3D Printing of Thermal Insulating Polyimide/Cellulose Nanocrystal Composite Aerogels with Low Dimensional Shrinkage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthetic Procedures of PMDA-ODA Polyamic Acid
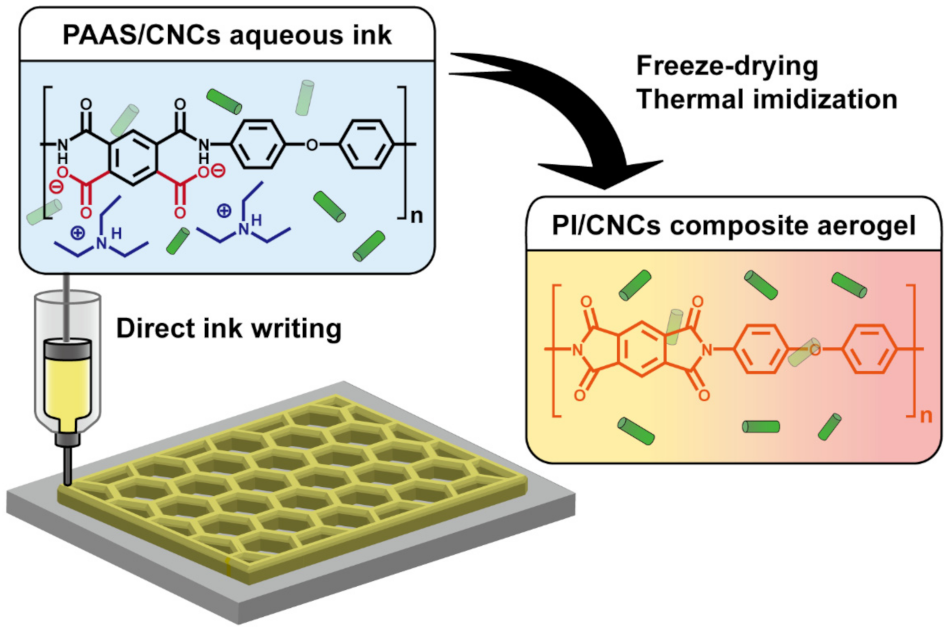
2.3. Preparation of PAAS/CNC Inks
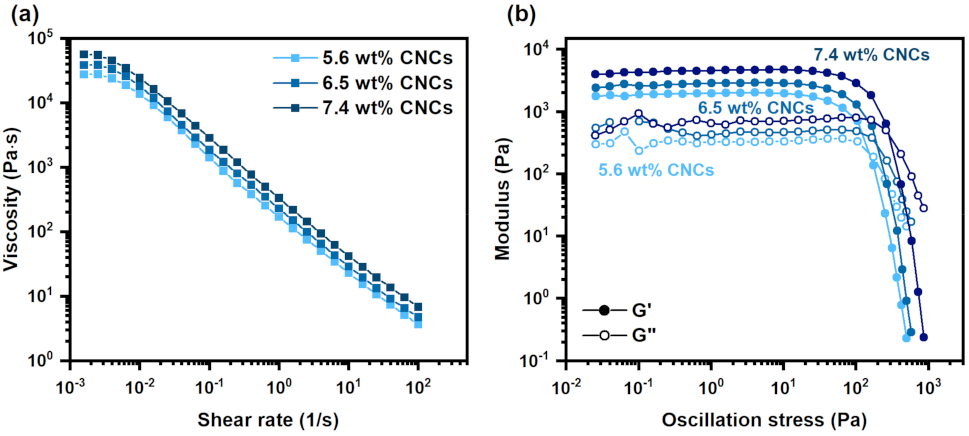
2.4. Rheological Test
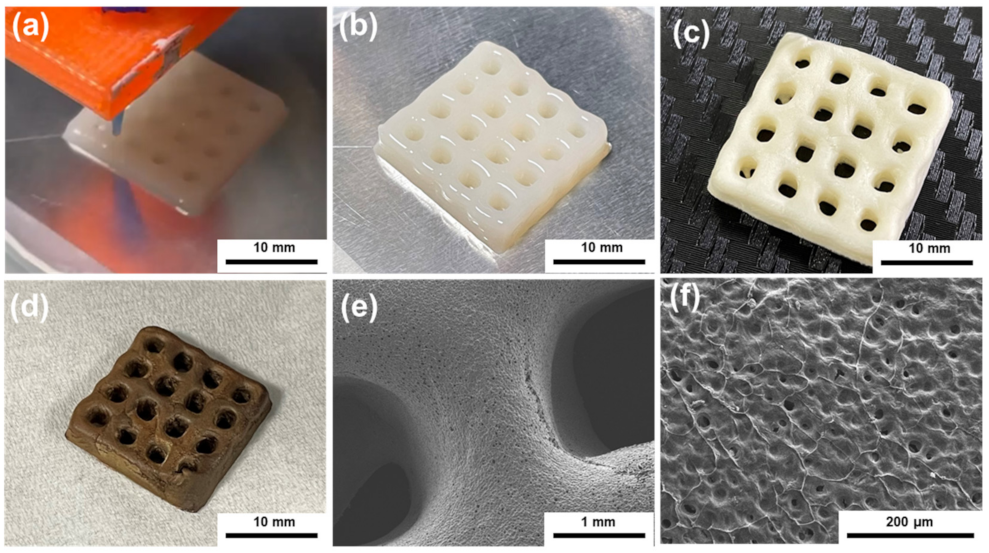
2.5. 3D Printing of PAAS/CNC Composite Ink
2.6. Preparation of PI, CNC, and PI/CNC Composite Aerogels
2.7. Characterization
3. Results and Discussions
3.1. Rheological Analysis of the PAAS/CNC Composite Inks
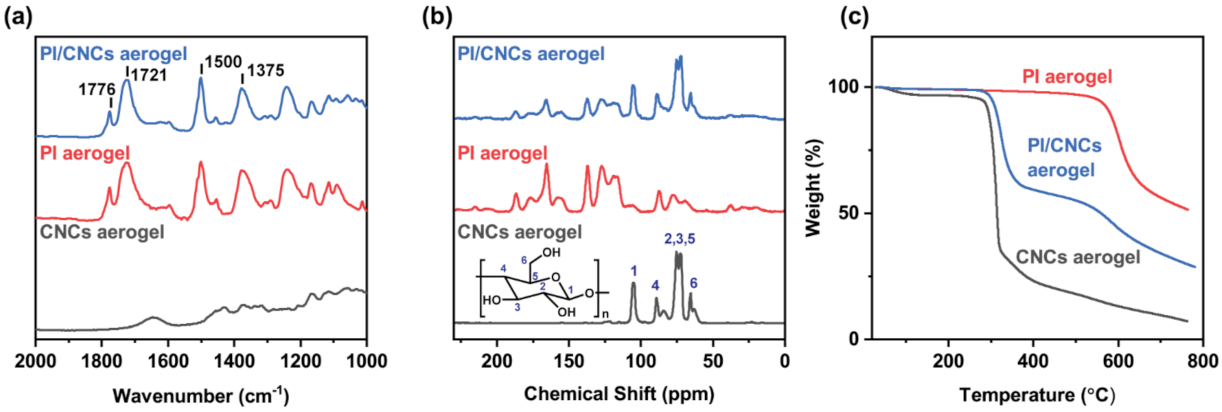
3.2. Chemical Analysis of the PI/CNC Nanocomposite Aerogels
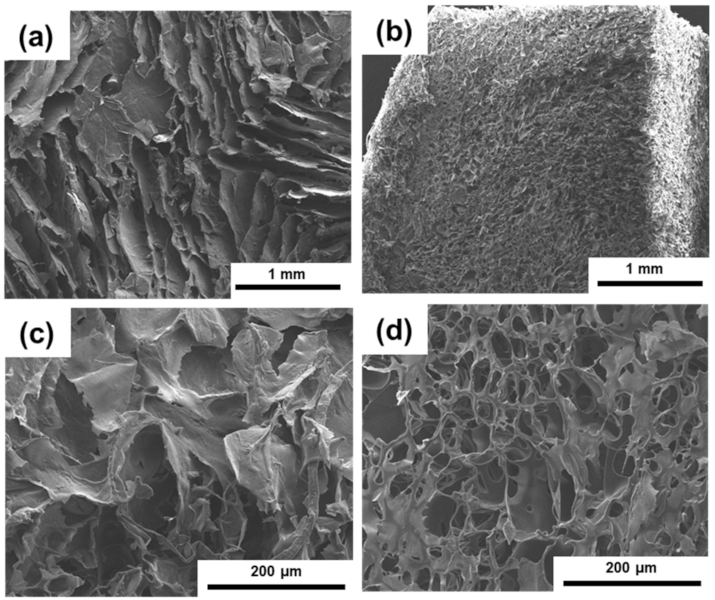
3.3. Internal Morphology of the PI/CNC Nanocomposite Aerogels
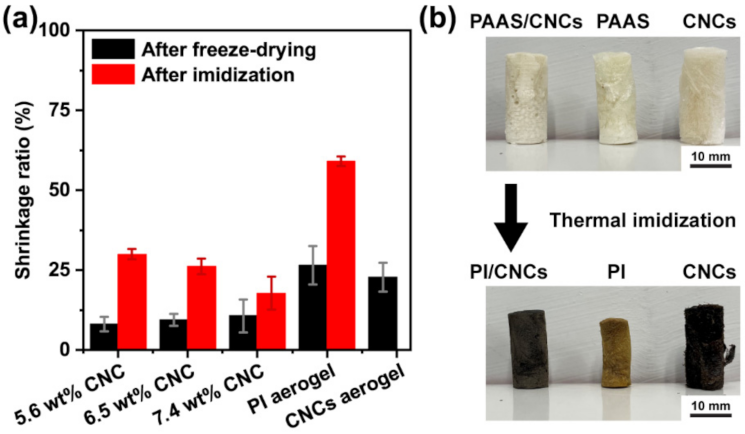
3.4. Performance of the PI/CNC Composite Aerogels
4. Conclusions
- High-resolution printing by DIW can be achieved by using the ink containing only 6.5 wt% CNCs.
- Chemical analysis showed that a significant amount of CNCs remained in the composite after thermal imidization, and CNCs displayed improved thermal stability.
- CNCs significantly reduce the dimensional shrinkage of the aerogels during preparation and enable the 3D printing of aerogels with high shape fidelity.
- The PI/CNC composite aerogels demonstrated increased mechanical stiffness and sufficient thermal insulating capability.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shi, B.; Ma, B.; Wang, C.; He, H.; Qu, L.; Xu, B.; Chen, Y. Fabrication and applications of polyimide nano-aerogels. Compos. Part A Appl. Sci. Manuf. 2021, 143, 106283. [Google Scholar] [CrossRef]
- Feng, J.; Su, B.-L.; Xia, H.; Zhao, S.; Gao, C.; Wang, L.; Ogbeide, O.; Feng, J.; Hasan, T. Printed aerogels: Chemistry, processing, and applications. Chem. Soc. Rev. 2021, 50, 3842–3888. [Google Scholar] [CrossRef] [PubMed]
- Liaw, D.-J.; Wang, K.-L.; Huang, Y.-C.; Lee, K.-R.; Lai, J.-Y.; Ha, C.-S. Advanced polyimide materials: Syntheses, physical properties and applications. Prog. Polym. Sci. 2012, 37, 907–974. [Google Scholar] [CrossRef]
- Ni, H.-J.; Liu, J.-G.; Wang, Z.-H.; Yang, S.-Y. A review on colorless and optically transparent polyimide films: Chemistry, process and engineering applications. J. Ind. Eng. Chem. 2015, 28, 16–27. [Google Scholar] [CrossRef]
