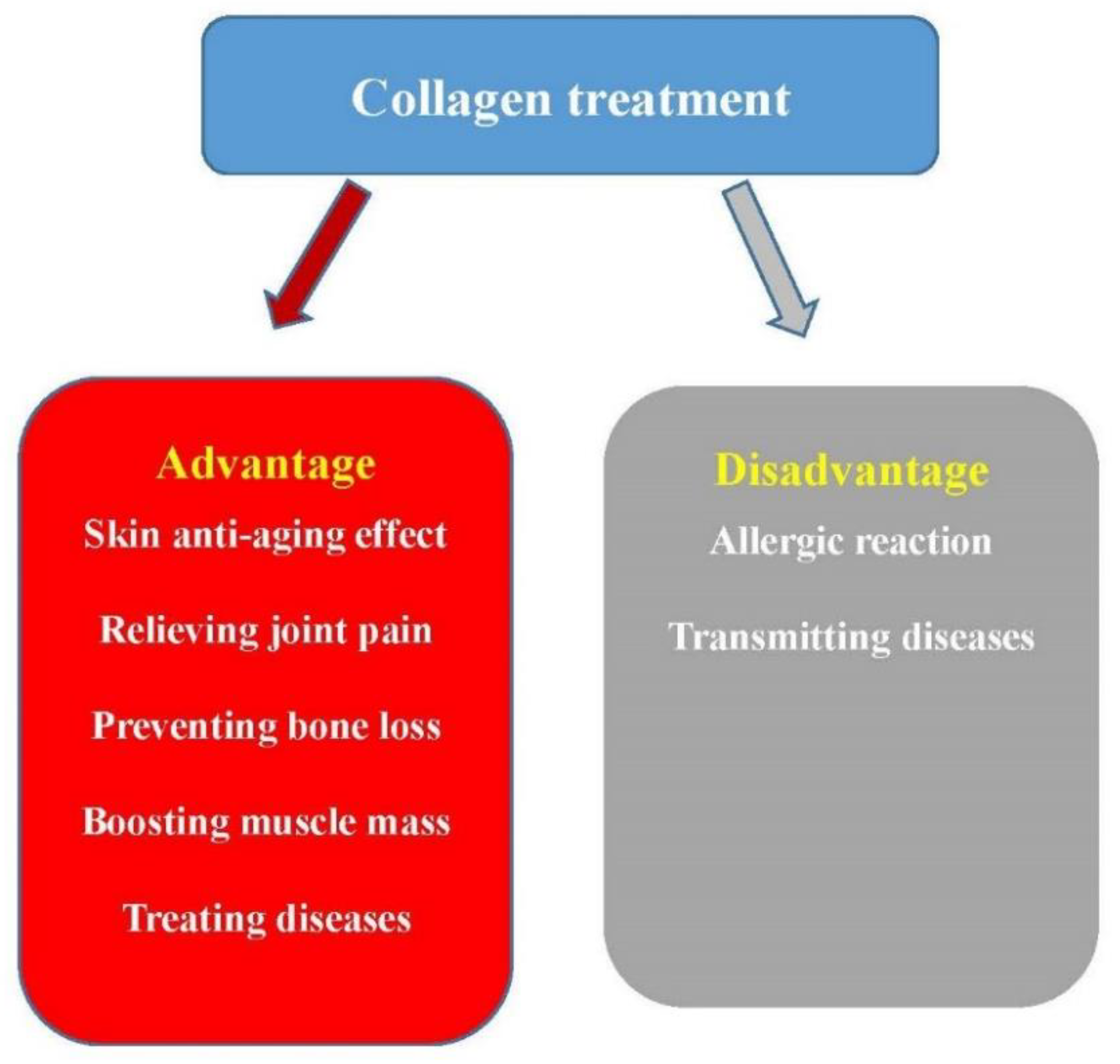
4.3. The Disadvantages of Collagen Treatment
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Meena, C.; Mengi, S.A.; Deshpande, S.G. Biomedical and industrial applications of collagen. Proc. Indian Acad. Sci. Chem. Sci. 1999, 111, 319–329. [Google Scholar]
- Sionkowska, A.; Adamiak, K.; Musiał, K.; Gadomska, M. Collagen Based Materials in Cosmetic Applications: A Review. Materials 2020, 13, 2417. [Google Scholar] [CrossRef]
- Lafarga, T.; Hayes, M. Bioactive peptides from meat muscle and by-products: Generation, functionality and application as functional ingredients. Meat Sci. 2014, 98, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.M.; Dornelles, R.C.P.; Mello, R.O.; Kubota, E.H.; Mazutti, M.A.; Kempka, A.P.; Demiate, I.M. Collagen extraction process. Int. Food Res. J. 2016, 23, 913–922. [Google Scholar]
- Theocharis, A.D.; Skandalis, S.S.; Gialeli, C.; Karamanos, N.K. Extracellular matrix structure. Adv. Drug Deliv. Rev. 2016, 97, 4–27. [Google Scholar] [CrossRef]
- Holmes, D.F.; Lu, Y.; Starborg, T.; Kadler, K.E. Collagen fibril assembly and function. Curr. Top. Dev. Biol. 2018, 130, 107–142. [Google Scholar] [PubMed]
- Gelse, K.; Poschl, E.; Aigner, T. Collagens--Structure, function, and biosynthesis. Adv. Drug Deliv. Rev. 2003, 55, 1531–1546. [Google Scholar] [CrossRef] [Green Version]
- Leon-Lopez, A.; Morales-Penaloza, A.; Martinez-Juarez, V.M.; Vargas-Torres, A.; Zeugolis, D.I.; Aguirre-Alvarez, G. Hydrolyzed Collagen-Sources and Applications. Molecules 2019, 24, 4031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheah, K.S. Collagen genes and inherited connective tissue disease. Biochem. J. 1985, 229, 287–303. [Google Scholar] [CrossRef] [Green Version]
- Silvipriya, K.; Kumar, K.K.; Bhat, A.; Kumar, B.D.; John, A.; Lakshmanan, P. Collagen: Animal sources and biomedical application. J. Appl. Pharm. Sci. 2015, 5, 123–127. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, M.I.A.; Barroso, L.G.R.; Sanchez, M.L. Collagen: A review on its sources and potential cosmetic applications. J. Cosmet. Dermatol. 2018, 17, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Boraschi-Diaz, I.; Wang, J.; Mort, J.S.; Komarova, S.V. Collagen Type I as a Ligand for Receptor-Mediated Signaling. Front. Phys. 2017, 5, 12. [Google Scholar] [CrossRef]
- Winterpacht, A.; Hilbert, M.; Schwarze, U.; Mundlos, S.; Spranger, J.; Zabel, B.U. Kniest and Stickler dysplasia phenotypes caused by collagen type II gene (COL2A1) defect. Nat. Genet. 1993, 3, 323–326. [Google Scholar] [CrossRef]
- Juan, L.; Xiao, Z.; Song, Y.; Zhijian, Z.; Jing, J.; Kun, Y.; Yuna, H.; Dongfa, D.; Lili, D.; Liuxin, T.; et al. Safety and immunogenicity of a novel therapeutic DNA vaccine encoding chicken type II collagen for rheumatoid arthritis in normal rats. Hum. Vaccines Immunother. 2015, 11, 2777–2783. [Google Scholar] [CrossRef] [Green Version]
- De Almagro, M.C. The Use of Collagen Hydrolysates and Native Collagen in Osteoarthritis. Am. J. Biomed. Sci. Res. 2020, 6, 530–532. [Google Scholar] [CrossRef]
- Wu, J.J.; Weis, M.A.; Kim, L.S.; Eyre, D.R. Type III collagen, a fibril network modifier in articular cartilage. J. Biol. Chem. 2010, 285, 18537–18544. [Google Scholar] [CrossRef] [Green Version]
- Gudmann, N.; Karsdal, M. Biochemistry of Collagens, Laminins and Elastin; Academic Press: Cambridge, MA, USA, 2016. [Google Scholar]
- Hamel, B.C.; Pals, G.; Engels, C.H.; van den Akker, E.; Boers, G.H.; van Dongen, P.W.; Steijlen, P.M. Ehlers-Danlos syndrome and type III collagen abnormalities: A variable clinical spectrum. Clin. Genet. 1998, 53, 440–446. [Google Scholar] [CrossRef]
- Sand, J.; Genovese, F.; Karsdal, M. Type IV collagen. In Biochemistry of Collagens, Laminins and Elastin; Elsevier: Amsterdam, The Netherlands, 2016; pp. 31–41. [Google Scholar]
- Karsdal, M. Biochemistry of Collagens, Laminins and Elastin: Structure, Function and Biomarkers; Academic Press: Cambridge, MA, USA, 2019. [Google Scholar]
- Nuytinck, L.; Freund, M.; Lagae, L.; Pierard, G.E.; Hermanns-Le, T.; De Paepe, A. Classical Ehlers-Danlos syndrome caused by a mutation in type I collagen. Am. J. Hum. Genet. 2000, 66, 1398–1402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, L.R.; Theato, P. Collagen and collagen mimetic peptide conjugates in polymer science. Eur. Polym. J. 2013, 49, 2986–2997. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Kurdtabar, M. Collagen-based highly porous hydrogel without any porogen: Synthesis and characteristics. Eur. Polym. J. 2007, 43, 877–889. [Google Scholar] [CrossRef]
- Song, W.K.; Liu, D.; Sun, L.L.; Li, B.F.; Hou, H. Physicochemical and Biocompatibility Properties of Type I Collagen from the Skin of Nile Tilapia (Oreochromis niloticus) for Biomedical Applications. Mar. Drugs 2019, 17, 137. [Google Scholar] [CrossRef] [Green Version]
