Bio-Zirconium Metal–Organic Framework Regenerable Bio-Beads for the Effective Removal of Organophosphates from Polluted Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
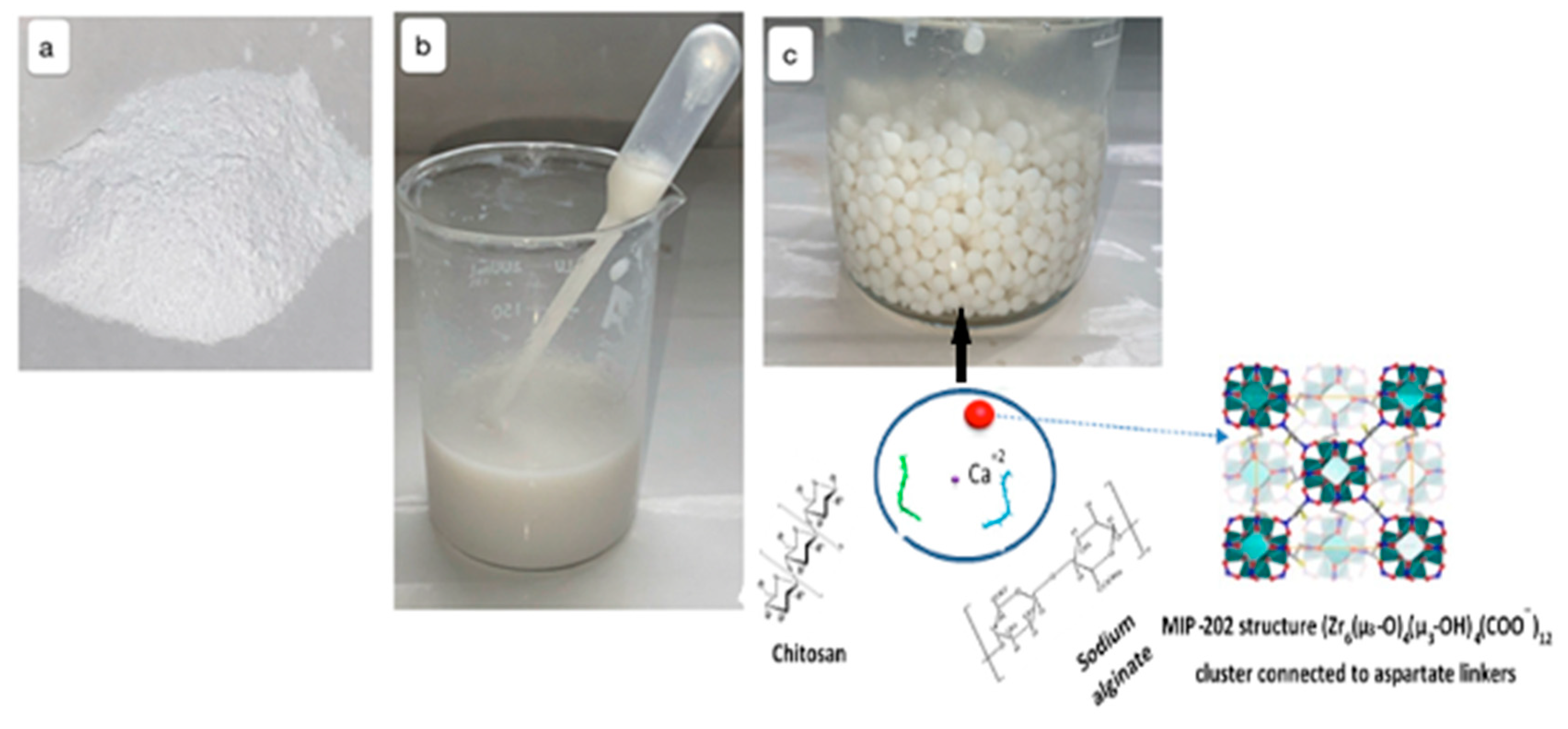
2.2.1. Synthesis of MIP-202 Powder
2.2.2. Fabrication of MIP-202/CA Beads Composite
2.2.3. Characterization Methods
2.2.4. Batch Adsorption of Diazinon from Polluted Water via MIP-202/CA Composite Beads
2.3. Analytical Methods
2.3.1. Equilibrium Isotherm Analysis for Diazinon Adsorption onto MIP 202/CA Composite Beads
2.3.2. Kinetic Models for Diazinon Adsorption onto MIP 202 Bio-MOF/CA Beads
2.4. Reusability Study of the Fabricated MIP-202/CA Composite Beads
3. Results
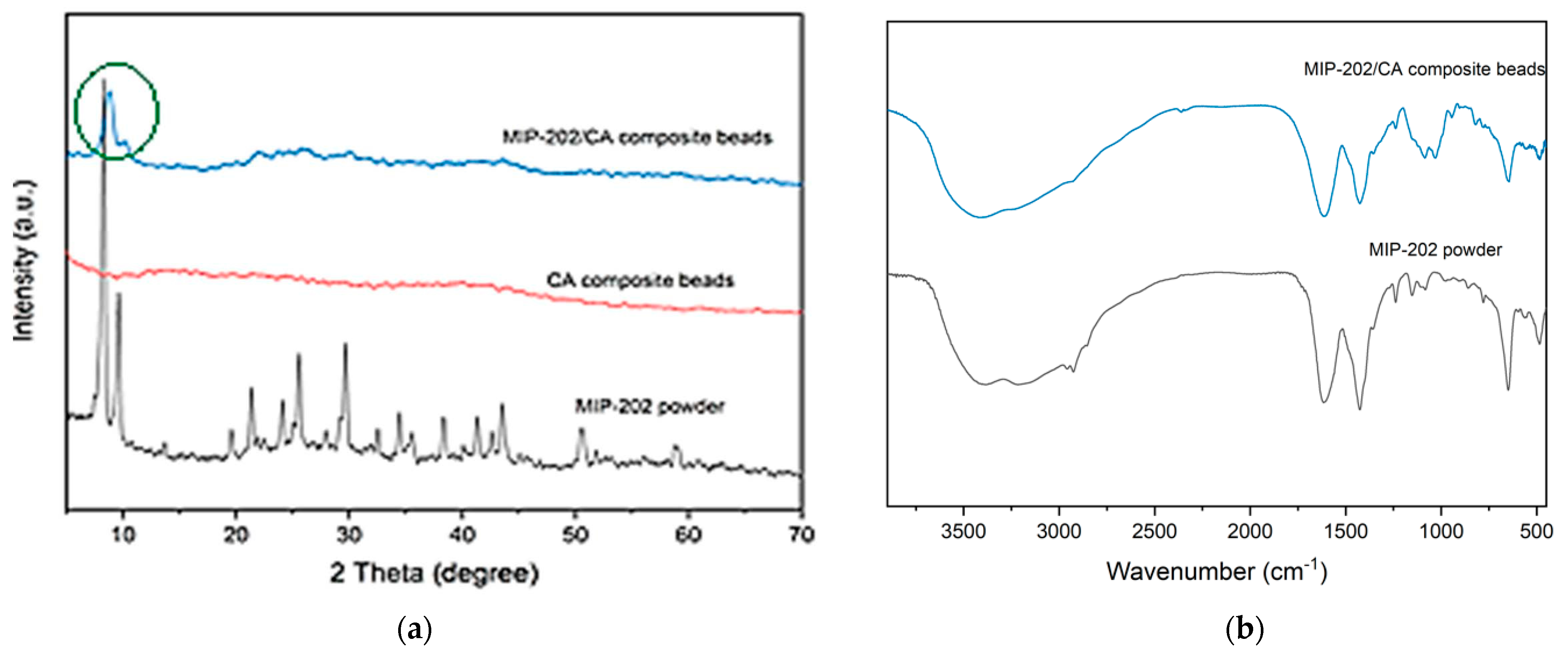
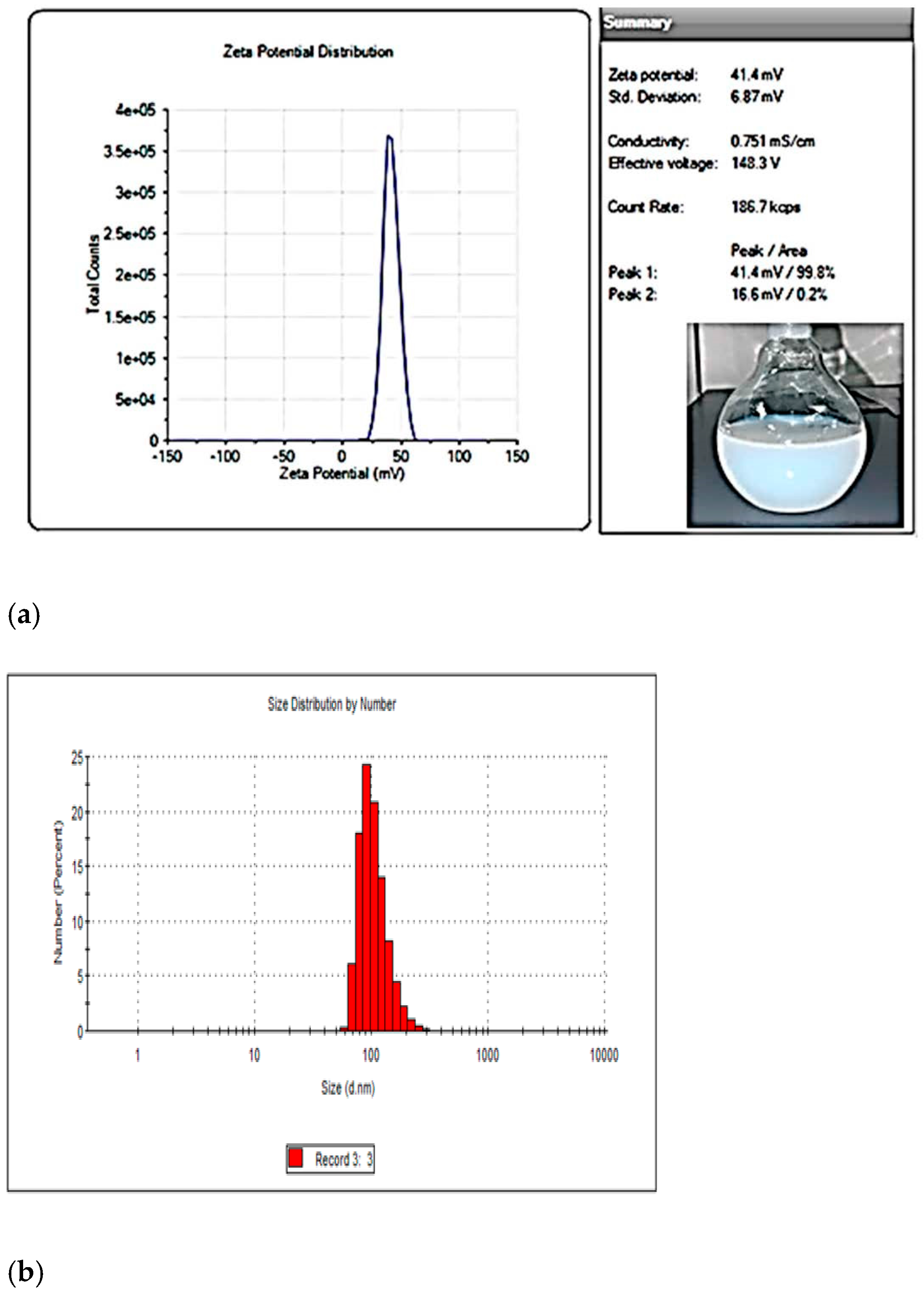
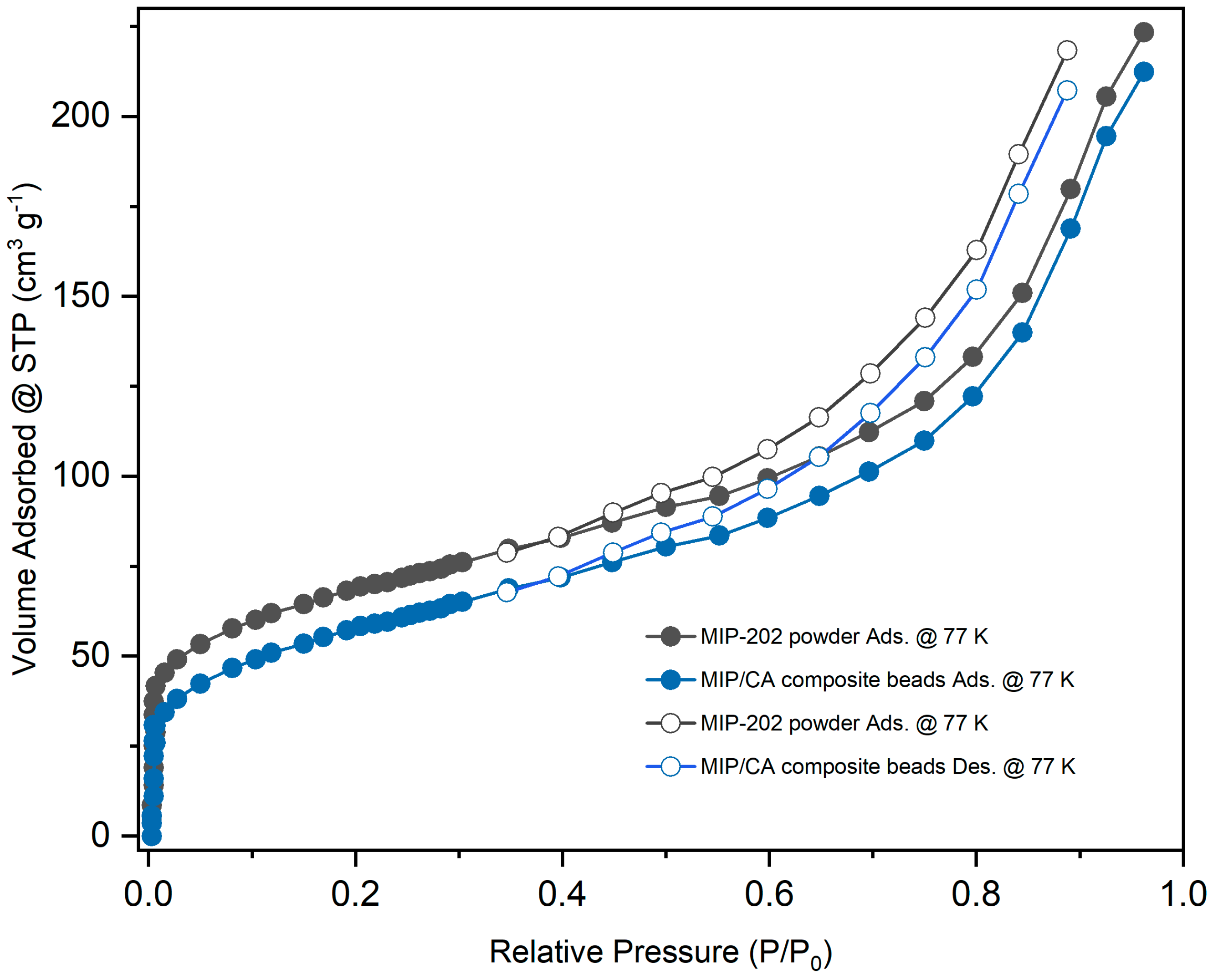
3.1. Optimization and Characterizations of MIP-202 Nanopwders and MIP-202/CA Beads Composite
3.2. Diazinon Decontamination Using the Fabricated MIP-202/CA Composite Beads
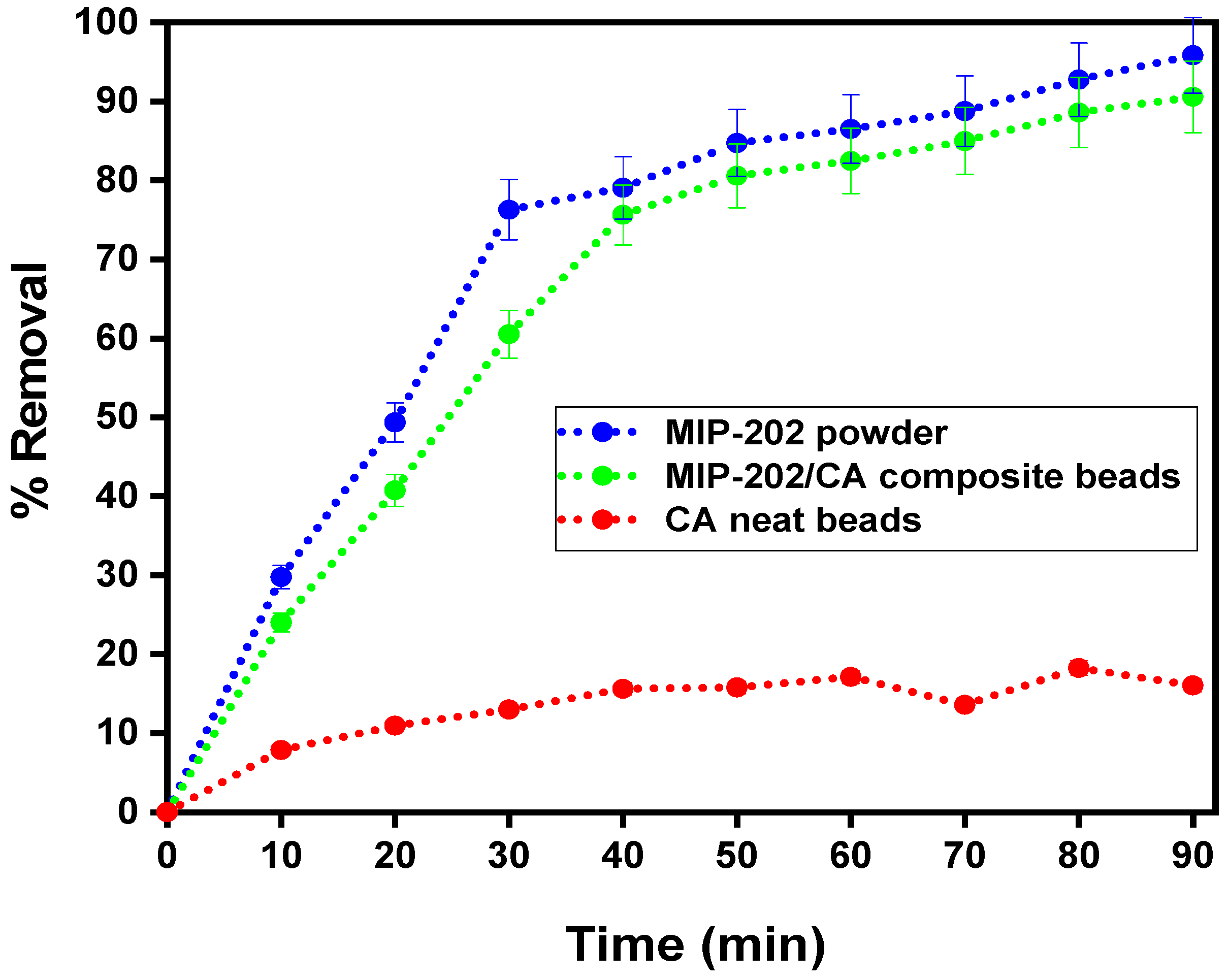
3.2.1. Influence of Contact Time on Diazinon Adsorption Processes
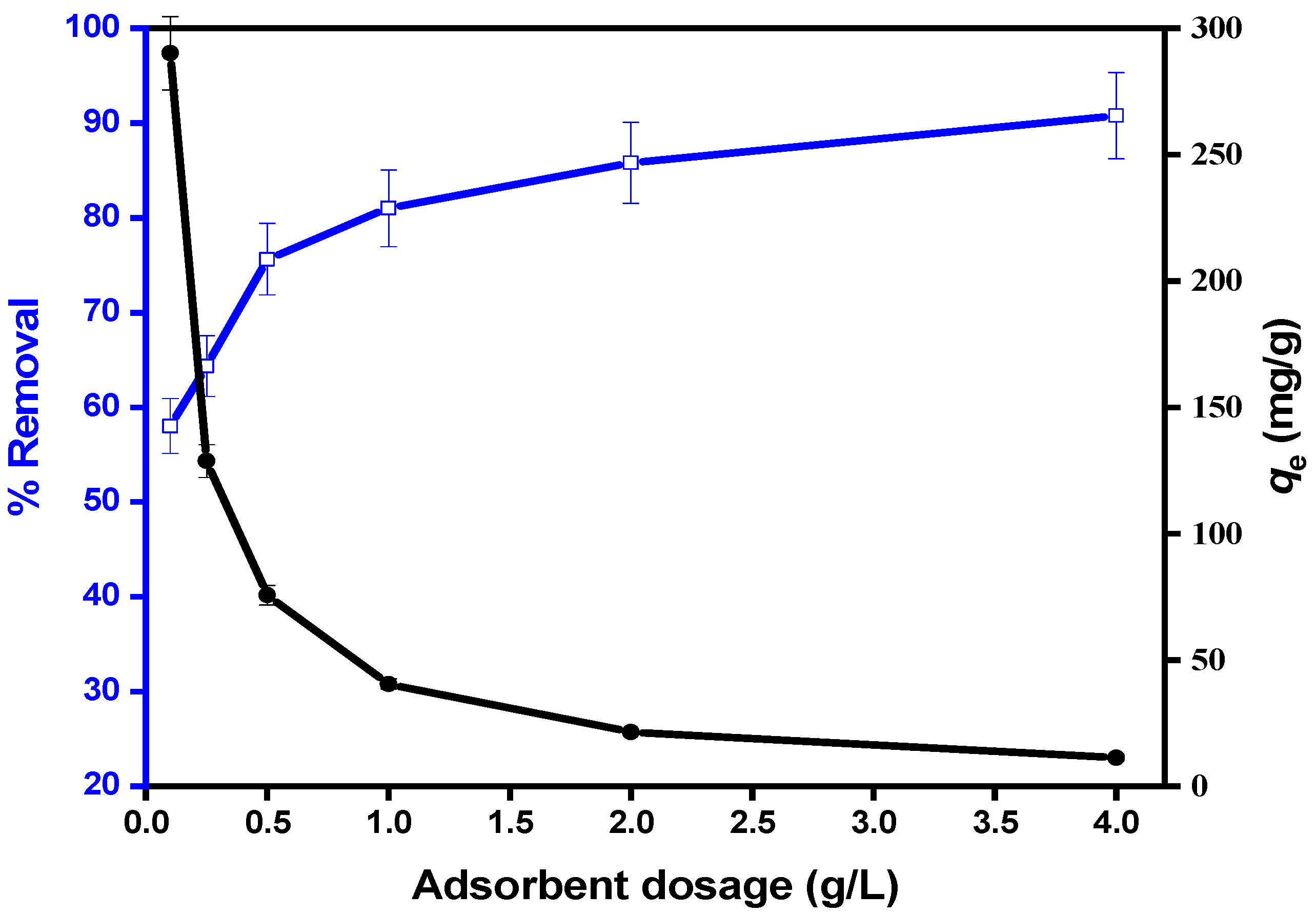
3.2.2. Influence of MIP-202/CA Composite Beads Dosage on the Diazinon Adsorption Processes
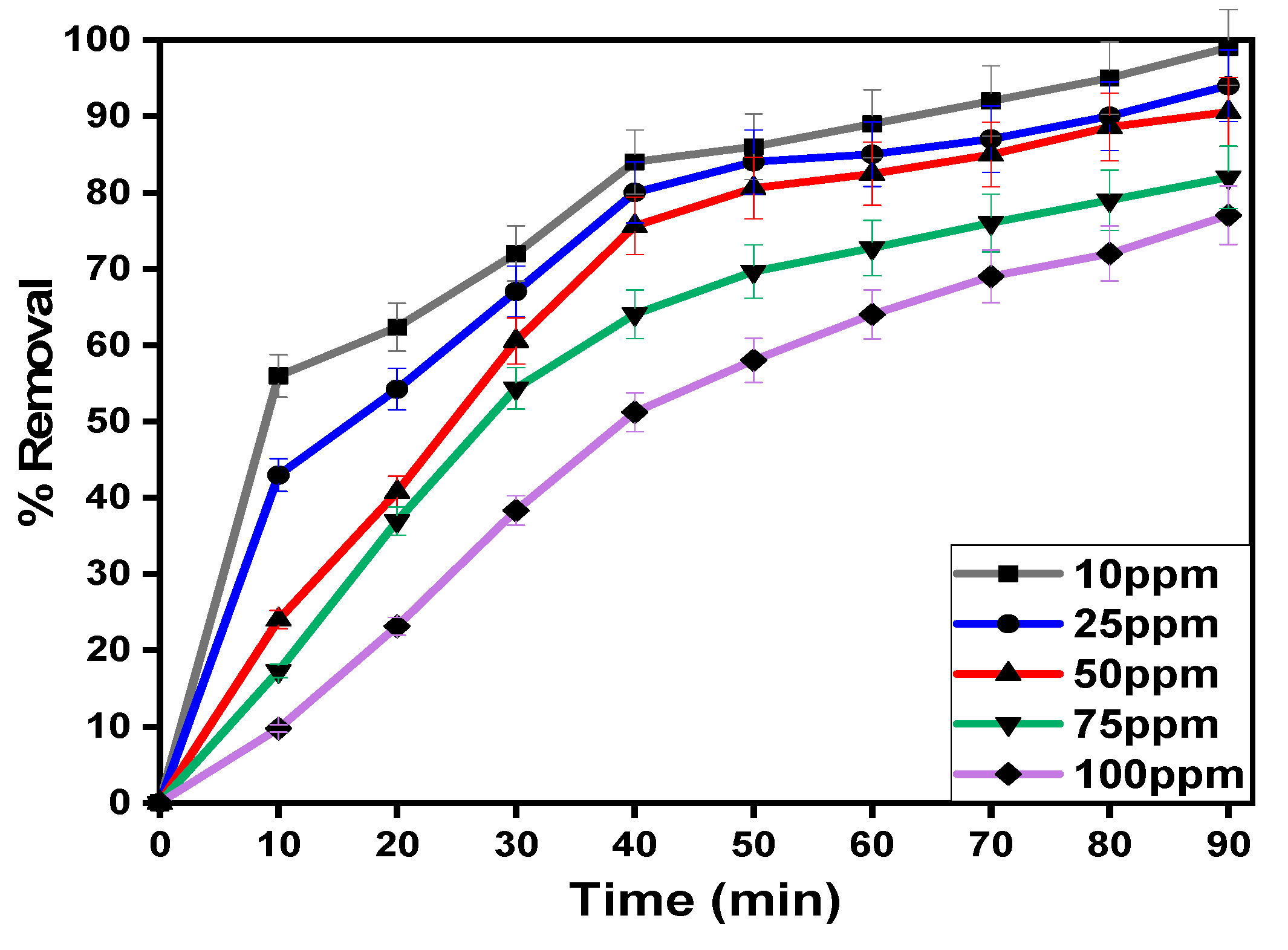
3.2.3. Influence of Initial Concentrations of Diazinon on the Adsorption Processes
3.3. Equilibrium Isotherm Analysis for Diazinon Adsorption onto MIP 202/CA Composite Beads
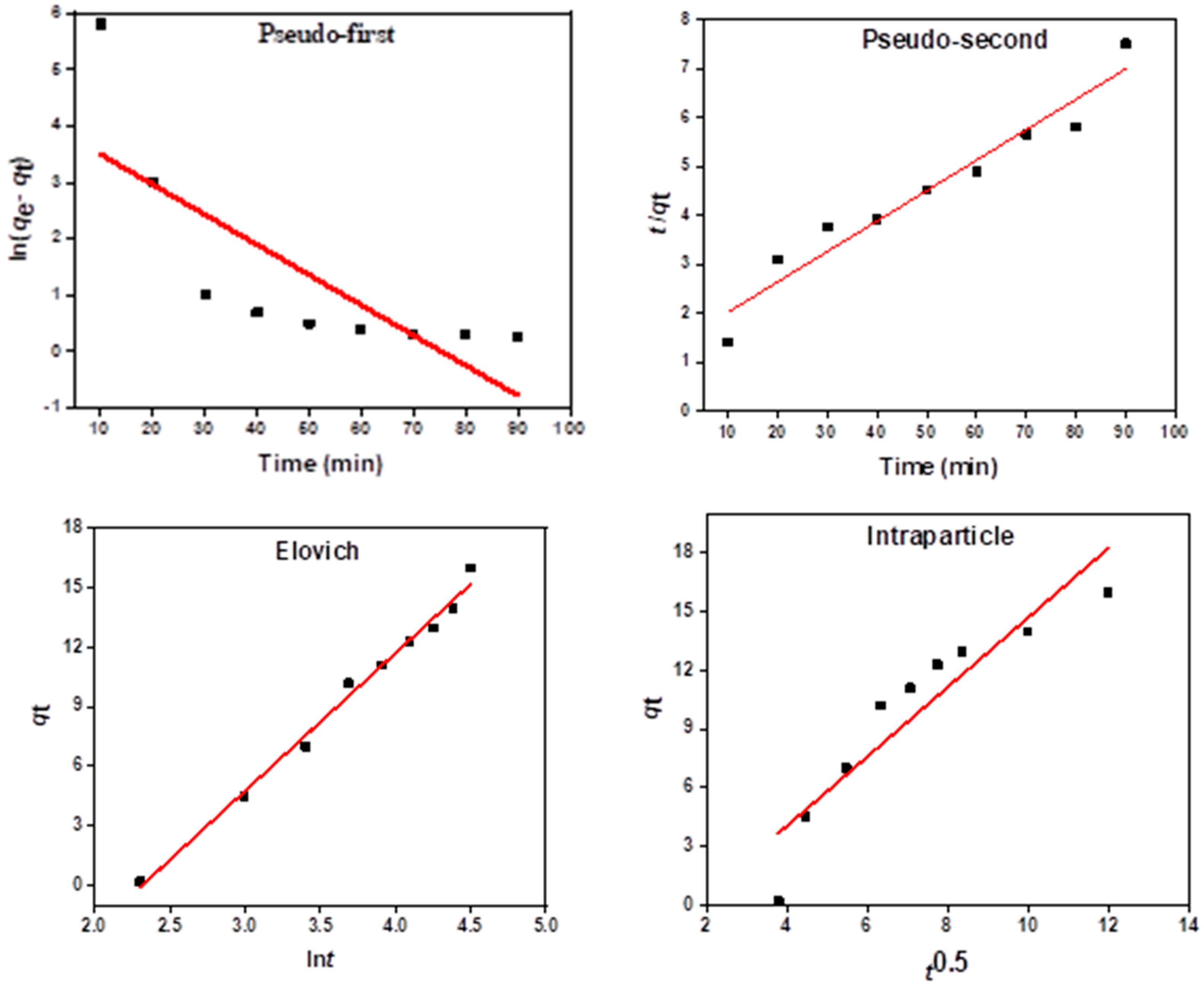
Kinetic Models of Daizinon Adsorption onto MIP 202/CA Composite Beads
3.4. Regeneration Studies of the Synthesized MIP-202/CA Beads
3.5. Comparison of Adsorption Capacity for MIP-202/CA Composite Beads with Other Adsorbent Materials
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elkady, M.F.; Shokry Hassan, H. Photocatalytic Degradation of Malachite Green Dye from Aqueous Solution Using Environmentally Compatible Ag/ZnO Polymeric Nanofibers. Polymers 2021, 13, 2033. [Google Scholar] [CrossRef] [PubMed]
- Schwarzenbach, R.P.; Egli, T.; Hofstetter, T.B.; von Gunten, U.; Wehrli, B. Global Water Pollution and Human Health. Annu. Rev. Environ. Resour. 2010, 35, 109–136. [Google Scholar] [CrossRef]