- Gu, W.; Wang, G.; Zhou, M.; Zhang, T.; Ji, G. Polyimide-based foams: Fabrication and multifunctional applications. ACS Appl. Mater. Interfaces 2020, 12, 48246–48258. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, X.; Xue, T.; Yang, F.; Fan, W.; Liu, T. Bidirectional anisotropic polyimide/bacterial cellulose aerogels by freeze-drying for super-thermal insulation. Chem. Eng. J. 2020, 385, 123963. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, Y.; Shao, Z.; Gao, W.; Fan, W.; Liu, T.; Bai, H. Multifunctional polyimide aerogel textile inspired by polar bear hair for thermoregulation in extreme environments. Chem. Eng. J. 2020, 390, 124623. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Zhou, Z.-H.; Zhou, C.-G.; Sun, W.-J.; Gao, J.-F.; Dai, K.; Yan, D.-X.; Li, Z.-M. Lightweight and robust carbon nanotube/polyimide foam for efficient and heat-resistant electromagnetic interference shielding and microwave absorption. ACS Appl. Mater. Interfaces 2020, 12, 8704–8712. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhang, X.; Qin, Y.; Dong, P.; Yao, W.; Matz, J.; Ajayan, P.M.; Shen, J.; Ye, M. Super-elasticity at 4 K of covalently crosslinked polyimide aerogels with negative poisson’s ratio. Nat. Commun. 2021, 12, 4092. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, W.; Wang, Z.; Wang, J.; Huang, T.; Dong, J.; Zhang, Q. Flexible PEDOT:PSS/polyimide aerogels with linearly responsive and stable properties for piezoresistive sensor applications. Chem. Eng. J. 2020, 395, 125115. [Google Scholar] [CrossRef]
- Zheng, Q.; Fang, L.; Guo, H.; Yang, K.; Cai, Z.; Meador, M.A.B.; Gong, S. Highly porous polymer aerogel film-based triboelectric nanogenerators. Adv. Funct. Mater. 2018, 28, 1706365. [Google Scholar] [CrossRef]
- Ligon, S.C.; Liska, R.; Stampfl, J.; Gurr, M.; Mülhaupt, R. Polymers for 3D printing and customized additive manufacturing. Chem. Rev. 2017, 117, 10212–10290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Truby, R.L.; Lewis, J.A. Printing soft matter in three dimensions. Nature 2016, 540, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.A. Direct ink writing of 3D functional materials. Adv. Funct. Mater. 2006, 16, 2193–2204. [Google Scholar] [CrossRef]
- Peng, E.; Zhang, D.; Ding, J. Ceramic robocasting: Recent achievements, potential, and future developments. Adv. Mater. 2018, 30, 1802404. [Google Scholar] [CrossRef] [PubMed]