- Charriere, G.; Bejot, M.; Schnitzler, L.; Ville, G.; Hartmann, D.J. Reactions to a bovine collagen implant: Clinical and immunologic study in 705 patients. J. Am. Acad. Dermatol. 1989, 21, 1203–1208. [Google Scholar] [CrossRef]
- Keefe, J.; Wauk, L.; Chu, S.; DeLustro, F. Clinical use of injectable bovine collagen: A decade of experience. Clin. Mater. 1992, 9, 155–162. [Google Scholar] [CrossRef]
- Aluko, R.E. Antihypertensive peptides from food proteins. Annu. Rev. Food Sci. Technol. 2015, 6, 235–262. [Google Scholar] [CrossRef]
- Fu, Y.; Young, J.F.; Løkke, M.M.; Lametsch, R.; Aluko, R.E.; Therkildsen, M. Revalorisation of bovine collagen as a potential precursor of angiotensin I-converting enzyme (ACE) inhibitory peptides based on in silico and in vitro protein digestions. J. Funct. Foods 2016, 24, 196–206. [Google Scholar] [CrossRef]
- Zhang, Y.; Olsen, K.; Grossi, A.; Otte, J. Effect of pretreatment on enzymatic hydrolysis of bovine collagen and formation of ACE-inhibitory peptides. Food Chem. 2013, 141, 2343–2354. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Xie, Y.; Wen, B.; Ye, M.; Liu, Y.; Imam, K.M.; Cai, H.; Zhang, C.; Wang, F.; Xin, F. Porcine bone collagen peptides promote osteoblast proliferation and differentiation by activating the PI3K/Akt signaling pathway. J. Funct. Foods 2020, 64, 103697. [Google Scholar] [CrossRef]
- Salamanca, E.; Tsai, C.Y.; Pan, Y.H.; Lin, Y.T.; Huang, H.M.; Teng, N.C.; Lin, C.T.; Feng, S.W.; Chang, W.J. In Vitro and In Vivo Study of a Novel Porcine Collagen Membrane for Guided Bone Regeneration. Materials 2016, 9, 949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santana, J.C.; Gardim, R.B.; Almeida, P.F.; Borini, G.B.; Quispe, A.P.; Llanos, S.A.; Heredia, J.A.; Zamuner, S.; Gamarra, F.; Farias, T.J.P. Valorization of chicken feet by-product of the poultry industry: High qualities of gelatin and biofilm from extraction of collagen. Polymers 2020, 12, 529. [Google Scholar] [CrossRef] [Green Version]
- Mokrejš, P.; Mrázek, P.; Gál, R.; Pavlačková, J.J.P. Biotechnological preparation of gelatines from chicken feet. Polymers 2019, 11, 1060. [Google Scholar] [CrossRef] [Green Version]
- Fauzi, M.B.; Lokanathan, Y.; Aminuddin, B.S.; Ruszymah, B.H.I.; Chowdhury, S.R. Ovine tendon collagen: Extraction, characterisation and fabrication of thin films for tissue engineering applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 68, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, I.; Mishra, D.; Das, T.; Maiti, S.; Maiti, T.K. Caprine (Goat) collagen: A potential biomaterial for skin tissue engineering. J. Biomater. Sci. Polym. Ed. 2012, 23, 355–373. [Google Scholar] [CrossRef]
- Silva, T.H.; Moreira-Silva, J.; Marques, A.L.P.; Domingues, A.; Bayon, Y.; Reis, R.L. Marine Origin Collagens and Its Potential Applications. Mar. Drugs 2014, 12, 5881–5901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadowska, M.; Kolodziejska, I.; Niecikowska, C. Isolation of collagen from the skins of Baltic cod (Gadus morhua). Food Chem. 2003, 81, 257–262. [Google Scholar] [CrossRef]
- Foegeding, E.; Lanier, T.; Hultin, H.J.F.C. Collagen. Food Chem. 1996, 3, 902–906. [Google Scholar]
- Kim, B.S.; Choi, J.S.; Kim, J.D.; Yoon, H.I.; Choi, Y.C.; Cho, Y.W. Human collagen isolated from adipose tissue. Biotechnol. Prog. 2012, 28, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wu, X.; Chen, J.; Lin, K. The development of collagen based composite scaffolds for bone regeneration. Bioact. Mater. 2018, 3, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Coppola, D.; Oliviero, M.; Vitale, G.A.; Lauritano, C.; D’Ambra, I.; Iannace, S.; de Pascale, D. Marine Collagen from Alternative and Sustainable Sources: Extraction, Processing and Applications. Mar. Drugs 2020, 18, 214. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.A. Collagen of Extracellular Matrix from Marine Invertebrates and Its Medical Applications. Mar. Drugs 2019, 17, 118. [Google Scholar] [CrossRef] [Green Version]
- Parisi, J.R.; Fernandes, K.R.; Avanzi, I.R.; Dorileo, B.P.; Santana, A.F.; Andrade, A.L.; Gabbai-Armelin, P.R.; Fortulan, C.A.; Triches, E.S.; Granito, R.N.; et al. Incorporation of Collagen from Marine Sponges (Spongin) into Hydroxyapatite Samples: Characterization and In Vitro Biological Evaluation. Mar. Biotechnol. 2019, 21, 30–37. [Google Scholar] [CrossRef]
- Lim, Y.S.; Ok, Y.J.; Hwang, S.Y.; Kwak, J.Y.; Yoon, S. Marine Collagen as A Promising Biomaterial for Biomedical Applications. Mar. Drugs 2019, 17, 467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Derkach, S.R.; Voron’ko, N.G.; Kuchina, Y.A.; Kolotova, D.S. Modified Fish Gelatin as an Alternative to Mammalian Gelatin in Modern Food Technologies. Polymers 2020, 12, 3051. [Google Scholar] [CrossRef] [PubMed]