- Hassan, H.; Elkady, M. Invention of hollow zirconium tungesto-vanadate at nanotube morphological structure for radionuclides and heavy metal pollutants decontamination from aqueous solutions. Nanoscale Res. Lett. 2015, 10, 474. [Google Scholar]
- Kosikowska, M.; Biziuk, M. Review of the determination of pesticide residues in ambient air. Trends Analyt. Chem. 2010, 29, 1064–1072. [Google Scholar] [CrossRef]
- Amooey, A.A.; Ghasemi, S.; Mirsoleimani-Azizi, S.M.; Gholaminezhad, Z.; Chaichi, M.J. Removal of Diazinon from aqueous solution by electrocoagulation process using aluminum electrodes. Korean J. Chem. Eng. 2014, 31, 1016–1020. [Google Scholar] [CrossRef]
- Wang, C.; Shih, Y. Degradation and detoxification of diazinon by sono-Fenton and sono-Fenton-like processes. Sep. Purif. Technol. 2015, 140, 6–12. [Google Scholar] [CrossRef]
- Mirsoleimani-Azizi, S.M.; Setoodeh, P.; Samimi, F.; Shadmehr, J.; Hamedi, N.; Rahimpour, M.R. Diazinon removal from aqueous media by mesoporous MIL-101(Cr) in a continuous fixed-bed system. J. Environ. Chem. Eng. 2018, 6, 4653–4664. [Google Scholar] [CrossRef]
- Elkady, M.; Hassan, H.S.; Hashim, A. Immobilization of magnetic nanoparticles onto amine-modified nano-silica gel for copper ions remediation. Materials 2016, 9, 460. [Google Scholar] [CrossRef]
- Shokry Hassan, H.; Kashyout, A.B.; Morsi, I.; Nasser, A.A.A.; Abuklill, H. Development of polypyrrole coated copper nanowires for gas sensor application. Sens. Biosens. Res. 2015, 5, 50–54. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Kaur, A. Various methods for removal of dyes from industrial effluents-a review. Indian. J. Sci. Technol. 2018, 11, 1–21. [Google Scholar] [CrossRef]
- Matouq, M.A.; Al-Anber, Z.A.; Tagawa, T.; Aljbour, S.; Al-Shannag, M. Degradation of dissolved diazinon pesticide in water using the high frequency of ultrasound wave. Ultrason. Sonochem. 2008, 15, 869–874. [Google Scholar] [CrossRef]
- Mirsoleimani-Azizi, S.M.; Amooey, A.A.; Ghasemi, S.; Salkhordeh-Panbechouleh, S. Modeling the Removal of Endosulfan from Aqueous Solution by Electrocoagulation Process Using Artificial Neural Network (ANN). Ind. Eng. Chem. Res. 2015, 54, 9844–9849. [Google Scholar] [CrossRef]