- Hegde, M.; Meenakshisundaram, V.; Chartrain, N.; Sekhar, S.; Tafti, D.; Williams, C.B.; Long, T.E. 3D printing all-aromatic polyimides using mask-projection stereolithography: Processing the nonprocessable. Adv. Mater. 2017, 29, 1701240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Y.; Xu, J.; Yan, C.; Chen, Y.; Zhang, X.; Jia, X.; Liu, Y.; Wang, X.; Zhou, F. Direct ink writing of high performance architectured polyimides with low dimensional shrinkage. Adv. Eng. Mater. 2019, 21, 1801314. [Google Scholar] [CrossRef]
- Guo, Y.; Ji, Z.; Zhang, Y.; Wang, X.; Zhou, F. Solvent-free and photocurable polyimide inks for 3D printing. J. Mater. Chem. A 2017, 5, 16307–16314. [Google Scholar] [CrossRef]
- Herzberger, J.; Meenakshisundaram, V.; Williams, C.B.; Long, T.E. 3D printing all-aromatic polyimides using stereolithographic 3D printing of polyamic acid salts. ACS Macro Lett. 2018, 7, 493–497. [Google Scholar] [CrossRef]
- Rau, D.A.; Herzberger, J.; Long, T.E.; Williams, C.B. Ultraviolet-assisted direct ink write to additively manufacture all-aromatic polyimides. ACS Appl. Mater. Interfaces 2018, 10, 34828–34833. [Google Scholar] [CrossRef]
- Wang, C.; Ma, S.; Li, D.; Zhao, J.; Zhou, H.; Wang, D.; Zhou, D.; Gan, T.; Wang, D.; Liu, C.; et al. 3D printing of lightweight polyimide honeycombs with the high specific strength and temperature resistance. ACS Appl. Mater. Interfaces 2021, 13, 15690–15700. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, J.; Sun, N.; Zhang, X.; Jiang, L. A novel self-healing poly(amic acid) ammonium salt hydrogel with temperature-responsivity and robust mechanical properties. J. Mater. Chem. A 2014, 2, 7666–7668. [Google Scholar] [CrossRef]
- Qin, S.; Jiang, Y.; Ji, Z.; Yang, C.; Guo, Y.; Zhang, X.; Qin, H.; Jia, X.; Wang, X. Three-dimensional printing of high-performance polyimide by direct ink writing of hydrogel precursor. J. Appl. Polym. Sci. 2021, 138, 50636. [Google Scholar] [CrossRef]
- Yang, C.; Jiang, P.; Qin, H.; Wang, X.; Wang, Q. 3D printing of porous polyimide for high-performance oil impregnated self-lubricating. Tribol. Int. 2021, 160, 107009. [Google Scholar] [CrossRef]
- Mariano, M.; El Kissi, N.; Dufresne, A. Cellulose nanocrystals and related nanocomposites: Review of some properties and challenges. J. Polym. Sci. Part B Polym. Phys. 2014, 52, 791–806. [Google Scholar] [CrossRef]