- Bayrak, A.; Pruger, P.; Stock, U.A.; Seifert, M. Absence of immune responses with xenogeneic collagen and elastin. Tissue Eng. Part A 2013, 19, 1592–1600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, D.; Gao, Y.; Wang, Y.; Yang, X.; He, C.; Zhu, M.; Zhang, S.; Mo, X. Evaluation of biocompatibility and immunogenicity of micro/nanofiber materials based on tilapia skin collagen. J. Biomater. Appl. 2019, 33, 1118–1127. [Google Scholar] [CrossRef]
- Jhawar, N.; Wang, J.V.; Saedi, N. Oral collagen supplementation for skin aging: A fad or the future? J. Cosmet. Dermatol. 2020, 19, 910–912. [Google Scholar] [CrossRef] [PubMed]
- Asserin, J.; Lati, E.; Shioya, T.; Prawitt, J. The effect of oral collagen peptide supplementation on skin moisture and the dermal collagen network: Evidence from an ex vivo model and randomized, placebo-controlled clinical trials. J. Cosmet. Dermatol. 2015, 14, 291–301. [Google Scholar] [CrossRef] [Green Version]
- Kang, M.C.; Yumnam, S.; Kim, S.Y. Oral Intake of Collagen Peptide Attenuates Ultraviolet B Irradiation-Induced Skin Dehydration In Vivo by Regulating Hyaluronic Acid Synthesis. Int. J. Mol. Sci. 2018, 19, 3551. [Google Scholar] [CrossRef] [Green Version]
- Bolke, L.; Schlippe, G.; Gerss, J.; Voss, W. A Collagen Supplement Improves Skin Hydration, Elasticity, Roughness, and Density: Results of a Randomized, Placebo-Controlled, Blind Study. Nutrients 2019, 11, 2494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.U.; Chung, H.C.; Choi, J.; Sakai, Y.; Lee, B.Y. Oral Intake of Low-Molecular-Weight Collagen Peptide Improves Hydration, Elasticity, and Wrinkling in Human Skin: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2018, 10, 826. [Google Scholar] [CrossRef] [Green Version]
- Evans, M.; Lewis, E.D.; Zakaria, N.; Pelipyagina, T.; Guthrie, N. A randomized, triple-blind, placebo-controlled, parallel study to evaluate the efficacy of a freshwater marine collagen on skin wrinkles and elasticity. J. Cosmet. Dermatol. 2021, 20, 825–834. [Google Scholar] [CrossRef]
- Tanaka, M.; Koyama, Y.; Nomura, Y. Effects of collagen peptide ingestion on UV-B-induced skin damage. Biosci. Biotechnol. Biochem. 2009, 73, 930–932. [Google Scholar] [CrossRef]
- Chan, B.P.; Leong, K.W. Scaffolding in tissue engineering: General approaches and tissue-specific considerations. Eur. Spine J. 2008, 17 (Suppl. 4), 467–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Padilla Colon, C.J.; Molina-Vicenty, I.L.; Frontera-Rodriguez, M.; Garcia-Ferre, A.; Rivera, B.P.; Cintron-Velez, G.; Frontera-Rodriguez, S. Muscle and Bone Mass Loss in the Elderly Population: Advances in diagnosis and treatment. J. Biomed. 2018, 3, 40–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuttappan, S.; Mathew, D.; Nair, M.B. Biomimetic composite scaffolds containing bioceramics and collagen/gelatin for bone tissue engineering—A mini review. Int. J. Biol. Macromol. 2016, 93, 1390–1401. [Google Scholar] [CrossRef] [PubMed]
- Roseti, L.; Parisi, V.; Petretta, M.; Cavallo, C.; Desando, G.; Bartolotti, I.; Grigolo, B. Scaffolds for bone tissue engineering: State of the art and new perspectives. Mater. Sci. Eng. C 2017, 78, 1246–1262. [Google Scholar] [CrossRef]
- Murphy, C.M.; Haugh, M.G.; O’Brien, F.J. The effect of mean pore size on cell attachment, proliferation and migration in collagen-glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials 2010, 31, 461–466. [Google Scholar] [CrossRef]
- Li, Z.; Du, T.; Ruan, C.; Niu, X. Bioinspired mineralized collagen scaffolds for bone tissue engineering. Bioact. Mater. 2021, 6, 1491–1511. [Google Scholar] [CrossRef]
- Dhand, C.; Ong, S.T.; Dwivedi, N.; Diaz, S.M.; Venugopal, J.R.; Navaneethan, B.; Fazil, M.H.; Liu, S.; Seitz, V.; Wintermantel, E.; et al. Bio-inspired in situ crosslinking and mineralization of electrospun collagen scaffolds for bone tissue engineering. Biomaterials 2016, 104, 323–338. [Google Scholar] [CrossRef]
- Xin, L.; Lin, X.; Pan, Y.; Zheng, X.; Shi, L.; Zhang, Y.; Ma, L.; Gao, C.; Zhang, S. A collagen scaffold loaded with human umbilical cord-derived mesenchymal stem cells facilitates endometrial regeneration and restores fertility. Acta Biomater. 2019, 92, 160–171. [Google Scholar] [CrossRef]
- Forte, A.J.V.; da Silva Freitas, R.; Alonso, N. Use of three-dimensional computed tomography to classify filling of alveolar bone grafting. Plast. Surg. Int. 2012, 2012, 259419. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Descamps, M.; Dejou, J.; Koubi, G.; Hardouin, P.; Lemaitre, J.; Proust, J.P. The biodegradation mechanism of calcium phosphate biomaterials in bone. J. Biomed. Mater. Res. 2002, 63, 408–412. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Bian, Y.; Zhou, L.; Feng, B.; Weng, X.; Liang, R. Biological evaluation of bone substitute. Clin. Chim. Acta 2020, 510, 544–555. [Google Scholar] [CrossRef] [PubMed]