- Sarkar, B.; Venkateswralu, N.; Rao, R.N.; Bhattacharjee, C.; Kale, V. Treatment of pesticide contaminated surface water for pro-duction of potable water by a coagulation-adsorption-nanofiltration approach. Desalination 2007, 212, 129–140. [Google Scholar] [CrossRef]
- Shokry, H.; Elkady, M.; Salama, E. Eco-friendly magnetic activated carbon nano-hybrid for facile oil spills separation. Sci. Rep. 2020, 10, 10265. [Google Scholar] [CrossRef]
- Elkady, M.; Shokry, H.; Hamad, H. New activated carbon from mine coal for adsorption of dye in simulated water or multiple heavy metals in real wastewater. Materials 2020, 13, 2498. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Li, X.; Li, Z. Adsorption equilibrium and kinetics of p-xylene on chromium-based metal organic framework MIL-101. Chem. Eng. J. 2011, 173, 150–157. [Google Scholar] [CrossRef]
- Marzbali, M.H.; Esmaieli, M.; Abolghasemi, H.; Marzbali, M.H. Tetracycline adsorption by H3PO4-activated carbon produced from apricot nut shells: A batch study. Process. Saf. Environ. Prot. 2016, 102, 700–709. [Google Scholar] [CrossRef]
- Marzbali, M.H.; Mir, A.A.; Pazoki, M.; Pourjamshidian, R.; Tabeshnia, M. Removal of direct yellow 12 from aqueous solution by adsorption onto spirulina algae as a high-efficiency adsorbent. J. Environ. Chem. 2017, 5, 1946–1956. [Google Scholar] [CrossRef]
- Gupta, V.K.; Saleh, T.A. Sorption of pollutants by porous carbon, carbon nanotubes and fullerene-An overview. Environ. Sci. Pollut. Res. Int. 2013, 20, 2828–2843. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, L.; Zhu, Y. Decontamination of Bisphenol A from Aqueous Solution by Graphene Adsorption. Langmuir 2012, 28, 8418–8425. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U. Arsenic removal from water/wastewater using adsorbents—A critical review. J. Hazard. Mater. 2007, 142, 1–53. [Google Scholar] [CrossRef] [PubMed]