- Frka-Petesic, B.; Guidetti, G.; Kamita, G.; Vignolini, S. Controlling the photonic properties of cholesteric cellulose nanocrystal films with magnets. Adv. Mater. 2017, 29, 1701469. [Google Scholar] [CrossRef]
- Nguyen, B.N.; Cudjoe, E.; Douglas, A.; Scheiman, D.; McCorkle, L.; Meador, M.A.B.; Rowan, S.J. Polyimide cellulose nanocrystal composite aerogels. Macromolecules 2016, 49, 1692–1703. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.-G.; Kim, G.-H.; Ha, C.-S. Polyimide/amine-functionalized cellulose nanocrystal nanocomposite films. Mater. Today Commun. 2017, 13, 275–281. [Google Scholar] [CrossRef]
- Siqueira, G.; Kokkinis, D.; Libanori, R.; Hausmann, M.K.; Gladman, A.S.; Neels, A.; Tingaut, P.; Zimmermann, T.; Lewis, J.A.; Studart, A.R. Cellulose nanocrystal inks for 3D printing of textured cellular architectures. Adv. Funct. Mater. 2017, 27, 1604619. [Google Scholar] [CrossRef]
- Lai, C.-W.; Yu, S.-S. 3D printable strain sensors from deep eutectic solvents and cellulose nanocrystals. ACS Appl. Mater. Interfaces 2020, 12, 34235–34244. [Google Scholar] [CrossRef] [PubMed]
- Heath, L.; Thielemans, W. Cellulose nanowhisker aerogels. Green Chem. 2010, 12, 1448–1453. [Google Scholar] [CrossRef]
- Yang, J.; Lee, M.-H. A water-soluble polyimide precursor: Synthesis and characterization of poly(amic acid) salt. Macromol. Res. 2004, 12, 263–268. [Google Scholar] [CrossRef]
- Smay, J.E.; Cesarano, J.; Lewis, J.A. Colloidal inks for directed assembly of 3-D periodic structures. Langmuir 2002, 18, 5429–5437. [Google Scholar] [CrossRef]
- Hausmann, M.K.; Rühs, P.A.; Siqueira, G.; Läuger, J.; Libanori, R.; Zimmermann, T.; Studart, A.R. Dynamics of cellulose nanocrystal alignment during 3D printing. ACS Nano 2018, 12, 6926–6937. [Google Scholar] [CrossRef]
- Lai, P.-C.; Yu, S.-S. Cationic cellulose nanocrystals-based nanocomposite hydrogels: Achieving 3D printable capacitive sensors with high transparency and mechanical strength. Polymers 2021, 13, 688. [Google Scholar] [CrossRef]
- Oguzlu, H.; Dobyrden, I.; Liu, X.; Bhaduri, S.; Claesson, P.M.; Boluk, Y. Polymer induced gelation of aqueous suspensions of cellulose nanocrystals. Langmuir 2021, 37, 3015–3024. [Google Scholar] [CrossRef]
- Oguzlu, H.; Boluk, Y. Interactions between cellulose nanocrystals and anionic and neutral polymers in aqueous solutions. Cellulose 2017, 24, 131–146. [Google Scholar] [CrossRef]