- Cuervo-Lozano, C.E.; Soto-Dominguez, A.; Saucedo-Cardenas, O.; Montes-de-Oca-Luna, R.; Alonso-Romero, S.; Del Consuelo Mancias-Guerra, M.; Alvarez-Lozano, E. Osteogenesis induced by a three-dimensional bioimplant composed of demineralised bone matrix, collagen, hydroxyapatite, and bone marrow-derived cells in massive bone defects: An experimental study. Tissue Cell 2018, 50, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Nathanael, A.J.; Oyane, A.; Nakamura, M.; Sakamaki, I.; Nishida, E.; Kanemoto, Y.; Miyaji, H. In Vitro and in Vivo Analysis of Mineralized Collagen-Based Sponges Prepared by a Plasma- and Precursor-Assisted Biomimetic Process. ACS Appl. Mater. Interfaces 2017, 9, 22185–22194. [Google Scholar] [CrossRef]
- Murakami, S.; Miyaji, H.; Nishida, E.; Kawamoto, K.; Miyata, S.; Takita, H.; Akasaka, T.; Fugetsu, B.; Iwanaga, T.; Hongo, H.; et al. Dose effects of beta-tricalcium phosphate nanoparticles on biocompatibility and bone conductive ability of three-dimensional collagen scaffolds. Dent. Mater. J. 2017, 36, 573–583. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, K.; Iwazawa, R.; Yoshioka, Y. Introduction to a new cell transplantation platform via recombinant peptide petaloid pieces and its application to islet transplantation with mesenchymal stem cells. Transpl. Int. 2016, 29, 1039–1050. [Google Scholar] [CrossRef] [Green Version]
- Akiyama, Y.; Ito, M.; Toriumi, T.; Hiratsuka, T.; Arai, Y.; Tanaka, S.; Futenma, T.; Akiyama, Y.; Yamaguchi, K.; Azuma, A.; et al. Bone formation potential of collagen type I-based recombinant peptide particles in rat calvaria defects. Regen. Ther. 2021, 16, 12–22. [Google Scholar] [CrossRef]
- Kaneko, M.; Shigeno, K.; Wakatsuki, M.; Nakada, A.; Inada, Y.; Nakamura, T. Bone regeneration utilizing a newly developed octacalcium phosphate/weakly denatured collagen scaffold. Int. J. Artif. Organs 2021, 44, 139–145. [Google Scholar] [CrossRef]
- Rosenberg, I.H. Sarcopenia: Origins and clinical relevance. J. Nutr. 1997, 127, 990S–991S. [Google Scholar] [CrossRef] [Green Version]
- Bauer, J.; Morley, J.E.; Schols, A.; Ferrucci, L.; Cruz-Jentoft, A.J.; Dent, E.; Baracos, V.E.; Crawford, J.A.; Doehner, W.; Heymsfield, S.B.; et al. Sarcopenia: A Time for Action. An SCWD Position Paper. J. Cachexia Sarcopenia Muscle 2019, 10, 956–961. [Google Scholar] [CrossRef]
- Argiles, J.M.; Campos, N.; Lopez-Pedrosa, J.M.; Rueda, R.; Rodriguez-Manas, L. Skeletal Muscle Regulates Metabolism via Interorgan Crosstalk: Roles in Health and Disease. J. Am. Med. Dir. Assoc. 2016, 17, 789–796. [Google Scholar] [CrossRef] [Green Version]
- Zdzieblik, D.; Oesser, S.; Baumstark, M.W.; Gollhofer, A.; Konig, D. Collagen peptide supplementation in combination with resistance training improves body composition and increases muscle strength in elderly sarcopenic men: A randomised controlled trial. Br. J. Nutr. 2015, 114, 1237–1245. [Google Scholar] [CrossRef] [Green Version]
- Centner, C.; Zdzieblik, D.; Roberts, L.; Gollhofer, A.; Konig, D. Effects of Blood Flow Restriction Training with Protein Supplementation on Muscle Mass And Strength in Older Men. J. Sports Sci. Med. 2019, 18, 471–478. [Google Scholar]
- Kallis, P.J.; Friedman, A.J. Collagen Powder in Wound Healing. J. Drugs Dermatol. 2018, 17, 403–408. [Google Scholar]
- Chattopadhyay, S.; Raines, R.T. Collagen-based biomaterials for wound healing. Biopolymers 2014, 101, 821–833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silcock, D. Collagen-based dressings as therapeutic agents for wound healing. In Drug-Device Combination Products; Elsevier: Amsterdam, The Netherlands, 2010; pp. 280–310. [Google Scholar]
- Fleck, C.A.; Simman, R. Modern collagen wound dressings: Function and purpose. J. Am. Col. Certif. Wound Spec. 2010, 2, 50–54. [Google Scholar] [CrossRef] [Green Version]
- Ge, B.; Wang, H.; Li, J.; Liu, H.; Yin, Y.; Zhang, N.; Qin, S. Comprehensive Assessment of Nile Tilapia Skin (Oreochromis niloticus) Collagen Hydrogels for Wound Dressings. Mar. Drugs 2020, 18, 178. [Google Scholar] [CrossRef] [Green Version]
- Felician, F.F.; Yu, R.H.; Li, M.Z.; Li, C.J.; Chen, H.Q.; Jiang, Y.; Tang, T.; Qi, W.Y.; Xu, H.M. The wound healing potential of collagen peptides derived from the jellyfish Rhopilema esculentum. Chin. J. Traumatol. 2019, 22, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, J.; Ding, Y.; Dai, X.; Li, Y. Oral administration of marine collagen peptides from Chum Salmon skin enhances cutaneous wound healing and angiogenesis in rats. J. Sci. Food Agric. 2011, 91, 2173–2179. [Google Scholar] [CrossRef]