- Akpinar, I.; Drout, R.J.; Islamoglu, T.; Kato, S.; Lyu, J.; Farha, O.K. Exploiting π–π Interactions to Design an Efficient Sorbent for Atrazine Removal from Water. ACS Appl. Mater. Interfaces 2019, 11, 6097–6103. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, G.M.; Eduardo, C., Jr.; Babasaheb, M.M.; Jongbeom, N.; Yusuke, Y.; Kevin, C.W.; Shiao-Wei, K. Construction Hierarchically Mesoporous/Microporous Materials Based on Block Copolymer and Covalent Organic Framework. J. Taiwan Inst. Chem. Eng. 2020, 112, 180–192. [Google Scholar] [CrossRef]
- Elkady, M.F.; Hassan, H.S.; Salama, E. Sorption Profile of Phosphorus Ions onto ZnO Nanorods Synthesized via Sonic Technique. J. Eng. 2016, 2016, 2308560. [Google Scholar] [CrossRef] [Green Version]
- Shokry Hassan, H. Role of preparation technique in the morphological structures of innovative nano-cation exchange. J. Mater. Res. Technol. 2019, 8, 2854–2864. [Google Scholar] [CrossRef]
- Langmi, H.W.; Ren, J.; North, B.; Mathe, M.; Bessarabov, D. Hydrogen Storage in Metal-Organic Frameworks: A Review. Electrochim. Acta 2014, 128, 368–392. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, X.; Mian, M.R.; Son, F.A.; Zhang, K.; Cao, R.; Chen, Z.; Lee, S.-J.; Idrees, K.B.; Goetjen, T.A.; et al. Structural Diversity of Zirconium Metal–Organic Frameworks and Effect on Adsorption of Toxic Chemicals. J. Am. Chem. Soc. 2020, 142, 21428–21438. [Google Scholar] [CrossRef]
- Lázaro, I.A.; Forgan, R.S. Application of zirconium MOFs in drug delivery and biomedicine. Coord. Chem. Rev. 2019, 380, 230–259. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Hu, M.; Huang, X.; Wang, M.; He, L.; Song, Y.; Jia, Q.; Zhou, N.; Zhang, Z.; Du, M. Multicomponent zirconium-based metal-organic frameworks for impedimetric aptasensing of living cancer cells. Sens. Actuators B Chem. 2020, 306, 127608. [Google Scholar] [CrossRef]
- Drout, R.J.; Robison, L.; Chen, Z.; Islamoglu, T.; Farha, O.K. Zirconium Metal–Organic Frameworks for Organic Pollutant Adsorption. Trends Chem. 2019, 1, 304–317. [Google Scholar] [CrossRef]
- Rangwani, S.; Howarth, A.J.; DeStefano, M.R.; Malliakas, C.D.; Platero-Prats, A.E.; Chapman, K.W.; Farha, O.K. Adsorptive removal of Sb(V) from water using a mesoporous Zr-based metal–organic framework. Polyhedron 2018, 151, 338–343. [Google Scholar] [CrossRef]