- Oguzlu, H.; Danumah, C.; Boluk, Y. Colloidal behavior of aqueous cellulose nanocrystal suspensions. Curr. Opin. Colloid Interface Sci. 2017, 29, 46–56. [Google Scholar] [CrossRef]
- Laurati, M.; Egelhaaf, S.U.; Petekidis, G. Nonlinear rheology of colloidal gels with intermediate volume fraction. J. Rheol. 2011, 55, 673–706. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Liu, Z.; Gao, S.; You, Q.; Zou, L.; Chen, J.; Liu, J.; Liu, X. Polyimide film with low thermal expansion and high transparency by self-enhancement of polyimide/sic nanofibers net. RSC Adv. 2018, 8, 19034–19040. [Google Scholar] [CrossRef] [Green Version]
- Li, M.-C.; Mei, C.; Xu, X.; Lee, S.; Wu, Q. Cationic surface modification of cellulose nanocrystals: Toward tailoring dispersion and interface in carboxymethyl cellulose films. Polymer 2016, 107, 200–210. [Google Scholar] [CrossRef] [Green Version]
- Rahnamoun, A.; Engelhart, D.P.; Humagain, S.; Koerner, H.; Plis, E.; Kennedy, W.J.; Cooper, R.; Greenbaum, S.G.; Hoffmann, R.; van Duin, A.C.T. Chemical dynamics characteristics of kapton polyimide damaged by electron beam irradiation. Polymer 2019, 176, 135–145. [Google Scholar] [CrossRef]
- Waters, J.F.; Likavec, W.R.; Ritchey, W.M. 13C CP–MAS NMR study of absorbed water in polyimide film. J. Appl. Polym. Sci. 1994, 53, 59–70. [Google Scholar] [CrossRef]
- Qiu, W.; Leisen, J.E.; Liu, Z.; Quan, W.; Koros, W.J. Key features of polyimide-derived carbon molecular sieves. Angew. Chem. Int. Ed. 2021, 60, 22322–22331. [Google Scholar] [CrossRef]
- Cui, Y.; Jin, R.; Zhang, Y.; Yu, M.; Zhou, Y.; Wang, L.-Q. Cellulose nanocrystal-enhanced thermal-sensitive hydrogels of block copolymers for 3D bioprinting. Int. J. Bioprinting 2021, 7, 397. [Google Scholar] [CrossRef]
- Chen, W.; Chen, W.; Zhang, B.; Yang, S.; Liu, C.-Y. Thermal imidization process of polyimide film: Interplay between solvent evaporation and imidization. Polymer 2017, 109, 205–215. [Google Scholar] [CrossRef]
- Li, V.C.-F.; Dunn, C.K.; Zhang, Z.; Deng, Y.; Qi, H.J. Direct ink write (DIW) 3D printed cellulose nanocrystal aerogel structures. Sci. Rep. 2017, 7, 8018. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Dai, T.; Yuan, S.; Zou, H.; Liu, P. Electromagnetic interference shielding performance of anisotropic polyimide/graphene composite aerogels. ACS Appl. Mater. Interfaces 2020, 12, 30990–31001. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Meador, M.A.B.; McCorkle, L.; Quade, D.J.; Guo, J.; Hamilton, B.; Cakmak, M. Tailoring properties of cross-linked polyimide aerogels for better moisture resistance, flexibility, and strength. ACS Appl. Mater. Interfaces 2012, 4, 5422–5429. [Google Scholar] [CrossRef]