- Wang, J.; Xu, M.; Liang, R.; Zhao, M.; Zhang, Z.; Li, Y. Oral administration of marine collagen peptides prepared from chum salmon (Oncorhynchus keta) improves wound healing following cesarean section in rats. Food Nutr. Res. 2015, 59, 26411. [Google Scholar] [CrossRef] [PubMed]
- Knefeli, H.-C.J. Improved bone healing after oral application of specific bioactive collagen peptides. Int. J. Nutraceuticals Funct. Foods Nov. Foods 2018, 17, 185–188. [Google Scholar]
- Sato, K.; Asai, T.T.; Jimi, S. Collagen-Derived Di-Peptide, Prolylhydroxyproline (Pro-Hyp): A New Low Molecular Weight Growth-Initiating Factor for Specific Fibroblasts Associated With Wound Healing. Front. Cell Dev. Biol. 2020, 8, 548975. [Google Scholar] [CrossRef]
- Cortellini, P.; Tonetti, M.S. Clinical concepts for regenerative therapy in intrabony defects. Periodontology 2000 2015, 68, 282–307. [Google Scholar] [CrossRef]
- Crea, A.; Deli, G.; Littarru, C.; Lajolo, C.; Orgeas, G.V.; Tatakis, D.N. Intrabony defects, open-flap debridement, and decortication: A randomized clinical trial. J. Periodontol. 2014, 85, 34–42. [Google Scholar] [CrossRef]
- Mazzoni, E.; Iaquinta, M.R.; Lanzillotti, C.; Mazziotta, C.; Maritati, M.; Montesi, M.; Sprio, S.; Tampieri, A.; Tognon, M.; Martini, F. Bioactive Materials for Soft Tissue Repair. Front. Bioeng. Biotechnol. 2021, 9, 613787. [Google Scholar] [CrossRef]
- Sheikh, Z.; Sima, C.; Glogauer, M. Bone Replacement Materials and Techniques Used for Achieving Vertical Alveolar Bone Augmentation. Materials 2015, 8, 2953–2993. [Google Scholar] [CrossRef]
- Rosa, C.D.D.R.D.; de Luna Gomes, J.M.; de Moraes, S.L.D.; Lemos, C.A.A.; da Fonte, T.P.; de Oliveira Limirio, J.P.J.; Pellizzer, E.P.J.T.S.D.J. Use of chlorhexidine chip after scaling and root planning on periodontal disease: A systematic review and meta-analysis. Saudi Dent. J. 2021, 33, 1. [Google Scholar] [CrossRef]
- Sheikh, Z.; Qureshi, J.; Alshahrani, A.M.; Nassar, H.; Ikeda, Y.; Glogauer, M.; Ganss, B. Collagen based barrier membranes for periodontal guided bone regeneration applications. Odontology 2017, 105, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ausenda, F.; Rasperini, G.; Acunzo, R.; Gorbunkova, A.; Pagni, G. New Perspectives in the Use of Biomaterials for Periodontal Regeneration. Materials 2019, 12, 2197. [Google Scholar] [CrossRef] [Green Version]
- Tizzoni, R.; Tizzoni, M.J. How do GTR and GBR Differ? A periodontitis case treated using an equine-derived, enzyme-deantigenic, collagen-preserving bone graft, and collagen membranes. J. Contemp. Dent. Pract. 2019, 20, 639–644. [Google Scholar] [CrossRef]
- Qian, Y.; Zhou, X.; Zhang, F.; Diekwisch, T.G.; Luan, X.; Yang, J. Triple PLGA/PCL scaffold modification including silver impregnation, collagen coating, and electrospinning significantly improve biocompatibility, antimicrobial, and osteogenic properties for orofacial tissue regeneration. ACS Appl. Mater. Interfaces 2019, 11, 37381–37396. [Google Scholar] [CrossRef] [PubMed]
- Pradeep, K.; Rajababu, P.; Satyanarayana, D.; Sagar, V. Gingival recession: Review and strategies in treatment of recession. Case Rep. Dent. 2012, 2012, 563421. [Google Scholar] [CrossRef] [Green Version]
- Naomi, R.; Ardhani, R.; Hafiyyah, O.A.; Fauzi, M.B. Current Insight of Collagen Biomatrix for Gingival Recession: An Evidence-Based Systematic Review. Polymers 2020, 12, 2081. [Google Scholar] [CrossRef] [PubMed]
- Cardaropoli, D.; Tamagnone, L.; Roffredo, A.; Gaveglio, L. Treatment of Gingival Recession Defects Using Coronally Advanced Flap With a Porcine Collagen Matrix Compared to Coronally Advanced Flap With Connective Tissue Graft: A Randomized Controlled Clinical Trial. J. Periodontol. 2012, 83, 321–328. [Google Scholar] [CrossRef]
- Schuh, C.; Benso, B.; Naulin, P.A.; Barrera, N.P.; Bozec, L.; Aguayo, S. Modulatory Effect of Glycated Collagen on Oral Streptococcal Nanoadhesion. J. Dent. Res. 2021, 100, 82–89. [Google Scholar] [CrossRef]
- Howell, T.H.; Fiorellini, J.; Jones, A.; Alder, M.; Nummikoski, P.; Lazaro, M.; Lilly, L.; Cochran, D. A feasibility study evaluating rhBMP-2/absorbable collagen sponge device for local alveolar ridge preservation or augmentation. Int. J. Periodontics Restor. Dent. 1997, 17, 124–139. [Google Scholar]
- Cho, H.; Jung, H.D.; Kim, B.J.; Kim, C.H.; Jung, Y.S. Complication rates in patients using absorbable collagen sponges in third molar extraction sockets: A retrospective study. J. Korean Assoc. Oral Maxillofac. Surg. 2015, 41, 26–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, S.J.; Chen, M.H.; Lin, H.Y.; Lin, C.P.; Chang, H.H. Pure type-1 collagen application to third molar extraction socket reduces postoperative pain score and duration and promotes socket bone healing. J. Formos. Med. Assoc. 2019, 118, 481–487. [Google Scholar] [CrossRef]