- Yang, J.; Trickett, C.A.; Alahmadi, S.B.; Alshammari, A.S.; Yaghi, O.M. Calcium l-Lactate Frameworks as Naturally Degradable Carriers for Pesticides. J. Am. Chem. Soc. 2017, 139, 8118–8121. [Google Scholar] [CrossRef] [PubMed]
- Hashem, T.; Ibrahim, A.H.; Wöll, C.; Alkordi, M.H. Grafting Zirconium-Based Metal–Organic Framework UiO-66-NH2 Nano-particles on Cellulose Fibers for the Removal of Cr(VI) Ions and Methyl Orange from Water. ACS Appl. Nano Mater. 2019, 2, 5804–5808. [Google Scholar] [CrossRef]
- Wang, S.; Wahiduzzaman, M.; Davis, L.; Tissot, A.; Shepard, W.; Marrot, J.; Martineau-Corcos, C.; Hamdane, D.; Maurin, G.; Devautour-Vinot, S.; et al. A robust zirconium amino acid metal-organic framework for proton conduction. Nat. Commun. 2018, 9, 4937. [Google Scholar] [CrossRef] [PubMed]
- Wahiduzzaman, M.; Wang, S.; Sikora, B.J.; Serre, C.; Maurin, G. Computational structure determination of novel metal–organic frameworks. Chem. Commun. 2018, 54, 10812–10815. [Google Scholar] [CrossRef]
- Diab, K.E.; Salama, E.; Hassan, H.S.; Abd El-Moneim, A.; Elkady, M.F. Biocompatible MIP-202 Zr-MOF tunable sorbent for cost-effective decontamination of anionic and cationic pollutants from waste solutions. Sci. Rep. 2021, 11, 6619. [Google Scholar] [CrossRef]
- Yang, S.; Peng, L.; Syzgantseva, O.A.; Trukhina, O.; Kochetygov, I.; Justin, A.; Sun, D.T.; Abedini, H.; Syzgantseva, M.A.; Oveisi, E.; et al. Preparation of Highly Porous Metal–Organic Framework Beads for Metal Extraction from Liquid Streams. J. Am. Chem. Soc. 2020, 142, 13415–13425. [Google Scholar] [CrossRef]
- Valizadeh, B.; Nguyen, T.N.; Smit, B.; Stylianou, K.C. Porous Metal-Organic Framework@Polymer Beads for Iodine Capture and Recovery Using a Gas-Sparged Column. Adv. Funct. Mater. 2018, 28, 1801596. [Google Scholar] [CrossRef]
- Ren, J.; Musyoka, N.M.; Langmi, H.W.; Swartbooi, A.; North, B.C.; Mathe, M. A more efficient way to shape metal-organic frame-work (MOF) powder materials for hydrogen storage applications. Int. J. Hydrogen Energy 2015, 40, 4617–4622. [Google Scholar] [CrossRef]