- Feng, J.; Wang, X.; Jiang, Y.; Du, D.; Feng, J. Study on thermal conductivities of aromatic polyimide aerogels. ACS Appl. Mater. Interfaces 2016, 8, 12992–12996. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.J.Y.; Lee, C.P.; Hashimoto, M. Preheating of gelatin improves its printability with transglutaminase in direct ink writing 3D printing. Int. J. Bioprint. 2020, 6, 296. [Google Scholar] [CrossRef] [PubMed]
- Goh, G.L.; Agarwala, S.; Yeong, W.Y. Aerosol-jet-printed preferentially aligned carbon nanotube twin-lines for printed electronics. ACS Appl. Mater. Interfaces 2019, 11, 43719–43730. [Google Scholar] [CrossRef] [PubMed]
- Ben Azouz, K.; Ramires, E.C.; Van den Fonteyne, W.; El Kissi, N.; Dufresne, A. Simple method for the melt extrusion of a cellulose nanocrystal reinforced hydrophobic polymer. ACS Macro Lett. 2012, 1, 236–240. [Google Scholar] [CrossRef]
- Nagalakshmaiah, M.; El Kissi, N.; Dufresne, A. Ionic compatibilization of cellulose nanocrystals with quaternary ammonium salt and their melt extrusion with polypropylene. ACS Appl. Mater. Interfaces 2016, 8, 8755–8764. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, C.; Yu, S.-S. 3D Printing of Thermal Insulating Polyimide/Cellulose Nanocrystal Composite Aerogels with Low Dimensional Shrinkage. Polymers 2021, 13, 3614. https://doi.org/10.3390/polym13213614
Feng C, Yu S-S. 3D Printing of Thermal Insulating Polyimide/Cellulose Nanocrystal Composite Aerogels with Low Dimensional Shrinkage. Polymers. 2021; 13(21):3614. https://doi.org/10.3390/polym13213614
Chicago/Turabian StyleFeng, Chiao, and Sheng-Sheng Yu. 2021. "3D Printing of Thermal Insulating Polyimide/Cellulose Nanocrystal Composite Aerogels with Low Dimensional Shrinkage" Polymers 13, no. 21: 3614. https://doi.org/10.3390/polym13213614
APA StyleFeng, C., & Yu, S.-S. (2021). 3D Printing of Thermal Insulating Polyimide/Cellulose Nanocrystal Composite Aerogels with Low Dimensional Shrinkage. Polymers, 13(21), 3614. https://doi.org/10.3390/polym13213614