- Maiorana, C.; Pivetti, L.; Signorino, F.; Grossi, G.B.; Herford, A.S.; Beretta, M. The efficacy of a porcine collagen matrix in keratinized tissue augmentation: A 5-year follow-up study. Int. J. Implant. Dent. 2018, 4, 1. [Google Scholar] [CrossRef] [Green Version]
- Arnal, M.J.D.; Arenas, Á.F.; Arbeloa, Á.L. Esophageal cancer: Risk factors, screening and endoscopic treatment in Western and Eastern countries. World J. Gastroenterol. WJG 2015, 21, 7933. [Google Scholar] [CrossRef]
- Chen, Y.H.; Wang, H. The Association between Depression and Gastroesophageal Reflux based on Phylogenetic Analysis of miRNA Biomarkers. Curr. Med. Chem. 2020, 27, 6536–6547. [Google Scholar] [CrossRef]
- Locke, G., 3rd; Talley, N.J.; Fett, S.L.; Zinsmeister, A.R.; Melton, L., 3rd. Prevalence and clinical spectrum of gastroesophageal reflux: A population-based study in Olmsted County, Minnesota. Gastroenterology 1997, 112, 1448–1456. [Google Scholar] [CrossRef]
- Åsling, B.; Jirholt, J.; Hammond, P.; Knutsson, M.; Walentinsson, A.; Davidson, G.; Agréus, L.; Lehmann, A.; Lagerström-Fermer, M. Collagen type III alpha I is a gastro-oesophageal reflux disease susceptibility gene and a male risk factor for hiatus hernia. Gut 2009, 58, 1063–1069. [Google Scholar] [CrossRef] [Green Version]
- Von Diemen, V.; Trindade, E.N.; Trindade, M.R. Hiatal hernia and gastroesophageal reflux: Study of collagen in the phrenoesophageal ligament. Surg. Endosc. 2016, 30, 5091–5098. [Google Scholar] [CrossRef]
- Oconnor, K.W.; Lehman, G.A. Endoscopic Placement of Collagen at the Lower Esophageal Sphincter to Inhibit Gastroesophageal Reflux—A Pilot-Study of 10 Medically Intractable Patients. Gastrointest. Endosc. 1988, 34, 106–112. [Google Scholar] [CrossRef]
- Lemperle, G.; Lemperle, S.M. Gastroesophageal Reflux Disease (GERD): An Overview of Current Minimal-Invasive Treatment Potentials. Am. J. Biomed. Sci. Res. 2019, 2, 253–264. [Google Scholar] [CrossRef]
- Martin, K.; Emil, S.; Bernard, C.; Gaied, F.; Blumenkrantz, M.; Laberge, J.M.; Morinville, V.; Nguyen, V.H. Dextranomer hyaluronic acid copolymer effects on gastroesophageal junction. J. Pediatr. Gastroenterol. Nutr. 2014, 58, 593–597. [Google Scholar] [CrossRef]
- Felson, D.T.; Zhang, Y.; Hannan, M.T.; Naimark, A.; Weissman, B.N.; Aliabadi, P.; Levy, D. The incidence and natural history of knee osteoarthritis in the elderly. The Framingham Osteoarthritis Study. Arthritis Rheum. 1995, 38, 1500–1505. [Google Scholar] [CrossRef]
- Abramoff, B.; Caldera, F.E. Osteoarthritis Pathology, Diagnosis, and Treatment Options. Med. Clin. N. Am. 2020, 104, 293. [Google Scholar] [CrossRef] [PubMed]
- Di Cesare Mannelli, L.; Micheli, L.; Zanardelli, M.; Ghelardini, C. Low dose native type II collagen prevents pain in a rat osteoarthritis model. BMC Musculoskelet. Disord. 2013, 14, 228. [Google Scholar] [CrossRef] [Green Version]
- Scarpellini, M.; Lurati, A.; Vignati, G.; Marrazza, M.G.; Telese, F.; Re, K.; Bellistri, A. Biomarkers, type II collagen, glucosamine and chondroitin sulfate in osteoarthritis follow-up: The “Magenta osteoarthritis study”. J. Orthop. Traumatol. 2008, 9, 81–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakilan, F.; Armagan, O.; Ozgen, M.; Tascioglu, F.; Bolluk, O.; Alatas, O. Effects of Native Type II Collagen Treatment on Knee Osteoarthritis: A Randomized Controlled Trial. Eurasian J. Med. 2016, 48, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Lugo, J.P.; Saiyed, Z.M.; Lane, N.E. Efficacy and tolerability of an undenatured type II collagen supplement in modulating knee osteoarthritis symptoms: A multicenter randomized, double-blind, placebo-controlled study. Nutr. J. 2016, 15, 14. [Google Scholar] [CrossRef] [Green Version]
- Isaka, S.; Someya, A.; Nakamura, S.; Naito, K.; Nozawa, M.; Inoue, N.; Sugihara, F.; Nagaoka, I.; Kaneko, K. Evaluation of the effect of oral administration of collagen peptides on an experimental rat osteoarthritis model. Exp. Ther. Med. 2017, 13, 2699–2706. [Google Scholar] [CrossRef]
- Dar, Q.A.; Schott, E.M.; Catheline, S.E.; Maynard, R.D.; Liu, Z.Y.; Kamal, F.; Farnsworth, C.W.; Ketz, J.P.; Mooney, R.A.; Hilton, M.J.; et al. Daily oral consumption of hydrolyzed type 1 collagen is chondroprotective and anti-inflammatory in murine posttraumatic osteoarthritis. PLoS ONE 2017, 12, e0174705. [Google Scholar] [CrossRef]
- Van der Kraan, P.M.; van den Berg, W.B. Chondrocyte hypertrophy and osteoarthritis: Role in initiation and progression of cartilage degeneration? Osteoarthr. Cartil. 2012, 20, 223–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loeser, R.F. Aging and osteoarthritis: The role of chondrocyte senescence and aging changes in the cartilage matrix. Osteoarthr. Cartil. 2009, 17, 971–979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xin, W.; Heilig, J.; Paulsson, M.; Zaucke, F. Collagen II regulates chondroycte integrin expression profile and differentiation. Connect. Tissue Res. 2015, 56, 307–314. [Google Scholar] [CrossRef]