- Baysal, K.; Aroguz, A.Z.; Adiguzel, Z.; Baysal, B.M. Chitosan/alginate crosslinked hydrogels: Preparation, characterization and application for cell growth purposes. Int. J. Biol. Macromol. 2013, 59, 342–348. [Google Scholar] [CrossRef]
- Elkady, M.F.; Shokry Hassan, H.; El-Sayed, E.M. Basic Violet Decolourization Using Alginate Immobilized Nanozirconium Tungestovanadate Matrix as Cation Exchanger. J. Chem. 2015, 2015, 385741. [Google Scholar] [CrossRef] [Green Version]
- Elkady, M.; Hassan, H.; Amer, W.; Salama, E.; Algarni, H.; Shaaban, E. Novel magnetic zinc oxide nanotubes for phenol adsorption mechanism modeling. Materials 2017, 10, 1355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Üner, O.; Geçgel, Ü.; Bayrak, Y. Adsorption of Methylene Blue by an Efficient Activated Carbon Prepared from Citrullus la-natus Rind: Kinetic, Isotherm, Thermodynamic, and Mechanism Analysis. Water Air Soil Pollut. 2016, 227, 247. [Google Scholar] [CrossRef]
- Liu, G.; Li, L.; Huang, X.; Zheng, S.; Xu, X.; Liu, Z.; Zhang, Y.; Wang, J.; Lin, H.; Xu, D. Adsorption and removal of organophosphorus pesticides from environmental water and soil samples by using magnetic multi-walled carbon nanotubes @ organic framework ZIF-8. J. Mater. Sci. 2018, 53, 10772–10783. [Google Scholar] [CrossRef]
- Ouznadji, Z.B.; Sahmoune, M.N.; Mezenner, N.Y. Adsorptive removal of diazinon: Kinetic and equilibrium study. Desalination Water Treat. 2016, 57, 1880–1889. [Google Scholar] [CrossRef]
- Baharum, N.A.; Nasir, H.M.; Ishak, M.Y.; Isa, N.M.; Hassan, M.A.; Aris, A.Z. Highly efficient removal of diazinon pesticide from aqueous solutions by using coconut shell-modified biochar. Arab. J. Chem. 2020, 13, 6106–6121. [Google Scholar] [CrossRef]
- Barazandeh, A.; Jamali, H.A.; Karyab, H. Equilibrium and kinetic study of adsorption of diazinon from aqueous solutions by nano-polypropylene-titanium dioxide: Optimization of adsorption based on response surface methodology (RSM) and central composite design (CCD). Korean J. Chem. Eng. 2021, 38, 1–10. [Google Scholar] [CrossRef]
- Nikzad, S.; Amooey, A.A.; Alinejad-Mir, A. Adsorption of diazinon from aqueous solutions by magnetic guar gum-montmorillonite. Chem. Data Collect. 2019, 20, 100187. [Google Scholar] [CrossRef]
- Esfandian, H.; Samadi-Maybodi, A.; Parvini, M.; Khoshandam, B. Development of a novel method for the removal of diazinon pesticide from aqueous solution and modeling by artificial neural networks (ANN). J. Ind. Eng. Chem. 2016, 35, 295–308. [Google Scholar] [CrossRef]
- Moussavi, G.; Hosseini, H.; Alahabadi, A. The investigation of diazinon pesticide removal from contaminated water by adsorption onto NH4Cl-induced activated carbon. Chem. Eng. J. 2013, 214, 172–179. [Google Scholar] [CrossRef]
- Bayat, M.; Alighardashi, A.; Sadeghasadi, A. Fixed-bed column and batch reactors performance in removal of diazinon pesticide from aqueous solutions by using walnut shell-modified activated carbon. Environ. Technol. Innov. 2018, 12, 148–159. [Google Scholar] [CrossRef]
- Phuong, N.M.; Chu, N.C.; Van Thuan, D.; Ha, M.N.; Hanh, N.T.; Viet, H.D.T.; Thanh Truc, N.T. Novel removal of diazinon pesticide by adsorption and photocatalytic degradation of visible light-driven Fe-TiO2/Bent-Fe photocatalyst. J. Chem. 2019, 2019, 2678927. [Google Scholar] [CrossRef] [Green Version]


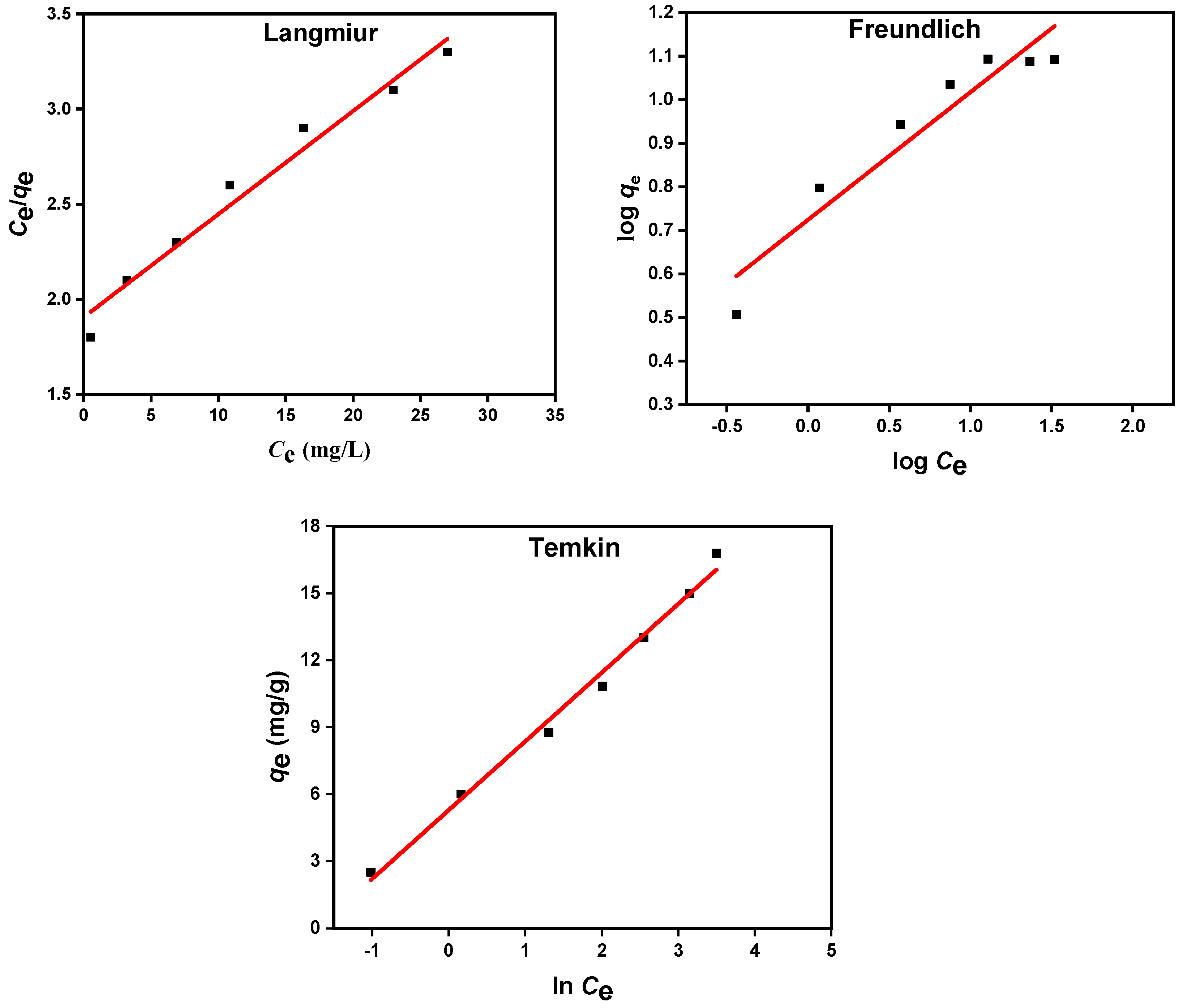

| Langmuir Parameters | Freundlich Parameters | Temkin Parameters | ||||||
|---|---|---|---|---|---|---|---|---|
| qm (mg/g) | KL (L/mg) | R2 | KF (mg/g) | nF | R2 | A (L/g) | B (J/mo) | R2 |
| 17.776 | 0.030 | 0.973 | 0.533 | 0.357 | 0.793 | 0.133 | 32.626 | 0.993 |
| Kinetic Model | Parameter | Value |
|---|---|---|
| Pseudo-first order | qexp.(mg/g) | 80.71 |
| qtheor (mg/g) | 34.12 | |
| K1 (min−1) | 0.011 | |
| R2 | 0.743 | |
| Pseudo-second order | qexp (mg/g) | 80.71 |
| qtheor (mg/g) | 75.62 | |
| K2 (g/mg·min) | 0.081 | |
| R2 | 0.935 | |
| Elovich kinetic model | α (mg/g·min) | −16.125 |
| β (g/mg) | 9.221 | |
| R2 | 0.921 | |
| Intraparticle diffusion kinetic model | C (mg/g·min) | 0.554 |
| ki (g/mg) | 0.041 | |
| R2 | 0.843 |
| Pollutant | Adsorbent Material | Optimized Conditions | Adsorption Capacity (mg/g) | Reference |
|---|---|---|---|---|
| Diazinon | MIP-202/CA beads | Dosage = 0.5 g/L Conc. = 50 mg/L Time = 40 min | 17.77 | Present work |
| Magnetic multi-walled carbon nanotubes | Dosage = 0.1 g/L Conc. = 8 mg/L Time = 30 min | 3.89 | [43] | |