- Lian, C.; Wang, X.; Qiu, X.; Wu, Z.; Gao, B.; Liu, L.; Liang, G.; Zhou, H.; Yang, X.; Peng, Y.; et al. Collagen type II suppresses articular chondrocyte hypertrophy and osteoarthritis progression by promoting integrin β1−SMAD1 interaction. Bone Res. 2019, 7, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Crowley, D.C.; Lau, F.C.; Sharma, P.; Evans, M.; Guthrie, N.; Bagchi, M.; Bagchi, D.; Dey, D.K.; Raychaudhuri, S.P. Safety and efficacy of undenatured type II collagen in the treatment of osteoarthritis of the knee: A clinical trial. Int. J. Med. Sci. 2009, 6, 312–321. [Google Scholar] [CrossRef] [Green Version]
- Caplazi, P.; Baca, M.; Barck, K.; Carano, R.A.; DeVoss, J.; Lee, W.P.; Bolon, B.; Diehl, L. Mouse Models of Rheumatoid Arthritis. Vet. Pathol. 2015, 52, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Barnett, M.L.; Kremer, J.M.; St Clair, E.W.; Clegg, D.O.; Furst, D.; Weisman, M.; Fletcher, M.J.; Chasan-Taber, S.; Finger, E.; Morales, A.; et al. Treatment of rheumatoid arthritis with oral type II collagen. Results of a multicenter, double-blind, placebo-controlled trial. Arthritis Rheum. 1998, 41, 290–297. [Google Scholar] [CrossRef]
- Wei, W.; Zhang, L.L.; Xu, J.H.; Xiao, F.; Bao, C.D.; Ni, L.Q.; Li, X.F.; Wu, Y.Q.; Sun, L.Y.; Zhang, R.H.; et al. A multicenter, double-blind, randomized, controlled phase III clinical trial of chicken type II collagen in rheumatoid arthritis. Arthritis Res. Ther. 2009, 11, R180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, H.; Liang, F.; Liu, N.; Song, X.; Yuan, F.; Luo, Y.; Zhao, X.; Long, J.; Sun, Y.; Xi, Y. Potent antirheumatic activity of a new DNA vaccine targeted to B7-2/CD28 costimulatory signaling pathway in autoimmune arthritis. Hum. Gene Ther. 2011, 22, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Liang, F.; Liu, N.; Luo, Y.; Xue, H.; Yuan, F.; Tan, L.; Sun, Y.; Xi, C.; Xi, Y. Construction and characterization of a novel DNA vaccine that is potent antigen-specific tolerizing therapy for experimental arthritis by increasing CD4+ CD25+ Treg cells and inducing Th1 to Th2 shift in both cells and cytokines. Vaccine 2009, 27, 690–700. [Google Scholar]
- Hata, T.; Kavanaugh, A. Rheumatoid arthritis in dermatology. Clin. Dermatol. 2006, 24, 430–437. [Google Scholar] [CrossRef]
- Chua-Aguilera, C.J.; Moller, B.; Yawalkar, N. Skin Manifestations of Rheumatoid Arthritis, Juvenile Idiopathic Arthritis, and Spondyloarthritides. Clin. Rev. Allergy Immunol. 2017, 53, 371–393. [Google Scholar] [CrossRef]
- Sayah, A.; English, J.C., 3rd. Rheumatoid arthritis: A review of the cutaneous manifestations. J. Am. Acad. Dermatol. 2005, 53, 191–209. [Google Scholar] [CrossRef]
- Mianehsaz, E.; Mirzaei, H.R.; Mahjoubin-Tehran, M.; Rezaee, A.; Sahebnasagh, R.; Pourhanifeh, M.H.; Mirzaei, H.; Hamblin, M.R. Mesenchymal stem cell-derived exosomes: A new therapeutic approach to osteoarthritis? Stem Cell Res. Ther. 2019, 10, 340. [Google Scholar] [CrossRef] [Green Version]
- Kovari, E.; Kaposi, A.; Bekes, G.; Kiss, Z.; Kurucz, R.; Mandl, P.; Balint, G.P.; Poor, G.; Szendroi, M.; Balint, P.V. Comorbidity clusters in generalized osteoarthritis among female patients: A cross-sectional study. Semin. Arthritis Rheu. 2020, 50, 183–191. [Google Scholar] [CrossRef]
- Geisinger, M.L.; Holmes, C.M.; Vassilopoulos, P.J.; Geurs, N.C.; Reddy, M.S. Is Laser Disinfection an Effective Adjunctive Treatment to Bone Augmentation for Peri-Implantitis? A Review of Current Evidence. Clin. Adv. Periodontics 2014, 4, 274–279. [Google Scholar] [CrossRef] [Green Version]
- Akyuz, F.; Soyer, O.M. Which diseases are risk factors for developing gastroesophageal reflux disease? Turk. J. Gastroenterol. 2017, 28, S44–S47. [Google Scholar] [CrossRef] [PubMed]
- Miura, Y.; Fukuda, K.; Maeda, T.; Kurosaka, M. Gastroesophageal reflux disease in patients with rheumatoid arthritis. Mod. Rheumatol. 2014, 24, 291–295. [Google Scholar] [CrossRef]
- Nampei, A.; Shi, K.; Ebina, K.; Tomita, T.; Sugamoto, K.; Yoshikawa, H.; Hirao, M.; Hashimoto, J. Prevalence of gastroesophageal reflux disease symptoms and related factors in patients with rheumatoid arthritis. J. Clin. Biochem. Nutr. 2013, 52, 179–184. [Google Scholar] [CrossRef]
- Lu, M.C.; Liu, K.C.; Lai, N.S.; Koo, M. Higher incidence of rheumatoid arthritis in patients with symptomatic osteoarthritis or osteoarthritis-related surgery: A nationwide, population-based, case-control study in Taiwan. BMJ Open 2015, 5, e008513. [Google Scholar] [CrossRef] [Green Version]
- Starodubtseva, I.A. Prevalence of secondary osteoarthritis in patients with rheumatoid arthritis and risk factors for its progression. Adv. Gerontol. 2014, 27, 693–698. [Google Scholar]