| Activated bentonite | Dosage = 0.5 g/L Conc. = 10 mg/L Time = 120 min | 5.56 | [44] | |
| Phosphoric acid coconut shell biochar | Dosage = 1 g/L Conc. = 10 mg/L Time = 120 min | 10.33 | [45] | |
| Nano-TiO2 and polypropylene | Dosage = 2.25 g/L Conc. = 10 mg/L Time = 32.2 min | 1.24 | [46] | |
| Magnetic guar gum | Dosage = 1 g/L Conc. = 5 mg/L Time = 60 min | 7.03 | [47] | |
| Magnetic guar gum-montmorillonite | Dosage = 1 g/L Conc. = 5 mg/L Time = 60 min | 9.73 | [48] | |
| Acid treated zeolite | Dosage = 3 g/L Conc. = 50 mg/L Time = 20 min | 15.10 | [49] | |
| Modified zeolite by Cu2O | Dosage = 2 g/L Conc. = 50 mg/L Time = 20 min | 61.73 | [49] | |
| NH4Cl-induced activated carbon | Dosage = 0.3 g/L Conc. = 20 mg/L Time = 30 min | 25.00 | [50] | |
| Walnut shell-modified activated carbon | Dosage = 1 g/L Conc. = 40 mg/L Time = 60 min | 34.31 | [51] | |
| Fe-TiO2/Bent-Fe | Dosage = 0.5 g/L Conc. = 16.74 mg/L Time = 30 min | 27.03 | [52] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diab, K.E.; Salama, E.; Hassan, H.S.; El-moneim, A.A.; Elkady, M.F. Bio-Zirconium Metal–Organic Framework Regenerable Bio-Beads for the Effective Removal of Organophosphates from Polluted Water. Polymers 2021, 13, 3869. https://doi.org/10.3390/polym13223869
Diab KE, Salama E, Hassan HS, El-moneim AA, Elkady MF. Bio-Zirconium Metal–Organic Framework Regenerable Bio-Beads for the Effective Removal of Organophosphates from Polluted Water. Polymers. 2021; 13(22):3869. https://doi.org/10.3390/polym13223869
Chicago/Turabian StyleDiab, Kamal E., Eslam Salama, Hassan Shokry Hassan, Ahmed Abd El-moneim, and Marwa F. Elkady. 2021. "Bio-Zirconium Metal–Organic Framework Regenerable Bio-Beads for the Effective Removal of Organophosphates from Polluted Water" Polymers 13, no. 22: 3869. https://doi.org/10.3390/polym13223869
APA StyleDiab, K. E., Salama, E., Hassan, H. S., El-moneim, A. A., & Elkady, M. F. (2021). Bio-Zirconium Metal–Organic Framework Regenerable Bio-Beads for the Effective Removal of Organophosphates from Polluted Water. Polymers, 13(22), 3869. https://doi.org/10.3390/polym13223869