- Miedel, E.L.; Brisson, B.K.; Hamilton, T.; Gleason, H.; Swain, G.P.; Lopas, L.; Dopkin, D.; Perosky, J.E.; Kozloff, K.M.; Hankenson, K.D.; et al. Type III collagen modulates fracture callus bone formation and early remodeling. J. Orthop. Res. 2015, 33, 675–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.H.; Wang, H. Exploring Diversity of COVID19 Based on Substitution Distance. Infect. Drug Resist. 2020, 13, 3887–3894. [Google Scholar] [CrossRef] [PubMed]
- Ejaz, H.; Alsrhani, A.; Zafar, A.; Javed, H.; Junaid, K.; Abdalla, A.E.; Abosalif, K.O.A.; Ahmed, Z.; Younas, S. COVID-19 and comorbidities: Deleterious impact on infected patients. J. Infect. Public Health 2020, 13, 1833–1839. [Google Scholar] [CrossRef]
- Lee, S.W.; Ha, E.K.; Yeniova, A.O.; Moon, S.Y.; Kim, S.Y.; Koh, H.Y.; Yang, J.M.; Jeong, S.J.; Moon, S.J.; Cho, J.Y.; et al. Severe clinical outcomes of COVID-19 associated with proton pump inhibitors: A nationwide cohort study with propensity score matching. Gut 2021, 70, 76–84. [Google Scholar] [CrossRef]
- Almario, C.V.; Chey, W.D.; Spiegel, B.M.R. Increased Risk of COVID-19 Among Users of Proton Pump Inhibitors. Am. J. Gastroenterol. 2020, 115, 1707–1715. [Google Scholar] [CrossRef]
- Jiang, G.; Cai, Y.; Yi, X.; Li, Y.; Lin, Y.; Li, Q.; Xu, J.; Ke, M.; Xue, K. The impact of laryngopharyngeal reflux disease on 95 hospitalized patients with COVID-19 in Wuhan, China: A retrospective study. J. Med. Virol. 2020, 92, 2124–2129. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Madhurapantula, R.S.; Kalyanasundaram, A.; Sabharwal, T.; Antipova, O.; Bishnoi, S.W.; Orgel, J.P.R.O. Ultrastructural Location and Interactions of the Immunoglobulin Receptor Binding Sequence within Fibrillar Type I Collagen. Int. J. Mol. Sci. 2020, 21, 4166. [Google Scholar] [CrossRef]
- Sakaguchi, M.; Toda, M.; Ebihara, T.; Irie, S.; Hori, H.; Imai, A.; Yanagida, M.; Miyazawa, H.; Ohsuna, H.; Ikezawa, Z.; et al. IgE antibody to fish gelatin (type I collagen) in patients with fish allergy. J. Allergy Clin. Immunol. 2000, 106, 579–584. [Google Scholar] [CrossRef]
- Mullins, R.J.; Richards, C.; Walker, T. Allergic reactions to oral, surgical and topical bovine collagen: Anaphylactic risk for surgeons. Aust. N. Z. J. Ophthalmol. 1996, 24, 257–260. [Google Scholar] [CrossRef] [PubMed]
- Koutsoumanis, K.; Allende, A.; Bolton, D.J.; Bover-Cid, S.; Chemaly, M.; Davies, R.; De Cesare, A.; Herman, L.M.; Hilbert, F.; EFSA Panel on Biological Hazards (BIOHAZ). Potential BSE risk posed by the use of ruminant collagen and gelatine in feed for non-ruminant farmed animals. EFSA J. 2020, 18, e06267. [Google Scholar] [PubMed]
- Kim, Y.; Nowzari, H.; Rich, S.K. Risk of Prion Disease Transmission through Bovine-Derived Bone Substitutes: A Systematic Review. Clin. Implant. Dent. R 2013, 15, 645–653. [Google Scholar] [CrossRef] [PubMed]


| Collagen | Function or Application | Tissue or Organ | Molecular Composition * |
|---|---|---|---|
| Type I | the organic part of the bone, membranes for guided tissue regeneration | Skin, bone, teeth, tendon, ligament, vascular ligature | |
| Type II | the main constituent of cartilage, cartilage repair, and arthritis treatment | cartilage | |
| Type III | the main constituent of reticular fibers, hemostats, and tissue sealants | muscle, blood vessels | |
| Type IV | the major component of the basement membrane, attachment enhancer of cell culture, and diabetic nephropathy indicator | basal lamina, the epithelium-secreted layer of the basement membrane | |
| Type V | feedstock for biomaterials in corneal treatments | Hair, cell surfaces, and placenta. |
| Disease | Collagen Treatment References |
|---|---|
| Skin aging | [48,49,50,51,52,53,54] |
| Bone defects | [41,58,59,60,62,64,65,66,67,68,70,71] |
| Sarcopenia | [75,76] |
| Wound healing | [78,79,80,81,82,83,84,85,86] |
| Periodontal disease | [86,93,94,95,96,97,98,99,100,102] |
| Gastroesophageal reflux | [107,108,109] |
| Osteoarthritis | [15,112,113,114,115,116,117,120,121,122,124] |
| Rheumatoid arthritis | [14,125,126,127,128,129] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H. A Review of the Effects of Collagen Treatment in Clinical Studies. Polymers 2021, 13, 3868. https://doi.org/10.3390/polym13223868
Wang H. A Review of the Effects of Collagen Treatment in Clinical Studies. Polymers. 2021; 13(22):3868. https://doi.org/10.3390/polym13223868
Chicago/Turabian StyleWang, Hsiuying. 2021. "A Review of the Effects of Collagen Treatment in Clinical Studies" Polymers 13, no. 22: 3868. https://doi.org/10.3390/polym13223868
APA StyleWang, H. (2021). A Review of the Effects of Collagen Treatment in Clinical Studies. Polymers, 13(22), 3868. https://doi.org/10.3390/polym13223868






